Abstract
Burkholderia pseudomallei, causative agent of the often fatal disease melioidosis, dwells in tropical soils and has been found in freshwater bodies. To investigate whether rivers are potential habitats or carriers for B. pseudomallei and to assess its geographical distribution in Laos, we studied 23 rivers including the Mekong, applying culture-based detection methods and PCR to water filters and streambed sediments. B. pseudomallei was present in 9% of the rivers in the dry season and in 57% in the rainy season. We found the pathogen exclusively in Southern and Central Laos, and mainly in turbid river water, while sediments were positive in 35% of the B. pseudomallei-positive sites. Our results provide evidence for a heterogeneous temporal and spatial distribution of B. pseudomallei in rivers in Laos with a clear north-south contrast. The seasonal dynamics and predominant occurrence of B. pseudomallei in particle-rich water suggest that this pathogen is washed out with eroded soil during periods of heavy rainfall and transported by rivers, while river sediments do not seem to be permanent habitats for B. pseudomallei. Rivers may thus be useful to assess the distribution and aquatic dispersal of B. pseudomallei and other environmental pathogens in their catchment area and beyond.
Introduction
Knowledge of the distribution and dispersal of pathogens in natural environments is crucial to understand the epidemiology of the diseases they cause, improve risk models and develop effective health management strategies1,2, particularly in countries with limited economic resources. Dispersal of microbes, including pathogenic species, is facilitated by transport in water and air, on particles or passive carriers (e.g. migrating birds) or in vectors and hosts3. While most research on the fate and transport of water-borne pathogens focuses on enteric bacteria4, studies addressing dispersal mechanisms of pathogens with environmental reservoirs, for example Burkholderia pseudomallei, are rare. The soil-dwelling bacterium B. pseudomallei is an emerging human pathogen and causative agent of melioidosis, an underdiagnosed infectious disease with an estimated global incidence of 165,000 cases per year of whom approximately 50% die5. Mainly known in Southeast Asia and Northern Australia, a recent environmental suitability model predicted a widespread occurrence of B. pseudomallei in tropical soils throughout the world. Consequently, melioidosis is probably endemic in many countries where it has never been reported6. In soil, B. pseudomallei is spatially heterogeneously distributed across different scales, ranging from geographical regions to localised patches of a rice field7, which makes its detection challenging. In addition to soil, B. pseudomallei has been found in a range of freshwater sources, including drinking water in Thailand8 and Australia9–11 and a river in Lao People’s Democratic Republic (Laos)12,13, where the distribution of melioidosis remains uncertain. B. pseudomallei in freshwater bodies are potential sources of infection9, particularly if they live permanently in these habitats. Moreover, rivers may transport B. pseudomallei from sources in the watershed and thereby indicate the presence of B. pseudomallei in the catchment and act as carriers for its environmental dispersal.
The aims of this pilot study were to investigate (i) the geographical distribution of B. pseudomallei in Laos and (ii) whether rivers are potential reservoirs and/or carriers for B. pseudomallei. For this purpose, we used two independent methods, conventional culture and PCR after enrichment, to detect B. pseudomallei in river water and, for the first time, in streambed sediments, and assessed the distribution data in an environmental context to explain spatiotemporal variations.
Results
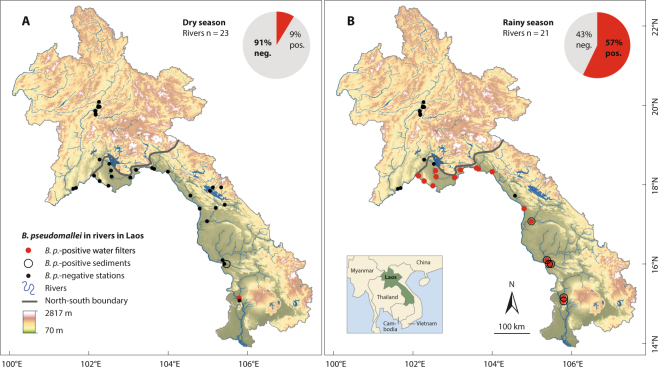
We investigated 23 rivers (36 sampling sites, hereafter stations) in Laos between 15 °N and 20 °N, including the Mekong (Table 1). B. pseudomallei was present in 9% (2/23) of the rivers (2/36 stations) in the dry season. In contrast, we found the pathogen in 57% (12/21) of the rivers (17/31 stations) in the rainy season, detected on at least one water filter (pre- or main filter) by at least one detection method (conventional culture or PCR after enrichment; Table 1). Apart from one filter-negative, sediment-positive station in the dry season, we only found B. pseudomallei in the sediment when it was present in the water, i.e. in 35% (6/17) of the B. pseudomallei-positive stations in the rainy season. All B. pseudomallei-positive stations were situated in the centre and south of Laos, and B. pseudomallei-positive sediments were only detectable in the southern-most rivers (Fig. 1). The north-south trend was also observable in B. pseudomallei-positive rivers with sampling sites in both regions, i.e. the Mekong (six sites) and Nam Ngum (four sites), where the northernmost 1–2 stations were negative and the 3–4 southernmost stations positive. The seasonal and regional contrast regarding the presence of B. pseudomallei was statistically significant when comparing all stations or all rivers, as well as stations or rivers in the rainy season, and stations or rivers in the south (Fisher’s exact test, p ≤ 0.001).
Table 1.
Sampled rivers and stations in Laos.
| River | Tributary of | Stations | Region | Geographical coordinates | B. pseudomallei | ||
|---|---|---|---|---|---|---|---|
| Latitude | Longitude | D | R | ||||
| Mekong | S. China Sea | 6 | N | 19.95601 | 102.24113 | − | − |
| N* | 17.89870 | 101.62397 | − | − | |||
| S* | 17.97276 | 102.50410 | − | + | |||
| S* | 17.39714 | 104.79999 | − | + | |||
| S* | 16.00503 | 105.42449 | − | + | |||
| S | 15.10721 | 105.79878 | − | + | |||
| Nam Ou | Mekong | 1 | N | 20.08642 | 102.26406 | − | − |
| Nam Suang | Nam Pa | 1 | N | 19.97931 | 102.24728 | − | − |
| Nam Pa | Mekong | 1 | N | 19.96049 | 102.28289 | − | − |
| Nam Khan | Mekong | 1 | N | 19.78600 | 102.18311 | − | − |
| Houay Khan | Nam Khan | 1 | N | 19.75995 | 102.18103 | − | − |
| Houay Pano | Nam Khan | 3 | N | 19.86034 | 102.17262 | − | − |
| N | 19.85903 | 102.17061 | − | − | |||
| N | 19.85263 | 102.16901 | − | − | |||
| Nam Lik | Nam Ngum | 1 | N* | 18.63280 | 102.28104 | − | − |
| Nam Mi | Mekong | 1 | N* | 17.91917 | 101.68856 | − | − |
| Nam Ngum | Mekong | 4 | S* | 18.52502 | 102.52631 | − | − |
| S* | 18.35581 | 102.57204 | − | + | |||
| S* | 18.20269 | 102.58588 | − | + | |||
| S* | 18.17879 | 103.05593 | − | + | |||
| Nam Thon | Mekong | 1 | S* | 18.09152 | 102.28159 | − | + |
| Nam Sang | Mekong | 1 | S* | 18.22284 | 102.14222 | − | + |
| Nam Mang | Mekong | 1 | S* | 18.37019 | 103.19846 | − | + |
| Nam Gniep | Mekong | 1 | S* | 18.41756 | 103.60217 | − | + |
| Nam Xan | Mekong | 1 | S* | 18.39523 | 103.65408 | − | + |
| Nam Kading | Mekong | 1 | S* | 18.32517 | 103.99924 | − | + |
| Nam Hinboun | Mekong | 1 | S* | 17.72699 | 104.56798 | − | − |
| Nam Xot | Nam Theun | 1(0) | S* | 17.93148 | 105.13257 | − | nd |
| Nam Theun | Mekong or Xe Bangfai† | 1(0) | S* | 17.84229 | 105.05841 | − | nd |
| Xe Bangfai | Mekong | 3(1) | S* | 17.49436 | 105.42959 | − | nd |
| S* | 17.41563 | 105.20320 | − | nd | |||
| S* | 17.07782 | 104.98496 | − | + | |||
| Xe Banghieng | Mekong | 1 | S* | 16.09804 | 105.37625 | − | + |
| Xe Bangnouan | Mekong | 1 | S | 16.00290 | 105.47937 | + | + |
| Xe Don | Mekong | 1 | S | 15.12390 | 105.80748 | + | + |
Stations: number of sampled stations in the dry season (rainy season in brackets if different). Region: geographical classification based on38,39; stations marked * belong to the centre of Laos (reference: Department of Tourism Marketing, Ministry of Information, Laos). B. pseudomallei: presence of B. pseudomallei by at least one detection method in river water and/or sediment. N = north, S = south, D = dry season, R = rainy season, nd = no data. †Flow direction depends on water level regulations of the Nam Theun dam lake. Geographical coordinates in degrees (WGS 1984) (dry season).
Figure 1.
B. pseudomallei (B.p.)-positive and -negative stations and rivers in the dry season (A) and rainy season. (B) North-south boundary based on38,39, map background based on elevation data (U.S. Geological Survey, https://earthexplorer.usgs.gov; Central Intelligence Agency, https://www.cia.gov/library/publications/the-world-factbook/index.html) and rivers/lakes/country shapefiles provided by the Centre for Development and Environment (CDE), CDE Lao Country Office, Laos. Geographic coordination system: WGS 1984, latitude and longitude in degrees; altitude of highest and lowest point in meters above mean sea level.
Almost as many B. pseudomallei-positive stations were identified by conventional culture as by molecular techniques (Table 2). However, PCR revealed a higher number of positive samples per station than culture, and the only two B. pseudomallei-positive stations in the dry season were detected by PCR. All culture-positive sediments resulted from direct incubation of the highest volume of sediment fluid (500 µL) on Ashdown’s agar. B. pseudomallei-positive main filters (23/38) outnumbered pre-filters (15/38).
Table 2.
Number of B. pseudomallei positive units comparing different detection methods and sample types (pre-filters, main filters, sediment).
| B. pseudomallei positive units | Direct culture | Post-enrichment PCR | Both methods positive | Total |
|---|---|---|---|---|
| Stations (only by respective method) | 15 (3) | 16 (4) | 12 | 19 |
| All samples | 16 | 31 | 10 | 47 |
| Pre-filters | 3 | 12 | 1 | 15 |
| Main filters | 10 | 13 | 7 | 23 |
| Stations with positive pre- and main filter | 0 | 10 | 0 | 10 |
| Sediment samples | 3 | 6 | 2 | 9 |
| Filter-positive, sediment-negative stations | 11 | 10 | 7 | 11 |
| Sediment-positive, filter-negative stations | 2 | 1 | 0 | 3 |
| Stations where all samples were positive | 0 | 4 | 0 | 4 |
The characteristics of physico-chemical water parameters measured on-site (turbidity, temperature, acidity, electrical conductivity as a proxy for salinity, dissolved oxygen, redox potential, altitude of the station) are shown in Table 3. Water temperature correlated moderately, and salinity, altitude, turbidity and pH weakly with the presence of B. pseudomallei on water filters (undirectional correlation). However, all physico-chemical parameters were functions of season and/or of region and correlated with at least one other parameter (Table 3). For example, water temperature was higher in the rainy season and in the south, and correlated negatively with altitude, while salinity showed the opposite pattern. As a result, none of the parameters was a significant independent predictor of the presence of B. pseudomallei in multivariate logistic regression models restricted to conditions under which B. pseudomallei was most common (in the water of southern river stations in the rainy season).
Table 3.
Characteristics of physico-chemical water parameters and altitude.
| Physico-chemical parameters | Median | Min – Max | Seasonal differences, mean (SD) | Regional differences in rainy season, mean (SD) | Corr. ratio | Bravais-Pearson correlations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry season | Rainy season | North | South | B. p. | Tur | Temp | pH | EC | DO | ORP | |||
| Altitude (m.a.s.l.) | 175 | 74–513 | 322 (106) | 140 (38)* | 0.15* | 0.01 | −0.39* | 0.02 | 0.52* | −0.47* | 0.33 | ||
| Turbidity (NTU) | 18 | 2–730 | 12 (11) | 227 (218)* | 257 (242) | 206 (206) | 0.13* | 0.35* | −0.21 | −0.12 | −0.07 | −0.10 | |
| Temperature (°C) | 26.5 | 19.5–30.0 | 25 (1.9) | 27.6 (1.5)* | 26.8 (1.8) | 28.2 (1.1)* | 0.29* | −0.17 | −0.10 | −0.04 | −0.23 | ||
| Acidity (pH) | 7.6 | 6.5–9.0 | 7.8 (0.5) | 7.3 (0.4)* | 7.4 (0.4) | 7.3 (0.4) | 0.09* | 0.40* | 0.34* | 0.14 | |||
| Electrical conductivity (µS/cm) | 152 | 10–623 | 194 (139) | 152 (92)* | 231 (80) | 101 (58)* | 0.15* | −0.08 | 0.43* | ||||
| Dissolved oxygen (%) | 85 | 25–138 | 92 (18) | 73 (16)* | 72 (17) | 73 (16) | 0.06 | −0.05 | |||||
| Redox potential (mV) | 86 | −39–275 | 118 (79) | 85 (28) | 75 (11) | 92 (34) | 0.00 | ||||||
Abbreviations: m.a.s.l. = meters above mean sea level, NTU = nephelometric turbidity units, mean = arithmetic mean, SD = standard deviation, Corr. ratio = correlation ratio, B. p. = B. pseudomallei, Tur = turbidity, Temp = temperature, pH = acidity, EC = electrical conductivity (proxy for salinity), DO = dissolved oxygen, ORP = redox potential. N = 67; exceptions: turbidity (n = 66), median, minimum and maximum of altitude in the rainy season (n = 31). The Bravais-Pearson correlation coefficient (r) is given for directional correlations between physico-chemical parameters, the correlation ratio (η2) for undirectional correlations between the presence of B. pseudomallei and physico-chemical parameters, range from 0 (no correlation) to 1 (perfect correlation). Statistical tests: seasonal comparison: paired t-test (n = 31 pairs, for turbidity n = 30 pairs), regional comparison: t-test, correlation ratio: t-test, Bravais-Pearson correlations: Pearson test; *statistically significant correlations or differences between groups, p < 0.01.
Discussion
We detected B. pseudomallei in more than half (57%) of the investigated rivers, which indicates a widespread distribution of the pathogen in Laos. To characterise rivers as potential reservoirs or carriers for B. pseudomallei, we analysed the seasonal dynamics of its occurrence in both river water and superficial near-riparian sediments. If rivers were reservoirs, i.e. permanent habitats for B. pseudomallei, we would expect to find the pathogen primarily and perennially in the uppermost streambed sediments which harbor the majority of bacterial biomass in rivers14, and resuspended in the water column under conditions of increased turbulence, e.g. during floods. However, in accordance with the highest seasonal incidence of melioidosis15, we detected B. pseudomallei predominantly in the rainy season while B. pseudomallei-positive sediments were rare and usually linked to B. pseudomallei-positive water samples. These findings suggest that rivers are potential carriers for B. pseudomallei, and streambed sediments do not seem to be permanent habitats for this bacterium although the occurrence of B. pseudomallei in deeper midstream sediments is unknown. Nevertheless, the role of rivers and other freshwater bodies16 in the seasonal transmission of melioidosis might be underestimated, despite the fact that melioidosis cases have rarely been associated with exposure to river water17.
The most likely source of B. pseudomallei in rivers are its known reservoir, tropical soils6. Being present down to at least 90 cm depth18, the pathogen is likely to be mobilised with eroded soil particles in surface and subsurface runoff and ultimately channeled into rivers. As suggested by B. pseudomallei-positive filters of different pore sizes, the pathogen may be transported free-floating or attached to suspended particles of various sizes. Under conditions of high discharge, B. pseudomallei may be washed onto the soil of flood plains or infiltrate alluvial banks and aquifers downriver19 and be washed away again, especially during periods of heavy rainfall. In the Mekong basin, 90% of the annual precipitation (~1000 to 2800 mm) occurs during the southwest monsoon20,21, when B. pseudomallei was most common. Rain and, consequently, runoff are the main erosional forces of climatic origin in humid tropical regions, and intensive rainfall has been associated with increased erosion and suspended sediment load in the Mekong area22,23. Accordingly, we detected B. pseudomallei predominantly in particle-rich water, as observed in previous studies10,12,13.
However, B. pseudomallei was absent in the turbid rivers of the Northern Highlands, where sloping lands are particularly susceptible to erosion due to extensive land-use changes23,24. We can only speculate about the reasons why we detected the pathogen exclusively in the Mekong plain, although samples from melioidosis patients have been referred to the Mahosot Hospital Microbiology Laboratories from almost all Lao provinces (unpublished observations). Methodological considerations include the definition of the north-south boundary, which was based on limited sources, but classifying the southern-most northern stations as southern stations did not change the statistical significance of the north-south contrast regarding the presence of B. pseudomallei. Bias caused by non-random sampling (for reasons of accessibility) and bacterial loads below the detection limits of our methods cannot entirely be excluded. However, we applied two independent detection methods including post-enrichment PCR, which previously proved to be the most sensitive method for the detection of B. pseudomallei in environmental samples25. The absence or low numbers of B. pseudomallei may be a consequence of contrasting climate, geological substrates, soil types, and land-use in the Northern Highlands compared to the Mekong plains in southern Laos. The higher proportion of irrigated rice cultivation (paddy rice) and industrial agricultural plantations in the Mekong plain in contrast to slash-and-burn cultivation in the north24, for instance, as well as regionally distinctive parameters such as lower temperature or higher salinity values of northern river water (own data and26), might be aspects of a non-permissive environment for B. pseudomallei. However, direct conclusions cannot be drawn based on single water samples from rivers with large catchment areas, as B. pseudomallei might originate from various sources upriver, having been associated with a broad range of soil types and land-covers12,27–30. For this reason, analyses of relationships between B. pseudomallei in rivers and environmental factors in a catchment area are considered to be most conclusive at the sub-catchment or meso-scale (10–100 km2)12,31, and remain to be investigated in Laos and elsewhere.
We provide evidence that rivers are potential carriers for B. pseudomallei, as has been shown for other soil organisms32, but likely not permanent reservoirs for this pathogen. Rivers facilitate the dispersal of B. pseudomallei in the environment, possibly over long distances and to previously non-endemic areas. Thus, rivers are potential sentinels to explore the presence of B. pseudomallei in catchment areas, particularly during periods of intensive erosion and high discharge. Moreover, rivers may be useful to track potential sources and monitor the spatiotemporal dynamics of aquatic dispersal of B. pseudomallei and other environmental pathogens in a watershed and beyond.
Methods
Sample collection and processing
We investigated 36 stations at 23 perennial rivers, including the Mekong, in Laos between 15°N and 20°N in the dry (March) and rainy (July) seasons in 2016. The choice of rivers and sites was based on a broad geographical coverage of Laos and a range of differently sized direct or indirect tributaries to the Mekong. Several rivers were sampled at multiple sites along their course (Table 1). We collected unreplicated surface water samples from the riverside (near-riparian zone) using 1.5 L PET drinking water bottles (triple-rinsed with water from the sampling site), and from a mixed composite sample across the river at two southern Mekong stations. Wherever feasible, we collected bulk samples from the top 10 cm of near-riparian streambed sediment using a 102 cm3 hand-held steel cylinder, and kept them in sterile, ziplocked plastic bags. On-site physico-chemical measurements included altitude and geographical coordinates using a GPS device (Garmin Oregon 650t), water turbidity using a nephelometric turbidity meter (Eutech TN100), and water temperature, acidity (pH), electrical conductivity (a proxy for salinity), dissolved oxygen, and redox potential using a portable multi-probe (YSI-556). All samples were transported in a cool box with ice packs. One to four days post-sampling, we manually homogenised the sediment samples and conducted vacuum filtration at the Mahosot Hospital Microbiology Laboratories with 500 mL (dry season) and 250 mL (rainy season) of water, using an electrical pump, 1-L glass flasks, a stainless-steel funnel (Whatman) and two membrane filters applied in succession: a pre-filter (5.0 µm pore size) and a main filter (0.2 µm pore size) (cellulose acetate, 47 mm diameter, Sartorius). The equipment was cleaned with 70% ethanol and sterile water between samples.
Microbiological methods
To detect B. pseudomallei on water filters and in sediment, we applied two independent methods: conventional culture techniques and PCR after an enrichment step, a sensitive approach for the detection of B. pseudomallei in low-abundance environments25. All microbiological analyses were conducted at the Mahosot Hospital Microbiology Laboratories in Class II Biosafety Cabinets.
Culture
Water filters (one pre-filter and one main filter per sampling site) were placed surface-up on Ashdown’s agar while sediment samples were prepared as described previously for soil33. In short, 100 g of homogenised sediment were mixed with 100 mL of sterile water in sterile, ziplocked plastic bags and left to settle at room temperature overnight before different volumes of supernatant (10, 100 and 500 µL) were spread on Ashdown’s agar. In addition, 1 mL supernatant was enriched with 9 mL of selective TBSS-C5034 at 40 °C for 48 h, and 10 µL of the enriched fluid incubated on Ashdown’s agar. All samples were incubated at 40 °C in air for up to 4 days with daily inspection (median 3 days, range 2–4 days). Suspect colonies were tested by agglutination with a latex reagent specific for the 200-kDa exopolysaccharide of B. pseudomallei35 resistance to colistimethate and susceptibility to amoxicillin-clavulanic acid, and latex-positive isolates with these characteristics were confirmed by API 20NE (BioMérieux, Basingstoke, UK)36 and a specific PCR based on37 with the following modifications: 20 µL reaction mixture containing final concentrations of 0.5 µM primers LPW13372 and LPW13373, 2 mM MgCl2, 200 µM each dNTP, 1 U Platinum Taq (Invitrogen) and 1x Platinum PCR buffer. Thermocycler conditions were 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 30 seconds, 60 °C for 45 seconds and 72 °C for 60 seconds, and a final extension of 72 °C for 10 minutes.
Pre-enrichment and DNA extraction
Pre-enrichment and DNA extraction were conducted as described previously25 with some modifications: Entire pre- and main filters and 20 g of homogenised sediment were immersed separately in 20 mL of modified Ashdown’s broth, and, after shaking the sediment samples at 12 × g for 2 h, vortexed and incubated at 37 °C in air for 42 h. The enriched samples were kept at −20 °C, defrosted and vortexed shortly before DNA extraction. After settling for 20 min, the liquid phase of the enriched sediments was centrifuged at 700 × g for 2 min and mixed with 150 µL of 3.5 mg/L aurintricarboxylic acid. Then, all enriched samples were centrifuged at 3220 × g for 45 min and DNA extracted from the sedimentation using the MoBio PowerSoil DNA isolation kit according to the manufacturer’s instructions with an additional cell lysis step (incubation with proteinase K at 55 °C for 30 min)25.
PCR
We applied a specific real-time PCR assay targeting a 115-base-pair region in the open-reading-frame 2 of the type III secretion system gene cluster (TTS1) of B. pseudomallei as described in25 with 500 nM primers BpTT4176F and BpTT4290R, 250 nM probe BpTT4208P (Biosearch Technologies) and 1 U Platinum Taq (Invitrogen), using a Rotor-Gene 6000 system (Qiagen) with 45 amplification cycles. Two positive controls (103 and 104 genome equivalents) and negative controls were included in every PCR run and showed the expected results. To control for PCR inhibition, 105 copies of Orientia tsutsugamushi 47-kDa plasmid was amplified with O. tsutsugamushi specific primers and probe25. Inhibition was assumed to be absent if the spiked DNA amplified within ±2 Ct values from the positive inhibition controls which was the case for all samples (occasionally after dilution).
Mapping and statistics
Maps were created with ArcGIS 10.3 and Adobe Illustrator CS6 using GPS coordinates of the sampling sites, elevation data (U.S. Geological Survey, https://earthexplorer.usgs.gov; Central Intelligence Agency, https://www.cia.gov/library/publications/the-world-factbook/index.html) and rivers/lakes/country shapefiles provided by the Centre for Development and Environment (CDE), CDE Lao Country Office. The geographical categories north (Northern Highlands) and south (Mekong plain and Annamite mountains, corresponding to the political centre and south) were based on a physio-geographical classification38, a geological map39 and topographic features. Statistical analyses were computed with Stata 14 and R 3.4.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We are grateful to the Lao Department of Agricultural Land Management and Dr. B. Bounxouei for facilitating this study at the Mahosot Hospital and in the field, to Dr. M. Vongsouvath and the staff of the Mahosot Hospital Microbiology Laboratory, Dr. S. Dittrich, A. Rachlin, Dr. P. Nawtaisong, A. Chanthongthip, Dr. E. Rochelle-Newall, Prof M. Lehmann, Dr H. Niemann, Dr. T. Kuhn, J. Kobler-Waldis, and R. Strunk for their help with laboratory work, technical inputs or logistical support, to V. Roth and the Centre for Development and Environment for providing map shapefiles, and to the Nam Theun 2 Power Company (NTPC) for providing logistical support and access to their site. This study was funded by the US Defence Threat Reduction Agency Cooperative Biological Engagement Programme (contract HDTRA-16-C-0017) (main funding), the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit funded by the Wellcome Trust of Great Britain (Grant number 089275/H/09/Z), the French National Research Agency (TecItEasy project, ANR-13-AGRO-0007), and the Institut de Recherche pour le Développement.
Author Contributions
R.E.Z., D.A.B.D., O.R., A.P., J.Z. and P.N.N. conceived and designed the study, R.E.Z., O.R., A.P. and S.R. undertook the fieldwork, R.E.Z., V.D., M.T.R. and D.A.B.D. conducted and supervised laboratory analyses, R.E.Z. and Y.A. conducted statistical analyses, R.E.Z. wrote the manuscript, and all authors reviewed and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho KH, et al. Modeling fate and transport of fecally-derived microorganisms at the watershed scale: state of the science and future opportunities. Water Res. 2016;100:38–56. doi: 10.1016/j.watres.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 2.Millennium Ecosystem Assessment. Ecosystems and human well-being: synthesis. Island Press, Washington DC, USA (2005).
- 3.Martiny JBH, et al. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 2006;4:102–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- 4.Rochelle-Newall, E., Nguyen, T. M. H., Le, T. P. Q., Sengtaheuanghoung, O. & Ribolzi, O. A short review of fecal indicator bacteria in tropical aquatic ecosystems: knowledge gaps and future directions. Front. Microbiol. 6, 10.3389/fmicb.2015.00308 (2015). [DOI] [PMC free article] [PubMed]
- 5.Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Resp. Crit. Care. 2015;36:111–125. doi: 10.1055/s-0034-1398389. [DOI] [PubMed] [Google Scholar]
- 6.Limmathurotsakul D, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 7.Limmathurotsakul D, et al. Burkholderia pseudomallei is spatially distributed in soil in northeast Thailand. PLoS Neglect. Trop. D. 2010;4:e694. doi: 10.1371/journal.pntd.0000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limmathurotsakul, D. et al. Activities of daily living associated with acquisition of melioidosis in Northeast Thailand: a matched case-control study. PLoS Neglect. Trop. D. 710.1371/journal.pntd.0002072 (2013). [DOI] [PMC free article] [PubMed]
- 9.Baker A, et al. Groundwater seeps facilitate exposure to Burkholderia pseudomallei. Appl. Environ. Microb. 2011;77:7243–7246. doi: 10.1128/AEM.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper ADK, et al. Association of the melioidosis agent Burkholderia pseudomallei with water parameters in rural water supplies in Northern Australia. Appl. Environ. Microb. 2010;76:5305–5307. doi: 10.1128/AEM.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglis TJJ, et al. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol. Infect. 2004;132:813–820. doi: 10.1017/S0950268804002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribolzi O, et al. Land use and soil type determine the presence of the pathogen Burkholderia pseudomallei in tropical rivers. Environ. Sci. Pollut. R. 2016;23:7828–7839. doi: 10.1007/s11356-015-5943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vongphayloth K, et al. Burkholderia pseudomallei detection in surface water in Southern Laos using Moore’s swabs. Am. J. Trop. Med. Hyg. 2012;86:872–877. doi: 10.4269/ajtmh.2012.11-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer H, Pusch M. Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshwater Biol. 2001;46:1335–1348. doi: 10.1046/j.1365-2427.2001.00753.x. [DOI] [Google Scholar]
- 15.Suputtamongkol Y, et al. The epidemiology of melioidosis in Ubon Ratchatani, Northeast Thailand. Int. J. Epidemiol. 1994;23:1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 16.Chuah CJ, Tan EKH, Sermswan RW, Ziegler AD. Hydrological connectivity and Burkholderia pseudomallei prevalence in wetland environments: investigating rice-farming community’s risk of exposure to melioidosis in North-East Thailand. Environ. Monit. Assess. 2017;189:287. doi: 10.1007/s10661-017-5988-1. [DOI] [PubMed] [Google Scholar]
- 17.Warner JM, et al. The epidemiology of melioidosis in the Balimo region of Papua New Guinea. Epidemiol. Infect. 2008;136:965–971. doi: 10.1017/S0950268807009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manivanh L, et al. Burkholderia pseudomallei in a lowland rice paddy: seasonal changes and influence of soil depth and physico-chemical properties. Sci. Rep. 2017;7:3031. doi: 10.1038/s41598-017-02946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunke M, Gonser TOM. The ecological significance of exchange processes between rivers and groundwater. Freshwater Biol. 1997;37:1–33. doi: 10.1046/j.1365-2427.1997.00143.x. [DOI] [Google Scholar]
- 20.Chen CJ, Senarath SUS, Dima-West IM, Marcella MP. Evaluation and restructuring of gridded precipitation data over the Greater Mekong Subregion. Int. J. Climatol. 2017;37:180–196. doi: 10.1002/joc.4696. [DOI] [Google Scholar]
- 21.Kingston DG, Thompson JR, Kite G. Uncertainty in climate change projections of discharge for the Mekong River Basin. Hydrol. Earth Syst. Sc. 2011;15:1459–1471. doi: 10.5194/hess-15-1459-2011. [DOI] [Google Scholar]
- 22.Colin C, Siani G, Sicre MA, Liu Z. Impact of the East Asian monsoon rainfall changes on the erosion of the Mekong River basin over the past 25,000 yr. Mar. Geol. 2010;271:84–92. doi: 10.1016/j.margeo.2010.01.013. [DOI] [Google Scholar]
- 23.Valentin C, et al. Runoff and sediment losses from 27 upland catchments in Southeast Asia: Impact of rapid land use changes and conservation practices. Agr. Ecosyst. Environ. 2008;128:225–238. doi: 10.1016/j.agee.2008.06.004. [DOI] [Google Scholar]
- 24.Kityuttachai, K., Heng, S. & Sou, V. Land cover map of the Lower Mekong Basin. Technical paper no. 59. Mekong River Commission, Phnom Penh, Cambodia (2016).
- 25.Knappik M, et al. Evaluation of molecular methods to improve the detection of Burkholderia pseudomallei in soil and water samples from Laos. Appl. Environ. Microb. 2015;81:3722–3727. doi: 10.1128/AEM.04204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ly, K. & Larsen, H. 2014 Lower Mekong regional water quality monitoring report. Technical paper no. 60. Mekong River Commission, Vientiane, Lao PDR (2014).
- 27.Corkeron ML, Norton R, Nelson PN. Spatial analysis of melioidosis distribution in a suburban area. Epidemiol. Infect. 2010;138:1346–1352. doi: 10.1017/S0950268809991634. [DOI] [PubMed] [Google Scholar]
- 28.Kaestli, M. et al. Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in Northern Australia. PLoS Neglect. Trop. D. 310.1371/journal.pntd.0000364 (2009). [DOI] [PMC free article] [PubMed]
- 29.Palasatien S, Lertsirivorakul R, Royros P, Wongratanacheewin S, Sermswan RW. Soil physicochemical properties related to the presence of Burkholderia pseudomallei. T. Roy. Soc. Trop. Med. Hyg. 2008;102(Suppl 1):S5–9. doi: 10.1016/S0035-9203(08)70003-8. [DOI] [PubMed] [Google Scholar]
- 30.Wuthiekanun V, Smith MD, Dance DA, White NJ. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. T. Roy. Soc. Trop. Med. Hyg. 1995;89:41–43. doi: 10.1016/0035-9203(95)90651-7. [DOI] [PubMed] [Google Scholar]
- 31.Uhlenbrook S, Roser S, Tilch N. Hydrological process representation at the meso-scale: the potential of a distributed, conceptual catchment model. J. Hydrol. 2004;291:278–296. doi: 10.1016/j.jhydrol.2003.12.038. [DOI] [Google Scholar]
- 32.Deiner K, Fronhofer EA, Machler E, Walser JC, Altermatt F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat. Commun. 2016;7:1–9. doi: 10.1038/ncomms12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wuthiekanun V, et al. Detection of Burkholderia pseudomallei in soil within the Lao People’s Democratic Republic. J. Clin. Microbiol. 2005;43:923–924. doi: 10.1128/JCM.43.2.923-924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galimand M, Dodin A. World evaluation of melioidosis. B. Soc. Pathol. Exot. 1982;75:375–383. [PubMed] [Google Scholar]
- 35.Duval BD, et al. Evaluation of a latex agglutination assay for the identification of Burkholderia pseudomallei and Burkholderia mallei. Am. J. Trop. Med. Hyg. 2014;90:1043–1046. doi: 10.4269/ajtmh.14-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amornchai P, et al. Accuracy of Burkholderia pseudomallei identification using the API 20NE system and a latex agglutination test. J. Clin. Microbiol. 2007;45:3774–3776. doi: 10.1128/JCM.00935-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho C-C, et al. Novel pan-genomic analysis approach in target selection for multiplex PCR identification and detection of Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia cepacia complex species: a proof-of-concept study. J. Clin. Microbiol. 2011;49:814–821. doi: 10.1128/JCM.01702-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duckworth, J. W., Salter, R. E. & Khounboline, K. (Compilers). Wildlife in Lao PDR: 1999 status report. IUCN, Vientiane, Lao PDR (1999).
- 39.United Nations Economic and Social Commission for Asia and the Pacific. Geological map of Lao People’s Democratic Republic, 1:1500000. In: Atlas of mineral resources of the ESCAP region. United Nations (1990).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.