Abstract
Probiotic Lactobacillus casei Shirota (LcS) is a potential decontaminating agent of aflatoxin B1 (AFB1). However, few studies have investigated the influence of diet, especially a high protein (HP) diet, on the binding of AFB1 by probiotics. This research was conducted to determine the effect of HP diet on the ability of LcS to bind AFB1 and reduce aflatoxin M1 (AFM1) in AFB1-induced rats. Sprague Dawley rats were randomly divided into three groups: A (HP only), B (HP + 108 CFU LcS + 25 μg AFB1/kg BW), and C (HP + 25 μg AFB1/kg BW). Levels of AST and ALP were higher in all groups but other liver function's biomarkers were in the normal range, and the liver's histology showed no structural changes. The urea level of rats in group B (10.02 ± 0.73 mmol/l) was significantly lower (p < 0.05) than that of rats in group A (10.82 ± 0.26 mmol/l). The presence of carcinoma in the small intestine and colon was more obvious in group C than in group B. Moreover, rats in group B had significantly (p < 0.05) lower AFM1 concentration (0.39 ± 0.01 ng/ml) than rats in group C (5.22 ± 0.28 ng/ml). Through these findings, LcS supplementation with HP diet alleviated the adverse effects of AFB1 by preventing AFB1 absorption in the small intestine and reducing urinary AFM1.
1. Introduction
Aflatoxin is produced by Aspergillus flavus and Aspergillus parasiticus [1]. This food contaminant can be found commonly in agricultural and food commodities such as maize, grains, peanuts, cereal, and animal feeds [1]. Food legislation and food processing are becoming more advanced, yet they are still unable to prevent the occurrence of aflatoxin in food commodities. Due to high stability of aflatoxin, it has become a problem not only during harvesting but also at every stage of food production, starting from harvesting of raw material, storage, and processing until the food reaches the consumers [2]. Aflatoxin should be removed, as long-term consumption of aflatoxin-contaminated food can cause carcinogenic and toxicity effects on humans and animals [3].
One of the main adverse effects of dietary aflatoxin exposure is aflatoxicosis. Aflatoxicosis is a foodborne disease due to aflatoxin ingestion in the diet and it can be categorized into acute and chronic aflatoxicosis. Acute aflatoxicosis results in death, whereas chronic aflatoxicosis can cause immune suppression, cancer, and other “slow” pathohistological conditions [4]. The liver is the main target of aflatoxicosis. In fact, one of the metabolites of aflatoxin, aflatoxin B1 (AFB1), has been classified by the International Agency for Research on Cancer (IARC) as group 1 carcinogen and is linked to the development of the liver cancer [5].
The recent alternative approach to remove AFB1 from the body is through the consumption of probiotic bacteria, as some studies have shown that probiotic bacteria might be a potential adsorbent of aflatoxin in the gastrointestinal tract by reducing aflatoxin bioavailability [6, 7]. World Health Organization (WHO) defined probiotics as “live microorganisms” which are able to provide advantages to the host when consumed in an adequate amount [8]. It is evident that probiotic consumption improved gastrointestinal health and immune system [9]. As an adsorbent of aflatoxin, probiotic bacteria remove AFB1 through noncovalent binding of AFB1 molecule to the bacterial cell wall [10]. Besides, polysaccharides and peptidoglycan components as well as teichoic acids of bacterial cell wall are involved in the binding process of AFB1 by probiotics [7]. The authors indicated also that teichoic acids play a major role in the binding of AFB1 by Lactobacillus reuteri and Lactobacillus casei Shirota (LcS) [7].
A randomized, double-blind, cross-over, placebo-controlled study with two 4-week intervention phases was conducted [11] to investigate the effectiveness of probiotics in reducing circular production of aflatoxin biomarkers in a population exposed to aflatoxin. The authors found that probiotic intervention reduced AFB1-lysine adduct (AFB1-lys) and urinary aflatoxin M1 (AFM1) in certain subjects [11]. Interestingly, it was found that diets may be one of the confounding factors that can affect the ability of probiotic LcS to bind AFB1 in the gastrointestinal tract. In addition, high intake of macronutrients can influence the metabolism of aflatoxin and subsequently affect the circular production of AFB1 metabolites [11].
In a recent review article [12], the authors mentioned that a high protein (HP) diet can affect aflatoxicosis. For example, rats fed with HP diet and exposed to AFB1 had no focal hyperplasia and less ductular reaction of liver [13]. These symptoms are commonly observed in the early stages of hepatocellular carcinoma (HCC). As such, the findings [13] may explain the effect of diets on the metabolism of aflatoxin as reported by Mohd Redzwan et al. [11] and to some extent slow the progression of HCC associated with aflatoxin exposure. On the other hand, it is still unclear whether diet manipulation can have influence on the activity of probiotics in reducing AFB1 bioavailability. A study by Nikbakht Nasrabadi et al. [14] found that LcS supplementation in aflatoxin-induced rats reduced serum AFB1. Nevertheless, the authors [14] did not take into consideration the influence of diet in the study protocol. Furthermore, diet was found as a confounding factor that can affect LcS activity and aflatoxin metabolism [11]. Therefore, this research was conducted to elucidate the effect of HP diet on the ability of probiotic LcS to reduce urinary AFM1 and certain aflatoxicosis symptoms in AFB1-induced rats.
2. Materials and Methods
Yakult fermented milk drink was purchased from a local supermarket in Serdang, Selangor, Malaysia. AFB1 was acquired from Trilogy Analytical Laboratory, Inc. (Vossbrink Drive, Washington). MRS broth, MRS agar, and sodium chloride were purchased from Merck (Darmstadt, Germany). Glycerol solution was procured from Sigma-Aldrich (St. Louis, MO, USA). ELISA kit for the detection of AFM1 in urine was purchased from Helica Biosystems, Inc. (Santa Ana, CA, USA).
2.1. Culturing Probiotic Bacteria
The source of LcS was from the Yakult cultured milk drink (contained live LcS) [14]. One hundred microliters (100 μL) of Yakult was aseptically spread onto MRS agar and incubated for 48 hours aerobically at 37°C. Then, one colony of LcS was transferred into MRS broth and further cultured for another 24 hours aerobically at 37°C. The growth of LcS was recorded every two hours, and the corresponding CFU was monitored optically at 600 nm. One hundred microliters (100 μL) was withdrawn from the broth and spread on the MRS agar to acquire the CFU. LcS's ability to bind AFB1 also depends on bacterial concentration. For a significant removal of 50% of aflatoxin, the minimum concentration of Lactobacillus bacteria needed is 2 × 109 CFU/ml [14]. Based on the absorbance value (OD) and CFU that were recorded during the 24 h of incubation, the bacterial cell was incubated at least 19 h in order to reach concentration of 109 CFU. The bacterial cells were harvested via centrifugation at 2200g using Kubota 2810 centrifuge (Tokyo, Japan) for 15 minutes and the supernatant was discarded. The bacterial pellet was resuspended with 50% (v/v) glycerol [14]. Prior to the usage, the glycerol liquid was replaced with saline solution. The identity of LcS cultured from Yakult cultured milk drink was confirmed with the 16S rRNA sequencing service provided by First BASE Laboratories Sdn Bhd (Seri Kembangan, Selangor, Malaysia).
2.2. Animals
Twenty-four (n = 24) male Sprague Dawley rats (7-8 weeks old, 290–300 g) were purchased from the Animal Resource Unit (ARU), Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, University Putra Malaysia (UPM). The rats were kept at room temperature under standard conditions of light (12 h light-dark cycle) and regulated temperature (20–22°C) and ventilation in the animal research house of Comparative Medicine and Technology Unit (COMeT), Institute of Bioscience, UPM. Two rats were housed in a cage with wood shavings. The cleaning process of the cages was performed two times per week and water supply was changed on a daily basis. Ethical approval for this animal study was given by the Institutional Animal Care and Use Committee, UPM (UPM/IACUC/AUP-R098/2016).
2.3. Preparation of Diet
A high protein (HP) diet was prepared based on Envigo recipe [15]. This diet was approximately 40% protein in terms of calories.
2.4. Experimental Study
Twenty-four rats (n = 24) were randomly divided into three groups of diets. Rats in group A (n = 8) received HP diet only (HP only); rats in group B (n = 8) were fed with HP diet supplemented with LcS (108-109 CFU) and AFB1 (LcS + HP + AFB1), while rats in group C (n = 8) were only provided with HP diet and AFB1 (HP + AFB1). For rats in group B, immediately after the fifth probiotic dose, AFB1 was given at a complete dosage. The complete dosage of AFB1 for rats in group B was 25 μg AFB1/kg body weight (BW). The dose selected in this study is equivalent to 0.03 to 0.45 mg/kg (30–450 ppb) of AFB1 in food. This range is commonly found in contaminated foods that are consumed daily by many populations, especially in developing countries [16, 17]. The rats were dosed five days per week and sacrificed 24 h following the last dose [16]. As for the rats in group C (n = 8), they were fed with HP diet and given the same AFB1 dose as previously described. Since rats of group A and C were not supplemented with LcS, they were gavaged with a saline solution. The repeated dose of AFB1 given in this study is a standard protocol for an animal study [18]. Overall, the experiment ran for 25 days and HP diet and water were provided ad libitum. Water and diet consumption as well as the body weight of all rats were recorded at the beginning and every three days. The body weight of rats was recorded using an electronic balance (A&D Co., Ltd., Tokyo, Japan). At the end of the intervention, all rats were anesthetized using Ketamine and Xylazine and blood sample was taken by cardiac puncture from the artery.
2.5. Urine Collection
Following the last dose of AFB1, all rats were kept individually in metabolic cage for the collection of urine. The urine samples were then stored at −80°C until analysis.
2.6. Blood Withdrawal
About 3–10 ml of blood was withdrawn and collected using blood collection tube with serum separator (Becton, Dickinson and Company (BD), Plymouth, UK). The blood serum was separated using Kubota 2810 centrifuge (Tokyo, Japan) at 4°C for 13 min at 2000g. Serum was collected and stored at −80°C until analysis.
2.7. Liver and Kidney Function Test
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein, and albumin were measured for liver function, while the levels of urea (UREA) and blood creatinine (CREA) were measured to assess kidney function. These tests were analyzed using fully automated clinical analyzer BiOLiS 24i Premium at the Haematology and Biochemistry Clinical Laboratory of Faculty of Veterinary Medicine, UPM.
2.8. Analysis of Urinary AFM1
Urine was analyzed for the presence of AFM1 using ELISA kit, specifically for the determination of urinary AFM1 (Helica Biosystems, Inc., Santa Ana, CA, USA) [19].
2.9. Histopathological Examination
The entire small intestine, colon, liver, and spleen were removed and fixed in formalin solution 10%, Neutral Buffered (R&M Chemicals, UK), for 3 days at room temperature. Fixed tissue samples were washed several times with 80–95% ethanol, followed by dehydration in absolute ethanol before clearing with xylene and embedding in paraffin. The paraffin-embedded tissues were sectioned serially at 4 μm thickness. The sections were stained with hematoxylin and eosin (H&E) for qualitative histological analysis.
2.10. Statistical Analysis
Data were analyzed using SPSS version 20 software (SPSS Inc., Chicago, IL). The mean differences of liver and kidney biomarkers were analyzed using ANOVA between groups and post hoc analysis (Tukey's test) was conducted for every significant ANOVA output. On the other hand, the difference of urinary AFM1 level was determined by independent t-test. Results were expressed in terms of mean ± SD. The level of significance was assigned at p < 0.05.
3. Result and Discussion
3.1. Body Weight Gain
Rats in group A gained weight throughout the study. Conversely, rats in groups B and C that were gavaged with AFB1 had lower weight gain compared to rats in group A (Figure 1). This observation is in agreement with other studies [14, 20]. AFB1 can cause weight loss and reduce food consumption by reducing the level of leptin [21], which directly affects the energy balance and body weight gain [22]. In the present study, no significant difference on the body weight gain was observed between group A (58.5 ± 4.6 g) and group C (53.33 ± 7.9 g), as the HP diet enhances detoxification of AFB1 [12]. However, rats in groups B (47.67 ± 5.5 g) had significantly lower weight gain (p < 0.05) compared to rats in group A. This observation is consistent with a study, where rats supplemented with probiotic Lactobacillus plantarum (Lp) had lower weight gain compared to group of rats that consumed high-energy-dense diet only [23]. Besides, it is postulated that rats supplemented with probiotic VSL#3 might have low weight gain due to the production of satiety hormone GLP-1, as the increment of this hormone assists in calories and fat burning [24]. In another animal study, the supplementation of Lactobacillus paracasei spp. paracasei F19 (F19) had a higher level of Angiopoietin-like 4 (ANGPTL4), which is likely to decrease fat storage [25] and subsequently lead to a reduction in body weight. It is supported by findings from a human intervention study, where a significant decrease in BMI, subcutaneous fat, visceral fat, and waist circumference was observed among subjects in the group supplemented with milk that contained 2 × 108 CFU of probiotics [26]. In addition, supplementation of Lactobacillus species caused a significant decrease in body weight and body fat in female subjects [27].
Figure 1.
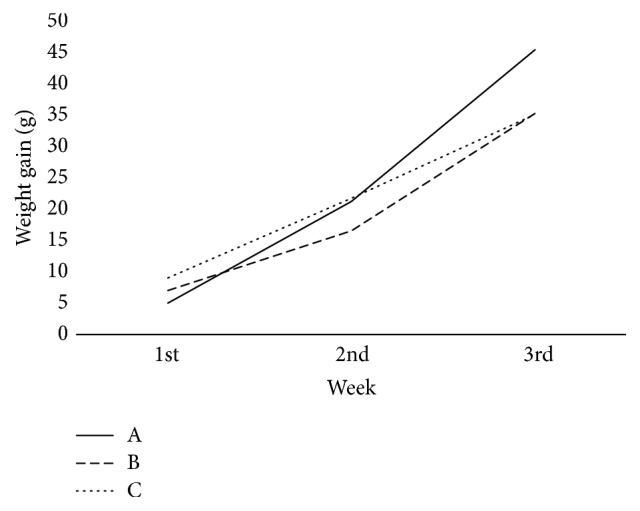
Comparison of rat's body weight gain between three groups. A: high protein only (HP), B: high protein, Lactobacillus casei Shirota, and aflatoxin B1 (HP + LcS + AFB1), C: high protein and aflatoxin B1 (HP + AFB1).
Besides that, probiotic supplementation affects the energy metabolism of the host through the production of short chain fatty acids (SCFA) [28]. A study [29] showed that supplementation of Lactobacillus salivarius ssp. salicinius JCM 1230 and Lactobacillus. agilis JCM 1048 during 24 h in a simulated chicken cecum significantly increased propionate and butyrate formation. In fact, L. acidophilus was able to increase SCFAs concentration in SHIME (Simulator of Human Microbial Ecosystem) reactor [30]. An increase of SCFAs is associated with the increment of the circulating concentrations of anorectic gut hormones such as peptide (PYY) and glucagon-like peptide-1 (GLP-1), and these gut hormones have been shown to cause a reduction in energy intake [31]. Other than that, SCFA reduces weight by increasing energy expenditure and enhances fat oxidation and thermogenesis by increasing the rate of oxygen consumption [32].
3.2. Liver Function Test
AST and ALT are enzymes of liver function, and elevated activities of these enzymes beyond a certain limit indicate liver lesions or other kinds of damage [33]. Rats in group A had high level of AST and the level was significantly different, compared to rats in group C (Table 1). This result is contradicted with a previous study as AFB1 exposure increased AST level [14]. The dosage of AFB1 given to the rats in the present study was similar to that in a study conducted by Nikbakht Nasrabadi et al. [14]. A possible explanation of this finding could be due to the type of diet. As previously mentioned, HP diet enhances detoxification of AFB1 [12]. HP diet also has effect on the hepatic enzymes as found in an animal study [34]. Following an HP diet, the liver enzymes enhance the catabolism of amino acid [35]. As the diet has high content of protein, the liver will have to secrete more enzymes for the catabolism of amino acid. Indeed, the increment of AST level between the groups was paralleled to the consumption of food, as rats in group A had higher food consumption than rats in group B and group C.
Table 1.
Biochemistry analysis of rat's blood sample for liver function test.
| Parameter | ALT (U/L) |
p value | AST (U/L) | p value | ALP (U/L) |
p value | ALB (g/L) | p value | TP (g/L) | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Group | ||||||||||
| A | 48.60 ± 12.13a | 0.987 | 196.40 ± 45.23b | 0.049 | 164.60 ± 28.43a | 0.385 | 31.52 ± 0.89a | 0.255 | 69.96 ± 3.70a | 0.394 |
| B | 47.60 ± 10.62a | 163.80 ± 2.26ab | 168.40 ± 10.92a | 30.90 ± 0.65a | 71.90 ± 1.81a | |||||
| C | 48.00 ± 7.54a | 142.60 ± 19.62a | 181.80 ± 15.96a | 30.32 ± 1.51a | 69.54 ± 2.56a |
A: high protein only (HP), B: high protein, Lactobacillus casei Shirota, and aflatoxin B1 (HP + LcS + AFB1), C: high protein and aflatoxin B1 (HP + AFB1), ALT: alanine transaminase, AST: aspartate transaminase, ALP: alkaline phosphatase, ALB: albumin, TP: total protein. p values were obtained from analysis of variance (ANOVA). Values are expressed as mean ± SD. Values with different superscript letter are significantly different (p < 0.05).
Despite the higher ALP level in all groups, there were no significant differences between groups (Table 1). Both AST and ALP levels were higher than the normal range (AST: ≤40 IU/L, [36], ALP: 44 to 147 IU/L, [37]) but no liver damage was observed. Additionally, other liver function's biomarkers such as ALT, total protein, and albumin remained in the normal range. The increased level of AST and ALP observed in this study could be the adaptation mechanism of the rats to the HP diet. Adaptation is an intentional response that performs by a body system to a new type of diet consumed, which causes changes in a functional state for better body performance [38]. This was evident in an animal study conducted by Johnson et al. [39], as monkeys that were provided with a high protein diet had elevated ALP level. Hence, changes of liver function's biomarkers, especially AST and ALP, are caused by the consumption of HP diet and are not due to liver damage. However, the effect of HP diet on liver enzymes depends on the percentage of macronutrients used in the experimental diet [40].
3.3. Kidney Function Test
The urea levels of the rats in groups A, B, and C were slightly higher than normal range of 5.4 to 7.9 mmol/L, as reported in rats [41] (Table 2). Protein intake causes changes in urea enzyme's cycle activities [35]. Dietary protein will be metabolized to essential and nonessential amino acids [42]. In addition, amino acid will be used to synthesize protein or converted to urea in the liver [42]. The production of urea depends on the amount of dietary protein consumed [42, 43].
Table 2.
Biochemistry analysis of rat's blood sample for kidney function test.
| Parameter | Urea (mmol/l) |
p value | Creatinine (umol/L) | p value |
|---|---|---|---|---|
| Group | ||||
| A | 10.82 ± 0.26b | 0.032 | 46.2 ± 3.34a | 0.772 |
| B | 10.02 ± 0.73a | 45.0 ± 2.54a | ||
| C | 10.10 ± 0.14ab | 45.0 ± 3.08a |
A: high protein only (HP), B: high protein, Lactobacillus casei Shirota, and aflatoxin (HP + LcS + AFB1), C: high protein and aflatoxin (HP + AFB1). p values were obtained from analysis of variance (ANOVA). Values are expressed as mean ± SD. Values with different superscript letter are significantly different (p < 0.05).
In the present study, there was a significant decrease (p < 0.05) of urea levels of rats in group B compared to rats in group A. This result agrees with another study [14] that found that the supplementation of LcS reduced the urea level. In contrast, there was no significant difference of urea level between group A and group C, demonstrating that AFB1 given to the rats did not affect the kidney function. Slight increase of urea level in this study does not represent kidney disease associated with AFB1. In addition, the changes that occurred in renal structure and function are typically a physiological effect and usually happen with increasing dietary protein consumption. A previous study showed that Wistar rats fed with 50% protein showed no abnormalities in renal function or pathology [44]. Besides, the finding by Lacroix et al. [44] showed no adverse effects to sclerotic glomeruli of rat after long-term consumption of diet with 60% of protein. In addition, no association was found between diet and structural changes in the kidney after four years of feeding dogs with 56, 27, or 19% protein [45]. Hence, the changes that occur in renal function are a normal adaptive mechanism in an organism with health kidney [46].
Creatinine is usually used to measure kidney function. Diet plays a vital role that affects creatinine value [47]. In an animal study, HP intake accelerated the progression of renal insufficiency [48]. Another study showed that high daily protein intake will increase the creatinine clearance [49]. With a normal diet, there is a significant increase in creatinine level when the AFB1 dose given is 25 ug/kg [16]. However, the present study did not find statistically significant effect on the creatinine level between all groups with the similar dose. In fact, the creatinine value was in the normal range of 30.5 to 114 umol/L [41].
3.4. Analysis of AFM1 in Urine
AFM1 was not detected in urine samples collected from the rats in group A. However, a statistically significant reduction of urinary AFM1(p > 0.05) was found in group B's rats compared to the rats in group C (Table 3). LcS supplemented to the rats in group B bind AFB1 and reduce its absorption in the small intestine [11]. In fact, the reduction of AFM1 was about 93% as compared to the rats in group B. In a study by Nikbakht Nasrabadi et al. [14], LcS supplementation caused 85% reduction of an aflatoxin biomarker. The changes in dietary intake can affect the bacteria composition [50]. Diet is the main environmental factor that can influence bacteria diversity and functionality [50–52]. For example, dietary protein affects the overall microbial diversity [53] by increasing beneficial microbiota in the gut [54]. Protein intake provides nitrogen sources for the microbial growth in the colon [55], so there will be sufficient amount of beneficial microorganisms as well as probiotic LcS in group B to adsorb AFB1.
Table 3.
The concentration of AFM1 in urine of aflatoxin-induced rats.
| Parameter | AFM1 (ng/ml) | p value |
|---|---|---|
| Group | ||
| B | 0.39 ± 0.01 | <0.001 |
| C | 5.22 ± 0.28 |
B: high protein, Lactobacillus casei Shirota, and aflatoxin B1 (HP + LcS + AFB1), C: high protein and aflatoxin B1 (HP + AFB1). Values are expressed as mean ± SD. p value was obtained by independence t-test.
Besides, HP intake stimulates the process of hepatic β-oxidation [56], which involves CYP2E1 and CYP4A enzymes [54], and a high level of the CYP2E1 enzyme was observed in the hepatocyte-derived cell lines with elevated glutathione (GSH) level. GSH plays a significant role for the detoxification process of AFB1 [11] and increased excretion of AFB1 from the body. Therefore, HP diet provided to the rats in the present study favored the detoxification of AFB1, which was further enhanced by the supplementation of probiotic LcS.
3.5. Histopathological Examination
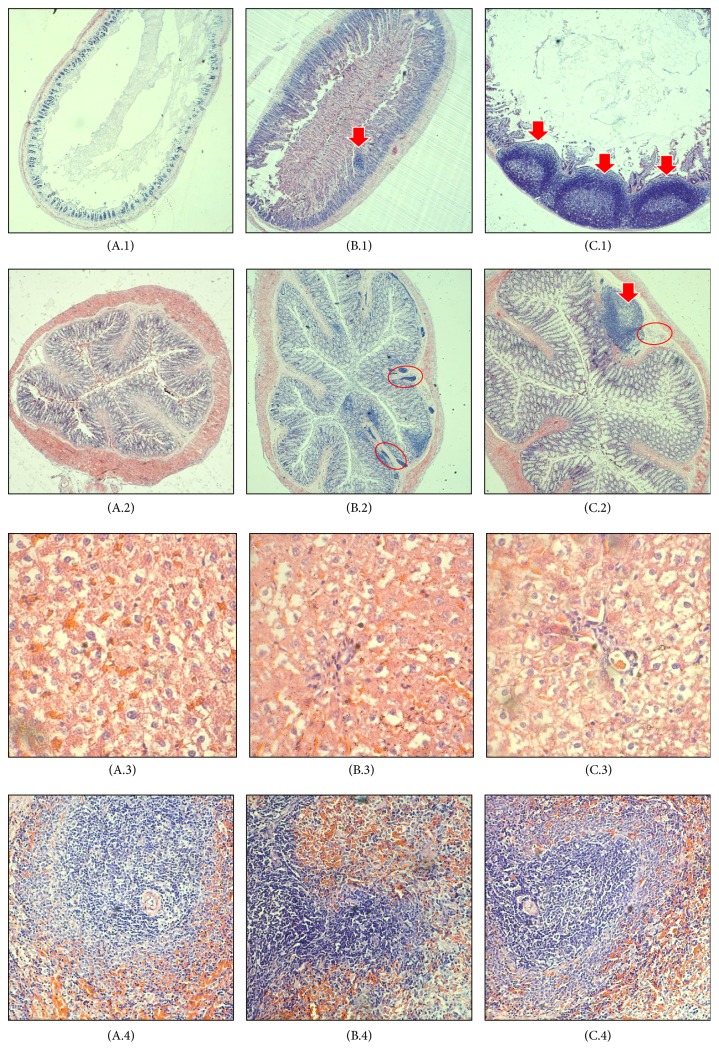
Histological analysis of H&E stained tissue revealed the histological changes, particularly in small intestine and colon (Figure 2). The small intestine of group A was in a healthy state (A.1) based on the histological observation. After AFB1 treatment for 4 weeks, large carcinoma was observed in the small intestine of the AFB1-treated group (C.1). Similar carcinoma was observed in group B (B.1), but the carcinoma growth was less in number and smaller in size compared to group C. In the colon, lymphocytes accumulation was observed in both AFB1-treated (C.2) and probiotic/AFB1-treated (B.2) groups. Lymphocytes accumulation indicates the occurrence of inflammation. However, carcinoma growth is only found in the AFB1-treated group (C.2). The results demonstrated the negative effects of AFB1 towards small intestine and colon, while such effects can be greatly reversed by the LcS treatment. AFB1 is commonly linked to liver cancer. However, in this study, no changes were found in H&E stained liver among the three different groups (A.3, B.3, and C.3). On the other hand, immune dysfunction is also one of the negative impacts from AFB1 contamination. Yet, no changes were observed in spleen of all groups (A.4, B.4, and C.4).
Figure 2.
Haematoxylin and eosin staining of small intestine (1), colon (2), liver (3), and spleen (4). A: high protein only (HP), B: high protein, Lactobacillus casei Shirota, and aflatoxin B1 (HP + LcS + AFB1), C: high protein and aflatoxin B1 (HP + AFB1). In small intestine, tumor-like growth (carcinoma) can be observed in group B and group C. In colon, tumor-like growth (carcinoma) and lymphocytes accumulation (inflammation) can be observed in group C. Group B only showed lymphocytes accumulation (inflammation). However, no prevalent changes have been observed in both liver and spleen. Arrow indicates tumor-like growth; red circle indicates lymphocytes accumulation. n = 5.
As mentioned above, the small intestine is the main site of aflatoxin absorption [57]. Few studies to date have reported the effect of AFB1 on intestinal epithelium. Similar to hepatocytes, intestinal epithelial cells express CYPs capable of converting AFB1 into the reactive epoxide; therefore AFB1 exposure might also promote weight loss through enteropathic effects. It is likely that environmental enteropathy is common in areas where dietary AFB1 exposure is endemic [58]. Such enteropathy is associated with histological changes in the small intestine, particularly inflammation and abnormal growth of intestinal cells. Jiang et al. [59] found a decrease in the percentages of T-cells and mRNA expression of cytokines in the intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) of the intestine in the AFB1 group compared to the control group. In human Caco-2 cells, AFB1 affects cell viability and growth, increases lactate dehydrogenase (LDH) activity release, and causes DNA damage [60]. Besides, the number of apoptotic cells in the jejunum and expression levels of Bax and caspase-3 genes were elevated in chickens fed AFB1-contaminated diet in comparison with controls [61]. In rodents, AFB1 induced intestinal lesions in the duodenum and ileum, which are characterized by a leucocytic and lymphocytic infiltration [62]. The current study demonstrated a significant toxin-induced gut dysfunction syndrome as shown in Figure 2 (C.1). Thus, future studies using this model should monitor gut absorptive and barrier functions of the animals.
Probiotics protect the intestine from xenobiotics, including AFB1 [63]. Besides their binding ability towards AFB1, probiotics also produce bioactive compounds that provide a significant protection against aflatoxicosis. Bioactive compounds produced by probiotics include antioxidant, anti-inflammatory, anticarcinogenic, and antibacterial compounds [64]. In the case of intestinal cancer prevention, probiotics provide strong antitumor activity via production of SCFA, reduction of colon carcinogenesis enzymes, and reduction of pH [4]. The presence of probiotics also provokes modulation immune system in the gut through alteration of gene expression. Generally, probiotics bind to Toll-like receptors (TLRs) to exert their immunomodulation effects, especially through TLR 2- and 4-dependent manner [65]. AFB1 is well known as hepatotoxicant and genotoxicant. However, in this study, no obvious changes were found in the liver of AFB1-treated rats (Figure 2: C.3). The immunosuppressive effect of AFB1 in this study also could not be observed in H&E stained spleen, as per Figure 2 (C.4). This phenomenon can be explained by the adsorption of AFB1 by LcS first occurring in the small intestine, as mentioned earlier. Therefore, intestinal injuries are most prevalent in this study. The intestinal adsorption of AFB1 will subsequently reduce systematic exposure [66].
4. Conclusion
Overall, this study found that LcS had the ability to bind AFB1 following an HP diet and alleviated the adverse effect of AFB1 on body weight and liver and kidney function. In addition, the consumption of LcS in the HP diet also increased the excretion of AFB1 metabolite, as AFM1 was greatly reduced in the urine. This was confirmed through the reduction of carcinoma occurrence in small intestine and colon for group of rats fed with AFB1 and LcS, compared to those fed with AFB1 alone. However, this study was limited by the lack of a normal diet group. In addition, broader research is needed to determine the effect of different percentage of protein on the ability of LcS and other probiotics to reduce the negative effect of AFB1 as this study only provided the rats with 40% of HP diet. Different macronutrients such as carbohydrates and fat may also have effect on probiotics and aflatoxin metabolism and hence warrant further investigation to determine the efficiency of probiotics as an adsorbent of aflatoxin.
Acknowledgments
This research was funded by Putra Research Grants from Universiti Putra Malaysia (UPM) [GP-IPS/2017/9517000 and GP-IPM/2016/9480100]. Z. Nurul Adilah and Winnie-Pui-Pui Liew are recipients of Graduate Research Fellowship (GRF) from School of Graduate Studies, UPM. Winnie-Pui-Pui Liew would like to acknowledge Ministry of Higher Education (MoHE), Malaysia, for the MyBrain15 Scholarship.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Z. Nurul Adilah and Winnie-Pui-Pui Liew conducted the experiment, carried out data analysis, and wrote the manuscript. S. Mohd Redzwan and I. Amin contributed by providing technical support for the project and proofread the final manuscript.
References
- 1.Tola M., Kebede B., Yildiz F. Occurrence, importance and control of mycotoxins: A review. Cogent Food & Agriculture. 2016;2(1):1–12. doi: 10.1080/23311932.2016.1191103. [DOI] [Google Scholar]
- 2.Udomkun P., Wiredu A. N., Nagle M., Müller J., Vanlauwe B., Bandyopadhyay R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application – A review. Food Control. 2017;76:127–138. doi: 10.1016/j.foodcont.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zain M. E. Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society. 2011;15(2):129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 4.Siva Kumar K. Colon cancer prevention through probiotics: An overview. Journal of Cancer Science & Therapy. 2015;7(3):81–92. doi: 10.4172/1948-5956.1000329. [DOI] [Google Scholar]
- 5.World Health Organization. some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Vol. 82. IARC Press; 2002. IARC monographs on the evaluation of carcinogenic risks to humans; pp. 1–556. [PMC free article] [PubMed] [Google Scholar]
- 6.Gratz S., Täubel M., Juvonen R. O., et al. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion, and toxicily of aflatoxin B1 in rats. Applied and Environmental Microbiology. 2006;72(11):7398–7400. doi: 10.1128/AEM.01348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Mendoza A., Guzman-De-Peña D., Garcia H. S. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. Journal of Applied Microbiology. 2009;107(2):395–403. doi: 10.1111/j.1365-2672.2009.04217.x. [DOI] [PubMed] [Google Scholar]
- 8.FAO/WHO. Food safety risk analysis: A guide for national food safety authorities. FAO Food and Nutrition Paper. 2006;87:1–119. [PubMed] [Google Scholar]
- 9.Amara A. A., Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceutical Journal. 2013;23(2):107–114. doi: 10.1016/j.jsps.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damayanti E., Istiqomah L., Saragih J. E., Purwoko T., Sardjono T. Characterization of lactic acid bacteria as poultry probiotic candidates with aflatoxin B1 binding activities. IOP Conference Series: Earth and Environmental Science. 2017;101 doi: 10.1088/1755-1315/101/1/012030.012030 [DOI] [Google Scholar]
- 11.Mohd Redzwan S., Abd Mutalib M. S., Wang J.-S., et al. Effect of supplementation of fermented milk drink containing probiotic Lactobacillus casei Shirota on the concentrations of aflatoxin biomarkers among employees of Universiti Putra Malaysia: A randomised, double-blind, cross-over, placebo-controlled study. British Journal of Nutrition. 2016;115(1):39–54. doi: 10.1017/S0007114515004109. [DOI] [PubMed] [Google Scholar]
- 12.Nurul Adilah Z., Mohd Redzwan S. Effect of dietary macronutrients on aflatoxicosis: a mini-review. Journal of the Science of Food and Agriculture. 2017;97(8):2277–2281. doi: 10.1002/jsfa.8234. [DOI] [PubMed] [Google Scholar]
- 13.Rogers A. E., Newberne P. M. Diet and aflatoxin B1 toxicity in rats. Toxicology and Applied Pharmacology. 1971;20(1):113–121. doi: 10.1016/0041-008X(71)90095-0. [DOI] [PubMed] [Google Scholar]
- 14.Nikbakht Nasrabadi E., Jamaluddin R., Abdul Mutalib M. S., Khaza'ai H., Khalesi S., Mohd Redzwan S. Reduction of aflatoxin level in aflatoxin-induced rats by the activity of probiotic Lactobacillus casei strain Shirota. Journal of Applied Microbiology. 2013;114(5):1507–1515. doi: 10.1111/jam.12148. [DOI] [PubMed] [Google Scholar]
- 15.ENVIGO: Custom Research Diet. Envigo.com, 2017. http://www.envigo.com.
- 16.Qian G., Wang F., Tang L., et al. Integrative toxicopathological evaluation of aflatoxin B. Toxicologic Pathology. 2013;41(8):1093–1105. doi: 10.1177/0192623313477256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel J. H., Lewis L. W., Redwood Y. A., et al. Comprehensive assessment of maize aflatoxin levels in eastern Kenya, 2005-2007. Environmental Health Perspectives. 2011;119(12):1794–1799. doi: 10.1289/ehp.1003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Tang L., Glenn T. C., Wang J.-S. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicological Sciences. 2016;150(1):54–63. doi: 10.1093/toxsci/kfv259.kfv259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redzwan S. M., Rosita J., Sokhini A. M. M., Aqilah A. R. N. Association between aflatoxin M1 excreted in human urine samples with the consumption of milk and dairy products. Bulletin of Environmental Contamination and Toxicology. 2012;89(6):1115–1119. doi: 10.1007/s00128-012-0853-y. [DOI] [PubMed] [Google Scholar]
- 20.Hathout A. S., Mohamed S. R., El-Nekeety A. A., Hassan N. S., Aly S. E., Abdel-Wahhab M. A. Ability of Lactobacillus casei and Lactobacillus reuteri to protect against oxidative stress in rats fed aflatoxins-contaminated diet. Toxicon. 2011;58(2):179–186. doi: 10.1016/j.toxicon.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Wahhab M. A., Aly S. E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. Journal of Agricultural and Food Chemistry. 2003;51(8):2409–2414. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- 22.Monalisa R. Role of leptin in obesity. Research Journal of Pharmacy and Technology. 2015;8(8):1073–1076. doi: 10.5958/0974-360X.2015.00185.7. [DOI] [Google Scholar]
- 23.Karlsson C. L. J., Molin G., Fak F., et al. Effects on weight gain and gut microbiota in rats given bacterial supplements and a high-energy-dense diet from fetal life through to 6 months of age. British Journal of Nutrition. 2011;106(6):887–895. doi: 10.1017/S0007114511001036. [DOI] [PubMed] [Google Scholar]
- 24.Yadav H., Lee J. H., Lloyd J., Walter P., Rane S. G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. The Journal of Biological Chemistry. 2013;288(35):25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronsson L., Huang Y., Parini P., et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4) PLoS ONE. 2010;5(9):1–7. doi: 10.1371/journal.pone.0013087.e13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadooka Y., Sato M., Ogawa A., et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. British Journal of Nutrition. 2013;110(9):1696–1703. doi: 10.1017/S0007114513001037. [DOI] [PubMed] [Google Scholar]
- 27.Park S., Bae J.-H. Probiotics for weight loss: A systematic review and meta-analysis. Nutrition Research. 2015;35(7):566–575. doi: 10.1016/j.nutres.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc J. G., Chain F., Martín R., Bermúdez-Humarán L. G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microbial Cell Factories. 2017;16(1, article no. 79):1–10. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meimandipour A., Soleimani A. F., Houshmand M., et al. Effects of rough handling on short chain fatty acid production and gastrointestinal pH in broilers and modulatory role of Lactobacilli. African Journal of Biotechnology. 2011;10(74):17030–17037. doi: 10.5897/AJB10.1592. [DOI] [Google Scholar]
- 30.Sivieri K., Morales M. L. V., Adorno M. A. T., Sakamoto I. K., Saad S. M. I., Rossi E. A. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME® reactor. BMC Gastroenterology. 2013;13(1, article no. 100) doi: 10.1186/1471-230X-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne C. S., Chambers E. S., Morrison D. J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. International Journal of Obesity. 2015;39(9):1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura I., Inoue D., Maeda T., et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proceedings of the National Acadamy of Sciences of the United States of America. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeringa M., Hastings A., Yamazaki S., De Koning P. Serum biomarkers in nonalcoholic steatohepatitis: Value for assessing drug effects? Biomarkers in Medicine. 2012;6(6):743–757. doi: 10.2217/bmm.12.87. [DOI] [PubMed] [Google Scholar]
- 34.Michael M., Anyakudo C., Onuwabhagbe O. F., Ademidun O. O. Ec nutrition research article hepatotoxicity of high protein diet in diabetic rats: An indication for necessary dietary precaution, vol. 5, pp. 195–202, 2017
- 35.Jean C., Rome S., Mathé V., et al. Metabolic evidence for adaptation to a high protein diet in rats. The Journal of Nutrition. 2001;131(1):91–98. doi: 10.1093/jn/131.1.91. [DOI] [PubMed] [Google Scholar]
- 36.Cacciola I., Scoglio R., Alibrandi A., et al. Evaluation of liver enzyme levels and identification of asymptomatic liver disease patients in primary care. Internal and Emergency Medicine. 2017;12(2):181–186. doi: 10.1007/s11739-016-1535-2. [DOI] [PubMed] [Google Scholar]
- 37.Schröder F. H., Tombal B., Miller K., et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: Results from a 12-month, comparative, phase III study. BJU International. 2010;106(2):182–187. doi: 10.1111/j.1464-410X.2009.08981.x. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa A., Kobayashi T., Sakai F., Kadooka Y., Kawasaki Y. Lactobacillus gasseri SBT2055 suppresses fatty acid release through enlargement of fat emulsion size in vitro and promotes fecal fat excretion in healthy Japanese subjects. Lipids in Health and Disease. 2015;14(1) doi: 10.1186/s12944-015-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson Q., Veith W. J., Mouton T. The impact of dietary protein intake on serum biochemical and haematological profiles in vervet monkeys. Journal of Medical Primatology. 2001;30(1):61–69. doi: 10.1111/j.1600-0684.2001.300108.x. [DOI] [PubMed] [Google Scholar]
- 40.Pesta D. H., Samuel V. T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Journal of Nutrition and Metabolism. 2014;11(1):1–8. doi: 10.1186/1743-7075-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker H. J., Lindsey J. R., Wesibroth S. H. The Laboratory Rat: Biology and diseases. Vol. 1. New York: Academic Press Inc; 2013. [Google Scholar]
- 42.Weiner I. D., Mitch W. E., Sands J. M. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clinical Journal of the American Society of Nephrology. 2015;10(8):1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sales M. C., de Azevedo Paiva A., de Queiroz D., Costa R. A. F., da Cunha M. A. L., Pedraza D. F. Nutritional status of iron in children from 6 to 59 months of age and its relation to vitamin A deficiency. Nutrición Hospitalaria. 2013;28(3):734–740. doi: 10.3305/nh.2013.28.3.6396. [DOI] [PubMed] [Google Scholar]
- 44.Lacroix M., Gaudichon C., Martin A., et al. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004;287(4):R934–R942. doi: 10.1152/ajpregu.00100.2004. [DOI] [PubMed] [Google Scholar]
- 45.Robertson G., Leclercq I., Farrell G. C. Nonalcoholic Steatosis and Steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;281(5):G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 46.Martin W. F., Armstrong L. E., Rodriguez N. R. Dietary protein intake and renal function. Nutrition Metabolism. 2005;2(1):p. 25. doi: 10.1186/1743-7075-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banfi G., Del Fabbro M. Serum creatinine values in elite athletes competing in 8 different sports: Comparison with sedentary people. Clinical Chemistry. 2006;52(2):330–331. doi: 10.1373/clinchem.2005.061390. [DOI] [PubMed] [Google Scholar]
- 48.Lentine K., Wrone E. M. New insights into protein intake and progression of renal disease. Current Opinion in Nephrology and Hypertension. 2004;13(3):333–336. doi: 10.1097/00041552-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Juraschek S. P., Appel L. J., Anderson C. A. M., Miller E. R., III Effect of a high-protein diet on kidney function in healthy adults: Results from the omniheart trial. American Journal of Kidney Diseases. 2013;61(4):547–554. doi: 10.1053/j.ajkd.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Queipo-Ortuño M. I., Boto-Ordóñez M., Murri M., et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. American Journal of Clinical Nutrition. 2012;95(6):1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 51.Nadal I., Santacruz A., Marcos A. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. International Journal of Obesity. 2009;33(7):758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Hurtado I., Santacruz A., Peiró G., et al. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl 4-Induced fibrosis. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0023037.e23037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lescheid D. W. Probiotics as regulators of inflammation: A review. Functional Foods in Health and Disease. 2014;4(7):299–311. [Google Scholar]
- 54.Lopez-Legarrea P., Fuller N. R., Zulet M. A., Martinez J. A., Caterson I. D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pacific Journal of Clinical Nutrition. 2014;23(3):360–368. doi: 10.6133/apjcn.2014.23.3.16. [DOI] [PubMed] [Google Scholar]
- 55.Duncan S. H., Lobley G. E., Holtrop G., et al. Human colonic microbiota associated with diet, obesity and weight loss. International Journal of Obesity. 2008;32(11):1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 56.Bortolotti M., Kreis R., Debard C., et al. High protein intake reduces intrahepatocellular lipid deposition in humans. American Journal of Clinical Nutrition. 2009;90(4):1002–1010. doi: 10.3945/ajcn.2008.27296. [DOI] [PubMed] [Google Scholar]
- 57.Dogi C., Cristofolini A., González Pereyra M. L., et al. Aflatoxins and Saccharomyces cerevisiae: Yeast modulates the intestinal effect of aflatoxins, while aflatoxin B1 influences yeast ultrastructure. World Mycotoxin Journal. 2017;10(2):171–181. doi: 10.3920/WMJ2016.2115. [DOI] [Google Scholar]
- 58.Knipstein B., Huang J., Barr E., et al. Dietary aflatoxin-induced stunting in a novel rat model: Evidence for toxin-induced liver injury and hepatic growth hormone resistance. Pediatric Research. 2015;78(2):120–127. doi: 10.1038/pr.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang M., Peng X., Fang J., Cui H., Yu Z., Chen Z. Effects of aflatoxin B1 on t-cell subsets and mRNA expression of cytokines in the intestine of broilers. International Journal of Molecular Sciences. 2015;16(4):6945–6959. doi: 10.3390/ijms16046945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J., Zheng N., Liu J., Li F. D., Li S. L., Wang J. Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food and Chemical Toxicology. 2015;83:54–60. doi: 10.1016/j.fct.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Peng X., Zhang K., Bai S., et al. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. International Journal of Environmental Research and Public Health. 2014;11(8):8567–8580. doi: 10.3390/ijerph110808567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akinrinmade F. J., Akinrinde A. S., Amid A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Research. 2016;32(2):53–60. doi: 10.1007/s12550-016-0239-9. [DOI] [PubMed] [Google Scholar]
- 63.Ahlberg S. H., Joutsjoki V., Korhonen H. J. Potential of lactic acid bacteria in aflatoxin risk mitigation. International Journal of Food Microbiology. 2015;207:87–102. doi: 10.1016/j.ijfoodmicro.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 64.Di Cerbo A., Palmieri B., Aponte M., Morales-Medina J. C., Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. Journal of Clinical Pathology. 2016;69(3):187–203. doi: 10.1136/jclinpath-2015-202976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finamore A., Roselli M., Imbinto A., Seeboth J., Oswald I. P., Mengheri E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0094891.e94891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gambacorta L., Pinton P., Avantaggiato G., Oswald I. P., Solfrizzo M. Grape Pomace, an Agricultural Byproduct Reducing Mycotoxin Absorption: In Vivo Assessment in Pig Using Urinary Biomarkers. Journal of Agricultural and Food Chemistry. 2016;64(35):6762–6771. doi: 10.1021/acs.jafc.6b02146. [DOI] [PubMed] [Google Scholar]