Abstract
Exposure to stressful environments in early childhood can cause a toxic stress response and lead to poor health outcomes, including obesity, cardiac disease, diabetes, and mental illness. In animals and maltreated children, the presence of a nurturing caregiver can buffer against the physiological disruptions associated with a toxic stress response; however, the specific caregiver and parenting characteristics that best promote a protective relationship in humans remain largely unexplored, particularly in families living in high-risk environments. In this study, framed in an ecobiodevelopmental (EBD) model, a cross-sectional design is being used to study 54 multi-ethnic, urban maternal-child dyads with children at early school age (4–9 years). Mothers’ past experiences, mental health, and caregiving patterns and children’s hair cortisol, C-reactive protein, pro-inflammatory cytokines, blood pressure, BMI, behavior, and school performance are being analyzed to identify maternal characteristics that may protect against children’s toxic stress response in families at high risk for exposure to stressors such as poverty, trauma, or exposure to violence.
Keywords: emotional states/feelings, family health, health promotion/wellness behaviors, parent-child relationships, prevention, stress and coping
1 | INTRODUCTION
A toxic stress response can result from exposure to extreme stressors in early childhood, such as poverty, violence, or parental mental illness. Without the protection of a supportive caregiver, a child’s stress response system can become persistently elevated, leading to excessive release of glucocorticoids (cortisol), catecholamines (epinephrine, norepinephrine), and inflammatory cytokines (interleukin-6, tumor necrosis factor-alpha; Juster, McEwen, & Lupien, 2010; McEwen, 2008). These disruptions result in alterations to brain architecture and neuroendocrine, immune, metabolic, and cardiovascular functioning, and can lead to impaired health and developmental outcomes in childhood including obesity, growth delay and impaired cognitive, language, social, and emotional skills (Garner, 2013; Johnson, Riley, Granger, & Riis, 2013; McEwen, 2008). A toxic stress response can also have consequences that extend into adulthood, including depression, alcoholism, obesity, diabetes, and cardiovascular disease (Garner, 2013).
In the United States, 14.7 million children live in poverty, 30.6% of female-headed households live below the poverty level, and two-thirds of adults report having experienced at least one traumatic event during childhood (DeNavas-Walt & Proctor, 2014; Felitti et al., 1998).
Because exposure to toxic stress can set the stage for poor health outcomes across the lifespan, an examination of protective mechanisms over the course of development is needed. A gap in the literature is particularly notable for school-aged children, a developmental stage requiring advanced cognitive, social, and emotional capacities (Janus & Duku, 2007). The preschool period also requires examination, as younger children may be both more susceptible to the effects of toxic stress and more highly influenced by caregiving behaviors (Lupien, McEwen, Gunnar, & Heim, 2009). At the intersection of these age groups, this study is designed to explore associations among maternal characteristics, current care-giving patterns, and indicators of a toxic stress response in a multi-ethnic, urban sample of mothers and early-school-age children.
1.1 | Protective factors in childhood
In the ecobiodevelopmental (EBD) framework, a child’s biology interacts with the early social environment (ecology) to influence long-term health and development (Shonkoff, 2010; Shonkoff et al., 2012). The most important element of the social environment is the child’s relationship with his or her primary caregiver (Bronfenbrenner & Ceci, 1994; Shonkoff et al., 2012). In animals and maltreated children, nurturing caregivers can buffer against the physiological disruptions associated with a toxic stress response (Hostinar, Sullivan, & Gunnar, 2014), but the specific positive caregiving characteristics that best promote a protective relationship in humans remain largely unexplored. These characteristics may include positive parenting behaviors like praise, comfort, and play, as well as parental reflective functioning (PRF), which describes caregivers’ abilities to understand their own mental states andthe underlying mental states of their child (Slade, 2005). Racial socialization behaviors, which include caregivers’ strategies to prepare children to succeed in a racially biased society, are also protective, but the relationship between racial socialization and a toxic stress response in children has not been explored (Yasui, 2015).
Mothers’ own childhood experiences may also influence their ability to protect their children from multiple stressors. Caregivers with a history of adverse childhood experiences (ACEs), such as abuse, neglect, or household dysfunction, are more likely to use corporal punishment, report parenting stress, and demonstrate less sensitive parenting (Ammerman et al., 2013; Belsky, Conger, & Capaldi, 2009; Chung et al., 2009; Steele et al., 2016). By contrast, a history of family strengths, such as support and cohesion, can lead to improved outcomes for caregivers and their children (Benzies & Mychasiuk, 2009; Hillis et al., 2010).
Past maternal experiences of discrimination may also influence caregiving behaviors and child health, as these experiences are often associated with depression, substance use, and harsh disciplinary practices in ethnic minority mothers (Murry, Brody, Simons, Cutrona, & Gibbons, 2008). Additionally, mothers who have symptoms of post-traumatic stress disorder (PTSD) or psychological distress may be unpredictable, unreliable, or emotionally unavailable, creating a significant source of stress for their children (Davies, Slade, Wright & Stewart, 2008; Yehuda et al., 2005). Despite the importance of these maternal characteristics, relationships among maternal characteristics, current caregiving patterns, and toxic stress response in children have not yet been explored.
1.2 | Markers of toxic stress
Indicators of a toxic stress response include physiological, health, and behavioral outcomes associated with exposure to persistent stressors in early childhood. Non-invasive biomarkers for stress, such as hair cortisol, salivary cytokines, and salivary c-reactive protein (CRP), enable safe and feasible measurement of the toxic stress response. Hair cortisol, for example, provides a measure of HPA axis functioning over time, and thus offers a major advantage over cortisol sources like serum and saliva that provide only a snapshot of real-time cortisol levels (Russell, Koren, Rieder, & Van Uum, 2012).
Other biomarkers also hold promise for measurement of the toxic stress response in children. C-reactive protein (CRP), for example, is a sensitive marker of systemic inflammation and immune dysfunction and is correlated with several inflammatory markers in children, including fibrinogen, arterial endothelial functioning, and white blood cell counts (Cook et al., 2000; Järvisalo et al., 2002; Visser, Bouter, McQuillan, Wener, & Harris, 2001).
Pro-inflammatory cytokines represent a complex network of intercellular protein messengers responsible for inducing inflammation and mediating neuroimmune-endocrine interactions, and may play an important role in the toxic stress response (Dinarello, 2000; Haddad, Saadé, & Safieh-Garabedian, 2002). However, measurement of these biomarkers has been extremely limited in children, particularly in ethnic minority or low-income populations (Juster et al., 2010).
A study of non-invasive biomarkers, paired with traditional measures of health and behavior in a sample of ethnically diverse children at preschool and early school age, offers an innovative approach to examining a toxic stress response in children at risk. Aligned with the National Institute of Nursing Research’s strategic plan to promote health and prevent illness in minority and underserved populations, the findings of this study will provide preliminary data that will be used to design future studies to investigate causal relationships between maternal characteristics and toxic stress in children. This foundational work will ultimately inform the development and testing of interventions designed to buffer the toxic stress response, and provide an essential foundation for a program of research focused on promoting health and reducing disparities in vulnerable families at risk for toxic stress.
1.3 | Study aims
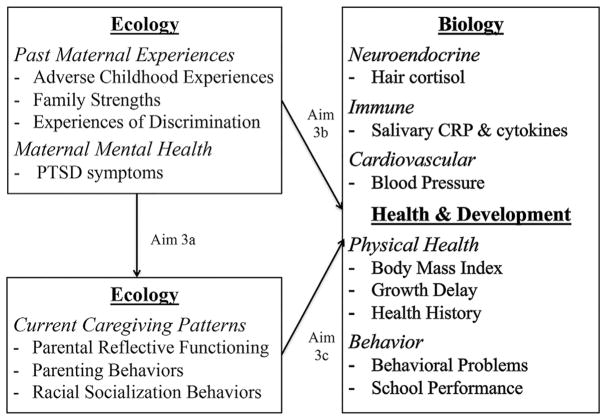
The purpose of this study is to describe and examine associations among maternal characteristics, current caregiving patterns, and indicators of a toxic stress response (Figure 1) in a multi-ethnic, urban sample of mothers and children at early school age (4–9 years). The primary aims of the study are to:
FIGURE 1.
Hypothesized relationships among study variables, based on the ecobiodevelopmental framework (Shonkoff et al., 2012) in which social environment (ecology) interacts with a child’s biology to influence long-term health and development
Describe physiological (hair cortisol, salivary C-reactive protein and inflammatory cytokines, blood pressure), health (body mass index, growth delay), and behavioral (internalizing/externalizing behaviors) indicators of a toxic stress response in a multi-ethnic, urban sample of children at early school age.
Describe maternal characteristics, including past maternal experiences (ACEs, family strengths, experiences of discrimination) and maternal mental health (PTSD symptoms), and current caregiving patterns (parental reflective functioning, parenting behaviors, racial socialization), in a multi-ethnic, urban sample of mothers with children at early school age.
-
Examine the associations among maternal characteristics (past maternal experiences, maternal mental health), current caregiving patterns, and children’s physiological, health and behavioral indicators of a toxic stress response.
-
3a
Examine the associations between maternal characteristics and current caregiving patterns.
-
3b
Examine the associations between maternal characteristics and children’s indicators of a toxic stress response.
-
3c
Examine the associations between current patterns of caregiving in mothers and children’s indicators of a toxic stress response.
-
3a
2 | METHODS
This study employs a cross-sectional design with data obtained from maternal-child dyads with children who are 4–9 years of age. The study sample includes maternal-child dyads recruited from the control group arm of Minding the Baby® (MTB), a randomized controlled trial (RCT) of a home visiting intervention for first-time mothers with risk factors including poverty, a history of trauma or young maternal age (Ordway et al., 2014; Sadler et al., 2013). Families participated in the MTB RCT from mid-pregnancy until children were 2 years of age. The RCT was completed in 2015. In the present follow-up study, data were collected from October 2016 through August 2017. Preliminary data analysis began in September 2017 and is ongoing.
2.1 | Sample
In the current study, participants were eligible for inclusion if (a) the child was between 4–9 years of age at the time of data collection; (b) the child’s mother had custody or regular contact with the child; (c) the dyad was residing within the state of CT; and (d) the dyad participated in the control group arm of the MTB RCT. During the data collection period, 83 maternal-child dyads were eligible for inclusion. Based on the recruitment success of a previous follow-up study of the MTB cohort, we estimated that 70% of the eligible sample would participate in the current study and thus expected a final sample of 58 mother-child dyads.
2.2 | Procedures
2.2.1 | Recruitment
Permission for future contact was included in the MTB RCT informed consent process. Since completion of the RCT in 2015, the MTB research assistant (RA) has kept up-to-date contact information on former participants and routinely sends holiday cards (Mother’s Day and winter holidays). To recruit participants for the current study, invitation letters and flyers were sent in colorful envelopes to all eligible families, and these letters were followed up with a text message from the follow-up study’s principal investigator (PI) approximately 1 week later. Although 15 families responded to the invitation sent by mail, text messages by the PI proved to be the most effective recruitment method. Forty families responded to an initial text message sent by the PI, and 12 responded when a second text message was sent. The remaining families were contacted through alternative strategies, including email invitations sent by the PI or phone calls made by the MTB RA, who was known to participants.
Using the above recruitment strategies, 70 out of 83 eligible families were successfully reached. Two families declined to participate, seven did not meet eligibility criteria due to loss of child custody or moving out of state, and seven expressed interest but never scheduled a visit. At the completion of data collection, 54 maternal-child dyads were enrolled in the study (Table 1), representing 65% of all eligible families.
TABLE 1.
Demographic characteristics of mothers and children in the study sample
| n (%) | Mean (SD) | |
|---|---|---|
| Mothers | ||
| Age in years | 26.8 (3.3) | |
| Race | ||
| White | 14 (25.9) | |
| Black | 26 (48.1) | |
| Other | 14 (25.9) | |
| Hispanic ethnicity | 27 (50.0) | |
| Hispanic cultural group | ||
| Puerto Rican | 20 (74.1) | |
| Mexican | 2 (7.4) | |
| Dominican | 1 (3.7) | |
| Other/multiple groups | 4 (14.8) | |
| Children | ||
| Age in years | 6.7 (2.1) | |
| Female gender | 25 (46.3) | |
| Race | ||
| White | 9 (16.7) | |
| Black | 27 (50.0) | |
| Asian | 1 (1.9) | |
| Other | 17 (31.5) | |
| Hispanic ethnicity | 28 (51.9) | |
| Hispanic cultural group | ||
| Puerto Rican | 20 (71.4) | |
| Mexican | 1 (3.5) | |
| Other/multiple groups | 7 (25.0) | |
| Grade level | ||
| Not enrolled | 3 (5.6) | |
| Pre kindergarten | 14 (25.8) | |
| Kindergarten | 9 (16.6) | |
| First grade | 4 (7.6) | |
| Second grade | 3 (5.6) | |
| Third grade | 9 (16.6) | |
| Fourth grade | 9 (16.6) | |
| Other | 3 (5.6) | |
SD, standard deviation.
2.2.2 | Data collection
Institutional review board approval for the study was obtained prior to data collection. The PI collected all of the data with assistance from the MTB RA. Parents provided informed consent and permission to talk with the child, and children ages 7–9 provided assent (Kohrman et al., 1995; National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1978).
Data were collected at a private location convenient for the participants, including participants’ homes (n = 21), the Yale School of Nursing’s biobehavioral laboratory (n = 25), and community libraries (n = 8). Visits lasted approximately 60–90 min and were scheduled with as much flexibility as possible to be convenient for busy families with many other obligations and stressors. All visits were conducted in English, as English-language proficiency was an inclusion criterion for participation in the original MTB RCT. Also consistent with the original MTB study, mothers were compensated $50 for their time, and an additional stipend was provided if travel costs were incurred. Children were given an educational book in recognition of their participation.
2.3 | Measures (Table 2)
TABLE 2.
Theoretical concepts, variables, and measures
| EBD component | Concept | Variable | Measure | Subject |
|---|---|---|---|---|
| Ecology | Maternal history of childhood adversity | Adverse childhood experiences | Childhood Trauma Questionnaire | Mother |
| Maternal history of family strengths | Family strengths | Childhood Trauma Questionnaire | Mother | |
| Maternal experiences of discrimination | Experiences of discrimination | Experiences of Discrimination Scale | Mother | |
| Maternal trauma history | PTSD symptoms | PTSD Checklist—Civilian | Mother | |
| Reflective capacity | Parental reflective functioning | Parental Reflective Functioning Questionnaire | Mother | |
| Supportive caregiving | Parenting behavior | Parent Behavior Inventory | Mother | |
| Protective parenting related to racial discrimination | Racial socialization behaviors | Racial Socialization Scale | Mother | |
| Biology | Physiological response | Neuroendocrine functioning | Hair cortisol | Child |
| Immune functioning | Salivary c-reactive protein, salivary cytokines | Child | ||
| Cardiovascular functioning | Blood pressure percentile | Child | ||
| Health and development | Endocrine disruption | Obesity | BMI percentile, z-score | Child |
| Metabolic demand | Growth delay | Height-for-age percentile | Child | |
| Behavioral development | Internalizing and externalizing behaviors | Child Behavior Checklist | Mother reports on child | |
| Physiological disruption | Health history | Demographic form | Mother reports on child | |
| Learning and cognition | School performance | Child Behavior Checklist | Mother reports on child |
EBD, ecobiodevelopmental model.
2.3.1 | Demographic characteristics
Participants provided demographic information for mothers and children, including age, ethnicity, education level, and employment.
2.3.2 | Past maternal experiences
Adverse childhood experiences and family strengths
The 28-item Childhood Trauma Questionnaire (CTQ) is a retrospective self-report measure to identify history of trauma and protective factors in adolescents and adults across five domains: physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect. The CTQ also includes seven items to assess family strengths. It is reliable and valid across a wide range of samples and has high internal consistency (α =.91). The CTQ takes 5 min to complete (Bernstein et al., 1994).
Experiences of discrimination
The Experiences of Discrimination (EOD) Scale is a 9-item self-report instrument to measure past exposure to discrimination in diverse domains, including at school, getting hired or getting a job, at work, getting housing, getting medical care, getting service in a store or restaurant, getting credit, bank loans, or a mortgage, on the street or in a public setting, and from the police or in the courts. The EOD also includes items to assess respondents’ responses to unfair treatment. The EOD has adequate internal consistency (α = .74–.86) and test-retest reliability (r = .70), and was significantly associated with psychological distress in a sample of black, Latino, and white working class adults (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005).
2.3.3 | Maternal mental health
PTSD symptoms
The PTSD Checklist—Civilian Version (PCL-C) is a 17-item self-report instrument with a four-factor structure (intrusions, avoidance, dysphoria, and hyperarousal) that corresponds to the key symptoms of PTSD (Krause, Kaltman, Goodman, & Dutton, 2007; Weathers, Litz, Herman, Huska, & Keane, 1993). The PCL-C can be used generally to evaluate the presence of PTSD symptoms over the past month related to any traumatic event (Weathers et al., 1993). The PCL-C has high test-retest reliability (r = .88), high internal consistency (α = .81–.96), and takes approximately 5 min to complete.
2.3.4 | Current patterns of caregiving
Parental reflective functioning
The Parental Reflective Functioning Questionnaire (PRFQ), is an 18-item questionnaire to assess a mother’s interest in and ability to recognize her child’s mental states (Luyten, Mayes, Nijssens, & Fonagy, 2017; Slade, 2005). The PRFQ has adequate internal consistency (α = .77–.85) and construct validity in socioeconomically diverse mothers (Luyten et al., 2017). The PRFQ takes 5 min to complete.
Parenting behaviors
The Parent Behavior Inventory (PBI) includes 20 items that measure parenting behaviors in parents of early school-aged children. The PBI includes two subscales with high internal consistency: supportive/engaged (α = .83) and hostile/coercive (α = .81). The PBI has adequate test-retest reliability (r = .69–.74) in an ethnically diverse sample of mothers and takes 5 min to complete (Lovejoy, Weis, O’Hare, & Rubin, 1999).
Racial socialization behaviors
The 16-item Racial Socialization Scale developed by Hughes and Chen (1997) includes three subscales with adequate internal reliability: preparation for bias (α = .91), cultural socialization (α = .84), and promotion of mistrust (α = .68). This measure has been used in studies with African American and Latino families and is appropriate for parents of children age 4–14 years (Hughes & Chen, 1997; Hughes, 2003).
2.3.5 | Children’s indicators of toxic stress
Neuroendocrine functioning
Hair cortisol is a noninvasive indicator of long-term HPA axis activity (Russell et al., 2012). Increased hair cortisol in children is associated with parenting stress, maternal depression, and cumulative psychosocial exposures (Karlén et al., 2015; Simmons et al., 2016). A sample of hair was cut from the posterior vertex of the child’s scalp, 1 cm in diameter and 3 cm in length, representing the most recent 3 months of systemic cortisol exposure. Information on hair color, hair product use, frequency of hair washing, and medication use was collected (Russell et al., 2012).
Immune functioning
Saliva was collected to measure C-reactive protein (CRP) and a panel of cytokines to assess children’s immune functioning (Cook et al., 2000; Haddad et al., 2002). Salivary CRP is highly correlated with serum CRP (r = .72), and elevated salivary CRP in children is associated with obesity, allergic asthma, and poor cardio-respiratory fitness (Naidoo, Konkol, Biccard, Dubose, & Mckune, 2012). Based on recent studies in children, a panel of four proinflammatory cytokines (interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha) was measured (Riis et al., 2016). Salivary cytokine levels are associated with oral immune activity in children; and elevated salivary cytokine levels are also associated with maternal distress, behavioral problems and self-reported depression in children, suggesting a relationship between salivary cytokines and systemic inflammation that requires further investigation (Keller, El-Sheikh, Vaughn, & Granger, 2010; Riis et al., 2016).
Saliva samples were collected with passive drool devices according to the procedure outlined by Salimetrics® labs (Salimetrics LLC, 2015). The PI conducted a gross oral exam prior to saliva collection, and information on mouth sores, dental caries, and loose/missing teeth was recorded (Riis et al., 2016). Salimetrics® salivary CRP analyses have acceptable precision and sensitivity data, with intra-assay coefficients of variability (CV) ranging from 1.9–5.9%, and inter-assay CVs ranging from 3.7–11.2% (Salimetrics LLC, 2015). Salivary cytokines were analyzed using a 4-plex electrochemiluminescence immunoassay manufactured by Meso Scale Discovery with acceptable intra-assay CVs (2.1–6.6; Riis et al., 2016).
Cardiovascular functioning
Blood pressure measurement is a valid and noninvasive method for assessing cardiovascular risk in children (Muntner, He, Cutler, Wildman, & Whelton, 2004). Per evidence-based guidelines, three seated blood pressure measurements at least 1 min apart were obtained with a calibrated manual sphygmomanometer after 5 min of rest. To account for diurnal variations in blood pressure, efforts were made to obtain measurements at the same time of day for each child (late afternoon after school), and the time of measurement was recorded (Bird & Michie, 2008). Percentiles were calculated based on age, height, and gender (Muntner et al., 2004).
2.3.6 | Children’s health and development
Body mass index (BMI)
Elevated BMI is a validated measure of obesity, and is associated with cardiovascular and metabolic risk factors in children (Freedman & Sherry, 2009). Children’s height and weight were measured with a calibrated stadiometer and scale. The child’s BMI (kg/m2) was calculated, and a BMI percentile and z-score were determined based on age and gender-appropriate CDC growth charts (Kuczmarski et al., 2002).
Growth delay
Growth delay is a promising indicator of exposure to chronic stress in children, as impaired bone growth has been associated with chronic stress and a lack of supportive care (Johnson, Bruce, Tarullo, & Gunnar, 2011). Children’s height percentiles were calculated based on age and gender-appropriate CDC growth charts (Kuczmarski et al., 2002).
Internalizing/externalizing behaviors
The Child Behavior Checklist (CBCL) is a widely used 99-item questionnaire designed to assess for behavioral and emotional problems in children based on parent report. There are two versions of the CBCL, selected according to age (CBCL/1½-5 and CBCL/6-18). Both assess internalizing (anxious, depressive) and externalizing (aggressive, hyperactive) behaviors. The CBCL/1½-5 and CBCL/6-18 have high internal consistency (α = .92 and .94) and test-retest reliability (r = .89 and .92). The CBCL is written at a fifth-grade reading level and takes 10 min to complete (Achenbach & Ruffle, 2000).
Health history
Mothers reported on children’s health history, including chronic illnesses (asthma, allergies, diabetes mellitus, food intolerance, gastrointestinal disorders), medications and number of emergency department visits, injuries/accidents, and hospitalizations.
School performance
Mothers reported on children’s academic performance, including attention or learning problems, need for special education services, accomplishments in school, and repeated grades. Questions were based on the academic background section of the CBCL (Achenbach & Ruffle, 2000).
2.4 | Laboratory procedures
Hair and salivary biomarkers were collected using protocols outlined by Salimetrics® (Salimetrics LLC, 2015). Prior to collection, the PI completed all biosafety training programs as required by Yale University. World Health Organization standard precautions for infection control were followed, including hand hygiene, personal protective equipment, and waste disposal procedures (World Health Organization, 2007). Hair and saliva samples were placed in de-identified (ID #s), sealed vials and stored in a secure file cabinet and office at room temperature. Saliva samples were stored in a secure subzero freezer at <20 degrees until shipment to the laboratory. Saliva was packed in dry ice and hair and saliva samples were shipped in sealed envelopes via FedEx to the University of Massachusetts and Salimetrics® laboratories, respectively, for analysis.
2.5 | Data analysis
Data were cleaned and imported into SAS 9.4® for analysis. Univariate analyses (means, medians, ranges, frequencies, standard deviations, histograms) were used to describe demographic characteristics of mothers and children. Univariate statistics were also used to address Aim 1 (to describe the physiological, health, and behavioral indicators of a toxic stress response in children) and Aim 2 (to describe maternal characteristics and current caregiving patterns).
In future analyses, Pearson and Spearman correlations will be used to describe the strength and direction of relationships between variables as displayed in Figure 1. For Aim 3a, correlations between each maternal characteristic and each current caregiving pattern will be explored. For Aim 3b, correlations between each maternal characteristic and each child indicator of toxic stress will be explored. For Aim 3c, correlations between each current caregiving pattern and each child indicator of toxic stress will be explored.
For each correlation that is statistically significant, linear regression analyses will be performed, with the independent and dependent variables occurring in the direction as indicated in Figure 1. We plan to treat each variable as continuous but will use different regression modeling if indicated by data distribution patterns. As this study is exploratory in nature and our goal is to determine effect sizes to inform the development of future studies, we will not adjust for multiple hypothesis testing due to the risk of missing an association.
3 | DISCUSSION
Data collection has been completed and analysis is ongoing. After the primary analyses are completed, future analyses may include exploration of variations by child age (preschool vs. early school age), gender, or race/ethnicity. Though the sample size is small, we expect that these analyses will provide insight into factors that may moderate the identified protective mechanisms and help to generate future hypotheses regarding the relationships among maternal characteristics, caregiving patterns, and child indicators of toxic stress. Using the noninvasive biomarkers collected in this study, future analyses may also include the development of a composite index for toxic stress, which would further advance understanding of the complex physiological mechanisms involved in a toxic stress response.
Ongoing efforts by the MTB parent study to maintain contact with families through newsletters, holiday cards, and yearly reunions proved to be essential for successful recruitment of this mobile community cohort. Although the participants were recruited up to 7 years after completion of the parent study, many families expressed enthusiasm and willingness to participate in both current and future follow-up studies. As described, text messages sent by the PI were most effective for contacting families, although multiple messages often were required.
Scheduling research visits also often was challenging, requiring the PI to be flexible and available for last-minute changes in meeting days, times, or locations. The Yale School of Nursing Biobehavioral Laboratory proved to be a valuable resource, allowing families to meet at a nearby, private location that was outside of their own homes. As visits typically lasted over an hour, the availability of toys and the assistance of the MTB RA were essential for keeping the child entertained, allowing the PI to assist mothers with the self-report questionnaires as necessary.
Although most mothers completed the instruments independently, when concerns regarding comprehension or literacy arose, the PI would read each question aloud and explain further as needed. To compensate for their time and efforts, participants received $50 in cash, which was determined to be fair compensation for up to 90 min of time and completion of numerous measures.
We anticipated that biomarker collection in children might have presented concerns for parents in the current study, particularly for ethnic minority families who may mistrust medical research or attribute specific cultural meanings to hair or saliva collection in children (Corbie-Smith, Thomas, & George, 2002; Ford, Boch, & McCarthy, 2016). Thus, detailed field notes regarding feasibility and strategies used for hair and saliva collection in children were recorded after every visit. Parents’ responses to two open-ended questions, intended to assess acceptability and cultural beliefs surrounding biomarker collection, were also recorded. These included “what questions do you have about our collection of hair or saliva for this study?” and “what advice do you have for myself or other researchers who are collecting hair and saliva samples in children? What can we do to improve this process?”
Ongoing assessment of the open-ended responses and field notes was used to identify concerns and improve our approach to biomarker collection methods throughout the study. For example, we found that children less than 5 years old often had difficulty with passive drool collection, and thus the instructions, language, and demonstration strategies used were adjusted for this age group accordingly. Additionally, many boys in the sample had hair less than 3 cm in length, so our data collection forms and procedures were updated to account for hair length differences. In the future, we plan to evaluate the field notes and parent responses using conventional content analysis in order to identify themes and ultimately offer recommendations for researchers including hair and salivary biomarkers in research with young children and ethnic minority families (Hsieh & Shannon, 2005).
Limitations of the current study should be acknowledged. Importantly, the best indicators of a toxic stress response in children remain poorly defined, and biomarkers in the current study were selected based on available knowledge and feasibility (Juster et al., 2010). The results of this study may contribute to a notable gap in the literature on noninvasive biomarkers of stress in ethnic minority and low-income children. Another limitation is the study’s small sample size, which may not be adequate to detect small effect sizes or to use multivariate statistical methods to study the relationships among variables. However, this exploratory study will provide an important foundation for hypothesis generation for future phases of research.
4 | CONCLUSION
Toxic stress prevention is critical for health promotion and reduction of health disparities among families living in socially and economically marginalized communities. If, as we expect, maternal characteristics and caregiving patterns are associated with indicators of toxic stress in children, this research will lead to future investigations designed to test the causal relationships among the phenomena, future clinical trials of caregiving interventions, and studies of the effects of caregiving on long-term health outcomes associated with exposure to chronic stress.
The findings of this study also have the potential to inform clinical practice, as understanding the association of maternal characteristics with child physiology, health, and development may encourage pediatric clinicians to take a broader ecological approach to addressing health promotion, encouraging healthy development, and preventing and treating childhood illnesses. Identification of specific caregiving characteristics associated with indicators of lesser exposure to chronic stress in children will also make a valuable contribution to pediatric clinical care, as these caregiving strategies can be encouraged both in the primary care setting and as part of in-depth interventions.
As this literature builds over time, findings may play an important role in the development of social and economic policies that aim to reduce maternal stressors, promote family strengths, prevent intergenerational transmission of trauma, and empower socioeconomically disadvantaged communities. This may include micro-level policy approaches, such as greater investment in early home visiting programs or other interventions designed to promote positive caregiving in young families (MacMillan et al., 2009), as well as macro-level approaches, such as comprehensive and innovative strategies to eliminate social segregation, improve media portrayal of ethnic minority groups, and reduce race-based discrimination in housing, education, healthcare, and employment (Ford & Airhihenbuwa, 2010; Gee & Ford, 2011). Given the lifelong consequences associated with a toxic stress response in childhood, this study has the potential to lay important groundwork for future research on toxic stress prevention that may ultimately drive population-level policy changes.
Acknowledgments
Funding information
National Institute of Nursing Research, Grant number: F31NR016385; Connecticut Nurses Foundation; NAPNAP Foundation; Alpha Nu Chapter of Sigma Theta Tau International; Jonas Nurse Leaders Scholars Program
The author would like to acknowledge Arietta Slade, Nancy Redeker, and Margaret Holland for their contributions in the development of this research protocol. This work was supported by the by the National Institute of Nursing Research of the National Institutes of Health (F31NR016385), the NAPNAP Foundation, the Connecticut Nurses Foundation, the Alpha Nu Chapter of Sigma Theta Tau International and the Jonas Nurse Leaders Scholars Program.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review. 2000;21:265–271. doi: 10.1542/pir.21-8-265. https://doi.org/10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Ammerman RT, Shenk CE, Teeters AR, Noll JG, Putnam FW, Van Ginkel JB. Multiple mediation of trauma and parenting stress in mothers in home visiting. Infant Mental Health Journal. 2013;34:234–241. https://doi.org/10.1002/imhj.21383. [Google Scholar]
- Belsky J, Conger R, Capaldi DM. The intergenerational transmission of parenting: Introduction to the special section. Developmental Psychology. 2009;45:1201–1204. doi: 10.1037/a0016245. https://doi.org/10.1037/a0016245. [DOI] [PubMed] [Google Scholar]
- Benzies K, Mychasiuk R. Fostering family resiliency: A review of the key protective factors. Child and Family Social Work. 2009;14:103–114. https://doi.org/10.1111/j.1365-2206.2008.00586.x. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, … Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bird C, Michie C. Measuring blood pressure in children. BMJ. 2008;336:1321. doi: 10.1136/bmj.a150. https://doi.org/10.1136/bmj.a150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Chung EK, Mathew L, Rothkopf AC, Elo IT, Coyne JC, Culhane JF. Parenting attitudes and infant spanking: The influence of childhood experiences. Pediatrics. 2009;124:e278–e286. doi: 10.1542/peds.2008-3247. https://doi.org/10.1542/peds.2008-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, … Strachan DP. C-reactive protein concentration in children: Relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. https://doi.org/10.1016/S0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, George DM. Distrust, race, and research. Archives of Internal Medicine. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. https://doi.org/10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- Davies J, Slade P, Wright I, Stewart P. Posttraumatic stress symptoms following childbirth and mothers’ perceptions of their infants. Infant Mental Health Journal. 2008;29:537–554. doi: 10.1002/imhj.20197. https://doi.org/10.1002/imhj.20197. [DOI] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor B. Income and poverty in the United States: 2013. 2014 Retrieved from https://www.census.gov/content/dam/Census/library/publications/2014/./p60-249.pdf.
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. https://doi.org/10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ford CL, Airhihenbuwa CO. Critical race theory, race equity, and public health: Toward antiracism praxis. American Journal of Public Health. 2010;100:S30–S35. doi: 10.2105/AJPH.2009.171058. https://doi.org/10.2105/AJPH2009.171058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JL, Boch SJ, McCarthy DO. Feasibility of hair collection for cortisol measurement in population research on adolescent health. Nursing Research. 2016;65:249–255. doi: 10.1097/NNR.0000000000000154. https://doi.org/10.1097/NNR0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–S34. doi: 10.1542/peds.2008-3586E. https://doi.org/10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- Garner AS. Home visiting and the biology of toxic stress: Opportunities to address early childhood adversity. Pediatrics. 2013;132:S65–S73. doi: 10.1542/peds.2013-1021D. https://doi.org/10.1542/peds.2013-1021D. [DOI] [PubMed] [Google Scholar]
- Gee GC, Ford CL. Structural racism and health inequities. Du Bois Review: Social Science Research on Race. 2011;8:115–132. doi: 10.1017/S1742058X11000130. https://doi.org/10.1017/S1742058x11000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuro-immune- endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. Journal of Neuroimmunology. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. https://doi.org/10.1016/S0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hillis S, Anda R, Dube S, Felitti V, Marchbanks P, Macaluso M, Marks J. The protective effect of family strengths in childhood against adolescent pregnancy and its long-term psychosocial consequences. The Permanente Journal. 2010;14(3):18–27. doi: 10.7812/tpp/10-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256–282. doi: 10.1037/a0032671. https://doi.org/10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15:1277–1288. doi: 10.1177/1049732305276687. https://doi.org/10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Hughes D, Chen L. When and what parents tell children about race: An examination of race-related socialization among African American families. Applied Developmental Science. 1997;1:200–214. [Google Scholar]
- Hughes D. Correlates of African American and Latino parents’ messages to children about ethnicity and race: A comparative study of racial socialization. American Journal of Community Psychology. 2003;31:15–33. doi: 10.1023/a:1023066418688. [DOI] [PubMed] [Google Scholar]
- Janus M, Duku E. The school entry gap: Socioeconomic, family, and health factors associated with children’s school readiness to learn. Early Education and Development. 2007;18:375–403. https://doi.org/10.1080/10409280701610796a. [Google Scholar]
- Järvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, … Raitakari OT. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. https://doi.org/10.1161/01.ATV.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development and Psychopathology. 2011;23:859–871. doi: 10.1017/S0954579411000356. https://doi.org/10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131:319–327. doi: 10.1542/peds.2012-0469. https://doi.org/10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. https://doi.org/10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Karlén J, Ludvigsson J, Hedmark M, Faresjö Å, Theodorsson E, Faresjö T. Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics. 2015;135:e1450–e1457. doi: 10.1542/peds.2014-2561. https://doi.org/10.1542/peds.2014-2561. [DOI] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children’s mental health: The role of child sex. Physiology and Behavior. 2010;101:705–712. doi: 10.1016/j.physbeh.2010.08.012. https://doi.org/10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrman A, Clayton EW, Frader JE, Grodin MA, Moseley KL, Porter IH, Wagner VM. Informed consent, parental permission, and assent in pediatric practice. Pediatrics. 1995;95:314–317. [PubMed] [Google Scholar]
- Krause ED, Kaltman S, Goodman LA, Dutton MA. Longitudinal factor structure of posttraumatic stress symptoms related to intimate partner violence. Psychological Assessment. 2007;19(2):165. doi: 10.1037/1040-3590.19.2.165. [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine. 2005;61:1576–1596. doi: 10.1016/j.socscimed.2005.03.006. https://doi.org/10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL. 2000 CDC growth charts for the United States: Methods and development. Vital and Health Statistics Series 11. 2002;2002(246):1–190. [PubMed] [Google Scholar]
- Lovejoy CM, Weis R, O’Hare E, Rubin EC. Development and initial validation of the Parent Behavior Inventory. Psychological Assessment. 1999;11:534–545. https://doi.org/10.1037//1040-3590.11.4.534. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. https://doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luyten P, Mayes LC, Nijssens L, Fonagy P. The Parental Reflective Functioning questionnaire: Development and preliminary validation. PLoS ONE. 2017;12(5):e0176218. doi: 10.1371/journal.pone.0176218. https://doi.org/10.1371/journal.pone.0176218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Wathen CN, Barlow J, Fergusson DM, Leventhal JM, Taussig HN. Interventions to prevent child maltreatment and associated impairment. The Lancet. 2009;373:250–266. doi: 10.1016/S0140-6736(08)61708-0. https://doi.org/10.1016/S0140-6736(08)61708-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. https://doi.org/10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. Journal of the American Medical Association. 2004;291:2107–2113. doi: 10.1001/jama.291.17.2107. https://doi.org/10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- Murry VM, Brody GH, Simons RL, Cutrona CE, Gibbons FX. Disentangling ethnicity and context as predictors of parenting within rural African American families. Applied Development Science. 2008;12:202–210. doi: 10.1080/10888690802388144. https://doi.org/10.1080/10888690802388144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo T, Konkol K, Biccard B, Dubose K, Mckune AJ. Elevated salivary C-reactive protein predicted by low cardio-respiratory fitness and being overweight in african children. Cardiovascular Journal of Africa. 2012;23:501–506. doi: 10.5830/CVJA-2012-058. https://doi.org/10.5830/CVJA-2012-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont report: Ethical principles and guidelines for the protection of human subjects of research. Washington, DC: US Government Print Office; 1978. [PubMed] [Google Scholar]
- Ordway MR, Sadler LS, Dixon J, Close N, Mayes L, Slade A. Lasting effects of an interdisciplinary home visiting program on child behavior: Preliminary follow-up results of a randomized trial. Journal of Pediatric Nursing. 2014;29:3–13. doi: 10.1016/j.pedn.2013.04.006. https://doi.org/10.1016/j.pedn.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Granger DA, Minkovitz CS, Bandeen-Roche K, DiPietro JA, Johnson SB. Maternal distress and child neuroendocrine and immune regulation. Social Science & Medicine. 2016;151:206–214. doi: 10.1016/j.socscimed.2015.12.043. https://doi.org/10.1016/j.socscimed.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. https://doi.org/10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Sadler LS, Slade A, Close N, Webb DL, Simpson T, Fennie K, Mayes LC. Minding the Baby: Enhancing reflectiveness to improve early health and relationship outcomes in an interdisciplinary home-visiting program. Infant Mental Health Journal. 2013;34:391–405. doi: 10.1002/imhj.21406. https://doi.org/10.1002/imhj.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics LLC. SalivaBio collection methods. 2015 Retrieved from https://www.salimetrics.com/collection-systems.
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81:357–367. doi: 10.1111/j.1467-8624.2009.01399.x. https://doi.org/10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, … Wegner LM. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. https://doi.org/10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Simmons JG, Badcock PB, Whittle SL, Byrne ML, Mundy L, Patton GC, … Allen NB. The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology. 2016;63:276–281. doi: 10.1016/j.psyneuen.2015.10.004. https://doi.org/10.1016/j.psyneuen.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Slade A. Parental reflective functioning: An introduction. Attachment and Human Development. 2005;7:269–281. doi: 10.1080/14616730500245906. https://doi.org/10.1080/14616730500245906. [DOI] [PubMed] [Google Scholar]
- Steele H, Bate J, Steele M, Danskin K, Knafo H, Nikitiades A, … Murphy A. Adverse childhood experiences, poverty, and parenting stress. Canadian Journal of Behavioural Science. 2016;48:32–38. https://doi.org/10.1037/cbs0000034. [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1):e13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility; Proceedings from: Annual convention of the international society for traumatic stress studies; San Antonio, TX. 1993. [Google Scholar]
- World Health Organization. Standard precautions in health care. 2007 Retrieved from http://www.who.int/csr/resources/publications/EPR_AM2_E7.pdf.
- Yasui M. A review of the empirical assessment of processes in ethnic-racial socialization: Examining methodological advances and future areas of development. Developmental Review. 2015;37:1–40. https://doi.org/10.1016/j.dr.2015.03.001. [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of Clinical Endocrinology & Metabolism. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. https://doi.org/10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]