Abstract
Salivary cortisol is considered to be a safe and noninvasive measure of hypothalamic-pituitary-adrenal axis functioning, and is a commonly measured biomarker of the human stress response in pediatric research. However, cortisol is highly variable and sensitive to a wide range of factors, creating a challenge for reliable salivary cortisol collection in the community setting. Furthermore, the acceptability of salivary cortisol collection in community samples of children is largely unknown. The purpose of this integrative review was to investigate current evidence on the acceptability and feasibility of salivary cortisol collection in community samples of children. In an analysis framed by the Theory of Planned Behavior, data extracted from 31 studies revealed six categories of psychosocial influences on acceptability and feasibility: uncertainty and misconceptions, cultural and ethnic values, family rules and values, difficulty following protocols and procedures, burden of multiple samples, and child refusal or resistance. Further research is required to fully understand the factors that influence acceptability and feasibility of salivary cortisol collection in community samples of children. Understanding individual, family, and community perceptions of biobehavioral research will lead to more culturally sensitive and feasible community-based research methods.
Keywords: saliva, cortisol, biomarkers, stress, child, integrative review
Integrating biological measures into nursing research allows for a comprehensive, multilevel perspective that can be used to understand complex health and behavioral phenomena (Cicchetti & Gunnar, 2008). To understand complex phenomena like community responses to stress, pediatric researchers are increasingly measuring noninvasive biomarkers, such as salivary cortisol, in community samples of children.
Salivary cortisol is a safe, convenient and reliable measure of the human stress response and thus is widely measured in pediatric research (Granger, Johnson, Szanton, Out, & Schumann, 2012; Keil, 2012). However, despite the perception that noninvasive biomarkers such as salivary cortisol are easy to collect, there is little knowledge as to whether these measures are acceptable to participants and feasible to collect in community settings and among families with diverse racial and ethnic backgrounds. A greater understanding of the acceptability and feasibility of noninvasive biomarker collection will enhance researchers, decision-making on approaches to culturally sensitive and methodologically rigorous biobehavioral research in community settings. Therefore, the purpose of this integrative review was to investigate the available evidence on acceptability and feasibility of salivary cortisol collection in diverse community samples of children, in order to offer recommendations for future research involving noninvasive biomarker collection in community settings.
Salivary Cortisol Collection
Salivary cortisol is a sensitive and specific measure of biologically active, unbound cortisol, and reflects functioning of the hypothalamic-pituitary-adrenal (HPA) axis, a major physiological stress response system (Granger et al., 2007; Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006; Jessop & Turner-Cobb, 2008; Keil, 2012). In response to physical or psychological stressors, the HPA axis is activated and cortisol is released from the adrenal glands into the bloodstream. Cortisol levels in the bloodstream peak approximately 15–30 minutes after HPA axis activation, and cortisol levels in saliva peak approximately 2 minutes later (Hanrahan et al., 2006). Cortisol is also released in 15–30 short bursts over the course of the day (Jessop & Turner-Cobb, 2008; Keil, 2012). Cortisol follows a circadian pattern that is established in early infancy, with serum levels peaking 20–30 minutes after awakening and dropping lowest around midnight (Hanrahan et al., 2006; Keil, 2012; Kudielka, Gierens, Hellhammer, Wust, & Schlotz, 2012).
While serum and salivary cortisol levels are highly correlated, salivary cortisol levels are influenced by a number of other factors, including chronic diseases, medications, age, pubertal status, and lifestyle habits. Thus, while salivary cortisol is considered simple to collect, it is also highly variable and responsive to a wide range of factors that that may threaten the acceptability and feasibility of collection in community settings (Granger et al., 2007; Hanrahan et al., 2006).
When salivary cortisol is collected in children, there are a number of methodological issues that require consideration (Keil, 2012). First, the timing of salivary cortisol collection must be precise and consistent across samples in order to obtain accurate and valid results (Kudielka et al., 2012). Adam and Kumari (2009) outlined a variety of ways that cortisol patterns can be measured (see Table 1), each requiring precise timing and often multiple samples across more than 1 day. Many include sample collection at bedtime or upon awakening, and thus collection by the child’s caregiver at home is often the most feasible approach. Researchers also must consider variability in timing according to a child’s age or developmental status. In young children, individual and family variations in sleeping, waking or napping patterns will influence daily cortisol secretion (Keil, 2012). In older children, school schedules and extracurricular activities may impede the feasibility of salivary cortisol collection at certain times or days of the week (Jessop & Turner-Cobb, 2008; Rotenberg & McGrath, 2014).
Table 1.
Timing of Cortisol Patterns Commonly Measured in Community Settings
| Cortisol Measure | Description of Timing |
|---|---|
| Cortisol awakening response | 30–45 min post awakening |
| Diurnal cortisol slope | Degree of change in levels from morning to evening |
| Area under the daytime cortisol curve | Levels measured at multiple points across the day |
| Waking cortisol | Immediately upon awakening |
| Cortisol at specific time points across the waking day | Specific clock times (i.e., 10 am and 10 pm) or time points (i.e., afternoon) |
| Bedtime cortisol | Immediately prior to bedtime |
| Cortisol reactivity to momentary stressors | Elevation in typical diurnal level for that time for day for that person |
| Cortisol reactivity to daily stressors | Changes in cortisol levels from one day to the next |
Note. Adapted from Psychoneuroimmunology (2009), 34, 1423–1436, Adam & Kumari, Assessing salivary cortisol in large-scale, epidemiological research, with permission from Elsevier.
In addition to the timing of saliva collection, salivary cortisol levels may also be influenced by a number of other factors, including medications, substance use, certain medical diagnoses, lifestyle factors and demographic characteristics (See Table 2; Adam & Kumari, 2009; Hanrahan et al., 2006). Certain behaviors within 30 minutes of salivary cortisol collection may also influence results. For example, food or beverage ingestion may change the oral environment, or even activate the HPA axis, in the case of caffeine or protein (Hanrahan et al., 2006). Tooth-brushing must also be avoided for 30 minutes prior to saliva collection, as this may interfere with collection of the sample or the assay results (Keil, 2012). In 7–10-year-old children, participation in after-school activities, particularly sports, was associated with an increase in bedtime cortisol levels in boys (Kertes & Gunnar, 2004). While the presence of these factors may not justify exclusion of salivary cortisol samples, they must at minimum be evaluated and accounted for in the analysis of results.
Table 2.
Factors that Interfere With Cortisol Secretion or Measurement
| Category | Examples |
|---|---|
| Medication and | Herbal products, corticosteroids, sex |
| substances | hormones, antidepressants, caffeine, lithium, methadone, phenytoin, nicotine |
| Diagnoses | Cushing disease, Addison disease, depression, panic disorder, obesity, schizophrenia, personality disorders, stuttering, ADHD |
| Lifestyle factors | Smoking, alcohol use, caffeine, exercise/activity level, perceived stress, recent food ingestion or tooth brushing |
| Demographic characteristics | Gender, race, age, pubertal status |
Note. Adapted from Psychoneuroimmunology (2009), 34, 1423–1436, Adam & Kumari, Assessing salivary cortisol in large-scale, epidemiological research, and Applied Nursing Research (2006), 19, 95–101, Hanrahan et al., Strategies for salivary cortisol collection and analysis in research with children, with permissions from Elsevier.
Saliva collection devices and procedures also require methodological consideration (Hanrahan et al., 2006; Harmon, Hibel, Rumyantseva, & Granger, 2007; Keil, 2012). In children, selecting an appropriate device for saliva collection must be based on the age and developmental level of the participant (Keil, 2012). Saliva collection devices for children include cotton ropes, feeding tubes, filter paper, microsponges, sorbettes, swabs or a passive drool collection device (Tryphonopoulos, Letourneau, & Azar, 2014). (See Keil [2012] and Tryphonopoulos et al. [2014] for recommendations according to age.) Selecting an appropriate collection device is not only imperative for the comfort of the participant, but inappropriate selection may lead to insufficient sample volumes and inaccurate results (Gröschl & Rauh, 2006; Harmon et al., 2007; Poll et al., 2007; Tryphonopoulos et al., 2014). A clear explanation of collection procedures is also essential, particularly when caregivers collect the samples at home (Hanrahan et al., 2006). The procedures must include explicit instructions regarding timing, storage, and return of samples. A detailed questionnaire should also be included with each sample, so that information on recent tooth brushing, food ingestion, illnesses or other interfering factors can be obtained. (See Hanrahan et al. [2006] for examples of home-collection instructions and questionnaires.) While these procedures are necessary for accurate collection of salivary cortisol, the complexity of these procedures may be intimidating or confusing and may contribute to attrition of participants, particularly for those with low reading levels, limited English language proficiency, or limited resources.
There is evidence to suggest that the complexity of salivary cortisol collection leads to non-adherence to research protocols in community samples, and that this may have an impact on the validity of results (Kudielka, Broderick, & Kirschbaum, 2003; Kudielka et al., 2012; Michels et al., 2012; Smith & Dougherty, 2014). For example, Smith and Dougherty used electronic monitoring to measure parental compliance with saliva collection procedures and found that non-adherence was associated with higher waking cortisol and lower cortisol awakening response in children ages 3–5 years.
To overcome the challenges associated with salivary cortisol collection in children at home, a number of strategies to improve compliance have been suggested. These include the use of electronic monitoring devices (such as the MEMS® Medication Event Monitoring System), video or in-person demonstration of the collection technique, color-coded sample collection tubes, incentives, and reminders such as emails or text messaging (Jessop & Turner-Cobb, 2008; Kaitz, Sabato, Shalev, Ebstein, & Mankuta, 2012; Tryphonopoulos et al., 2014). Despite these strategies, however, the complexity of salivary cortisol collection in children remains a potential threat to the acceptability and feasibility of this method in community samples of children.
Theoretical Framework for Review
This review was informed by the Theory of Planned Behavior (Ajzen, 1991), in which an individual’s intention to perform a particular behavior is influenced by three central determinants: attitudes, social norms, and perceived behavioral control. Attitudes refer to the individual’s perception of the risks and benefits associated with the behavior. Social norms refer to the individual’s perceptions of social pressure or expectation to perform or not perform the behavior. Perceived behavioral control, similar to self-efficacy, is the individual’s perception of the ease or difficulty involved in performing a particular behavior. Thus, according to the theory, behavior is not predicted by general attitudes, but rather is a function of beliefs or perceptions relevant to the specific behavior in question (Ajzen, 1991).
Because influences on acceptability and feasibility of salivary cortisol collection are likely multifactorial, the use of this theoretical framework provides a conceptual structure for in-depth analysis of these types of influences on behavior (Torraco, 2005). The Theory of Planned Behavior was used to provide an in-depth understanding of the multifaceted perceptions that may influence salivary cortisol collection behavior in children and as a result, may significantly affect the acceptability and feasibility of these methods in community settings. Exploring individual, family and community influences on salivary cortisol collection is essential for understanding and enhancing the acceptability and feasibility of these methods in community samples of children.
Methods
This review was conducted following the integrative review approach proposed by Whittemore and Knaff (2005). It allows for inclusion of studies with a diverse range of methodologies in order to fully understand a phenomenon of interest, and includes five stages: (a) problem identification, (b) literature search, (c) data evaluation, (d) data analysis, and (e) presentation. Adherence to the procedures within each stage will enhance methodological rigor (Whittemore & Knaff, 2005).
Problem Identification
Salivary cortisol collection is a safe, reliable, and noninvasive method for measuring HPA axis functioning in children. However, the acceptability of salivary cortisol collection in community samples of children is largely unknown. Furthermore, the complexities of salivary cortisol collection in community settings pose a number of challenges that may threaten the acceptability and feasibility of this method. This integrative review, therefore, was an investigation of the following questions: (a) is salivary cortisol collection in children acceptable in the community setting? And (e) what factors influence acceptability and feasibility when collecting salivary cortisol samples from children at home? With an improved understanding of the acceptability and feasibility of salivary cortisol collection in community samples, researchers can develop strategies to improve collection of salivary cortisol in community-based research studies.
Literature Search
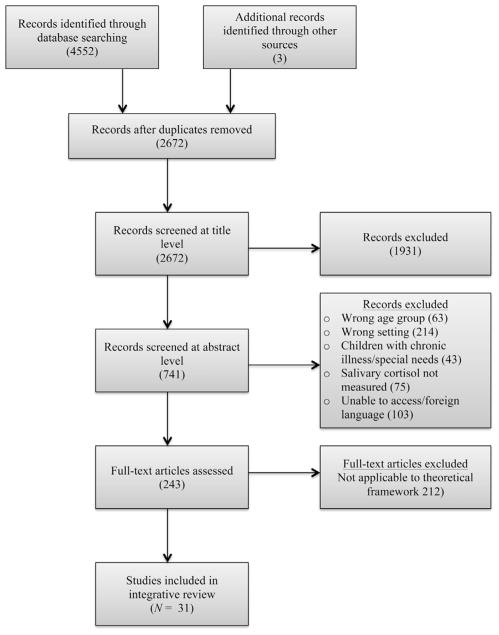
Electronic databases were searched using specific search terms and appropriate Boolean operators. Search terms included salivary, saliva, cortisol, hydrocortisone, stress, and physiologic stress. Electronic databases included MEDLINE, PsycINFO, EMBASE, and CINAHL. Results were limited to human studies, English language studies, and studies of children. Hand-searching of reference lists was also done, yielding three additional studies (see Fig. 1 for PRISMA flow diagram).
FIGURE 1.
Development of sample of articles for review (Moher et al., 2009).
At the title and abstract level, studies were screened for the following inclusion criteria: salivary cortisol collected in healthy children age 12 or younger, in the home setting, and by the child, caregiver, or member of the research team. Studies were excluded if saliva was collected in an inpatient or clinical setting (intensive care unit, dental procedures) or as part of a laboratory task (e.g., Tier social stress test, still face paradigm, Strange Situation Procedure). Additional exclusion criteria were children with chronic illnesses or a history of prematurity or developmental disorders because issues surrounding acceptability and feasibility likely differ in these special populations.
The resulting 243 full-text articles were reviewed for applicability to the three components of the Theory of Planned Behavior (attitudes, social norms, or perceived behavioral control). At least one component of the Theory of Planned Behavior was addressed in reports of 31 studies, and these were included in the integrative review.
Data Evaluation
The 31 reports in this integrative review were 27 quantitative studies, 1 qualitative study, and 3 review articles. Varying stages of childhood were also represented, with three studies of salivary cortisol in infancy, six in toddlerhood, seven at preschool age, and 12 at school age. As for applicability to the Theory of Planned Behavior, the vast majority of studies (n =28) addressed the third component of the theory: perceived behavioral control. Attitudes were addressed in two studies, and social norms were addressed in three. It is also important to note that acceptability and feasibility were primary objectives for discussion in only a handful of studies reviewed (n =4). For the remainder of studies (n =27), questions of acceptability and feasibility were secondary objectives, and in many cases they were subtly mentioned in the methods section on data collection.
Data Analysis and Presentation
Data from each full-text report were extracted and entered into a table for organization and synthesis. Information extracted included the study methodology, population assessed, primary findings, and component of the Theory of Planned Behavior that was addressed (See Table 3). The data were then evaluated for patterns and themes within each component of the theory, and comparisons were made according to age group, method, timing, and location of salivary cortisol collection (Whittemore & Knaff, 2005). Final analysis resulted in six categories of psychosocial influence on the acceptability and feasibility of salivary cortisol collection in community samples of children. Results are presented according to each component of the framework.
Table 3.
Characteristics of Studies Selected for Integrative Review, in Alphabetical Order by First Author
| Study | Method | Sample Characteristics | Pertinent Findings | Component of Theory of Planned Behavior |
|---|---|---|---|---|
| Bair-Merritt et al. (2012) | Pilot study | N =55; M age: 7.4 y; 38% girls | 29 families returned samples, typically within 2 weeks and before a reminder phone call was made. 5 provided info about why they had not returned the samples: 3 stated the child refused to provide saliva, 2 stated they kept forgetting. |
Perceived behavioral control |
| Davis, Donzella, Krueger, and Gunnar (1999) | Quantitative | N =70; M age: 8 y; range: 7–12y; 47% girls | 54 participants provided noon samples on at least 1 of 2 school days. Families sent collection materials to school with the child’s lunch, and children collected samples before eating. Data for evening and noon samples were analyzed separately over concerns that noon samples might be less reliable or reflect data from a group of more mature children. |
Perceived behavioral control |
| Dietrich et al. (2013) | Quantitative | N =2230 children; M age: 11.1 y; range: 10.0–12.6 y; 50.8% girls | 44 subjects excluded due to lack of compliance with the protocol (failing to take the first sample within 5 min of awakening or the second sample between 25 and 35 min after awakening). Non-compliant children had higher depression scores. |
Perceived behavioral control |
| Dougherty et al. (2009) | Quantitative | N =166; M age: 43.42 mo.; range: 3–4 y; 43.6% girls | In families who did not participate in cortisol collection, parents were rated as higher on parental hostility, and children had experienced more stressful life events, compared to participating families. | Perceived behavioral control |
| Dougherty et al. (2013) | Quantitative | N =470; M age: 6.23 y; range: 5.28–7.58 y; 46.5% girls | 255 families participated in salivary cortisol collection. Non-participating mothers had higher rates of lifetime depression compared to participating mothers. |
Perceived behavioral control |
| Egliston et al. (2007) | Review | N/A | Authors raised questions as to how “non-invasive” cortisol sampling really is when used with infants. Authors reported that 7–8 month old infants strongly resist cortisol sampling methods, which may threaten internal validity. Authors suggested that resistance to sampling may lead to selective attrition, with the most reactive infants being lost to the study, but engaging parents in sample collection may improve compliance. |
Perceived behavioral control |
| Fisher et al. (2007) | Quantitative | N =81; M age: 4.8 y; range: 2–6 y; 52% girls | 36 mothers and children completed the diurnal cortisol protocol. Diurnal sampling protocol described as time-consuming and challenging for parents and children. Younger children and children of single mothers were less likely to successfully complete the protocol. Participating and non-participating families did not differ in terms of family socioeconomic status, age of mother, number of children per family, maternal smoking, observed level of maternal emotional support, mothers’ childhood withdrawal, or child IQ. |
Perceived behavioral control |
| Granger et al. (2007) | Review | Included family life project (N =1193); range: 6–15 mo | In this study, white infants and those from middle to upper class families were less likely to be compliant than African-Americans or those from impoverished or low-income families. | Social norms |
| Groeneveld et al. (2010) | Quantitative | N =116; range: 20–40 mo.; 48% girls | Among other reasons, authors attributed low participation rate to childcare providers and parents disliking the idea of saliva samples being taken from the children. | Attitudes |
| Groschl et al. (2003) | Quantitative | N =252; range: 4 days–15 y; 50% girls | According to author experiences, salivettes are inappropriate for use with newborns due to danger of swallowing the swab, and refusal to keep the large swab inside their mouths. The authors report this procedure may cause stress to infants. | Perceived behavioral control |
| Gutteling et al. (2005) | Quantitative | N =29; M age: 5.31 y; 69% girls | 18.96% of the cortisol samples on the school days and 41.37% on weekend days were missing. Missing values due to the parents/children not having the opportunity to collect it, or samples not containing enough saliva for analysis. 13 children did not participate in any weekend saliva collection due to logistical problems. |
Perceived behavioral control |
| Hibel et al. (2014) | Quantitative | N =47; M age: 3.51 y; age range: 2–4 y | Differences in morning structure and routines on work and non-work days may affect sampling compliance. However, mothers did not self-report lower adherence on non-workdays, and analyses did not reveal a difference in children’s awakening cortisol on workdays compared to non-workdays. |
Perceived behavioral control |
| Jessop and Turner-Cobb (2008) | Review | N/A | Challenges in saliva sampling varied by stage of childhood and adolescence, including differences in school routines, dietary habits, and sleep patterns. Adherence may be more reliable in parent-controlled environment of early childhood, and less reliable during puberty and early teenage years. |
Perceived behavioral control |
| Kaitz et al. (2012) | Quantitative | N =174; M age: 45.26 mo.; 50.7% girls | 12% of the sample missed at least one collection time point. Children who were resistant but did not outright refuse saliva collection had higher cortisol levels than compliant children. Children who resisted were perceived as having more internalizing symptoms than compliant children. |
Perceived behavioral control |
| Laurent et al. (2014) | Quantitative | N =177; range: 3–6 y; 50% girls | 5.8% of samples were missing because the parent failed to collect and/or return the sample, 1.0% missing because the sampling time was incorrect, and 21.4% were missing for other reasons (caseworker or family refusal, lost sample, child unable to do the collection). | Perceived behavioral control |
| Marsman et al. (2012) | Quantitative | N =1594; M age: 11.08 y; 50.3% girls | Children who participated in salivary cortisol collection did not differ from non-participating children in terms of gender, behavioral problems, perceived parental rejection, or perceived parental emotional warmth. Slight difference in SES between participating and non-participating children. |
Perceived behavioral control |
| Marsman et al. (2008) | Quantitative | N =1768; M age: 11.09 y; 50.8% girls | Children who participated in collection did not differ from non-participating children in terms of gender or pubertal status. Children who did not participate were slightly older and had a higher mean body mass index than participating children. |
Perceived behavioral control |
| Michels et al. (2012) | Quantitative | N =323; M age: 8.41 y; 51% girls | Cortisol levels differed between parents who reported sampling at the appropriate time and those who reported other times. Families who were non-compliant did not differ from others in terms of parental education, family structure, age or sex. |
Perceived behavioral control |
| Michels et al. (2012) | Quantitative | N =444; M age: 8.4 y; range: 5–11 y; 50.7% girls | Most often missing were last two samples on second day of collection. Parents indicated that the specific timing and restrictions of sampling procedures were difficult, rather than sampling itself. Deviance from protocol often included food intake/tooth brushing prior to saliva collection, caffeine intake or physical activity, or use of influencing medications. |
Perceived behavioral control |
| O’Farrelly and Hennessy (2013). | Qualitative | N =17; range: 2–5 y | Caregivers may have uncertainties and misconceptions regarding use for biological data, which may be heightened when asked to make decisions for their child. Theme of “lost for words:” Caregivers unable to articulate concerns due to unfamiliarity of biobehavioral research. Encouraging child participation in procedures and ensuring collection from a familiar adult would be helpful in younger children. |
Attitudes social norms perceived behavioral control |
| Rotenberg and McGrath (2014) | Quantitative | N =201; M age: 12.67 y; range: 8–18 y; 45.3% girls | Both children and adolescents completed salivary cortisol collection upon awakening. Parents were highly involved with saliva collection in both age groups, with parents completing daily log for 97% of participants. |
Perceived behavioral control |
| Saridjan et al. (2010) | Quantitative | N =366; range: 11.7–19.3 mo.; 43.4% girls | 602/822 (68%) parents returned one or more saliva samples. Reported reasons for non-response: 1% too busy, 32% tried but failed to obtain saliva samples, 67% gave no reason. No difference in socioeconomic status, maternal smoking, or birth weight between participants and non-responders. Infants who successfully completed all saliva samples (n =366) were more likely to be boys. |
Perceived behavioral control |
| Smith and Dougherty (2014) | Quantitative | N =95; M age: 49.93 mo.; range: 36–71 mo.; 53.1% girls | Parental compliance with saliva sampling procedures assessed based on parent self-report and an electronic monitoring device. Self reported compliance (83.0%) was significantly higher than objective compliance (68.8%) for the 2-day sampling period. From the first to second day of sampling, self reported compliance dropped from 84.5% to 79.5% and objective compliance dropped from 72.9% to 64.6%. |
Perceived behavioral control |
| Sondeijker et al. (2008) | Quantitative | N =2935; M age: 11.09 y; 50.8% girls | 2,230 (76%) of children participated in salivary cortisol collection. Participants and non-participants did not differ on sociodemographic variables, mental health, or teacher-rated problem behaviors. Children with highly disruptive behaviors and low socioeconomic status were less likely to successfully complete sampling. |
Perceived behavioral control |
| Sondeijker et al. (2007) | Quantitative | N =2935; M age: 11.09 y; 50.8% girls | 1,768 children provided saliva samples. No difference in gender, pubertal development, or behavioral problems between completers and non-completers. Children who did not provide samples were slightly older and scored higher on antisocial behavior than those who provided samples. |
Perceived behavioral control |
| Stalder et al. (2013) | Quantitative | N =33; M age: 6.52 mo.; range: 2–12 mo | Number of missing study days was positively associated with infants’ current age, height and weight, suggesting that it was more difficult to obtain valid cortisol data from older and more physically developed infants. | Perceived behavioral control |
| Vermeer et al. (2010) | Quantitative | N =45; M age: 32 mo.; range: 18–40 mo | 43% of saliva samples missing: parents or caregivers forgot to collect the saliva or the child refused to cooperate (22%), the samples did not contain enough saliva for analysis (20%), or samples were not within the detection limit for cortisol (0.8%). Children with complete cortisol data did not differ with respect to age, gender, or temperament. |
Perceived behavioral control |
| Watamura et al. (2010) | Quantitative | N =79; M age: 4 y; range: 2–5 y; 46% girls | Discrepancy between reported and recorded collection times was positively correlated with cortisol levels on both weekend days and days when children attended childcare. | Perceived behavioral control |
| Watamura et al. (2003) | Quantitative | N =67; range: 2–38 mo.; 58% girls | Families of 36 children successfully collected samples at home. Children with and without saliva samples did not differ on ethnicity or maternal education. | Social norms perceived behavioral control |
| Watamura et al. (2004) | Quantitative | N =77; range: 12–36 mo | Difficulty with saliva collection reported in this young age range. In the first 51 families, nearly 60% of families were unable to provide any samples, and an additional 30% were missing some samples. After revising sampling procedures, 75% of families returned full saliva samples, and saliva sample volumes also increased. |
Perceived behavioral control |
| Waynforth (2007). | Quantitative | N =75; M age: 5.1 y; range: 3–8 y | Most commonly reported reasons for noncompliance were that participation took too long, or the family was too busy to successfully complete the saliva collection regime and/or daily diary. Six parents were unable to collect saliva from their children using the equipment provided, and two parents did not follow instructions closely enough for their data to be useful. Non-compliance not correlated with parent’s full-time employment or socioeconomic status. |
Perceived behavioral control |
Note. M =mean. Y =years, Mo. =months.
Results
Attitudes: Uncertainty and Misconceptions
Two studies provided insight into the perceptions and inhibitions that may influence parental decision-making involving salivary cortisol collection. In a quantitative study of 20–40-month-old children, authors attributed an overall low participation rate to parental attitudes, stating that some parents “disliked the idea of saliva samples taken from the children” (Groeneveld, Vermeer, van IJzendoorn, & Linting, 2010, p. 20). Additionally, in a study of preschool-aged children, researchers conducted qualitative interviews to better understand parental and child perceptions following salivary cortisol collection. While most parents reported no concerns about saliva collection, others were described as “lost for words,” citing that they had concerns about saliva sampling but found these concerns difficult to articulate. The authors speculated that this may be due to unfamiliarity with biobehavioral research in the community, and that these uncertainties and misconceptions may be heightened when biological measures are assessed outside of the clinical setting (O’Farrelly & Hennessy, 2013).
Social Norms: Cultural and Ethnic Values
Social perceptions of biobehavioral research are likely to influence participation in salivary cortisol collection, and these perceptions may differ by race, culture, or social class (Corbie-Smith, Thomas, & St. George, 2002). However, none of the studies in this review included evaluation of community-wide perceptions of salivary cortisol collection. Two teams did evaluate the relationship between ethnicity and adherence to sampling procedures and found conflicting results. In a study of infants and toddlers (N =67), researchers found that successful collection of salivary cortisol at home did not differ based on ethnicity in a sample reported as 76% Caucasian American, 12% Asian American, 9% African American, and 3% Hispanic American (Watamura, Donzella, Alwin, & Gunnar, 2003). In contrast, in a study of infants in the Family Life Project, a large epidemiological study of families living in rural areas of high child poverty, ethnicity was associated with non-adherence to collection protocols that resulted in high rates of missing data. In that study, white families (n =688) were more likely to be non-adherent to salivary cortisol collection protocols than were African American families (n =500; Granger et al., 2007). These findings suggest that cultural and ethnic values may influence the social norms surrounding participation in biobehavioral research, but further investigation is required.
Social Norms: Family Rules
Family rules and values are also likely to play a significant role in the decision to participate in research involving salivary cortisol collection. Family rules about spitting were reported as one reason for children to refuse participation (O’Farrelly & Hennessy, 2013). In a content analysis of home visitors, field notes in the Family Life Project, missing saliva samples were often related to caregivers, refusal to wake sleeping infants for saliva collection (Granger et al., 2007). Other family rules and values were not explicitly examined in the studies included in this review.
Perceived Behavioral Control: Difficulty Following Protocols and Procedures
The complexity of salivary cortisol collection can place a burden on caregivers and lead to perceived difficulty with the procedures. The precise timing required, and restrictions such as avoiding certain food products, are perceived as particularly difficult (Michels et al., 2012). Incorporating salivary cortisol collection into family routines is also perceived as a challenging task, with caregivers frequently reporting they were “too busy” or “kept forgetting” to collect the required samples (Bair-Merritt, Johnson, Okelo, & Page, 2012; Gutteling, de Weerth, & Buitelaar, 2005; Michels et al., 2012; Saridjan et al., 2010; Vermeer et al., 2010; Waynforth, 2007). The availability of family resources also appeared to play an important role, with teams reporting less adherence in families with low socioeconomic status, single-parent families, caregivers with a history of mental illness, and children experiencing more stressful life events (Dougherty, Klein, Olino, Dyson, & Rose, 2009; Dougherty et al., 2013; Fisher et al., 2007; Marsman et al., 2012; Sondeijker et al., 2008).
Perceived Behavioral Control: Burden of Multiple Samples
Collection of multiple samples placed an additional burden on family participants and may contribute to noncompliance or attrition. This was highlighted in two studies of sampling compliance over time. In a study of preschool-aged children, researchers used electronic monitoring to measure compliance, and found that objective compliance decreased from 72.9% to 64.6% from the first to second day of sampling (Smith & Dougherty, 2014). Similarly, in a study of school-aged children, researchers found that most missing samples were from the final two samples taken on the second day of sampling, suggesting that this burden leads to attrition over time (Michels et al., 2012).
The timing of samples may also be perceived as burdensome and influence compliance. In two studies, researchers noted less compliance and more missing data on weekends as opposed to weekdays, noting that this difficulty may be related to a lack of routine and structure on days without work or school (Gutteling et al., 2005; Hibel, Trumbell, & Mercado, 2014).
Perceived Behavioral Control: Child Refusal or Resistance
Child refusal or resistance to participation in saliva sampling was reported by a number of research teams, and refusal or resistance varied by age, sampling procedure, and child characteristics (Bair-Merritt et al., 2012; Fisher et al., 2007; Laurent, Gilliam, Bruce, & Fisher, 2014; Saridjan et al., 2010; Vermeer et al., 2010). Groschl, Rauh and Dorr (2003) noted that salivettes may be inappropriate for saliva collection in young infants because they are unable to keep a large swab in their small mouths. The authors reported that this not only led to infant stress but posed a major safety concern due to risk of swallowing the swab (Groschl et al., 2003). Two other teams reported that as infants became older and more physically developed, such as at age 7–8 months, they tended to resist more strongly, and obtaining valid data became much more difficult (Egliston, McMahon, & Austin, 2007; Stalder et al., 2013). In older children, parental supervision was essential to obtaining valid saliva samples. Jessop and Turner-Cobb (2008) suggested that adherence to collection protocol is better in the parent-controlled environment of early childhood and becomes much more challenging in older children with changing routines and less parental supervision. This is consistent with reports that that younger children were more likely than early adolescents to provide valid saliva samples, likely a result of parental vigilance during sampling procedures (Marsman et al., 2008; Rotenberg & McGrath, 2014; Sondeijker et al., 2007).
Family and child characteristics may also have an influence on refusal or resistance to salivary cortisol collection. In a study of preschool-aged children, researchers found that children who were resistant to sample collection had higher cortisol levels than their non-resistant peers. These children also displayed more internalizing symptoms, such as fearfulness and withdrawal, based on both parent and teacher reports (Kaitz et al., 2012). Researchers in other studies noted less participation by children with antisocial behaviors, highly disruptive behaviors, higher body mass index, or more stressful life events, and by caregivers with greater hostility, lifetime depression, and disorganization in the home environment (Dougherty et al., 2009, 2013; Marsman et al., 2008; Sondeijker et al., 2007, 2008; Watamura, Coe, Laudenslager, & Robertson, 2010).
Discussion
Salivary cortisol is a widely used measure of HPA axis functioning and offers a number of advantages over other biological measures because it is safe, reliable, and can be collected in large community samples (Keil, 2012; Tryphonopoulos et al., 2014). In this review, a number of factors related to perceived behavioral control were noted to influence acceptability and feasibility of salivary cortisol collection. The results of this review indicate that certain child and family characteristics, such as internalizing behaviors, experience of stressful life events, and mental health issues, not only influence participation and compliance, but may also be associated with higher cortisol levels. Thus, the children and families who are potentially most important to investigate may be selectively dropping out or ineffectively participating in the study, leading to an inaccurate or incomplete picture of cortisol levels within a particular sample. However, this information was often mentioned as an aside rather than being the main focus of research reports. Detailed information on the participant characteristics that lead to attrition, refusal, or non-adherence to sampling procedures will improve future research involving salivary cortisol collection. This information will not only improve recruitment and adherence in future studies, but also can be used to predict and respond to selective attrition within a sample.
While information on the acceptability and feasibility of salivary cortisol collection in community samples of children is limited, research conducted in other populations and with other biomarkers provides additional insight that may be used to improve salivary cortisol collection methods. In a study comparing saliva collection methods for inflammatory cytokines, researchers found that children age 9–17 years preferred the oral swab method to passive drool, reporting oral swabs were quicker and easier to use (Hiremath et al., 2015). In a qualitative study of parental receptivity to child biomarker testing for tobacco smoke, researchers found that opposition to testing was associated with reluctance to cause short-term discomfort, perceived powerlessness, and mistrust of research (Rosen et al., 2015). In a study of acceptability of in-home finger-stick blood samples in an urban, low-income sample of adults, researchers found that participants were comfortable with blood sampling but required reassurance that the blood would not be used for other purposes (Bryant Borders, Grobman, Amsden, Collins, & Holl, 2007).
Thus, while specific preferences regarding biomarker collection may differ by method or population, identification of recurrent themes in community samples may improve the acceptability of biobehavioral research on a larger scale. With better reporting, improved collaboration among biobehavioral pediatric researchers may lead to the development of strategies and best practices for salivary cortisol collection according to child age or developmental level. For example, Donzella, Kertes, and Gunnar (2004) described a change in strategy for saliva collection in toddlers after nearly 60% of families were unable to successfully collect samples at home. By sharing information on the success or failure of strategies used to improve adherence, the acceptability and feasibility of community-based biobehavioral research methods will be greatly enhanced.
The results of this integrative review indicate that despite its wide use, there is a dearth of available information on the attitudes, norms, and perceived control beliefs affecting acceptability and feasibility of collecting salivary cortisol in community samples of children. Of the 243 full-text articles reviewed in this study, only 31 addressed one or more components of the Theory of Planned Behavior. Issues surrounding acceptability and feasibility were explicitly examined in only four studies, while the remainder of reports required detailed examination of text to identify these issues. Further, the majority of studies addressed the third component of the theoretical framework, perceived behavioral control, and very little information was obtained regarding attitudes or social norms. Thus, in order to fully answer the questions posed in this review, further research on the acceptability and feasibility of salivary cortisol collection is required.
Caregiver and child attitudes towards salivary cortisol collection are likely to have a major influence on participation and engagement in biobehavioral research studies. Identifying misconceptions about salivary cortisol collection, particularly related to safety or intended use of the sample, will have important implications for enhancing recruitment, improving the informed consent process, properly educating participants, and increasing adherence to sampling procedures. It is important for researchers to more fully understand participants, misconceptions or fears regarding inappropriate use of biological measures, such as use of samples for drug or genetic testing without consent. This is especially important for research with families from ethnic minority backgrounds, particularly African American families, as the history of racial discrimination and unethical treatment of African Americans in the United States may justifiably lead to mistrust of biobehavioral research (Boulware, Cooper, Ratner, LaVeist, & Powe, 2003; Corbie-Smith et al., 2002; Gamble, 1997).
To address potential misconceptions regarding the use of biological measures in research studies, a detailed description of plans for current and future uses of biological specimens must be included in the informed consent process. In the Washington Heights Inwood Informatics Infrastructure for Comparative Effectiveness Research (WICER) project, researchers reported using a stepped approach to obtaining informed consent, in which participants consented to participate in both ongoing and future research activities with varying levels of specificity (Bakken & Reame, 2016). This stepped approach not only preserves participant autonomy but also is likely to enhance acceptability of biobehavioral studies and foster a relationship of trust between participants and the research team.
Identifying social norms, including cultural, ethnic, and family values that influence participation in salivary cortisol collection, will also improve acceptability of biomarker collection and allow researchers to build trust within communities. When the cultural or ethnic background of a research team differs from the community being studied, implicit cultural meanings associated with biomarker collection may not be readily apparent and thus should be respectfully examined. Families living within communities may represent multiple cultural or ethnic backgrounds, and thus researchers must be careful not to assume that a given set of social norms can be attributed to all individuals within that community. Social norms may differ according to gender, socioeconomic status, or geographic location, and thus attitudes and social norms must be carefully considered prior to each study. Assessing attitudes and social norms may best be accomplished through qualitative interviews, focus groups or community-based participatory research, so that researchers can fully explore perceptions and strategies for collection of biological measures with community members.
Limitations
This integrative review was limited by the lack of explicit discussion regarding acceptability and feasibility in the vast majority of reports reviewed. Although each full-text article was reviewed in great detail, it is possible that studies appropriate for inclusion were missed due to the brevity of discussion of acceptability and feasibility. This integrative review may also have been limited by use of a theoretical framework to guide the selection of studies and discussion of results, although it provided a conceptual structure to identify and organize pertinent information. While this may have led to inadvertent exclusion of studies that did in fact fit within the framework, this is considered unlikely, as each study was briefly reviewed in the initial stages of screening, and the theoretical framework was broadly defined. Finally, reporting bias within the literature may potentially influence the results of this review. Researchers who had little difficulty with salivary cortisol collection may have been unlikely to report issues or strategies for improvement, and thus the results of this review may only reflect studies with less successful salivary cortisol collection.
Conclusions
As nurses continue to incorporate biomarkers such as salivary cortisol into community-based pediatric research studies, it is essential to investigate the acceptability and feasibility of these methods in community samples. This is important for research with all noninvasive biomarkers, such as hair cortisol and salivary cytokines, as the simplicity of these collection methods may cause researchers to overlook potential challenges rooted in individual, family, or community attitudes and social norms related to biological data collection in the community setting. Identification of barriers to collection procedures will also enable development of strategies to improve feasibility and validity of community-based biobehavioral research methods, which may lead to greater engagement and trust amongst community members. Thus, the acceptability and feasibility of bio-marker sample collection in community-dwelling samples is an essential area for future research.
Acknowledgments
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR008346. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Funding for the author is also supported by The Jonas Nurse Leaders Scholars Program at Yale University. The Jonas Nurse Leaders Scholars Program at Yale University is made possible by a grant from the Jonas Center for Nursing and Veterans Healthcare. The author would like to acknowledge Lois Sadler for her helpful comments in the preparation of this manuscript.
References
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-t. [DOI] [Google Scholar]
- Bair-Merritt MH, Johnson SB, Okelo S, Page G. Intimate partner violence exposure, salivary cortisol, and childhood asthma. Child Abuse & Neglect. 2012;36:596–601. doi: 10.1016/j.chiabu.2011.12.002. doi: http://dx.doi.org/10.1016/j.chiabu.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken S, Reame N. The promise and potential perils of big data for advancing symptom management research in populations at risk for health disparities. Annual Review of Nursing Research. 2016;34:247–260. doi: 10.1891/0739-6686.34.247. [DOI] [PubMed] [Google Scholar]
- Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Reports. 2003;118:358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant Borders AE, Grobman WA, Amsden LB, Collins ET, Holl JL. Factors that influence the acceptability of collecting in-home finger stick blood samples in an urban, low-income population. Journal of Health Care for the Poor and Underserved. 2007;18:100–115. doi: 10.1353/hpu.2007.0004. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR. Integrating biological measures into the design and evaluation of preventive interventions. Development and Psychopathology. 2008;20:743. doi: 10.1017/s0954579408000357. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St George DMM. Distrust, race, and research. Archives of Internal Medicine. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35:188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Ormel J, Buitelaar JK, Verhulst FC, Hoekstra PJ, Hartman CA. Cortisol in the morning and dimensions of anxiety, depression, and aggression in children from a general population and clinic-referred cohort: An integrated analysis. The TRAILS study. Psychoneuroendocrinology. 2013;38:1281–1298. doi: 10.1016/j.psyneuen.2012.11.013. doi: http://dx.doi.org/10.1016/j.psyneuen.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Olino TM, Dyson M, Rose S. Increased waking salivary cortisol and depression risk in preschoolers: The role of maternal history of melancholic depression and early child temperament. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2009;50:1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x. doi: http://dx.doi.org/10.1111/j.1469-7610.2009.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Olino TM, Dyson MW, Bufferd SJ, Rose SA, Klein DN. Maternal psychopathology and early child temperament predict young children’s salivary cortisol 3 years later. Journal of Abnormal Child Psychology. 2013;41:531–542. doi: 10.1007/s10802-012-9703-y. doi: http://dx.doi.org/10.1007/s10802-012-9703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: Conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology. 2007;32:1–13. doi: 10.1016/j.psyneuen.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Fisher DB, Serbin LA, Stack DM, Ruttle PL, Ledingham JE, Schwartzman AE. Intergenerational predictors of diurnal cortisol secretion in early childhood. Infant and Child Development. 2007;16:151–170. [Google Scholar]
- Gamble VN. Under the shadow of Tuskegee: African Americans and health care. American Journal of Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Johnson SB, Szanton SL, Out D, Schumann LL. Incorporating salivary biomarkers into nursing research: An overview and review of best practices. Biological Research for Nursing. 2012;14:347–356. doi: 10.1177/1099800412443892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua G. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Groeneveld MG, Vermeer HJ, van IJzendoorn MH, Linting M. Children’s wellbeing and cortisol levels in home-based and center-based childcare. Early Childhood Research Quarterly. 2010;25:502–514. [Google Scholar]
- Groschl M, Rauh M, Dorr HG. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clinical Chemistry. 2003;49:1688–1691. doi: 10.1373/49.10.1688. [DOI] [PubMed] [Google Scholar]
- Gröschl M, Rauh M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids. 2006;71:1097–1100. doi: 10.1016/j.steroids.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, Granger DA. Measuring salivary cortisol in studies of child development: Watch out-what goes in may not come out of saliva collection devices. Developmental Psychobiology. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Trumbell JM, Mercado E. Work/non-workday differences in mother, child, and mother-child morning cortisol in a sample of working mothers and their children. Early Human Development. 2014;90:1–7. doi: 10.1016/j.earlhumdev.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath G, Olive A, Shah S, Davis CM, Shulman RJ, Devaraj S. Comparing methods to collect saliva from children to analyze cytokines related to allergic inflammation. Annals of Allergy, Asthma and Immunology. 2015;114:63–64. doi: 10.1016/j.anai.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: A focus on health and disease in children. Stress. 2008;11:1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Kaitz M, Sabato R, Shalev I, Ebstein R, Mankuta D. Children’s noncompliance during saliva collection predicts measures of salivary cortisol. Developmental Psychobiology. 2012;54:113–123. doi: 10.1002/dev.20580. doi: http://dx.doi.org/10.1002/dev.20580. [DOI] [PubMed] [Google Scholar]
- Keil MF. Salivary cortisol: A tool for biobehavioral research in children. Journal of Pediatric Nursing. 2012;27:287–289. doi: 10.1016/j.pedn.2012.02.003. doi: http://dx.doi.org/10.1016/j.pedn.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1016/j.lfs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Gierens A, Hellhammer DH, Wust S, Schlotz W. Salivary cortisol in ambulatory assessment-some dos, some don’ts, and some open questions. Psychosomatic Medicine. 2012;74:418–431. doi: 10.1097/PSY.0b013e31825434c7. doi: http://dx.doi.org/10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Gilliam KS, Bruce J, Fisher PA. HPA stability for children in foster care: Mental health implications and moderation by early intervention. Developmental Psychobiology. 2014;56:1406–1415. doi: 10.1002/dev.21226. doi: http://dx.doi.org/10.1002/dev.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman R, Nederhof E, Rosmalen JG, Oldehinkel AJ, Ormel J, Buitelaar JK. Family environment is associated with HPA-axis activity in adolescents. The TRAILS study. Biological Psychology. 2012;89:460–466. doi: 10.1016/j.biopsycho.2011.12.013. doi: http://dx.doi.org/10.1016/j.biopsycho.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Marsman R, Swinkels SH, Rosmalen JG, Oldehinkel AJ, Ormel J, Buitelaar JK. HPA-axis activity and externalizing behavior problems in early adolescents from the general population: The role of comorbidity and gender: The TRAILS study. Psychoneuroendocrinology. 2008;33:789–798. doi: 10.1016/j.psyneuen.2008.03.005. doi: http://dx.doi.org/10.1016/j.psyneuen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Michels N, Sioen I, De Vriendt T, Huybrechts I, Vanaelst B, De Henauw S. Children’s morning and evening salivary cortisol: Pattern, instruction compliance and sampling confounders. Hormone Research in Pediatrics. 2012;77:27–35. doi: 10.1159/000334412. doi: http://dx.doi.org/10.1159/000334412. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, … Tugwell P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6:7. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrelly C, Hennessy E. Considering the realities of salivary research with young children: What’s spit all about? International Journal of Social Research Methodology: Theory & Practice. 2013;16:323–335. doi:0.1080/13645579.2012.705640. [Google Scholar]
- Poll EM, Kreitschmann-Andermahr I, Langejuergen Y, Stanzel S, Gilsbach JM, Gressner A, Yagmur E. Saliva collection method affects predictability of serum cortisol. Clinica Chimica Acta. 2007;382:15–19. doi: 10.1016/j.cca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Rosen LJ, Tillinger E, Guttman N, Rosenblat S, Zucker DM, Stillman F, Myers V. Parental receptivity to child bio-marker testing for tobacco smoke exposure: A qualitative study. Patient Education and Counseling. 2015;98:1439–1445. doi: 10.1016/j.pec.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ. Sampling compliance for cortisol upon awakening in children and adolescents. Psychoneuroendocrinology. 2014;40:69–75. doi: 10.1016/j.psyneuen.2013.10.002. doi: http://dx.doi.org/10.1016/j.psyneuen.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, … Tiemeier H. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The generation R study. Hormones & Behavior. 2010;57:247–254. doi: 10.1016/j.yhbeh.2009.12.001. doi: http://dx.doi.org/10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Smith VC, Dougherty LR. Noisy spit: Parental noncompliance with child salivary cortisol sampling. Developmental Psychobiology. 2014;56:647–656. doi: 10.1002/dev.21133. doi: http://dx.doi.org/10.1002/dev.21133. [DOI] [PubMed] [Google Scholar]
- Sondeijker FE, Ferdinand RF, Oldehinkel AJ, Tiemeier H, Ormel J, Verhulst FC. HPA-axis activity as a predictor of future disruptive behaviors in young adolescents. Psychophysiology. 2008;45:398–404. doi: 10.1111/j.1469-8986.2008.00639.x. doi: http://dx.doi.org/10.1111/j.1469-8986.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Sondeijker FE, Ferdinand RF, Oldehinkel AJ, Veenstra, Tiemeier H, Ormel J, Verhulst FC. Disruptive behaviors and HPA-axis activity in young adolescent boys and girls from the general population. Journal of Psychiatric Research. 2007;41:570–578. doi: 10.1016/j.jpsychires.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Stalder T, Baumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: Ontogeny and associations with development-related variables. Psychoneuroendocrinology. 2013;38:552–559. doi: 10.1016/j.psyneuen.2012.07.015. doi: http://dx.doi.org/10.1016/j.psyneuen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Torraco R. Writing integrative literature reviews: Guidelines and examples. Human Resource Development Review. 2005;4:356–367. [Google Scholar]
- Tryphonopoulos PD, Letourneau N, Azar R. Approaches to salivary cortisol collection and analysis in infants. Biological Research for Nursing. 2014;16:398–408. doi: 10.1177/1099800413507128. doi: http://dx.doi.org/10.1177/1099800413507128. [DOI] [PubMed] [Google Scholar]
- Vermeer HJ, Groeneveld MG, Larrea I, van IJzendoorn MH, Barandiaran A, Linting M. Child care quality and children’s cortisol in Basque country and the Netherlands. Journal of Applied Developmental Psychology. 2010;31:339–347. doi: 10.1016/j.appdev.2010.05.001. [DOI] [Google Scholar]
- Watamura SE, Coe CL, Laudenslager ML, Robertson SS. Child care setting affects salivary cortisol and antibody secretion in young children. Psychoneuroendocrinology. 2010;35:1156–1166. doi: 10.1016/j.psyneuen.2010.02.001. doi: http://dx.doi.org/10.1016/j.psyneuen.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: Age differences and behavioral correlates. Child Development. 2003;74:1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Waynforth D. The influence of parent-infant cosleeping, nursing, and childcare on cortisol and SIgA immunity in a sample of British children. Developmental Psychobiology. 2007;49:640–648. doi: 10.1002/dev.20248. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Knafl K. The integrative review: Updated methodology. Journal of Advanced Nursing. 2005;52:546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]