Abstract
Bariatric surgery is increasingly employed to improve fertility and reduce obesity related co-morbidities in obese women. Surgical weight loss not only improves the chance of conception but reduces the risk of pregnancy complications including pre-eclampsia, gestational diabetes, and macrosomia. However, bariatric procedures increase the incidence of intrauterine growth restriction (IUGR), fetal demise, thromboembolism and other gestational disorders. Using our rodent model of vertical sleeve gastrectomy (VSG), we tested the hypothesis that VSG in diet-induced, obese dams would cause immune and placental structural abnormalities that may be responsible for fetal demise during pregnancy. VSG dams studied on gestational day (G) 19 had reduced circulating T cell (CD3+ and CD8+) populations compared to lean or obese controls. Further, local interleukin 1 β and interleukin 1 receptor antagonist mRNA were increased in placenta of VSG dams. Placental barrier function was also affected, with increased trans-placental permeability to small molecules, increased matrix metalloproteinase 9 expression, and increased apoptosis in VSG. Furthermore, we identified increased placental mTOR signaling that may contribute to preserving the body weight of the fetuses during gestation. These changes occurred in the absence of a macronutrient deficit or gestational hypertension in the VSG dams. In summary, previous VSG in dams may contribute to fetal demise by affecting maternal immune system activity and compromise placental integrity.
Keywords: VSG, bariatric surgery, pregnancy, placenta, development
INTRODUCTION
Obesity in women leads to reproductive difficulties that include oligo/anovulation, menstrual cycle disturbances and inappropriate sex hormone levels (3, 25, 29, 37, 56). In addition, obesity results in reduced fertility (22, 26), reduced ovarian reserve (3), and reduced quality/quantity of embryos during in vitro fertilization (IVF) (46). Pregnant obese women have a higher-than-normal risk for preeclampsia (13, 58), gestational diabetes (45, 58), macrosomic births (10, 13, 48) and still births (1, 4, 53). Weight loss prior to pregnancy can lead to improvements in these indices; however, long-term body weight loss is difficult to achieve.
Bariatric surgery can be extremely effective for long-term body weight loss and amelioration of the comorbidities of obesity (5, 7, 12, 14). About 80% of the recipients of surgical weight loss are women of whom approximately half are of child bearing age. (52). The major advantage to bariatric surgery during the child-bearing years is the potential for improving reproductive capacity as well as achieving a healthier pregnancy. Improvements after bariatric surgery include return of normal menstrual cycles (16, 55), improved levels of reproductive hormones (44), recovery of luteal function (41), increased spontaneous (unassisted) pregnancies (36, 54), improvements in assisted pregnancies (36), lower risk of gestational diabetes and preeclampsia (19, 27), and reduced risk of large-for-gestational age (LGA) babies (27, 47, 48, 59). Thus, bariatric surgery holds great promise to improve reproductive outcomes for obese women interested in child-bearing.
In contrast to the many potential benefits of bariatric surgery, a number of negative pregnancy outcomes have also been reported with bariatric surgery. These negative outcomes include an increased risk for abnormal glucose kinetics and hypoglycemia during pregnancy (17), increased risk for miscarriage (20), small-for-gestational age babies (SGA) or intrauterine growth restricted infants (IUGR) (2, 19, 38), birth at a reduced gestational age (20, 27), preterm birth (19, 51) and still-birth or neonatal death (21, 27). These adverse events may be due to maternal micronutrient deficiencies following surgical weight loss or the timing of pregnancy with respect to the bariatric surgery (24, 33). To date, however, there are no conclusive data to support these hypotheses.
Many of the positive metabolic benefits of bariatric surgery realized in humans are also observed in rodent models and thus rodents offer a relevant model for testing many aspects of the surgery. Obese rats that undergo bariatric surgery demonstrate body weight loss, body fat loss, improved glucose tolerance and improved lipid profiles (8, 23, 49). Furthermore, our previous studies in rats are consistent with reports of IUGR in human bariatric births as offspring born to rat dams that had received vertical sleeve gastrectomy (VSG) prior to pregnancy were smaller and shorter at postnatal day 2 (PND2). This finding was true irrespective of whether the dams were maintained on a low-fat chow or palatable high-fat diet (HFD) before and after surgery. However, the long term outcomes for offspring of dams consuming HFD after VSG were much worse (23), including the development of glucose intolerance, greater levels of adiposity, and less lean mass compared to the offspring of obese controls under the same conditions (23). This was despite no gross differences in macro- or micronutrients intake or levels.
In the present studies we tested the hypothesis that surgical weight loss impacted maternal immune function and placental physiology during gestation. Because we knew that offspring were smaller than controls at PND2, we reasoned that the insult(s) to development and growth occurred during gestation and that probing the in utero environment may uncover some of the contributing factors driving the IUGR phenotype. We used our female rat model of VSG to probe some of our hypotheses concerning the effects of maternal bariatric surgery on the morbidity and mortality of offspring. Here, we focused specifically on gestation and asked whether clinically-identified causes of reduced birth weight and fetal demise might be driving the negative issues in VSG offspring. We investigated macronutrient intake during pregnancy and pregnancy weight gain, whether blood pressure contributes to fetal loss and low weight as it does in pre-eclampsia and whether reproductive hormone levels were appropriate to sustain a pregnancy. In addition, to define any potential maternal immunologic deficits, plasma cytokine levels and various immune cell types were determined. Finally, placental molecular structure and function were also examined to identify systemic transcriptome changes that could lead to placental dysfunction. The present work suggests that maternal immune changes and local placental dysfunction may contribute to the problems identified with pregnancy post-VSG.
METHODS
Animals
All procedures for animal use complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Female and male Long Evans rats (200-225g) (Harlan, Indianapolis, IN) were initially multiply housed and maintained in a room on a 12/12-h light/dark cycle at 25 °C and 50-60% humidity with ad libitum access to water. After 1 week of acclimatization to the vivarium, female rats were placed on a palatable high-fat diet (HFD) (#D03082706, Research Diets, New Brunswick, NJ, 4.54 kCal/g; 40% fat, 46% carbohydrate, 15% protein) for 4 weeks prior to surgery. Animals were divided into sham-VSG or VSG groups. Following surgeries, one group of sham-VSG continued on HFD and were considered obese controls. VSG animals and one group of sham-VSG were switched to standard rat chow (Harlan 8640, Harlan SD) for the remainder of the study. The sham-VSG on chow become lean as a result of switch to chow (Lean controls), and thus became body weight controls. In total, we used four cohorts of female rats animals totaling 100 animals of which 65 animals successfully became pregnant (Lean, N = 23; VSG = 19; Obese controls = 23) and were included in the study. Of the non-pregnant females, N=12 were used as controls for blood pressure measurements. The general paradigm for the studies is shown in Table 1.
Table 1.
Plasma parameters from Lean, VSG and Obese G19 dams.
| Lean | VSG | Obese | Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| triglycerides | (mg/dL) | 558.4 | ± | 57.1 | 71.59 | ± | 18.9 | 804.9 | ± | 147.70 | P < 0.05 Lean vs. Obese; P < 0.05 Lean vs. VSG |
| cholesterol | (mg/dL) | 94.2 | ± | 4.0 | 83.70 | ± | 9.6 | 103.2 | ± | 4.73 | |
| insulin | (ng/dL) | 2.6 | ± | 0.5 | 2.584 | ± | 0.4 | 4.738 | ± | 0.57 | P < 0.05 Lean vs. Obese |
| leptin | (pg/mL) | 423.3 | ± | 54.8 | 228.90 | ± | 41.3 | 1154.00 | ± | 285.60 | P < 0.05 Lean vs. Obese; P < 0.05 Lean vs. VSG |
| testosterone | (pg/mL) | 0.6 | ± | 0.1 | 1.73 | ± | 0.5 | 0.55 | ± | 0.08 | P < 0.05 Lean vs. VSG |
| progesterone | (ng/mL) | 163.1 | ± | 10.6 | 222.90 | ± | 21.9 | 255.40 | ± | 31.98 | P < 0.05 Lean vs. Obese; |
P < 0.05,
P < 0.01,
P < 0.001.
N=4-13/group. Data are presented as mean ±SEM.
Surgical Procedures
Pre-operative care
Four days prior to surgery, body composition was assessed using EchoMRI analyzer (Houston, TX). Animals were fed Osmolite OneCal liquid diet (Abbott Laboratories, IL), but no solid-food for 24 h prior to surgery.
VSG
As previously described (61), VSG consisted of a midline laparotomy with exteriorization of the stomach. Ligaments and connective tissue were removed leaving an easily articulated stomach. The lateral 80% of the stomach was excised using an ENDO GIA Ultra Universal stapler (#EGIAUSHORT, Covidien, MA) coupled with an ENDO GIA Auto Suture Universal Articulating Loading Unit, 45 mm – 2.5 mm (#030454, Covidien, MA). A gastric tube in continuity with the esophagus and duodenum thus remained. This gastric sleeve was then reintegrated into the abdominal cavity and the abdominal wall was closed in layers using 4-0 coated vicryl suture (#VE494, Ethicon Endo-Surgery Inc., OH).
Post-operative care
Following surgery, all rats received care for 3 d, consisting of once-daily subcutaneous injections of 5 mL saline, 0.10 mL Buprenex® (0.05mg/kg), and 0.25 mL carprofen (5mg/kg) Animals were maintained on Osmolite until food was returned 3 d following surgery.
Body weight, composition and food intake
Body weight was measured daily during the first post-operative week and then weekly until the start of mating. Echo Magnetic resonance imaging (echoMRI) whole-body composition analysis (EchoMedical Systems, Houston, TX) was performed on all rats at 1 week prior to surgery and 5 weeks post-surgery to determine fat and lean body composition.
Glucose tolerance testing
During post-operative week 5, a subset of animals (Lean, N = 19; VSG, N = 17; Obese, N = 19) were fasted 6 h after the onset of the light cycle. Baseline tail vein blood glucose was measured by Accu-Chek Aviva Plus™ glucometer and glucose strips (Roche, Indianapolis, IN). Animals were then dosed with 50% dextrose (1.5 g/kg body weight) intraperitoneally at time (t) = 0 min and subsequently blood glucose measured at 15, 30, 45, 60 and 120 min post-injection. Tail vein blood was collected in heparinized tubes for insulin determination at 0 and 15 min. Insulin measurements were made using an Insulin ELISA (#90060, Crystal Chem INC., IL).
Breeding protocol
Beginning 6 weeks after sham or VSG surgery, females were placed in the cage of a male in a 1:1 or 2:1, female: male ratio. Vaginal smears were performed daily, and the presence of sperm was considered day 1 (G1) of pregnancy. Females were returned to their own cage on G1, and body weight and food intake measured on G6, G12 and G18.
Blood pressure (BP) measurement
On G18, a subset of rats (Non-pregnant: (Lean, N = 3; VSG, N = 3; Obese, N = 6). Pregnant: (Lean, N = 8; VSG, N = 6; Obese N= 7)) were anesthetized by isoflurane gas anesthesia, and a catheter was placed in the femoral artery for blood sampling and BP monitoring. The catheter was exteriorized at the back of the neck, as previously described (39). The next day, G19, conscious BP recordings were made in animals that were placed in restraining cages. Rats were habituated to the restraining cages for 1 h before catheter connection to instrumentation. Mean arterial pressure was monitored in conscious rats with a pressure transducer connected to a Grass recorder (model 7B-chart, Grass Instrument). After a 60-min stabilization period, recordings were made for two periods of 30 min each, and the data were averaged.
Tissue harvesting
All dams in the study were euthanized on G19. The uterus was exteriorized and placement and number fetuses were counted in the bi-horned uterus. The placentae were dissected and weighed, and fetuses were weighed individually. The fetus and placenta in the number 3 and 4 positions counting from the right, and the left ovary were preferentially collected for mRNA and protein processing to control for uterine position.
Cell Isolation Protocol
For a subset of dams (Lean, N = 6; VSG, N = 6; Obese, N= 6), total blood was collected for cell sorting following decapitation. Erythrocytes were lysed using 1X PharmLyse (BD Biosciences) according to the manufacturer’s instructions. Peripheral blood lymphocytes (PBL) were washed and resuspended in PBS pH 7.4 containing 2% FCS and 0.09% sodium azide (stain buffer). 5×105 cells were stained with immune cell specific antibodies (BD Biosciences, Franklin Lakes, NJ) as follows CD8a (#561611), CD4 (#554837), CD3 (#554833), CD161a (#555009) or CD45R (#554881) at a concentration of 1:100 diluted in stain buffer for 30 min on ice. Cells were then washed two times with 2 mL stain buffer and centrifuged at 350 ×g for 5 min at 4°C. Cells were resuspended in 400 μL of stain buffer and immediately analyzed using a Beckman Coulter Gallios analyzer at the UMMC Cancer Institute Flow Cytometry Core Facility. Data were analyzed using Kaluza software.
Paraffin embedding and TUNEL assay
Paraformaldehyde (PFA) post-fixed hemi-sectioned placentae (Lean, N = 13; VSG N = 9; Obese, N= 9) were subjected to standard paraffin-embedding, and then sectioned at 5 μm on to glass slides and processed for TUNEL staining with Click-iT Plus TUNEL Assay for In Situ Apoptosis Detection, Alexa Fluor® 488 dye (#C10617, Molecular Probes, Inc., Eugene, OR), according to the manufacturer’s specifications.
Measurements of analytes
The following analytes were measured in plasma according to the manufacturer’s specifications: cholesterol (#TR13421, ThermoFisher, VA), triglycerides (#TR22421, ThermoFisher, VA), leptin (#MOB00, R & D System, Minneapolis, MD), testosterone (#DSL-4900, Beckman Coulter, Danvers, MA), insulin (#80558, Crystal Chem, Downers Grove, IL), progesterone (#90060, Crystal Chem, Downers Grove, IL), and cytokines (#171K1001M, Bio-Plex Pro Rat Cytokine 24-Plex Immunoassay, BioRad, Laboratories Hercules, CA).
RNA processing and real-time PCR
Fresh tissue was flash frozen on methylbutane cooled dry ice and stored in −80 °C until further processing. Placentae (Lean, N = 11; VSG, N = 9; Obese, N = 11) were cut into 4 quadrants; one was used for RNA extraction, while the others were retained for additional analysis. RNA was extracted using a QIAGEN miniprep RNA kit (QIAGEN, Inc, Valencia, CA), and complementary DNA was transcribed using an iScript complementary DNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative polymerase chain reaction was performed on a BioRad CFX96 Touch Real-Time PCR Detection System using TaqMan inventoried gene expression assays (Life Technologies, Foster City, CA) listed in Table 2 and Supplemental Table 2.
Table 2.
Gene expression analysis of Lean, VSG and Obese G19 placenta using rtPCR.
| Lean | VSG | Obese | Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cldn5 | Rn01753146_s1 | 100 | ± | 11.5 | 209.1 | ± | 37.9 | 219.3 | ± | 80.5 | *P < 0.05 Lean vs. VSG |
| il1rn | Rn02586400_m1 | 100 | ± | 6.9 | 192.2 | ± | 34.9 | 117.2 | ± | 28.6 | **P < 0.01 Lean vs. VSG |
| Il1ß | Rn00580432_m1 | 100 | ± | 17.3 | 180.2 | ± | 29.0 | 155.8 | ± | 47.3 | *P < 0.05 Lean vs. VSG |
| mmp9 | Rn00579162_m1 | 100 | ± | 11.9 | 231.2 | ± | 81.1 | 97.6 | ± | 14.6 | P = 0.05 Lean vs. VSG |
| mmp12 | Rn00588640_m1 | 100 | ± | 7.8 | 82.1 | ± | 11.4 | 63.2 | ± | 9.5 | *P < 0.05 Lean vs. Obese |
| ar | Rn00560747_m1 | 100 | ± | 9.0 | 67.3 | ± | 13.2 | 68.4 | ± | 14.7 | *P < 0.05 Lean vs. VSG |
| lepr | Rn01433205_m1 | 100 | ± | 10.7 | 57.3 | ± | 8.7 | 95.8 | ± | 8.9 | *P < 0.05 Lean vs. VSG |
P < 0.05,
P < 0.01.
N=7-11/group. Data are presented as mean ±SEM.
Protein extraction and western blot procedure
Tissue was flash frozen on methylbutane cooled on dry ice then stored at −80°C until use. Protein was extracted from one quarter of a placenta (Lean, N = 5; VSG, N = 3; Obese, N= 5) using the Santa Cruz RIPA lysis buffer system (Santa Cruz Biotechnology, Dallas, TX). Concentrations were determined using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL), and spectrometry was performed with a Tecan Infinite 200 PRO. Protein was combined at a 1:1 ratio with Laemmli sample buffer (BioRad Laboratories, Hercules, CA) and denatured at 95° C for 5 min. Protein (40 μg) was loaded onto BioRad 4-20% polyacrylamide Mini Protean TGX gels, and electrophoresis was performed in a BioRad Tetra-Cell 2 gel system. Protein was then transferred to PVDF membranes using a BioRad Trans-Turbo transfer system. Membranes were blocked for 1 h at room temperature with Pierce Protein-Free (TBS) Blocking Buffer (Thermo Scientific, Rockford, IL). Primary antibodies used were as follows: rabbit MMP9 (1:2000, #38898, Abcam), rabbit mTOR (1:1,000, #2983, Cell Signaling) and mouse β-actin (1:10,000, #A5441, Sigma-Aldrich) overnight at 4°C. In between incubations, membranes were washed with TBS with 0.05% Tween. Anti-rabbit HRP conjugate (1:5000, #sc-2004, Santa Cruz Biotechnology, Dallas, TX) and anti-mouse HRP conjugate (1:5000, #sc-2005, Santa Cruz Biotechnology, Dallas, TX) was used to incubate membranes for 1 h at room temperature before applying Thermo Scientific SuperSignal West Femto HRP substrate. Images were taken with a BioRad ChemiDoc XRS system and analyzed using ImageJ 1.48 software.
Microarray analysis
Whole genome transcript analysis was performed using an Affymetrix platform from G19 placentae from (Lean, N = 6; VSG N = 6). RNA was isolated using TRIzol® and RNeasy Mini Kit (#74106, Qiagen, Hilden, Germany), and evaluated for quality and integrity (Bio-Rad Experion™ System), as done previously (60). Placental RNA was processed using manufacturer directions for specific application [GeneChip® 2.0 ST] using Affymetrix equipment (Scanner 3000 7G System). Hybridized chips were automatically washed, stained and scanned at the UMMC Institutional Molecular and Genomics Core using Affymetrix equipment. Data obtained from these gene expression studies are deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) with the GEO accession number GSE106883.
Analysis of microarray data was performed using software provided by Affymetrix (Affymetrix® Expression Console™ Software) and commercially available GeneSifter™ software platform (http://www.genesifter.net). In brief, differentially expressed genes evaluated by t-test using two methods: (1) FWER (family-wise error rate) procedure, P<0.05 and fold-change ±1.2 or greater; and/or (2) Benjamini and Hochberg FDR (false discovery rate) which corrects for multiple comparison, using P<0.05, and fold-change ±1.2 or greater. Gene networks and functional analysis were evaluated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com). Gene expression differences were confirmed using RT-PCR method described above.
Placental barrier testing
A subset of G19 pregnant animals (Lean, N = 8; VSG, N = 5; Obese, N = 4) were anaesthetized using isoflurane and received a bolus intravenous injection of a cocktail containing 1mg/kg neutral Texas Red tagged dextran, 3000 MW (#D3329, Molecular Probes, OR) and 0.5 mg/kg sodium fluorescein (#46960, Fluka, MO). Animals remained anesthetized for 60 min and were then euthanized and tissues harvested. Tissue fluorescence was determined by ex vivo whole organ fluorescence imaging using an IVIS Spectrum (Perkin Elmer, Waltham, MA) immediately after death. Tissues were dissected from the amniotic sac and placed in PBS and imaged in the IVIS using epi-illumination and 465 nm excitation/520 nm emission filters for fluorescein and 570 nm excitation/620 nm emission filters for Texas Red, f-stop 2, and auto exposure. Mean fluorescence radiant efficiency of each organ in each fluorescence channel was determined using a closely drawn region of interest with Living Image 4.3 software (Perkin Elmer). The mean fluorescence radiant efficiency of each fetus was divided by the mean fluorescence radiant efficiency of its placenta to calculate percentage transferred. The percentage transferred for each fetal-placental pair was averaged for all pups in each dam. Data represent the average placental transfer of (n=3-6 dams per group), each with an average of 7 pups/litter (range = 2-10). To generate images for publication, one representative image of placentae and pups from each group was utilized, and all images were displayed on the same scale to allow direct comparison of the fluorescence intensity among groups.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6.07 (GraphPad Software, San Diego, California) USA. Differences between groups were assessed by using one-way ANOVA and two-tailed distribution with a Tukey’s post hoc to determine significant comparisons. To observe time-wise differences, two-way ANOVA (variables: surgical group and time) with Bonferroni post hoc test was used. All results are given as means ± SEM. Results were considered statistically significant when P< 0.05.
RESULTS
Food intake and Body weights
All female rats given a HFD for 4 weeks prior to surgery increased body weight, and they all lost weight during the initial 7 post-operative days (POD), irrespective of the surgery (Sham or VSG) (Figure 2A). VSG rats lost the most weight in the first 7 days post-surgery (*P < 0.05) (Figure 2A). Obese sham controls remained on HFD and continued to gain weight following Sham-VSG over the next 5 weeks with body weights higher than the other two groups from POD21 until the end of the experiment at varying intervals (δP < 0.05, δδP < 0.01) (Figure 2A). Lean sham controls and VSG rats that were both switched from HFD to chow 3 days post-surgery regained similar amounts of body weight during the next 5 weeks, such that body weights were similar by POD21 (Figure 2A). Thus, in the present study, the Lean group serves as a weight-matched control for the VSG as well as a diet control.
Figure 2. Metabolic parameters post-surgery prior to pregnancy.
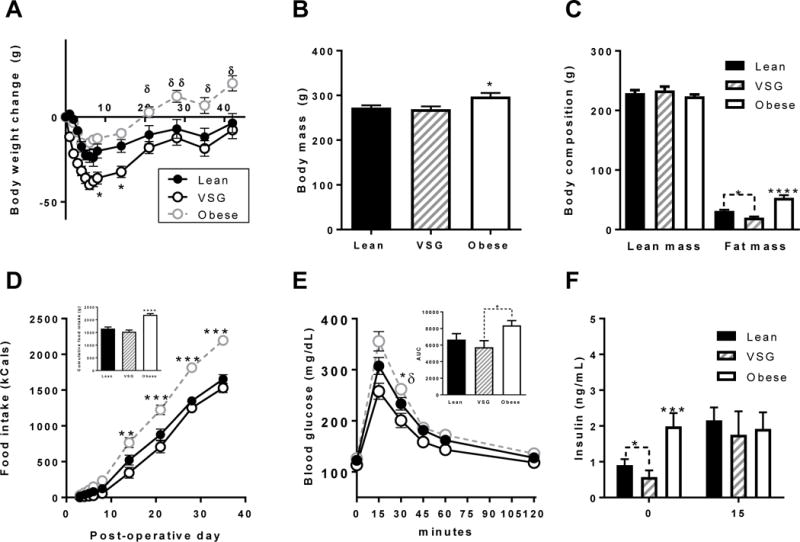
(A) Body weight change of Lean, VSG and obese female rats during the 35 d post-surgery period. (B) Body mass at 35 d post-surgery at the time of EchoMRI. (C) Lean and fat mass composition at 35 days post-surgery. (D) Cumulative calorie intake during the 35 d post-surgery. (E) Blood glucose during an intraperitoneal glucose tolerance test. Inset (Area-under-the-curve. (F) Baseline fasting insulin and 15 minutes after i.p. glucose injection at GTT. (*P < 0.05, **P < 0.01, ***P < 0.001). Lean vs. Obese, δP < 0.05, δδP < 0.01. Data are presented as mean ±SEM. N = 7-12/group
Obese controls consumed significantly more kCals cumulatively (Figure 2D inset) and per day than either Lean controls or VSG in the 5 weeks (***P<0.001) (Figure 2D). There was no difference between kCal consumption between Lean and VSG female rats after POD21.
Body composition
By post-operative week 5, body composition analysis by EchoMRI showed no impact of surgery on lean mass in any of the groups (Figure 2C). However, Obese controls had greater levels of fat mass than either Lean controls or VSG (P<0.0001), and VSG rats had significantly lower levels of fat mass than the Lean animals (*P<0.05) (Figure 2C).
Glucose and insulin tolerance tests
On POD37, an intraperitoneal glucose tolerance test (ipGTT) was performed on 6 h fasted rats. Baseline fasting glucose was similar between groups. As expected, Obese controls were significantly glucose intolerant over either Lean controls or VSG animals particularly at the 30 min time point (*P<0.05) and Obese rats exhibited significantly increased area-under the curve (AUC) over VSG rats (*P<0.05) (Figure 2E inset), and no difference between Lean and VSG female rats (Figure 2E inset). At baseline (t=0), Obese controls exhibited insulin resistance with two fold higher insulin levels than either Lean controls or VSG rats (***P<0.001) (Figure 2F). Furthermore, VSG animals had reduced insulin even over Lean controls (*P<0.05) (Figure 2F). At t=15 min post glucose injection, insulin levels were increased over baseline in Lean and VSG rats but not in Obese animals.
Pregnancy outcomes
Six weeks after sham or VSG surgery, animals were mated. Average food intake was measured during three successive time intervals gestation day (G) 1-6, G6-12 and G12-18, and no difference was measured in average daily kCal intake between Lean and VSG animals (Figure 3A). Obese animals consumed significantly more kCals during G1-6 and G12-18 than the other groups, and hence consumed more kCals overall during the entire pregnancy than the other groups (Figure 3A). There was no difference in body weight gain during the G1-6 or G6-12 for any of the 3 groups (data not shown). However by the G12-18, VSG animals showed a reduced weight gain compared to the other two groups (Figure 3B) which is reflected in a reduced total weight gain for the entire pregnancy (P<0.05).
Figure 3. Food intake, body weight gain and blood pressure during pregnancy.
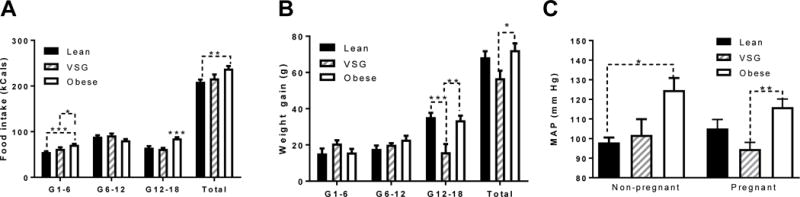
(A) Food intake binned into three 6-day intervals. Data shown as 1st-3rd intervals and total food intake (B) Body weight gain reported in three 6-day intervals and total. (C) Mean arterial blood pressure in non-pregnant female rats and G19 pregnant dams. *P<0.05, **P<0.01. Data are presented as mean ±SEM.
Blood pressure
In age-matched non-pregnant females, Obese controls had higher mean arterial pressure (MAP) than did Lean rats (P<0.05), while MAP was similar in non-pregnant Lean and VSG rats (Figure 3C). On G19 of pregnancy, Obese females also had a higher MAP than VSG rats, but not Lean rats (P<0.01).
Metabolic Parameters
As shown in Table 1 in plasma samples taken on G19, Obese controls had higher triglycerides, insulin and leptin levels than the other groups (P<0.05). Cholesterol levels were not different among the groups. Insulin was similar between Lean controls and VSG rats, and leptin levels were higher in Lean than VSG rats.
Steroid hormones and Placental Receptor Genes
On G19, testosterone levels were higher in VSG than either Obese or Lean controls (P<0.05) (Table 1). Progesterone was significantly higher in VSG and Obese dams in comparison to Lean rats (P<0.05) (Table 1). We probed specifically for androgen receptors (ar) expression in the placenta and found ar to be significantly reduced in VSG and Obese (P < 0.05) whereas there was no difference in the expression of estrogen receptors (essra) and progesterone receptors (pr) between groups (Supplemental Table 2).
Evaluation of Maternal Circulating T cells, Cytokines, and IgG
Plasma was obtained on G19 from all rat groups for flow cytometry analysis of CD3+ (marker of total T cells), CD4+ (marker of T helper cells), and CD8+ (marker of cytotoxic T cells), CD161+ cells (marker of natural killer (NK) cells), and CD45r+ (marker of B cells). VSG dams had lower levels of CD3+ (pan T cells) and CD8+ cytotoxic T cells than Lean controls (P<0.05) (Figure 4A,C), but similar to levels in Obese controls. There were no differences in CD4+ (T helper) (Figure 4B), CD116+ (NK) (Figure 4D), or CD45R+ (B cells) (Figure 4E) amongst the rat groups. Immunoassay for 24 cytokines was done on G19 plasma from rats. Differences amongst the groups were identified in only four of the cytokines measured (Figure 4F-I) (Supplemental Table 1). Interleukin 7 (IL7) was significantly increased in VSG dams in comparison with Lean dams (P<0.05), but was not different than Obese dams. Obese dams exhibited reduced IL-13 (P<0.05) and M-CSF (P<0.01) in comparison with Lean dams, but not different than VSG dams. Circulating VEGF was significantly reduced in VSG dams compared with the other Lean dams (P<0.05). The level of circulating total plasma IgG are an indicator of responsiveness of the immune system [58], and we found that plasma total IgG was two-fold higher in VSG rats than in either Lean (P<0.05) or Obese (P<0.01) controls (Figure 4J). There were no differences in levels of plasma IgG1, IgG2a or IgG2b amongst the groups (results not shown).
Figure 4. Cell sorting of blood from G19 pregnant dams.
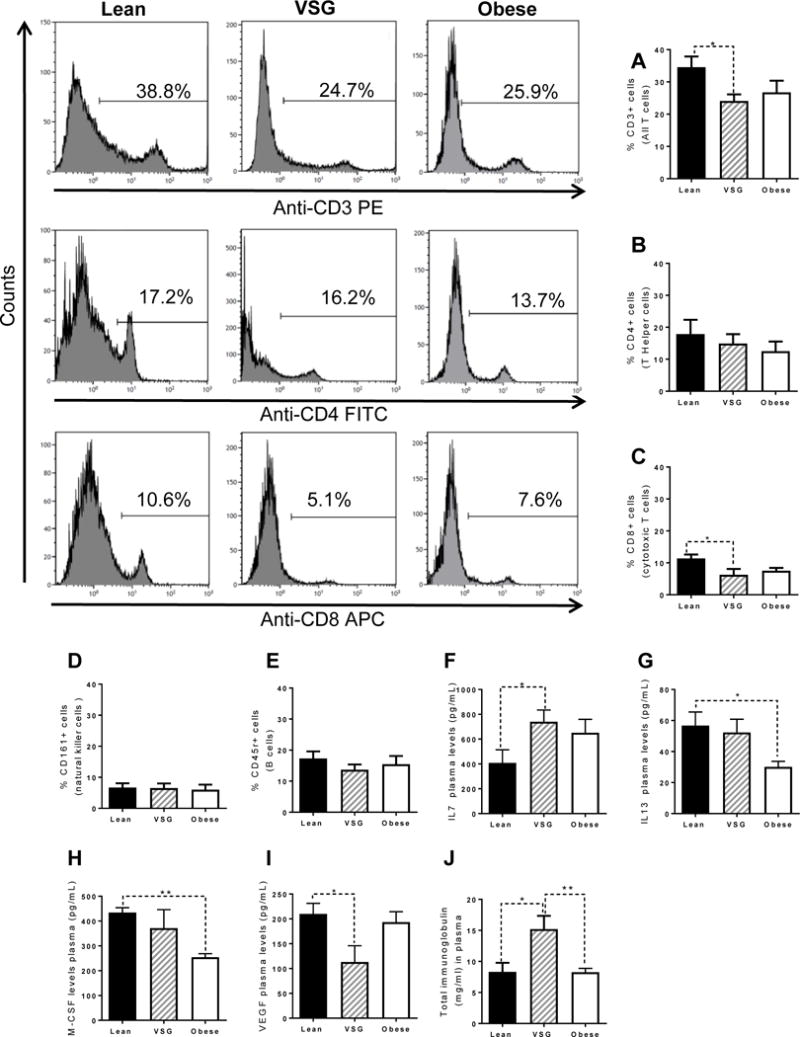
(A) Percentage of CD3+ cells which labels all T cells. (B) Percentage of CD4+ cells which labels T Helper cells. (C) Percentage of CD3+ cells which labels cytotoxic T cells. (D) Percentage of CD161+ cells which labels natural killer cells. (E) Percentage of CD45+ cells which labels B cells. (F) Maternal circulating IL7 levels during G19. (G) Maternal circulating IL13 levels during G19. (H) Maternal circulating M-CSF levels during G19. (I) Maternal circulating VEGF levels during G19. (J) Plasma levels of total immunoglobulins. *P<0.05, **P<0.01. N=6/group. Data are presented as mean+−SEM.
Feto-placental Outcomes
Pregnant animals were euthanized on G19 and tissues harvested for analysis. VSG dams had significantly reduced number of viable (non-resorbed) pups (P<0.05) in comparison to lean (Figure 5A) and an increased but not significant number of resorbed feto-placental units (Figure 5B). As such, total fetal weight for VSG dams was significantly reduced (P<0.05) (Figure 5C) in comparison to Lean pregnancies. However, the average live fetal weight showed no significant difference between groups (Figure 5D). Total placental weight for VSG dams was also significantly reduced compared to the other groups (P<0.05) (Figure 5E); however, average placental weight for VSG dams was not significantly different than the other groups (Figure 5F). Overall, the percentage of each pregnancy that experienced fetal demise was significantly increased in VSG (P<0.05) (Figure 5G). Hence, at G19 VSG dams on normal chow exhibited increased fetal death and reduced litter size with compensation for growth in the remainder of the fetuses. Our focus was to determine the potential reasons for reduced fetal viability, and whether there were any adverse consequences in the fetuses that remained.
Figure 5. Fetal-placental unit analysis.
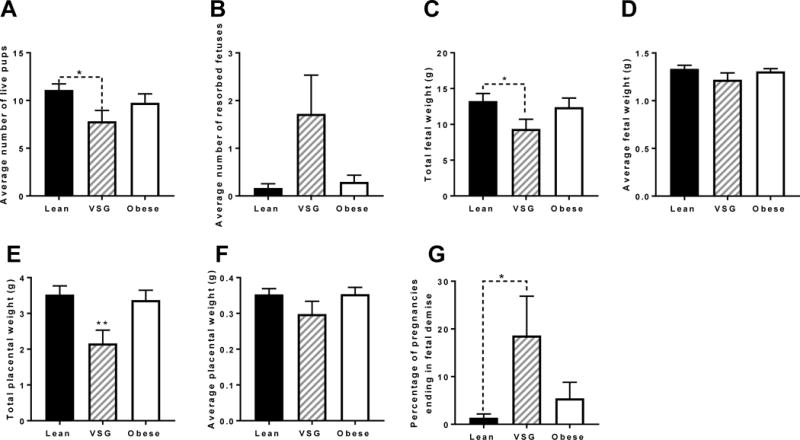
(A) Average number of live fetuses (B) Average number of resorbed fetuses (C) Total fetal weight (D) Average fetal weight (E) Total placental weight (F) Average placental weight (G) Percentage of pregnancies ending in fetal demise. N = 19-23. *P < 0.05, **P < 0.01. Data are presented as mean ±SEM.
Placental Transcriptome Analyses
To determine if gene expression patterns of the VSG placenta could explain the increased fetal demise from VSG dams, whole transcriptome analysis was performed to query changes between Lean and VSG placentae (Figure 6A). Using a Student’s t test comparison with Benjamini and Hochberg correction and a statistical cutoff of P<0.05, 745 placental genes were differentially expressed (Figure 6B), of which 275 were down regulated, and 470 up-regulated (Figure 6C). Using a statistical cutoff P<0.05 and Fold change (FC) of >1.3, 100 genes were differentially expressed between groups (Figure 6B). Of these 100 genes, 53 of the genes (34 upregulated and 19 downregulated) were for RNA-coding genes. In addition there were 47 genes (32 upregulated and 15 downregulated) that had absolute fold-change differences of >1.3 that were non-coding RNA or small nucleolar RNA.
Figure 6. Transcriptome analysis of Lean and VSG placenta.
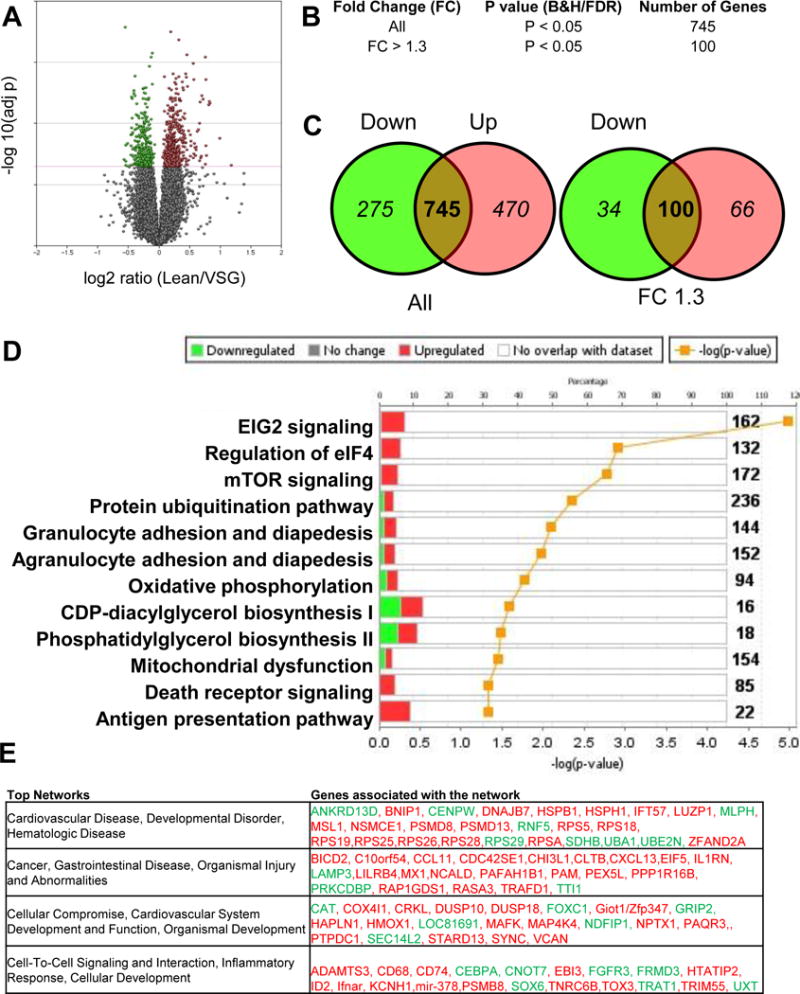
(A) Volcano plot of the log2 ratio of Lean and VSG microarray expression with green denoting down-regulation and red denoting up-regulation. (B) Fold change (FC) and absolute numbers of altered genes. (C) Venn diagrams demonstrating the apportioning of genes by directionality of change. (D) Pathway analysis chart using IPA demonstrating the top up and down regulated signaling families affected by VSG. (E) Top four networks affected by surgery in the placenta and the genes affected in the network organized by down-regulation in green and up-regulation in red. N=6/group.
The list of 745 genes were subjected to further analysis using Ingenuity Pathway Analysis (IPA) and the top 12 canonical pathways from this analysis are reported (Figure 6D). The Granulocyte and agranulocyte adhesion diapedesis, Antigen presentation pathway, Death receptor signaling and mTOR signaling are shown (Figure 5D).
Under this this category, interleukin 1 receptor antagonist (il1rn) was significantly up-regulated in VSG placenta in comparison to Lean (Table 1). We used RT-PCR to validate this VSG mRNA increase on an increased sample size. In addition to increases in il1rn, differences were observed in il1β (P<0.05) with no changes in il1α or the receptor il1r1.
To further validate the Death Receptor pathway, we performed TUNEL staining as marker for apoptotic cells. We determined a significant increase in TUNEL positive immunoreactivity for the Death receptor pathway in the VSG placenta in comparison to Lean (P<0.05) (Figure 6C). These data support an increased rate of cell death in the VSG placenta.
In addition, the top 4 networks dysregulated between Lean and VSG placenta include the following topical areas (Figure 6D): 1) Cardiovascular Disease, Developmental Disorder, Hematologic Disease, 2) Cancer, Gastrointestinal Disease, Organismal Injury and Abnormalities, 3) Cellular Compromise, Cardiovascular System Development and Function, Organismal Development and 4) Cell-To-Cell Signaling and Interaction, Inflammatory Response, Cellular Development. These networks include a host of genes that are inter-related and are functionally up or down-regulated as reported (Figure 6E).
We validated a number of the gene transcripts that are listed in these Networks using targeted rtPCR (Table 2). Mmp9 was up-regulated (P<0.05) and was specific; it did not impact expression levels of mmp2, 3 or 12 (Supplemental Table 2). Timp1 was also not altered nor were MMP partners, adam17 or bsg (Supplemental Table 2). Western blot verification on placental tissue samples determined increased levels of MMP9 protein as well (Figure 7).
Figure 7. Validation of three unique pathways altered by maternal VSG in the placenta.
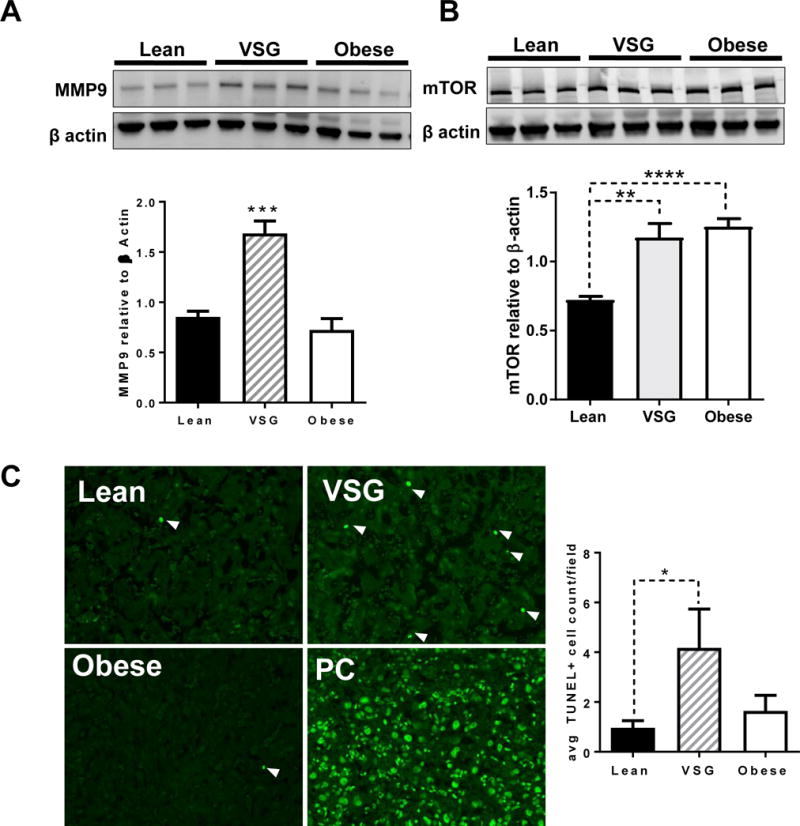
(A) Analysis of MMP9 protein by Western blot and normalized to β actin. (B) Protein levels of mTOR normalized to β actin by western blot. N = 3-5/group. (C) TUNEL staining of Lean, VSG and Obese placenta with positive control (PC) shown N=9-13/group. One way ANOVA with Tukey’s post hoc, *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ±SEM.
Since the microarray IPA analysis suggested that the mTOR signaling network was highlighted in our VSG placental samples (Figure 6D), western blot analysis was used to verify the mRNA data. mTOR protein was significantly increased in the placentae of VSG dams (Figure 7B). In addition, there was a significant increase in placental mTOR in the Obese group. Because the remaining VSG fetuses that did not succumb to resorption did not have reduced weight, we hypothesized that mTOR programming may be driving the growth, allowing the fetuses to remain on a normal growth trajectory despite potentially adverse circumstances.
Based on the fact that VEGF was reduced in plasma of VSG dams, soluble VEGF receptor, s-FLT1, expression was measured by western blot in plasma of G19 rats. However, there were no differences in s-FLT1 among the groups (data not shown). In addition, there were no differences in the placental mRNA expression of VEGF (vegf), placental growth factor (plgf), or the hypoxia-related genes (hif1a, hmox1, and hspe1)(Supplemental Table 2).
Placental permeability and evaluation of tight junctions
Considering the specific changes in mmp9 mRNA and MMP9 protein, we sought to determine whether there were functional changes to barrier function in the placenta as described in Methods. VSG dams demonstrated an approximately 20% increase in transfer of fluorescein from dam to fetus (P<0.05) (Figure 8). However there was no difference in the percent transfer of the higher molecular weight dextran (Figure 8). Taken together these data suggest there is a breakdown of the placental barrier in VSG pregnancies.
Figure 8. Imaging of G19 fetal placental units in Lean, VSG and Obese pregnancies.
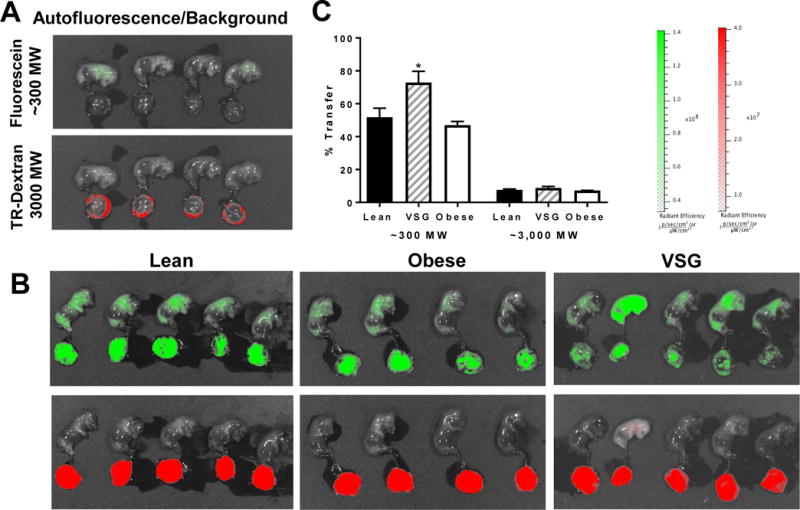
(A) Background/autofluorescence levels in each channel. (B) Images of fetuses and placenta in red and green channels after injection of ~300 Dalton fluorescein and 3000 Dalton Texas red labeled dextran. N =3-6/group, 3 saline controls. (C) Percent transfer of each of the two labelled chemicals. Student’s T test, *P < 0.05. Data are presented as mean ±SEM.
Expanding on the finding that placental permeability is increased, we sought to determine if members of the tight junction complex in the placenta were altered. Placental expression of claudins has been previously reported, so we probed for cldn1-6 to determine placental expression. Of the highly-prevalent claudins in the placenta, only cldn5 was significantly upregulated in VSG animals (P<0.05) (Table 2). Also occludin (ocln) was down-regulated in VSG animals compared to Lean (P<0.05) (Table 2). Cadherin1 (cdh1), connexin 43 (cx43) and zona occludins (zo1) were not altered (Supplemental Table 2).
DISCUSSION
In the present study we tested the hypothesis that surgical weight loss impacted maternal immune function and placental physiology during gestation that negatively impacted the fetus leading to IUGR. The most important findings in dams were that, compared to the lean and/or obese sham controls: 1) VSG improved body weight, reduced fat mass with no effect on lean mass; 2) reduced fasting insulin and improved other metabolic and lipidomic parameters; 3) reduced the number of circulating pan (CD3+) T cells and CD8+ T cells; 4) reduced levels of circulating VEGF with increased levels of inflammatory cytokines such as IL17; and 5) attenuated the elevated blood pressure in the dams. Unfortunately, VSG in dams reduced the number of live pups and increased the number of resorbed pups. To determine potential mechanisms that may account for the negative effect of VSG on the fetuses, we found that placental MMP9 and mTOR were elevated compared to lean sham control dams, and there was a higher level of placental apoptosis as measured by TUNEL assay. Placental permeability to dextran in VSG dams was also increased. Finally, placental transcriptome analyses identified numerous pro-inflammatory and pro-apoptosis pathways that can be evaluated further in the future to identify other potential mechanisms responsible for fetal demise in dams who have undergone VSG.
Women of childbearing age that are obese with other metabolic comorbidities frequently consider weight loss surgery to improve their chances of bearing children. Recent meta-analyses suggest that 58% of infertile women become spontaneously pregnant after bariatric surgery (36). The desire to improve reproductive potential is a great motivator for obtaining bariatric surgeries in this sub-population of women. Therefore, it is imperative that we understand the impact of bariatric surgery on the offspring of these women.
Though many gestation-related diseases that are exacerbated with obesity appear to be reduced after surgical weight loss (19, 27, 47, 48, 59), a serious cause for concern in pregnancies is the increase in delivery of small-for-gestational-age (SGA) babies. Intrauterine growth restriction (IUGR) is increased by 2-3 fold after bariatric surgery (15, 38). In addition, pregnancies in women who had undergone procedures such as Roux-en-Y gastric bypass or RYGB, had a greater risk for SGA in comparison to weight loss procedures that such as LAPBAND, including VSG (11). Though many of the human studies report IUGR with RYGB and not LAPBAND, few studies have determined the occurrence of IUGR by type of surgery. This is especially important since the numbers of VSG surgeries are presently surpassing RYGB, such that greater than 50% of the surgeries performed today are VSG, mainly due to the simplicity of the procedure and reduced complications in the women.
While there have been suggestions as to why there may be SGA births following bariatric surgery, these hypotheses have not been formally tested in the clinic. The most common hypothesis is that women who have undergone VSG are not following the directives on appropriate wait times to become pregnant following surgery, typically wait time is suggested to be 18 months. However, in a study where this notion was formally tested, timing did not appear to be a causative factor in SGA births (28, 50). Taken together the question remains as to whether the timing of pregnancy following bariatric surgery actually contributes to adverse outcomes in the fetus.
In the present studies, we systematically evaluated the common causes thought to elicit either fetal demise or reduced growth. Worldwide, the top causes of reduced birth weight include gestational malnutrition, drug, alcohol and cigarette consumption, pre-eclampsia/gestational hypertension, and maternal infection/immune complications. Using our rodent model of VSG, we eliminated socially-derived causes of fetal developmental harm (i.e. nicotine, alcohol and drug use). Here we build off the body of work that we previously reported during pregnancy (23). Our earlier work suggested that the hyperphagia of pregnancy and lactation were intact in the VSG dams in comparison to Lean or obese sham control dams. In the present studies, we also conducted more structured analyses of body weight distribution during gestation in 6 day increments. These data highlight that in the 3rd segment of rat pregnancy, there is a substantial drop in body weight in the dams subjected to VSG that is not substantiated with reduced food intake. This suggests that between days 12 and 18, the pregnancies are no longer progressing properly, and that this reduced body weight may signal fetuses that are not progressing developmentally. Importantly, it is clear from our data that reduced body weight in dams is not due to reduced or aberrant food intake.
As noted above, at G19, VSG dams have a reduced number of viable pups and increased number of resorbed placental fetal units. The average of the reduction in body weight is partially made up by the number of missing fetal placental units. This further suggests that there may be some factor(s) driving this phenomenon during pregnancy. One limitation of these studies is that the fetuses that appear most vulnerable (i.e. are resorbed) by G19, and thus we were not able to distinguish the output measures in the fetuses that survive and the ones that do not. Our future studies would necessitate euthanasia earlier in gestation, potentially, mid-gestation, to determine the time course and potential mechanisms during which their viability diminishes.
Though bariatric surgery in humans reduces gestational hypertension and preeclampsia [26, 27], we queried whether our rat model behaved similarly. Here we demonstrated that mean arterial pressure (MAP) in G19 VSG dams was similar to controls. Decreases in MAP/BP also occurred in non-pregnant, age-matched control VSG dams. To date, it is not known whether the mechanism for this reduced blood pressure after bariatric surgery is solely due to weight loss-dependent changes.
Abnormal levels of reproductive hormones, in particular low progesterone, is a source of loss of pregnancy and spontaneous abortion in humans (43). In our rat model progesterone in VSG pregnancy was higher than in lean sham (normal chow fed) control dams and similar to HFD fed obese control dams. Surprisingly, the VSG dams had higher than normal levels of testosterone with a concomitant increase in androgen receptor mRNA expression in the placenta, suggesting the potential influence of testosterone in somehow mediating fetal demise and/or IUGR. Higher levels of androgens during pregnancy have been linked to a variety of negative phenotypes in offspring including reproductive abnormalities in both male and female progeny, as well as some post-natal learning issues (6, 57). The cause of elevated testosterone and progesterone in the rat VSG dams is unknown, and has not been reported to date in pregnant women who have previously undergone VSG.
Infection and immune perturbations are significant drivers of fetal demise and failure-to-thrive pregnancies. The 24-cytokines panel we performed on G19 maternal rat plasma suggested no overt infection in the VSG pregnancies, as evidenced by the absence of a rise in circulation of IL6, TNFα and IL1β. The only significantly increased cytokine during G19 VSG pregnancy was interleukin 7. IL7 can be released with immune activation of the gut (31, 32) and globally works to increase maturation of T cells when lymphopenia is present (18). We hypothesized that IL7 may be increased to compensate for the reduced number of total T cells, especially cytotoxic T cells, in the cell sorting experiments. We found no differences in the total number of B cells among the three groups of pregnant dams. We did measure total immunoglobulin G (IgG) levels and found VSG dams had significantly increased levels of total IgG. Increases in IgGs suggest activation of immune system to induce pathogen elimination, toxin binding or in allergic hypersensitivity. IgG antibodies do cross the placental barrier and certain IgG antibodies are highly associated with still births (42). It is unknown whether the uninhibited gastric emptying of VSG (9) also alters the permeability of the endothelial barrier of the gut resulting in an acute “leaky gut” and potentially allowing short term entry of pathogens or toxins that cause measurable IgG increases, and may also account for the increase in IL7.
We previously showed significant morphologic abnormalities of the placenta from VSG pregnancies from dams maintained on HFD after their surgery (23). If they were maintained on normal chow during pregnancy, there were no noticeable changes (23). In the present study, we also report a significantly increased number of apoptotic cells as labelled by TUNEL in the placentae of VSG pregnant dams maintained on normal chow. The biological significance of this increased level of cellular death is not clear, and more work needs to be done to determine the direct cause of this loss of cells along with determining the phenotype of these cells. These data however support the microarray reports of.
Using gene expression microarray analyses, immune system and barrier function gene networks were found to be increased in VSG pregnancy. Taken together with the IVIS studies that show increased permeability of the placental barrier, we hypothesized that circulating vasoactive mediators may be causing breakdown of the placental barrier through MMP9 activation. VSG surgery changes a variety of gut-related peptides that have vasoactive properties, including vasoactive intestinal peptide (VIP) and bradykinin. These might be acting more directly during pregnancy to affect barrier function. In the current study, the circulating systemic pro-inflammatory cytokines are not driving this reduced barrier since they are not increased, though the increased placental il1β and ilrn mRNA suggest other potential local cellular mediators in the placenta of unknown origin. The source of the inflammation could be local immune (Hofbauer) cells or endothelial cells in origin. However, identification of the cell mediators for inflammation is beyond the scope of the current work and will be evaluated in future studies.
Finally, gene activation of the mTOR pathway found by microarray in the VSG placentae was validated using western blot. Activation of mTOR may be a compensatory mechanism to continue nutrient partition and growth and development of the fetus of VSG dams at a rate similar to fetuses of Lean control dams. The surviving VSG fetuses from which we are able to sample, may be spared, because mTOR activation propagates growth and improves nutrient availability. At the present time why some VSG fetuses were not able to thrive, and why the remaining ones have a tendency to reduced barrier function but still appear to grow at a rate equivalent to fetuses of lean controls is unknown. Our previous work showed that whether the VSG dam was maintained on chow or HFD after the surgery, the results were similar, i.e. pups were smaller after VSG at postnatal day 2. Thus we hypothesize that during the final days of gestation, the reduced placental barrier coupled with the other insults we identified may reduce the growth of these offspring. Indeed sparing of some of the VSG fetuses through placental mTOR activation to increase nutrient availability during this phase is an intriguing hypothesis.
The present work does have limitations as a pre-clinical model. While the female rats gain significant weight on the HFD prior to pregnancy, obesity in a rat model is relative and not as clear as the increases in body mass index (BMI) seen in women. In addition, by the time the female rats are ready to be mated, some have been on HFD for 8 weeks which can significantly reduce fertility just as found in obese women. If the animals were on diet for longer than that prior to surgery, infertility would detract from our ability to perform these studies (prior unpublished observation). Further, the immune system of the rat operates differently that in the human, and some of the differences we observe may be species specific.
In conclusion, the female rodent model of VSG largely mimics the metabolic improvements of VSG in women. There are a significant number of potentially negative consequences reported to date in humans who have undergone bariatric surgery that remain to be understood and whose mechanisms are unknown. Using our rodent model of VSG coupled with pregnancy, we report diverse changes in the VSG pregnancy that may provide insight into the adverse consequences on their offspring. While bariatric surgery is often contemplated in women who want to become pregnant, our work highlights that VSG surgery triggers significant changes to the maternal milieu during pregnancy, and suggests that women who are undergoing bariatric surgery with the hope of becoming pregnant should be counseled as to the potential for negative outcomes in their offspring.
Supplementary Material
Figure 1.
Timeline information for pregnancy studies in G19 rats.
Acknowledgments
B.E.G is partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM104357, and the Mississippi Center of Excellence in Perinatal Research COBRE (P20GM121334).
The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the National Institute of General Medical Sciences of the National Institutes of Health, including Mississippi INBRE (P20GM103476), Center for Psychiatric Neuroscience (CPN)-COBRE (P30GM103328), Obesity, Cardio-renal and Metabolic Diseases-COBRE (P20GM104357), and the Mississippi Center of Excellence in Perinatal Research-COBRE (P20GM121334).
The work of the Analytical and Assay Core is supported by Cardio-renal and Metabolic Diseases-COBRE (P20GM104357), and the Mississippi Center of Excellence in Perinatal Research COBRE (P20GM121334).
We are grateful to Drs. Gailen Marshall, Merry Lindsey, Padmanabhan R. Iyer and Denise Montgomery for use of instrumentation and assistance in performing the BIORAD cytokine array.
We are grateful to Dr. Merry Lindsey for sharing the MMP9 antibody.
Footnotes
No financial conflicts of interest exist.
References
- 1.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. Jama. 2014;311:1536–1546. doi: 10.1001/jama.2014.2269. [DOI] [PubMed] [Google Scholar]
- 2.Baker RW, Osman J, Bodnar RJ. Differential actions of dopamine receptor antagonism in rats upon food intake elicited by either mercaptoacetate or exposure to a palatable high-fat diet. Pharmacol Biochem Behav. 2001;69:201–208. doi: 10.1016/s0091-3057(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 3.Balkan F, Cetin N, Usluogullari CA, Unal OK, Usluogullari B. Evaluation of the ovarian reserve function in patients with metabolic syndrome in relation to healthy controls and different age groups. Journal of ovarian research. 2014;7:63. doi: 10.1186/1757-2215-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar LM, Parks WT, Perkins K, Pugh SJ, Platt RW, Feghali M, Florio K, Young O, Bernstein S, Simhan HN. Maternal prepregnancy obesity and cause-specific stillbirth. The American journal of clinical nutrition. 2015;102:858–864. doi: 10.3945/ajcn.115.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256 e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155:3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 7.Casella G, Soricelli E, Castagneto-Gissey L, Redler A, Basso N, Mingrone G. Changes in insulin sensitivity and secretion after sleeve gastrectomy. The British journal of surgery. 2015 doi: 10.1002/bjs.10039. [DOI] [PubMed] [Google Scholar]
- 8.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-Independent Changes in Blood Glucose Homeostasis After Gastric Bypass or Vertical Sleeve Gastrectomy in Rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D’Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. American journal of physiology Endocrinology and metabolism. 2014;306:E424–432. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Wei J, Tong M, Yu L, Lee AC, Gao YF, Zhao M. Associations between body mass index and maternal weight gain on the delivery of LGA infants in Chinese women with gestational diabetes mellitus. Journal of diabetes and its complications. 2015 doi: 10.1016/j.jdiacomp.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Chevrot A, Kayem G, Coupaye M, Lesage N, Msika S, Mandelbrot L. Impact of bariatric surgery on fetal growth restriction: experience of a perinatal and bariatric surgery center. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2014;10:396–403. doi: 10.1016/j.soard.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 13.Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Nguyen MT, Chiheb S, Valensi P, Carbillon L. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: A retrospective observational study. Diabetes & metabolism. 2015 doi: 10.1016/j.diabet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. Jama. 2013;310:2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell’Agnolo CM, Cyr C, de Montigny F, de Barros Carvalho MD, Pelloso SM. Pregnancy after Bariatric Surgery: Obstetric and Perinatal Outcomes and the Growth and Development of Children. Obesity surgery. 2015;25:2030–2039. doi: 10.1007/s11695-015-1668-9. [DOI] [PubMed] [Google Scholar]
- 16.Edison E, Whyte M, van Vlymen J, Jones S, Gatenby P, de Lusignan S, Shawe J. Bariatric Surgery in Obese Women of Reproductive Age Improves Conditions That Underlie Fertility and Pregnancy Outcomes: Retrospective Cohort Study of UK National Bariatric Surgery Registry (NBSR) Obesity surgery. 2016;26:2837–2842. doi: 10.1007/s11695-016-2202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feichtinger M, Stopp T, Hofmann S, Springer S, Pils S, Kautzky-Willer A, Kiss H, Eppel W, Tura A, Bozkurt L, Gobl CS. Altered glucose profiles and risk for hypoglycaemia during oral glucose tolerance testing in pregnancies after gastric bypass surgery. Diabetologia. 2017;60:153–157. doi: 10.1007/s00125-016-4128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. Journal of immunology (Baltimore, Md : 1950) 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 19.Galazis N, Docheva N, Simillis C, Nicolaides KH. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. European journal of obstetrics, gynecology, and reproductive biology. 2014;181:45–53. doi: 10.1016/j.ejogrb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Goldman RH, Missmer SA, Robinson MK, Farland LV, Ginsburg ES. Reproductive Outcomes Differ Following Roux-en-Y Gastric Bypass and Adjustable Gastric Band Compared with Those of an Obese Non-Surgical Group. Obesity surgery. 2016 doi: 10.1007/s11695-016-2158-4. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez I, Rubio MA, Cordido F, Breton I, Morales MJ, Vilarrasa N, Monereo S, Lecube A, Caixas A, Vinagre I, Goday A, Garcia-Luna PP. Maternal and perinatal outcomes after bariatric surgery: a Spanish multicenter study. Obesity surgery. 2015;25:436–442. doi: 10.1007/s11695-014-1387-7. [DOI] [PubMed] [Google Scholar]
- 22.Gosman GG, King WC, Schrope B, Steffen KJ, Strain GW, Courcoulas AP, Flum DR, Pender JR, Simhan HN. Reproductive health of women electing bariatric surgery. Fertility and sterility. 2010;94:1426–1431. doi: 10.1016/j.fertnstert.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Science translational medicine. 2013;5:199ra112. doi: 10.1126/scitranslmed.3006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jans G, Matthys C, Bogaerts A, Lannoo M, Verhaeghe J, Van der Schueren B, Devlieger R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: a systematic review. Advances in nutrition (Bethesda, Md) 2015;6:420–429. doi: 10.3945/an.114.008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in Relation to Body Mass and Menstrual Cycle Patterns. Epidemiology. 1999;10:422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. Journal of women’s health (2002) 2015;24:299–307. doi: 10.1089/jwh.2014.5000. [DOI] [PubMed] [Google Scholar]
- 27.Johansson K, Cnattingius S, Naslund I, Roos N, Trolle Lagerros Y, Granath F, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. The New England journal of medicine. 2015;372:814–824. doi: 10.1056/NEJMoa1405789. [DOI] [PubMed] [Google Scholar]
- 28.Kjaer MM, Nilas L. Timing of pregnancy after gastric bypass-a national register-based cohort study. Obesity surgery. 2013;23:1281–1285. doi: 10.1007/s11695-013-0903-5. [DOI] [PubMed] [Google Scholar]
- 29.Kopelman PG, Pilkington TR, White N, Jeffcoate SL. Abnormal sex steroid secretion and binding in massively obese women. Clinical endocrinology. 1980;12:363–369. doi: 10.1111/j.1365-2265.1980.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 30.Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. American journal of reproductive immunology (New York, NY : 1989) 2014;72:129–140. doi: 10.1111/aji.12234. [DOI] [PubMed] [Google Scholar]
- 31.Lefrancois L, Puddington L. Extrathymic intestinal T-cell development: virtual reality? Immunology today. 1995;16:16–21. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Zhu Z, Xiao H, Wakefield MR, Ding VA, Bai Q, Fang Y. The role of IL-7 in Immunity and Cancer. Anticancer research. 2017;37:963–967. doi: 10.21873/anticanres.11405. [DOI] [PubMed] [Google Scholar]
- 33.Manning S, Finer N, Elkalaawy M, Hashemi M, Jenkinson AD, Adamo M, O’Brien P, Batterham RL, Richens Y. Timing of pregnancy in obese women after bariatric surgery. Pregnancy hypertension. 2014;4:235. doi: 10.1016/j.preghy.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. The Journal of clinical endocrinology and metabolism. 2007;92:2672–2679. doi: 10.1210/jc.2006-2349. [DOI] [PubMed] [Google Scholar]
- 35.Mengesha B, Griffin L, Nagle A, Kiley J. Assessment of contraceptive needs in women undergoing bariatric surgery. Contraception. 2016 doi: 10.1016/j.contraception.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Milone M, De Placido G, Musella M, Sosa Fernandez LM, Sosa Fernandez LV, Campana G, Di Minno MN, Milone F. Incidence of Successful Pregnancy After Weight Loss Interventions in Infertile Women: a Systematic Review and Meta-Analysis of the Literature. Obesity surgery. 2016;26:443–451. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- 37.Moy V, Jindal S, Lieman H, Buyuk E. Obesity adversely affects serum anti-mullerian hormone (AMH) levels in Caucasian women. Journal of assisted reproduction and genetics. 2015;32:1305–1311. doi: 10.1007/s10815-015-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker MH, Berghella V, Nijjar JB. Bariatric surgery and associated adverse pregnancy outcomes among obese women. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–4. doi: 10.3109/14767058.2015.1060214. [DOI] [PubMed] [Google Scholar]
- 39.Reckelhoff JF, Zhang H, Srivastava K, Roberts LJ, 2nd, Morrow JD, Romero JC. Subpressor doses of angiotensin II increase plasma F(2)-isoprostanes in rats. Hypertension. 2000;35:476–479. doi: 10.1161/01.hyp.35.1.476. [DOI] [PubMed] [Google Scholar]
- 40.Rios DR, Alpoim PN, Godoi LC, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Increased Levels of sENG and sVCAM-1 and Decreased Levels of VEGF in Severe Preeclampsia. American journal of hypertension. 2015 doi: 10.1093/ajh/hpv170. [DOI] [PubMed] [Google Scholar]
- 41.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. Partial recovery of luteal function after bariatric surgery in obese women. Fertility and sterility. 2009;92:1410. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saccone G, Berghella V, Maruotti GM, Ghi T, Rizzo G, Simonazzi G, Rizzo N, Facchinetti F, Dall’Asta A, Visentin S, Sarno L, Xodo S, Bernabini D, Monari F, Roman A, Eke AC, Hoxha A, Ruffatti A, Schuit E, Martinelli P. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. American journal of obstetrics and gynecology. 2017;216:525.e521–525.e512. doi: 10.1016/j.ajog.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Saccone G, Schoen C, Franasiak JM, Scott RT, Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta-analysis of randomized, controlled trials. Fertility and sterility. 2017;107:430–438 e433. doi: 10.1016/j.fertnstert.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Sarwer DB, Spitzer JC, Wadden TA, Mitchell JE, Lancaster K, Courcoulas A, Gourash W, Rosen RC, Christian NJ. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA surgery. 2014;149:26–33. doi: 10.1001/jamasurg.2013.5022. [DOI] [PubMed] [Google Scholar]
- 45.Scifres C, Feghali M, Althouse AD, Caritis S, Catov J. Adverse Outcomes and Potential Targets for Intervention in Gestational Diabetes and Obesity. Obstetrics and gynecology. 2015;126:316–325. doi: 10.1097/AOG.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 46.Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstetrics and gynecology. 2011;118:63–70. doi: 10.1097/AOG.0b013e31821fd360. [DOI] [PubMed] [Google Scholar]
- 47.Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, Mazor M. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. American journal of obstetrics and gynecology. 2004;190:1335–1340. doi: 10.1016/j.ajog.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Biertho L, Simard S, Kral JG, Marceau P. Effects of Bariatric Surgical Weight Loss in Mothers on Intergenerational Transmission of Obesity. Endocrinology. 2009;150:4816. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 49.Stefater M, Perez-Tilve D, Chambers A, Wilson-Perez H, Sandoval D, Berger J, Toure M, Tscoep M, Woods S, Seeley R. Sleeve Gastrectomy Induces Loss of Weight and Fat Mass in Obese Rats, But Does Not Affect Leptin Sensitivity. Gastroenterology. 2010;138:2426–2436 e2423. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stentebjerg LL, Andersen LL, Renault K, Stoving RK, Jensen DM. Pregnancy and perinatal outcomes according to surgery to conception interval and gestational weight gain in women with previous gastric bypass. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016:1–7. doi: 10.1080/14767058.2016.1208746. [DOI] [PubMed] [Google Scholar]
- 51.Stephansson O, Johansson K, Näslund I, Neovius M. Bariatric Surgery and Preterm Birth. New England Journal of Medicine. 2016;375:805–806. doi: 10.1056/NEJMc1516566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stroh C, Weiner R, Wolff S, Knoll C, Manger T. Influences of gender on complication rate and outcome after Roux-en-Y gastric bypass: data analysis of more than 10,000 operations from the German Bariatric Surgery Registry. Obesity surgery. 2014;24:1625–1633. doi: 10.1007/s11695-014-1252-8. [DOI] [PubMed] [Google Scholar]
- 53.Stuber TN, Kunzel EC, Zollner U, Rehn M, Wockel A, Honig A. Prevalence and Associated Risk Factors for Obesity During Pregnancy Over Time. Geburtshilfe und Frauenheilkunde. 2015;75:923–928. doi: 10.1055/s-0035-1557868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan O, Carr BR. The impact of bariatric surgery on obesity-related infertility and in vitro fertilization outcomes. Seminars in reproductive medicine. 2012;30:517–528. doi: 10.1055/s-0032-1328880. [DOI] [PubMed] [Google Scholar]
- 55.Teitelman M, Grotegut C, Williams N, Lewis J. The Impact of Bariatric Surgery on Menstrual Patterns. Obesity surgery. 2006;16:1457. doi: 10.1381/096089206778870148. [DOI] [PubMed] [Google Scholar]
- 56.Valderhaug TG, Hertel JK, Nordstrand N, Dale PO, Hofso D, Hjelmesaeth J. The association between hyperandrogenemia and the metabolic syndrome in morbidly obese women. Diabetology & metabolic syndrome. 2015;7:46. doi: 10.1186/s13098-015-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walf AA, Frye CA. Gestational or acute restraint in adulthood reduces levels of 5alpha-reduced testosterone metabolites in the hippocampus and produces behavioral inhibition of adult male rats. Frontiers in cellular neuroscience. 2012;6:40. doi: 10.3389/fncel.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei YM, Yang HX, Zhu WW, Liu XY, Meng WY, Wang YQ, Shang LX, Cai ZY, Ji LP, Wang YF, Sun Y, Liu JX, Wei L, Sun YF, Zhang XY, Luo TX, Chen HX, Yu LJ. Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–5. doi: 10.3109/14767058.2015.1081167. [DOI] [PubMed] [Google Scholar]
- 59.Weintraub AY, Levy A, Levi I, Mazor M, Wiznitzer A, Sheiner E. Effect of bariatric surgery on pregnancy outcome. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2008;103:246–251. doi: 10.1016/j.ijgo.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Westbrook L, Johnson AC, Regner KR, Williams JM, Mattson DL, Kyle PB, Henegar JR, Garrett MR. Genetic susceptibility and loss of Nr4a1 enhances macrophage-mediated renal injury in CKD. Journal of the American Society of Nephrology : JASN. 2014;25:2499–2510. doi: 10.1681/ASN.2013070786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.William J, Lawson KS, Spann Redin A, Zamarripa Carlos A, Hosler Jonathan P, Grayson Bernadette E. Vertical sleeve gastrectomy improves metabolic disease in a rodent model of surgical menopause. Menopause. 2017 doi: 10.1097/GME.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


