Abstract
Background
In Creutzfeldt-Jakob disease (CJD) and other related transmissible spongiform encephalopathies it is critical to understand the various pathways by which the infectious agent spreads to different organs.
Methods
We injected a CJD agent into mice, either intracerebrally (ic) or intraperitoneally (ip) and monitored the progressive appearance of abnormal PrP in peripheral tissues over time.
Results
Abnormal PrP was detected in lymphoreticular tissues of the gastrointestinal tract as early as 28 to 32 days after infection by both routes. This change persisted until the terminal stages of disease. In contrast, abnormal PrP was not detected in brain or spinal cord until 80 to 120 days after ic inoculation, or until 170 days after ip inoculation.
Conclusions
Brain lacks significant lymphatic drainage, and has little infectivity before 40 days, even after ic inoculation. Thus the infectious inoculum must spread to the gut by a vascular route, a direction opposite to that generally assumed. This interpretation is consistent with previous studies demonstrating white blood cell infectivity as well as perivascular PrP accumulations in CJD. Notably, enteric infection at early as well as later stages of disease, and regardless of the route of agent entry, implicates potential environmental spread by fecal matter.
Background
Increasing evidence suggests that bovine spongiform encephalopathy (BSE) agents spread more rapidly and universally via contaminated food than was previously assumed. Experimentally, the oral route of infection is very inefficient with most strains of scrapie and Creutzfeldt-Jakob disease (CJD). Nevertheless, the infectious agent has been recovered from the distal ileum during the late stages of BSE (reviewed in [1]). This enteric region is replete with lymphoid Peyer's patches that are typically targeted by many penetrating viruses. Additional studies in non-human primates have shown that an abnormal form of host prion protein (PrP) accumulates in Peyer's Patches at ≥ 5 months after oral infection, a time when animals already had neurologic changes [2]. Other immunocytochemical studies have suggested that the infectious agent spreads through the body only after first targeting the gastrointestinal tract [3]. Since neuronal ganglia and fibers of the gut wall were interpreted to contain abnormal PrP histologically, neuronal fibers have often been assumed to be conduits by which the agent spreads from the periphery into the brain [4]. However, because Peyer's patches show similar changes after intraperitoneal (ip) inoculation with CJD agents [5,6], we questioned the validity of unidirectional flow of agent away from the gut, as well as whether infectious extension occurs exclusively via neurons.
Methods
We inoculated mice either ip or ic with 10 μl of isotonic saline containing 1% (v/v) of a mouse brain homogenate with the FU (or Fukuoka 2) strain of CJD, and the passaged brain contained ~109 infectious units/gm wet weight. Control mice received equivalent injections of homogenate from a normal mouse brain. At each time point indicated in Table 1, four mice were sacrificed and evaluated for PrP pathology. Ileal Peyer's patches, intraperitoneal lymph nodes, spleen, bladder, lung, kidney, pancreas, spinal cord and brain were fixed in either 10% formalin (2 each) or perfused (2 each) with buffered 4% paraformaldeyde before paraffin embedding. To confirm infectivity of the inoculum, four mice in each inoculated group (ip and ic) were kept until they displayed terminal signs and spongiform brain pathology and gliosis. Results with the ic route were the same as previously detailed for additional molecular markers [7]. The small size of individual gut associated lymphatic tissues (GALT) and the microscopic nature of neuronal plexi precluded analysis of PrP by Western blotting. Thus, in situ immunohistology was used to evaluate abnormal PrP in the gut as described [1]. Numerous larger tissue samples in our laboratory have been evaluated by both methods, and all cases of positive PrP histopathology have shown positive PrP-res by Western blotting. All tissues were treated and evaluated with equivalent specimen from uninfected mice.
Table 1.
Abnormal accumulation of PrP at various post inoculation stages.
| Abnormal PrP | CJD:days pi | NORMAL:days pi | ||||||||||
| TISSUE (route) | 2 | 4 | 14 | 28*–32 | 42 | 70* | 90*–120 | >170* | 2 | 32 | 240 | |
| central | ||||||||||||
| Brain (ic) | - | - | - | - | - | - | + | na | - | - | - | |
| Spinal cord (ic) | - | - | - | - | - | - | + | na | - | - | - | |
| Brain (ip) | - | - | - | - | - | - | - | + | - | - | - | |
| Spinal cord (ip) | - | - | - | - | - | - | - | + | - | - | - | |
| peripheral | ||||||||||||
| Peyer's Patch (ic) | - | - | - | + | + | + | + | na | - | - | - | |
| Lymph node (ic) | - | - | - | + | + | + | + | na | - | - | - | |
| Spleen (ic) | - | - | - | + | + | + | + | na | - | - | - | |
| Lymph nodes (ip) | - | - | - | + | nd | + | + | + | - | nd | - | |
| Spleen (ip) | - | - | - | + | + | + | + | + | - | - | - | |
Results
Abnormal PrP accumulation is a sign of agent invasion. Because ileal Peyer's patches were insufficient for Western blotting analysis of PrP change, and could not be cleanly dissected, we used in situ methods that abolish virtually all background PrP of normal animals in the majority of brain nuclei as previously depicted [1,7]. Larger PrP positive specimens invariably yielded positive PrP-res by Western blotting. However, some large neurons in the brainstem as well as islet cells of the pancreas typically maintained prominent PrP staining even after heat and limited proteolytic treatment. This inability to distinguish abnormal PrP in these few instances may not be resolvable by Western blotting since PrP from normal islet cells can also be maintained after proteolysis in electrophoretic analyses [8]. Similarly, the large neurons of gut showed equivocal PrP stain differences between normal inoculated and CJD infected mice beginning at day 2 after ip or ic injection. Therefore the PrP stain was not sufficiently specific to conclude the infectious agent associated with these enteric neurons. In particular, the assumption of infectivity in these neurons at early stages of infection is made even more tenuous by the finding that significant agent replication in the brain occurs only 40 days after ic inoculation [9].
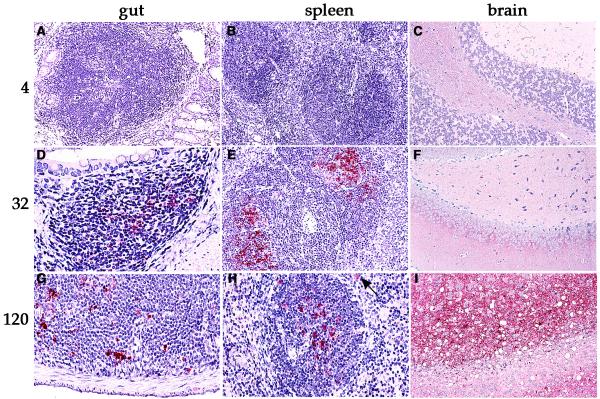
In all the other tissues, the differences in PrP between uninfected control and CJD mice were obvious. The time course of infection in brain and gastrointestinal organs is shown in Table 1. Within each group (n = 4), all mice consistently exhibited similar negative or positive changes in PrP. For comparison, the relevant data from sequential ip inoculation experiments are summarized in Table 1 (as indicated by asterisks). Between 28 and 32 days after infection, distinct changes in PrP were detected in all abdominal lymphoid tissues examined, including Peyer's patches, central and peripheral mesenteric lymph nodes, and the spleen. In Peyer's patches, PrP changes were found on follicular dendritic cells (Figure 1), as well as in myeloid cells peripheral to germinal centers. This pathology of PrP accumulation on follicular dendritic cells was similar to that demonstrated in spleen by confocal microscopy, as described previously [8]. Control mice showed no such changes (Figure 1). At 28–32 days, equivalent accumulations of abnormal PrP were also detected in abdominal lymphoid tissues, as represented in Table 1 by spleen and lymph nodes. These changes were comparable at this time, regardless of whether agent was inoculated peripherally (ip) or centrally (ic). Kidney and lung were negative for abnormal PrP. However, the bladder wall showed abnormal PrP in lymphoid regions, although epithelial cells were not positive.
Figure 1.
Representative sections of gut, spleen, and brain stained for abnormal PrP at sequential times after ic inoculation. (A – C) No pathologic PrP is observed four days after inoculation. (D – F) Only lymphoid tissues are positive 32 days after inoculation. (G – I) Clinically ill mice show abnormal PrP accumulation in brain as well as in lymphoid tissues 120 days after inoculation. The arrow in H shows a positive cell outside the germinal center that is morphologically consistent with a macrophage or dendritic cell. Methods for inoculation, fixation and detection of PrP detection have been described previously [1].
In contrast to gut and abdominal lymphoid tissues, no abnormalities in PrP were detected in brain or spinal cord until much later after infection. Abundant abnormal PrP became apparent in both the spinal cord and brain only between 90 to 120 days after ic inoculation and >170 days after ip inoculation.. Cerebral sections showed focal deposits of pathologic PrP in regions with spongiform changes at these more terminal stages as shown in Figure 1. Similarly, studies have shown that PrP change in the cerebrum switches from marginal to clear cut between 70–80 days, and other studies have shown significant increases in infectivity beginning at 60 days after ic inoculation [7,9].
Discussion
These experiments demonstrate abnormalities in PrP develop in the gut occur during early subclinical phases of infection, a time when PrP and spongiform change is not yet detectable in the central nervous system. Although infectious agent can sometimes elicit little pathologic PrP, and can be separated from most PrP during brain fractionation [10], abnormal PrP is produced only after or during exposure to the infectious agent. It is therefore remarkable that mice challenged with CJD via ic or ip routes exhibited the same early temporal pattern of PrP accumulation in the gastrointestinal tract. The presence of PrP-positive cells in the gut therefore does not implicate an oral route of infection, and furthermore indicates a common route of spread that does not involve neurons.
The gastrointestinal tract would appear to be the natural route of infection with CJD in response to oral exposure to the infectious agent. In orally delivered scrapie, there was immediate uptake of infectivity and agent replication in Peyer's patches as assayed by end-point dilution. This enteric increase in infectivity preceded infection in the spleen and spinal cord [3] and the favored concept has been that the agent spreads by lymphatics from the gut to the spleen, and into the spinal cord via the enteric and splenic sympathetic neurons. After ip scrapie, neuroinvasion may also be initiated through specific sites in the medulla, essentially excluding the lymphoreticular system as an important conduit for agent spread [4]. In the present experiments where agent was delivered intracerebrally, the lymphatic channels are probably irrelevant because the brain does not have significant lymphatic drainage. However, inoculation trauma to the brain allows most agent to be rapidly released through disrupted traumatized vessels or cleared via active cellular processes such as phagocytosis [9]. Many studies with visible tracers have shown inoculated materials travel out via vascular channels, and similarly CJD is detected in blood just after inoculation [11].
The present studies therefore indicate these agents spread via blood, with neural routes playing a secondary role. Original studies in CJD revealed a hematogenous route for agent spread after ic inoculation, and agent could also be detected in white blood cells later in disease [11,12]. For example, white blood cells from guinea pigs infected with CJD repeatedly caused disease in recipients, including the initial three weeks after ic inoculation [11]. This early appearance of blood infectivity coincided with the loss and/or clearance of most infectivity away from the brain [9]. At later stages of the disease, when the CJD agent had replicated substantially in brain, even higher levels of agent reappeared in the blood. In contrast, blood is claimed not to contain infectivity in a scrapie mouse model [13]. However mice do not survive inoculation with 20% white blood cell homogenates as was used to detect infectivity in guinea pigs. Furthermore others have found white blood infectivity in hamsters, mice and humans [14] and Houston and co-workers recently demonstrated that BSE could be transmitted by transfusion from a sheep that had been orally infected with BSE [5]. The wide dissemination of agent to many distant tissues and the perivascular PrP pathology in the brain after ip infection also support a hematogenous route of agent spread [6,8]. There is also a danger of over interpreting high levels of PrP in some neurons where the histologic picture may not be specific for infection, as in the myenteric plexus neurons noted here.
Additional arguments pointing to blood as the source of early infectivity, perhaps even more than neurons, were derived from direct analyses of dental tissue. After ip challenge with scrapie, infectivity was higher in gingival tissues than in dental pulp, and trigeminal ganglia lacked detectable infectivity until >50 days after inoculation [15]. Our current study adds further evidence suggesting that blood itself is the carrier of the infectious agent, as spinal cord showed no abnormal PrP after ic inoculation at times when clear signs of agent invasion were present in GALT. In the case of BSE, and potentially with vCJD in humans, it has now become accepted that transfused blood could be infectious [16,17]. The current studies reemphasize the probability of white blood cell infection in virtually all transmissible spongiform encephalopathies of animals and man.
While several types of white blood cells may effectively spread agent via blood to many organs including brain, macrophages and other myeloid cells like dendritic cells appear to be involved. Spleen myeloid cells 28–32 days after infection showed significant infectivity after extended culture [8]. In accord with this, we detected PrP-positive cells with a myeloid migratory morphology in enteric Peyer's patches here at the same early time. Moreover, in chronic studies of transgenic mice with specific immune cell deficiencies, similar migratory cells outside of germinal centers and around small vessels contained abnormal PrP in the submucosal interstitium of the gut. Superficial epithelial and M cells were labeled less frequently [6]. This data further supports the blood borne route of agent toward the gut. It is important to recognize this direction of agent invasion, because it is often assumed that early infection of gut, as in a diagnostic setting, is acquired only through feeding.
The widespread infection of gastrointestinal lymphoreticular tissues is important epidemiologically because it suggests there can be shedding of agent into the gut lumen during all stages of infection. This mechanism may be particularly important if common gastrointestinal infections and chemical irritations lead to superficial epithelial and submucosal shedding. Similarly, small amounts of agent might be shed through the urine under some circumstances. These findings raise the concern that if the agent is repeatedly shed into the environment, it may be extremely difficult to completely eradicate BSE in endemic areas where thousands of cows have been infected. The presence of any of these agents (BSE, scrapie, CJD) in excreted materials, including cow manure used as fertilizer could be a source for continuing human and animal spread.
Conclusions
In these studies, we provide evidence that blood can carry the CJD agent toward the gastrointestinal tract in a direction opposite to that commonly assumed. Peripheral PrP pathology can be detected months before significant agent replication in the brain. The fact that the agent can spread through blood into excretory organs such as gut implicates potential shedding of agent into the environment with greater epidemic spread.
Competing interests
None declared
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported by NTH grants NS12674 and NS34569. We thank Mark Chernyak for help with the histology.
Contributor Information
Klaus Radebold, Email: radebold@usa.net.
Mark Chernyak, Email: laura.manuelidis@yale.edu.
Daniel Martin, Email: laura.manuelidis@yale.edu.
Laura Manuelidis, Email: laura.manuelidis@yale.edu.
References
- Manuelidis L, Fritch W, Xi YG. Evolution of a strain of CJD that induces BSE-like plaques. Science. 1997;277:94–98. doi: 10.1126/science.277.5322.94. [DOI] [PubMed] [Google Scholar]
- Bons N, Mestre-Frances N, Belli P, et al. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH, Walker CA. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 1989;12:213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- Baldauf E, Beekes M, Diringer H. Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. J Gen Virol. 1997;78:1187–97. doi: 10.1099/0022-1317-78-5-1187. [DOI] [PubMed] [Google Scholar]
- Muramoto T, Kitamoto T, Tateishi J, Goto I. Accumulation of abnormal prion protein in mice infected with Creutzfeldt-Jacob disease via intraperitoneal route: a sequential study. Am J Pathol. 1993;143:1470–1479. [PMC free article] [PubMed] [Google Scholar]
- Shiomchik MJ, Radebold K, Duclos N, Manuelidis L. Neuroinvasion by a Creutzfeldt-Jakob disease agent in the absence of B cells and follicular dendritic cells: Proc Natl Acad Sci. 2001;98:9289–9294. doi: 10.1073/pnas.161055198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CA, Lu ZY, Zaitsev I, Manuelidis L. Microglial activation varies in different models of Creutzfeldt-Jakob disease. J Virol. 1999;73:5089–5097. doi: 10.1128/jvi.73.6.5089-5097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Zaitsev I, Koni P, Lu ZY, Flavell RA, Fritch W. Follicular dendritic cells and dissemination of Creutzfeldt-Jacob disease. J Virol. 2000;74:8614–8622. doi: 10.1128/JVI.74.18.8614-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Fritch W. Infectivity and host responses in Creutzfeldt-Jakob disease. Virology. 1996;216:46–59. doi: 10.1006/viro.1996.0033. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Beneath the emperor's clothes: The body of data in scrapie and CJD. Annales de L'Institut Pasteur. 1997;8 (4):311–326. doi: 10.1016/S0924-4204(97)86597-3. [DOI] [Google Scholar]
- Manuelidis EE, Gorgacz EJ, Manuelidis L. Viremia in experimental Creutzfeldt-Jacob disease. Science. 1978;200:1069–1071. doi: 10.1126/science.349691. [DOI] [PubMed] [Google Scholar]
- Manuelidis EE, Kim JH, Mericangas JR, Manuelidis L. Transmission to animals of Creutzfeldt-Jacob disease from human blood. Lancet. 1985;ii:896–897. doi: 10.1016/S0140-6736(85)90165-5. [DOI] [PubMed] [Google Scholar]
- Raeber AJ, Klein MA, Frigg R, Flechsig E, Aguzzi A, Weissmami C. PrP-dependent association of prions with splenic but not circulating lymphocytes of scrapie-infected mice. EMBO J. 1999;18:2702–2706. doi: 10.1093/emboj/18.10.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rowher RG, Dunstan BS, MacAuley C, Gadjusek DC, Drohan WN. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion. 1998;38:810–816. doi: 10.1046/j.1537-2995.1998.38998408999.x. [DOI] [PubMed] [Google Scholar]
- Ingrosso L, Pisani F, Pocchiari M. Transmission of the 263K scrapie strain by the dental route. J Gen Virol. 1999;80:3043–3047. doi: 10.1099/0022-1317-80-11-3043. [DOI] [PubMed] [Google Scholar]
- Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999–1000. doi: 10.1016/S0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- Brown P, Cervenakova L, Diringer H. Blood infectivity and the prospects for a diagnostic screening test in Creutzfeldt-Jacob disease. J Lab Clin Med. 2001;137:5–13. doi: 10.1067/mlc.2001.111951. [DOI] [PubMed] [Google Scholar]