Abstract
Background
Gout is a metabolic disease and is the most common form of inflammatory arthritis affecting men. However, the pathogenesis of gout is still uncertain, and novel biomarkers are needed for early prediction and diagnosis of gout. The aim of this study was to develop a systemic metabolic profile of patients with asymptomatic hyperuricemia (HUA) and gout by using a metabolomics approach, and find potential pathophysiological mechanisms of and markers of predisposition to gout.
Methods
Serum samples were collected from 149 subjects, including 50 patients with HUA, 49 patients with gout and 50 healthy controls. 1H nuclear magnetic resonance (NMR) spectroscopy combined with principal components analysis and orthogonal partial least squares-discriminant analysis were used to distinguish between samples from patients and healthy controls. Clinical measurements and pathway analysis were also performed to contribute to understanding of the metabolic change.
Results
By serum metabolic profiling, 21 metabolites including lipids and amino acids were significantly altered in patients with HUA or gout. The levels of identified biomarkers together with clinical data showed apparent alteration trends in patients with HUA or gout compared to healthy individuals. According to pathway analysis, three and five metabolic pathways were remarkably perturbed in patients with HUA or gout, respectively. These enriched pathways involve in lipid metabolism, carbohydrate metabolism, amino acids metabolism and energy metabolism.
Conclusions
Taken together, we identified the biomarker signature for HUA and gout, which provides biochemical insights into the metabolic alteration, and identified a continuous progressive axis of development from HUA to gout.
Electronic supplementary material
The online version of this article (10.1186/s13075-018-1600-5) contains supplementary material, which is available to authorized users.
Keywords: Metabolomics, NMR, Hyperuricemia, Gout, Biomarkers
Background
Gout is a type of common inflammatory arthritis in adults that is associated with excruciating pain, and the prevalence of gout has risen over the last few decades [1–3]. It reduces quality of life in patients, even causing disability due to excruciatingly painful acute attacks of gouty arthritis, malformation of joints, chronic joint damage and renal stone formation. Gout is triggered by the deposition of monosodium urate crystals in the joints and hyperuricemia (HUA) contributes to the development of gout [4, 5]. HUA has long been recognized as the key causal precursor in the development of gout and the prevalence of comorbidities tends to increase with serum uric acid (SUA) levels [6, 7]. HUA and gout are closely associated with components of metabolic syndrome, kidney injury and cardiovascular diseases [8–10]. However, the pathogenesis of gout seems to be complex because many individuals with HUA form monosodium urate crystals and develop acute attacks of gouty arthritis, but some of them do not follow this trend [11]. It is well-known that HUA and gout are metabolic diseases, but it is uncertain what the metabolic difference between them is and whether this difference promotes acute attacks of gouty arthritis. Therefore, it is important to recognize metabolism-related indicators in gout, which may contribute to understanding of the predisposition to gout. Moreover, due to the possibility that the diagnosis of gout based on SUA as a biomedical indicator in the clinic may be inaccurate, even if crystals are identified in the digits [12], the identification of novel biomarkers associated with the occurrence and development of gout is highly desirable to prevent the acute attacks of gouty arthritis and destruction of the joints.
Metabolomics is an emerging and rapidly developing field that offers an efficient approach to describe biomarkers or characterize perturbations of diseases via detection, identification and quantification of low-molecular-weight metabolites (< 1 kDa) in biological samples such as plasma and urine [13–15]. The metabolomics approach has been successfully applied in recent years to identify early signals or biomarkers of abnormalities [16], biological pathway characterization [17] and disease diagnosis [16, 18]. 1H nuclear magnetic resonance (NMR) spectroscopy is an attractive tool in metabolomics research because of several advantages, such as simple sample preparation, high reproducibility and fast analysis. Therefore, 1H NMR-based metabolomics is suitable for simultaneous and systemic analysis of multiple compounds of metabolite fingerprinting [19].
In the current study, we carried out a serum metabolomics study on a male population with normal SUA, hyperuricaemia and gout by using 1H NMR spectroscopy coupled with chemometric methods. The aim of this study was to explore the serum metabolic alteration in patients with asymptomatic hyperuricemia and patients with gout, to capture the metabolic alteration associated with the initiation and progression of gout.
Methods
Study subjects and sample collection
Asymptomatic patients with hyperuricemia (n = 50) and patients with gout (n = 49) were enrolled in the physical examination center of the Second Affiliated Hospital of Harbin Medical University between January 2013 and June 2015. An additional 50 healthy control samples were collected from healthy donors. All the diagnosis of these patients was confirmed by experienced doctors according to serum uric acid levels and joint swelling. Hyperuricemia in men was defined as SUA ≥ 416 mmol/L, which is a widely accepted diagnostic criterion [20–23]. All patients with gout fulfilled the 1977 preliminary American Rheumatism Association classification criteria for gout [24]. They had been newly diagnosed with gout within the last 2 years and had experienced no acute attacks of gout within the last 3 months, thus excluding the effects of inflammation and drug treatments. All participants were male adult residents in the Harbin regionand were did not have diabetes mellitus, heart disease, liver or renal dysfunction, gastrointestinal disease, pulmonary disease or cancer, and had taken no metabolic drugs or dietary supplements within the last 3 months. Each participant had been given a standardized diet plan for 3 days before blood was drawn, and the consumption of alcohol and caffeine products were forbidden during this period. Demographic data (age and gender) and anthropometric data (height, weight, diastolic blood pressure (DBP) and systolic blood pressure (SBP)) were obtained from all participants, and current medications and medical history were also recorded.
Venous blood was taken from participants after overnight fasting and allowed to clot for 30 min at room temperature. It was then centrifuged at 3000 rpm for 10 min and the supernatant was stored at − 80 °C until NMR analysis.
Clinical chemistry measurements
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, albumin, globulin, fasting glucose, creatinine, urea nitrogen, triglyceride, uric acid, cholesterol, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol were measured using an automatic biochemical analyzer (AUTOLAB PM4000, Rome, Italy). The values were expressed as mean ± SD. Student’s t test was conducted to compare the clinical biochemical data using SPSS 20 software (SPSS Inc., Chicago, IL, USA). A p value <0.05 was regarded as statistically significant.
Serum sample preparation
Serum samples were removed from − 80 °C storage and thawed at 4 °C. A volume of 200 μL serum and 350 μL of 0.9% NaCl (w/v) solution containing 20% D2O were mixed and then followed by centrifugation (10,000 g, 4 °C, 10 min). Finally 500 μL of the supernatant of each sample was transferred into individual 5-mm high-quality NMR tubes.
1H NMR spectroscopic analysis
NMR spectra of serum samples were recorded on a Bruker AVIII 500 spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5-mm inverse broadband probe at 300 K. The 1H NMR spectra were recorded with the relaxation edited Carr–Purcell–Meiboom–Gill (CPMG, RD-90°-(τ-180°-τ)n-acquisition) pulse sequence to detect low-molecular-weight metabolites over a spectral width of 20 ppm with 128 transients, 60 k data points, and 4 s relaxation delay. In order to facilitate the identification of metabolites, two dimensional (2D) J-resolved spectroscopy (JRES) spectra were acquired as previously reported [25, 26].
NMR data processing
NMR spectra were processed using TOPSPIN software package (version 3.2, Bruker Biospin, Germany). For 1H NMR spectra, an exponential window function was employed with a line broadening factor of 0.3 Hz and zero-filled to 128 k prior to Fourier transformation. Each spectrum was then manually phase-corrected and baseline-corrected and calibrated with the anomeric proton signal of α-glucose (δ 5.23 ppm). The spectra were segmented into regions with a width of 0.01 ppm (δ 0.5–9.0 ppm) using AMIX software package (V3.9.14, Bruker Biospin). The regions of imperfect water saturation signals (δ 4.50–5.15 ppm) and urea signals (δ 5.50–6.50 ppm) were discarded. The NMR resonances were assigned according to an electronic database (HMDB, http://www.hmdb.ca/) and data from the literature [27, 28], and were confirmed with 2D NMR results.
Multivariate statistical analysis
Multivariate data analysis was performed in order to establish a systemic overview of the discrimination of metabolic patterns in patients with HUA, patients with gout and controls. At first, principal components analysis (PCA) was used to observe the intrinsic metabolic variation in 1H NMR spectra data. Next, orthogonal partial least squares-discriminant analysis (OPLS-DA) was carried out to maximize the variation between groups and then detect significant metabolites that contribute to the variation. A coefficient of variation-analysis of variance (CV-ANOVA) approach was further applied to test the significance of intergroup differentiations (p < 0.05) in OPLS-DA models. Loadings plots of OPLS-DA models were generated using MATLAB 7.1 (Mathworks Inc., USA) with correlation coefficients. In these loadings plots, hot-colored metabolites have greater contribution in intergroup differentiations than cold-colored ones. The selection of significant metabolites was based on correlation coefficients (|r| > 0.6) and Student’s t test (p < 0.01). To visualize the alterations of remarkable metabolites in three groups, a heat map was created using MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/).
Pathway analysis
Pathway analysis of remarkably changed metabolites in patients with HUA and patients with gout was applied within MetaboAnalyst 3.0. Among all the perturbed pathways, the ones with impact value > 0.1 and p < 0.05 were selected as significantly perturbed metabolic pathways in HUA and patients with gout.
Results
Baseline characteristics of participants
The representative sample of this metabolomics study consisted of 149 male participants (50 controls, 50 patients with HUA and 49 patients with gout) whose basic characteristics and clinical variables are summarized in Table 1. Body mass index (BMI), DBP, SBP, ALT, AST, fasting glucose, uric acid, triglyceride, cholesterol and LDL-cholesterol were notably increased in HUA and in patients with gout compared to the control group (p < 0.05). Compared to the HUA group, the gout group had significant higher levels of DBP, SBP, fasting glucose, uric acid and HDL-cholesterol and a lower level of albumin.
Table 1.
Baseline characteristics (demographic, anthropometric and clinical data) for the HUA, gout, and control groups
| Parameter | Control (n = 50) | HUA (n = 50) | Gout (n = 49) |
|---|---|---|---|
| Basic characteristics | |||
| Age (years) | 43.8 ± 11.5 | 39.08 ± 10.4* | 45.6 ± 7.3## |
| Sex (female/male) | 0/50 | 0/50 | 0/50 |
| BMI (kg/m2) | 23.4 ± 3.2 | 27.09 ± 3.0** | 26.5 ± 3.3** |
| Smoker/non-smoker | 24/26 | 22/28 | 23/26 |
| Alcohol consumption (%) | 61 | 67 | 57 |
| Clinical variables | |||
| DBP (mmHg) | 78.6 ± 5.7 | 85.1 ± 11.1** | 91.8 ± 12.4**## |
| SBP (mmHg) | 114.8 ± 6.5 | 125.8 ± 14.4** | 136.3 ± 19.2**## |
| ALT(U/L) | 22.6 ± 11.3 | 43.8 ± 30.6** | 34.8 ± 18.9** |
| AST(U/L) | 20.5 ± 5.9 | 27.7 ± 14.8** | 26.8 ± 12.9** |
| Total protein (g/L) | 74.8 ± 4.3 | 75.5 ± 4.4 | 74.6 ± 4.5 |
| Albumin (g/L) | 49.4 ± 2.9 | 48.9 ± 2.2 | 47.8 ± 3.3**# |
| Globulin (g/L) | 25.4 ± 4.0 | 26.7 ± 3.5 | 26.9 ± 4.0 |
| Fasting glucose (mmol/L) | 5.0 ± 0.4 | 5.7 ± 0.7** | 6.3 ± 1.9**# |
| Urea nitrogen (mmol/L) | 5.6 ± 1.3 | 5.4 ± 1.2 | 5.5 ± 1.8 |
| Creatinine (μmol/L) | 80.6 ± 9.6 | 81.2 ± 13.9 | 91.8 ± 23.2**## |
| Uric acid (μmol/L) | 325.1 ± 60.6 | 470.6 ± 55.0** | 536.2 ± 131.4**## |
| Triglyceride (mmol/L) | 1.1 ± 0.4 | 2.3 ± 1.2** | 3.1 ± 2.1** |
| Cholesterol (mmol/L) | 4.7 ± 0.8 | 5.2 ± 0.8** | 5.4 ± 1.0** |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.3 | 1.1 ± 0.2** | 1.3 ± 0.3## |
| LDL-cholesterol (mmol/L) | 2.7 ± 0.5 | 3.2 ± 0.7** | 3.0 ± 0.6* |
Data are presented as mean ± SD except where stated otherwise
HUA hyperuricemia, BMI body mass index, DBP diastolic blood pressure, SBP systolic blood pressure, ALT alanine aminotransferase, AST aspartate aminotransferase, HDL high-density lipoprotein, LDL low-density lipoprotein
*p < 0.05, **p < 0.01, compared to control; #p < 0.05, ##p < 0.01, compared to the HUA group
1H NMR spectroscopy
Three CPMG 1H NMR spectra (Fig. 1) of serum samples obtained from control individuals (Fig. 1a), patients with HUA (Fig. 1b) and gout (Fig. 1c) show the average signals of metabolites. In total, 41 metabolites were identified in serum samples including lipids, glucose, amino acids and organic acids, as shown in Fig. 1 and Additional file 1.
Fig. 1.
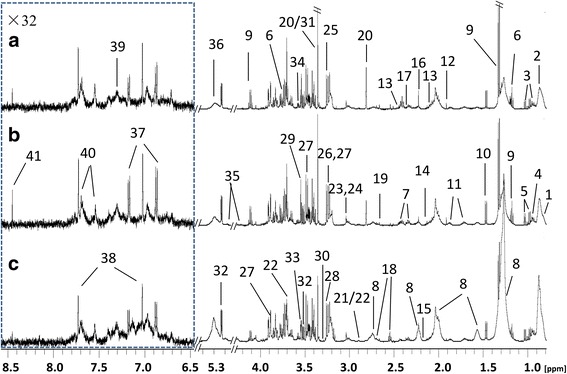
Typical 500-MHz Carr–Purcell–Meiboom–Gill (CPMG) 1H nuclear magnetic resonance spectra of human serum samples from controls (a), patients with hyperuricemia (b) and patients with gout (c). The dotted regions were vertically expanded 32 times. 1, high-density lipoprotien; 2, very low-density lipoprotein; 3, isoleucine; 4, leucine; 5, valine; 6, ethanol; 7, 3-hydroxybutytrate; 8, lipid; 9, lactate; 10, alanine; 11, lysine; 12, acetate; 13, glutamine; 14, methionine; 15, glycoprotein; 16, acetone; 17, glutamate; 18, citrate; 19, aspartate; 20, methylguanidine; 21, trimethylamine; 22, dimethylglycine; 23, creatine; 24, creatinine; 25, choline; 26, arginine; 27, β-glucose; 28, trimethylamine n-oxide; 29, myo-inositol; 30, proline; 31, scyllo-inositol; 32, α-glucose; 33, glycine; 34, threonine; 35, triglycerides; 36, unsaturated lipids; 37, tyrosine; 38, 1-methylhistidine; 39, phenylalanine; 40, tryptophan; 41, formate
Multivariate analysis of NMR data
Since SUA is an important factor in gout, and the range of SUA levels was large in subjects with gout in this study (Table 1), the NMR spectrum data from the two gout subgroups including gout with HUA (n = 32) and gout with normal SUA (n = 17) were analyzed by PCA and OPLS-DA, to determine whether SUA affected the metabolic profiles in patients with gout. The OPLS-DA scores plot (Additional file 2) showed no metabolic variation trend between the two subgroups in relation to SUA. This may be due to the complexity of gout pathogenesis, which cannot be explained by levels of uric acid, as aforementioned. Therefore, in the following multivariate data analysis, the two gout subgroups were processed and treated as one group.
To observe the clustering trends of samples obtained from patients with HUA or gout and control subjects, serum metabolic profiling was performed using PCA and OPLS-DA. The PCA scores plot for the first two components (R2X = 0.367, Q2 = 0.34) reflecting a separation trend in the gout, HUA and control groups (Fig. 2a). Furthermore, three distinct clusters of samples were observed in the OPLS-DA scores plot (R2X = 0.484, R2Y = 0.711 and Q2 = 0.566; Fig. 2b). To assess the risk that the current OPLS-DA model was spurious, the data were analyzed using the CV-ANOVA approach; a p value of 1.1E-26 showed that the OPLS-DA model was valid.
Fig. 2.
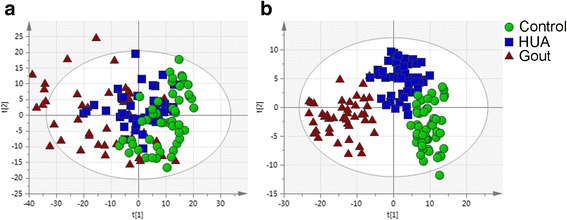
Principal components analysis (a) and orthogonal partial least squares-discriminant analysis (b) score plots based on 1H nuclear magnetic resonance data from serum samples obtained from controls, patients with hyperuricemia (HUA) and patients with gout
OPLS-DA was further performed to identify the significantly altered metabolites in the HUA and gout groups as shown in Fig. 3. The OPLS-DA scores plot (R2X = 0.516, Q2Y = 0.931, Q2 = 0.538, p = 2.4E-10) showed clear separation between the HUA and control groups (Fig. 3a). According to the corresponding loading plot, compared with control group, the HUA group had significantly higher levels of very low-density lipoprotein (VLDL), isoleucine, leucine, lipid, lactate, alanine, lysine, acetone, glutamate, creatinine, β-glucose, α-glucose, threonine, triglycerides, unsaturated lipids and tyrosine. The metabolic differences between the gout and control groups were visible in the OPLS-DA scores plot (R2X = 0.52, R2Y = 0.963, Q2 = 0.729, p = 1.5E-19; Fig. 3b). Compared with the control group, VLDL, isoleucine, leucine, lipid, glutamine, methionine, acetone, citrate, aspartate, β-glucose, creatinine, α-glucose, threonine, triglycerides, unsaturated lipids and phenylalanine were remarkably increased in the gout group. Moreover, there was a clear difference in metabolic profiles between the HUA and gout groups in the OPLS-DA scores plot (R2X = 0.518, R2Y = 0.945, Q2 = 0.641, p = 5.5E-15; Fig. 3c). Compared with the HUA group, the gout group had notably higher VLDL, lipid, acetone, citrate, aspartate, β-glucose and α-glucose. The significantly changed metabolites are summarized in Table 2. Among the 21 metabolites remarkably changed in patients with HUA and patients with gout, a total of 11 metabolites were disturbed in both groups (Fig. 4a). To further understand the metabolic changes in patients with HUA and patients with gout, a clustering heatmap was used to visualize changes in metabolites. The heatmap (Fig. 4b) of 21 significantly changed metabolites in patients with HUA and patients with gout, showed that there was a remarkable change of the metabolic profile in patients with HUA and a more greater difference in patients with gout.
Fig. 3.
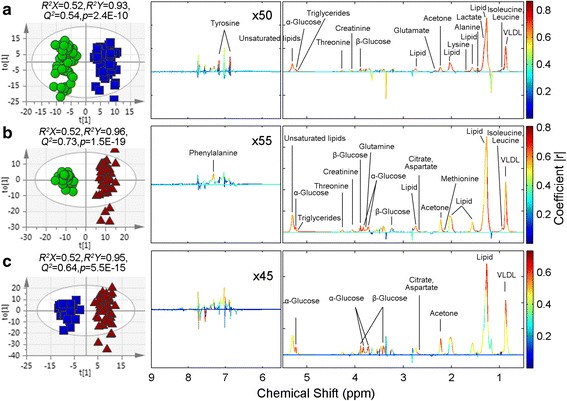
Orthogonal partial least squares-discriminant analysis score plots of samples (left panel) and corresponding coefficient loading plots (right panel) obtained from different pairwise groups: a hyperuricemia (HUA) (blue dots) and control groups (green dots); b gout (red dots) and control groups (green dots); c gout (red dots) and HUA (blue dots). The color bar on the right corresponds to the weight of a variable in the discrimination between sets of samples, beginning from weak (blue) to strong (red) correlation for the discrimination. VLDL, very low-density lipoprotein
Table 2.
Summary of significantly changed metabolites in the HUA and gout group
| Metabolites | Changes in HUA (vs control) |
Changes in gout (vs control) |
Changes in gout (vs HUA) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Trend | r a | p b | Trend | r a | p b | Trend | r a | p b | |
| VLDL | ↑ | 0.71 | 0.00 | ↑ | 0.61 | 0.00 | ↑ | 0.61 | 0.00 |
| Isoleucine | ↑ | 0.86 | 0.00 | ↑ | 0.72 | 0.00 | – | – | – |
| Leucine | ↑ | 0.83 | 0.00 | ↑ | 0.71 | 0.00 | – | – | – |
| Lipid | ↑ | 0.68 | 0.00 | ↑ | 0.72 | 0.00 | ↑ | 0.63 | 0.00 |
| Lactate | ↑ | 0.70 | 0.00 | – | – | – | – | – | – |
| Alanine | ↑ | 0.77 | 0.00 | – | – | – | – | – | – |
| Lysine | ↑ | 0.76 | 0.00 | – | – | – | – | – | – |
| Glutamine | – | – | – | ↑ | 0.60 | 0.00 | – | – | – |
| Methionine | – | – | – | ↑ | 0.67 | 0.00 | – | – | – |
| Acetone | ↑ | 0.64 | 0.00 | ↑ | 0.63 | 0.00 | ↑ | 0.65 | 0.00 |
| Glutamate | ↑ | 0.69 | 0.00 | – | – | – | – | – | – |
| Citrate | – | – | – | ↑ | 0.68 | 0.00 | ↑ | 0.65 | 0.00 |
| Aspartate | – | – | – | ↑ | 0.73 | 0.00 | ↑ | 0.63 | 0.00 |
| Creatinine | ↑ | 0.63 | 0.00 | ↑ | 0.65 | 0.00 | – | – | – |
| β-Glucose | ↑ | 0.65 | 0.00 | ↑ | 0.70 | 0.00 | ↑ | 0.65 | 0.00 |
| α-Glucose | ↑ | 0.63 | 0.00 | ↑ | 0.68 | 0.00 | ↑ | 0.66 | 0.00 |
| Threonine | ↑ | 0.65 | 0.00 | ↑ | 0.60 | 0.00 | – | – | – |
| Triglycerides | ↑ | 0.70 | 0.00 | ↑ | 0.61 | 0.00 | – | – | – |
| Unsaturated lipids | ↑ | 0.67 | 0.00 | ↑ | 0.63 | 0.00 | – | – | – |
| Tyrosine | ↑ | 0.75 | 0.00 | – | – | – | – | – | – |
| Phenylalanine | – | – | – | ↑ | 0.63 | 0.00 | – | – | – |
Increased levels are indicated by arrows (↑)
VLDL very low-density lipoprotein
aCorrelation coefficient (r) was obtained from the orthogonal partial least squares-discriminant analysis model
bThe p value was calculated using Student’s t test
Fig. 4.
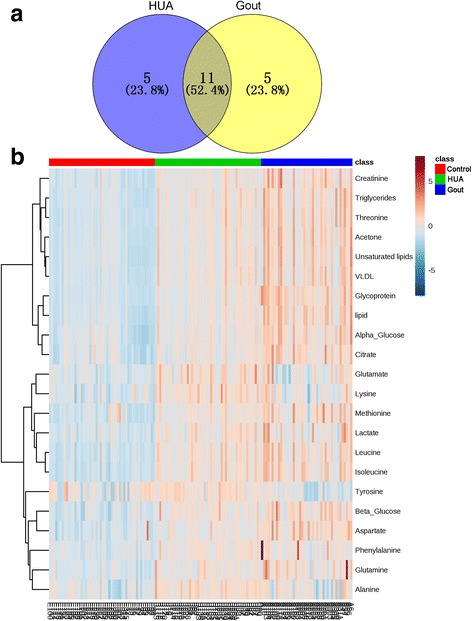
Significantly changed metabolites in patients with hyperuricemia (HUA) and patients with gout. a Numbers of significant metabolites. b Heatmap of significantly changed metabolites. The color of each section corresponds to a concentration value of each metabolite calculated by the peak area normalization method (red, upregulated; blue, downregulated)
Pathway analysis
According to the pathway analysis, 29 and 30 metabolic pathways were disturbed in patients with HUA and patients with gout, respectively. In the HUA group, three pathways were significantly perturbed including aminoacyl-transfer RNA (tRNA) biosynthesis, valine, leucine and isoleucine biosynthesis, and D-glutamine and D-glutamate metabolism were significantly perturbed. In patients with gout, five metabolic pathways were remarkably disturbed including aminoacyl-tRNA biosynthesis, valine, leucine and isoleucine biosynthesis, nitrogen metabolism, alanine, aspartate and glutamate metabolism, D-glutamine and D-glutamate metabolism (Fig. 5 and Additional file 3).
Fig. 5.
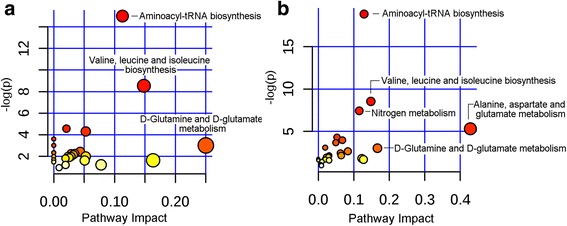
Pathway analysis of significantly changed metabolites in the hyperuricemia group (a) and gout group (b). tRNA, transfer RNA
Discussion
Gout is a worldwide public health problem. However, current research falls short in evaluating the metabolic change in gout and asymptomatic hyperuricemia. In the current study, we used 1H NMR-based metabolomics to analyze metabolites in serum from patients with asymptomatic hyperuricemia and gout, hoping to help gain understanding of the predisposition to gout.
Our research demonstrated that the 1H NMR-based metabolomics approach is feasible to examine metabolic change in patients with asymptomatic hyperuricemia and gout. Such an approach is also helpful in selecting metabolic pathways that play vital roles in the development of gout. The levels of some identified biomarkers showed a trend of an apparent increase in patients with HUA and patients with gout from levels in healthy individuals; together with clinical data this suggests an increase in metabolic disorders. In pathway analysis (Fig. 5 and Additional file 3), more metabolic pathways were notably affected in the gout groups (five pathways) than in the HUA groups (three pathways), which indicated that there was a more severe metabolic disorder in patients with gout.
Lipid metabolism
Altered lipid profiles were observed in patients with HUA, including increased levels of VLDL, fatty acids, triglyceride (TG) and unsaturated lipids; related variables in clinical chemistry results (Table 1) were also found to be significantly changed including increased TG, total cholesterol (TC), LDL-cholesterol and decreased HDL-cholesterol. Similarly, elevated VLDL, fatty acids, TG and unsaturated lipids were observed in patients with gout, which was consistent with clinical chemistry data showing that TC, TG and LDL-cholesterol increased in patients with gout. These results suggest that there was a lipid metabolism disorder in both patients with HUA and patients with gout. Several researchers have reported that HUA and gout are associated with cardiovascular and cerebrovascular diseases due to the correlation between serum uric acid and serum lipids [29, 30]. Moreover, our findings that lipid metabolism disorder and the elevated blood pressure, fasting plasma glucose and BMI in patients with HUA and patients with gout were consistent with the previous study showing that the prevalence of metabolic syndrome among individuals with HUA and gout is remarkably high [29, 31]. Our results indicate that lipid levels are highly linked with HUA and gout, thereby lipid-lowering therapy may provide a supplementary role to slow the development of gout.
Carbohydrate metabolism
Increased α-glucose and β-glucose were observed in samples from both patients with HUA and patients with gout, which suggests changes in carbohydrate metabolism. A large number of studies have shown that serum uric acid is positively related to elevated blood glucose due to insulin resistance [32–35]. Insulin is the only hormone in the body that promotes the uptake and utilization of glucose in tissues and lowers blood glucose. Although we did not measure insulin, the increased glucose verified the inhibition of glucose metabolism in both patients with HUA and patients with gout, and it was more severe in patients with gout because of the higher glucose in the samples from patients with gout than in those from patients with HUA. As the main product of glycolysis, lactate is typically interpreted as a marker of anaerobic metabolism, and its accumulation usually accounts for a high energy demand in the biological system [36]. Increased lactate was observed in HUA samples, indicating the energy demand in patients with HUA induced by low utilization of glucose. However, the trend of increased lactate was not observed in gout; this may be due to the accelerated gluconeogenesis in patients with gout for converting lactate to glucose to meet the more urgent energy demand. Increased citrate levels were seen in gout but not in HUA samples. Since citrate is an important intermediate in the tricarboxylic acid cycle (TCA) in mitochondria, the data may imply that altered mitochondrial function affected citrate handling and induced and imbalance in the global energy supply in patients with gout.
Aminoacyl-tRNA biosynthesis
Six and seven amino acids were significantly increased (p < 0.01) in patients the HUA (alanine, lysine, isoleucine, leucine, threonine and tyrosine) and the gout (phenylalanine, glutamine, aspartic acid, methionine, isoleucine, leucine, threonine) groups, respectively (Additional file 3), which indicates decreased protein syntheses or increased amino acid synthesis. Coincidentally, aminoacyl-tRNA biosynthesis was significantly affected in both patients with gout and patients with HUA. Aminoacyl-tRNA biosynthesis plays an important role in matching amino acids with tRNAs containing the corresponding anticodon for the messenger RNA (mRNA)-guided synthesis of proteins at the ribosome [37]. As we all know, amino acid metabolism is the biochemical basis in the regulation of both proteins and energy metabolisms. Greater involvement of amino acids and the greater impact value of aminoacyl-tRNA biosynthesis in gout compared to HUA suggests that translation was suppressed following the development of gout. Furthermore, aminoacyl-tRNA synthetases (AARSs) are essential enzymes in aminoacyl-tRNA biosynthesis, which have a family of twenty enzymes [38]. It is reported that mutations in AARSs have been identified in diverse human diseases, such as musculoskeletal, cardiovascular, and urinary diseases [39]. Therefore, AARSs maybe potential indicators for identifying HUA and gout.
Valine, leucine and isoleucine biosynthesis
Branched chain amino acids (BCAAs), including isoleucine, leucine and valine, are essential amino acids and act as important signaling molecules and substrate in protein synthesis. On the other hand, increasing evidence shows that perturbed amino acid metabolism, especially circulating metabolites such as high levels of blood BCAAs, are strongly associated with insulin resistance, obesity, diabetes mellitus and cardiovascular disease [40–42]. Mitochondrial branched chain aminotransferase (BCATm), one of the two BCAT isoforms and highly expressed in all tissues in the mitochondria of the cell, converts the BCAAs into their corresponding α-keto acids. Thus, the increased levels of BCAAs in our study can be attributed to reduced expression of BCATm. It indicates that the increase in BCAAs causes the accumulation of its byproducts that can impaire mitochondrial capacity, and the affected mitochondrial function is related to the development of insulin resistance [40, 43]. Wang et al. found that BCAAs are significantly related to obesity and risk factors for some metabolic diseases [44]. Another study followed 2422 normoglycemic individuals for 12 years and found that the BCAAs may presage the development of type 2 diabetes mellitus by up to a decade or more and thus, may be among the earliest detectable metabolic derangements on the route to diabetes mellitus [45]. Our findings are in agreement, as both the HUA and gout groups had higher levels of isoleucine and leucine, and elevated BMI and fasting glucose, suggesting that there is correlation between insulin resistance and gout development.
Furthermore, it is known that BCAAs can undergo transamination to generate nitrogen for synthesis of non-essential amino acids such as glutamine and alanine [46]. In our study, although BCAAs were increased in samples both from patients with HUA and patients with gout, increased glutamine was only observed in gout but not in HUA and increased alanine was only seen in HUA but not in gout. These may have resulted from differential consumption of these amino acids in HUA and in gout. Glutamine is the most abundant free amino acid in human blood; it is consumed by proliferating cells and converted to glutamate en route to producing other metabolic intermediates that contribute to cell growth [47, 48]. Therefore, the increased level of glutamine in gout may due to the lower cellular metabolic rate in patients with gout. Alanine is used in protein synthesis and as precursor for gluconeogenesis in the liver. Under periods of starvation, alanine is generated from muscle BCAAs and transported to the liver where it is used in the glucose alanine cycle to make glucose for energy needs [49]. Hence, the fact that increased alanine was only seen in HUA but not in gout may be due to its consumption in gluconeogenesis to meet the more urgent energy demand in patients with gout.
D-Glutamine and D-glutamate metabolism
The D-glutamine and D-glutamate metabolism is a major regulatory mechanism of glutamate and glutamine levels in organisms [50]. Glutamate is an excitatory neurotransmitter and glutamine is the precursor and storage form of glutamate. In this study, compared to controls, patients with HUA had a higher level of glutamate and patients with gout had a higher level of glutamine. Thus, our results indicate that the perturbation of D-glutamine and D-glutamate metabolism occurred in both patients with HUA and patients with gout. There is evidence to suggest that uric acid has a remarkable antioxidant effect on neurons [51, 52]. However, the protective effect of gout on the risk of neurological disease is a controversial issue [53, 54]. It is said that metabolic syndrome, a frequent comorbidity of HUA and gout, might offset the anti-oxidative benefit from the high uric acid level [55, 56]. Zheng et al. identified disturbance of the glutamate-glutamine cycle with an increased level of glutamine in the hippocampus of mice with diabetes-associated decline in cognition, and regarded this change as the underlying reason for diabetes-related neurological complications [57]. Although none of the patients with gout in the present study had diabetes mellitus, fasting glucose was significantly increased in these patients. Thus, glutamine may be an early biomarker of gout and its comorbidities.
Alanine, aspartate and glutamate metabolism and nitrogen metabolism
In our study, metabolites related to alanine, aspartate and glutamate metabolism (aspartic acid and glutamine) and nitrogen metabolism (phenylalanine, aspartic acid and glutamine) were increased in serum from patients with gout: this indicates the perturbation of amino acid metabolism and energy metabolism in patients with gout. Among all the significantly disturbed metabolic pathways, alanine, aspartate and glutamate metabolism and nitrogen metabolism were disturbed in patients with gout but were not detected in patients with HUA, which showed the aggravation of metabolic disorders in patients with gout.
Conclusion
In summary, we investigated the application of 1H NMR spectroscopy-based metabolomics to detect metabolic changes in serum from patients with HUA and patients with gout. Our results indicated significant dysregulation of metabolic pathways in patients with gout. The metabolic alterations were associated with the disturbance of lipid metabolism, carbohydrate metabolism, amino acids metabolism and energy metabolism. Clear metabolic differences were observed between patients with HUA, patients with gout and controls, indicating that the disease has a continuous progressive development axis. The combination of these metabolic alterations may corporately hold promise for early prediction and diagnosis of the progression of gout.
Additional files
Metabolite assignments of major resonances detected in 1H NMR spectra from human serum samples. (DOCX 29 kb)
PCA and OPLS-DA scores plots based on 1H NMR spectrum data of serum samples obtained from two gout subgroups including gout with HUA (n = 32) and gout with normal SUA (n = 17). (PDF 337 kb)
Metabolic pathways significantly altered in patients with HUA and patients with gout. (DOCX 18 kb)
Acknowledgments
Funding
This work was supported by the Danone Institute Nutrition Research and Education Fund (DIC2015–07).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AARSs
Aminoacyl-tRNA synthetases
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BCAAs
Branched chain amino acids
- BCATm
Mitochondrial branched chain aminotransferase
- BMI
Body mass index
- CPMG
Carr–Purcell–Meiboom–Gill
- DBP
Diastolic blood pressure
- HDL
High-density lipoprotein
- HUA
Asymptomatic hyperuricemia
- JRES
J-resolved spectroscopy
- LDL
Low-density lipoprotein
- NMR
Nuclear magnetic resonance
- OPLS-DA
Orthogonal partial least squares-discriminant analysis
- PCA
Principal components analysis
- SBP
Systolic blood pressure
- SUA
Serum uric acid
- TC
Total cholesterol
- tRNA
Transfer RNA
- TG
Triglyceride
- VLDL
Very low-density lipoprotein
Authors’ contributions
Authors’ contributions were as follows: conception and design of the study (YZ, HP, YY); collection of clinical samples (YZ, HZ, DC); metabolomics analysis and the multivariate statistics (YZ, FG); drafting the article (YZ, HP, YY) and critical revisions for important intellectual content (YZ, HZ, DC, FG, HP, YY). All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol was approved by the ethics committee of Shanghai University of Medical & Health Sciences, and written informed consent was obtained from all the participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13075-018-1600-5) contains supplementary material, which is available to authorized users.
Contributor Information
Yannan Zhang, Email: yannan_jn@163.com.
Huanzhen Zhang, Email: zhz318@163.com.
Dong Chang, Email: dongchang1969@163.com.
Fuchuan Guo, Email: guo2016fuchuan@163.com.
Hongzhi Pan, Email: panhongzhilaoshi@163.com.
Yuexin Yang, Email: yxyang@263.net.
References
- 1.Roddy E, Choi H. Epidemiology of gout. Rheum Dis Clin N Am. 2014;40(2):155–175. doi: 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanyan Z, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 3.Wallace KL, Riedel AA, Nancy JR, Robert W. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587. [PubMed] [Google Scholar]
- 4.Priyanka C, Edward R, Lorna C, Jane R, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology. 2013;52(11):2031–2040. doi: 10.1093/rheumatology/ket265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8(10):610–621. doi: 10.1038/nrrheum.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campion EW, Glynn RJ, Delabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421–426. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 7.Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, Nuki G. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Ann Rheum Dis. 2008;67(7):960–966. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15(3):175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puig JG, Martínez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol. 2008;20(2):187–191. doi: 10.1097/BOR.0b013e3282f4b1ed. [DOI] [PubMed] [Google Scholar]
- 10.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. doi: 10.1186/s12916-017-0890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlesinger N, Baker DG, Schumacher HR. Serum urate during bouts of acute gouty arthritis. J Rheumatol. 1997;24(11):2265–2266. [PubMed] [Google Scholar]
- 12.Vincent G, Liliane C, Jean-Charles G. Contribution of digit joint aspiration to the diagnosis of rheumatic diseases. Joint Bone Spine. 2002;69(1):58–61. doi: 10.1016/S1297-319X(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson JK, John C, Lindon JC, Elaine H. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1(2):153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 15.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134(5):714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Ju L, Hou Z, Deng H, Zhang Z, Wang L, Yang Z, Yin J, Zhang Y. Screening, verification, and optimization of biomarkers for early prediction of cardiotoxicity based on metabolomics. J Proteome Res. 2015;14(6):2437–2445. doi: 10.1021/pr501116c. [DOI] [PubMed] [Google Scholar]
- 17.Ratnasekhar C, Sonane M, Satish A, Mudiam MK. Metabolomics reveals the perturbations in the metabolome of Caenorhabditis elegans exposed to titanium dioxide nanoparticles. Nanotoxicology. 2015;9(8):994–1004. doi: 10.3109/17435390.2014.993345. [DOI] [PubMed] [Google Scholar]
- 18.Chan GH, Ho EN, Leung DK, Wong KS, Wan TS. Targeted metabolomics approach to detect the misuse of steroidal aromatase inhibitors in equine sports by biomarkers profiling. Anal Chem. 2015;88(1):764–72. doi: 10.1021/acs.analchem.5b03165. [DOI] [PubMed] [Google Scholar]
- 19.Larive CK, Barding GA, Jr, Dinges MM. NMR spectroscopy for metabolomics and metabolic profiling. Anal Chem. 2015;87(1):133–146. doi: 10.1021/ac504075g. [DOI] [PubMed] [Google Scholar]
- 20.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 21.Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, Takishita S. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27(11):835–841. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Doherty MT, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, Mccarthy G. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the standing committee for international clinical studies including therapeutics (ESCISIT) Ann Rheum Dis. 2006;65(10):1301–1311. doi: 10.1136/ard.2006.055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristóbal B. Uric acid and cardiovascular risk. N Engl J Med. 2009;360(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace SL, Robinson H, Masi HT. Decker JL, Mccarty DJ, TSF Y. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheumatol. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wang Y, Hao F, Zhou X, Han X, Tang H, Ji L. Human serum metabonomic analysis reveals progression axes for glucose intolerance and insulin resistance statuses. J Proteome Res. 2009;8(11):5188–5195. doi: 10.1021/pr900524z. [DOI] [PubMed] [Google Scholar]
- 26.Pechlivanis A, Kostidis S, Saraslanidis P, Petridou A, Tsalis G, Veselkov K, Mikros E, Mougios V, Theodoridis GA. 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum. J Proteome Res. 2013;12(1):470–480. doi: 10.1021/pr300846x. [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre DA, Jimenez B, Lewintre EJ, Martin CR, Schafer H, Ballesteros CG, Mayans JR, Spraul M, Garcia-Conde J, Pineda-Lucena A. Serum metabolome analysis by 1H-NMR reveals differences between chronic lymphocytic leukaemia molecular subgroups. Leukemia. 2010;24(4):788–797. doi: 10.1038/leu.2009.295. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995;67(5):793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 29.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism. 2006;55(10):1293–1301. doi: 10.1016/j.metabol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Bansal BC, Gupta RR, Bansal MR, Prakash C. Serum lipids and uric acid relationship in ischemic thrombotic cerebrovascular disease. Stroke. 1975;6(3):304–307. doi: 10.1161/01.STR.6.3.304. [DOI] [PubMed] [Google Scholar]
- 31.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 32.Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986;62(733):1001–1006. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han T, Lan L, Qu R, Xu Q, Jiang R, Na L, Sun C. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension. 2017;70(4):703–711. doi: 10.1161/HYPERTENSIONAHA.117.09508. [DOI] [PubMed] [Google Scholar]
- 34.Shani M, Vinker S, Dinour D, Leiba M, Twig G, Holtzman E, Leiba A. High normal uric acid levels are associated with an increased risk of diabetes in lean, normoglycemic healthy women. J Clin Endocrinol Metab. 2016;101(10):3772–3778. doi: 10.1210/jc.2016-2107. [DOI] [PubMed] [Google Scholar]
- 35.Bombelli M, Quarti-Trevano F, Tadic M, Facchetti R, Cuspidi C, Mancia G, Grassi G. Uric acid and risk of new-onset metabolic syndrome, impaired fasting glucose and diabetes mellitus in a general Italian population: data from the Pressioni Arteriose Monitorate E Loro Associazioni study. J Hypertens. 2018; 10.1097/HJH.0000000000001721. [DOI] [PubMed]
- 36.Kokushi E, Uno S, Harada T, Koyama J. (1)H NMR-based metabolomics approach to assess toxicity of bunker a heavy oil to freshwater carp, Cyprinus carpio. Environ Toxicol. 2012;27(7):404–414. doi: 10.1002/tox.20653. [DOI] [PubMed] [Google Scholar]
- 37.Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69(1):617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 38.Mirande M. The aminoacyl-tRNA synthetase complex. Subcell Biochem. 2017;83:505–522. doi: 10.1007/978-3-319-46503-6_18. [DOI] [PubMed] [Google Scholar]
- 39.Sissler M, Gonzalez-Serrano LE, Westhof E. Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol Med. 2017;23(8):693–708. doi: 10.1016/j.molmed.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Würtz P, Tiainen M, Mäkinen VP, Kangas AJ, Soininen P, Saltevo J, Keinänen-Kiukaanniemi S, Mäntyselkä P, Lehtimäki T, Laakso M. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35(8):1749. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T, Zhang Z, Kolwicz SC, Jr, Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25(2):374–385. doi: 10.1016/j.cmet.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martos-Moreno GA, Mastrangelo A, Barrios V, Garcia A, Chowen JA, Ruperez FJ, Barbas C, Argente J. Metabolomics allows the discrimination of the pathophysiological relevance of hyperinsulinism in obese prepubertal children. Int J Obes. 2017;41(10):1473–1480. doi: 10.1038/ijo.2017.137. [DOI] [PubMed] [Google Scholar]
- 44.Wang SM, Yang RY, Wang M, Ji FS, Li HX, Tang YM, Chen WX, Dong J. Identification of serum metabolites associated with obesity and traditional risk factors for metabolic disease in Chinese adults. Nutr Metab Cardiovasc Dis. 2018;28(2):112–118. doi: 10.1016/j.numecd.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darmaun D, Dechelotte P. Role of leucine as a precursor of glutamine alpha-amino nitrogen in vivo in humans. Am J Phys. 1991;260(2 Pt 1):E326–E329. doi: 10.1152/ajpendo.1991.260.2.E326. [DOI] [PubMed] [Google Scholar]
- 47.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26(9):877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felig P. The glucose-alanine cycle. Metabolism. 1973;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 50.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, Schwarzschild MA. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110(1):300–305. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakshi R, Zhang H, Logan R, Joshi I, Xu Y, Chen X, Schwarzschild M. Neuroprotective effects of urate are mediated by augmenting astrocytic glutathione synthesis and release. Neurobiol Dis. 2015;82:574–579. doi: 10.1016/j.nbd.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson's disease: a cohort study. Arthritis Rheum. 2008;59(11):1549–1554. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
- 54.Ungprasert P, Srivali N, Thongprayoon C. Gout is not associated with a lower risk of Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2015;21(10):1238–42. doi: 10.1016/j.parkreldis.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 55.Choi H, Ford E. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120(5):442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson's disease. Oxidative Med Cell Longev. 2014;2014:729194. doi: 10.1155/2014/729194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Y, Yang Y, Dong B, Zheng H, Lin X, Du Y, Li X, Zhao L, Gao H. Metabonomic profiles delineate potential role of glutamate-glutamine cycle in db/db mice with diabetes-associated cognitive decline. Mol Brain. 2016; 10.1186/s13041-016-0223-5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metabolite assignments of major resonances detected in 1H NMR spectra from human serum samples. (DOCX 29 kb)
PCA and OPLS-DA scores plots based on 1H NMR spectrum data of serum samples obtained from two gout subgroups including gout with HUA (n = 32) and gout with normal SUA (n = 17). (PDF 337 kb)
Metabolic pathways significantly altered in patients with HUA and patients with gout. (DOCX 18 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


