Abstract
Along with the intensive development of methods of livestock breeding, breeders’ expectations are growing concerning feed additives that would guarantee such results as accelerating growth rate, protection of health from pathogenic infections and improvement of other production parameters such as: absorption of feed and quality of meat, milk, eggs. The main reason for their application would be a strive to achieve some beneficial effects comparable to those of antibiotic-based growth stimulators, banned on 01 January 2006. High hopes are being associated with the use of probiotics, prebiotics and synbiotics. Used mainly for maintenance of the equilibrium of the intestinal microbiota of livestock, they turn out to be an effective method in fight against pathogens posing a threat for both animals and consumers. This paper discusses definitions of probiotics, prebiotics and synbiotics. Criteria that have to be met by those kinds of formulas are also presented. The paper offers a list of the most commonly used probiotics and prebiotics and some examples of their combinations in synbiotic formulas used in animal feeding. Examples of available study results on the effect of probiotics, prebiotics and synbiotics on animal health are also summarised.
Keywords: Animal health, Prebiotics, Probiotic bacteria, Synbiotics
Background
It is estimated that by 2050 the number of people in the world will reach 9 billion. Constant growth of the human population is inseparably associated with a growing demand for food of plant and animal origin. For that reason, scientists are looking for solutions allowing intensification of food production, with simultaneous reduction of production costs, and in compliance with high standards of quality and safety (for both people and the environment). Types of used feed additives affect animal health and increased production of high quality meat, eggs, milk and fish. Animal production is inseparable from nutrition and health of the consumer, and animal intestinal pathogens, such as Campylobacter, Salmonella, Listeria and Yersinia, are a direct source of food contamination and a cause of zoonoses. Therefore, new methods of animal breeding are introduced, aimed at increased quality and safety of meat, while taking animal welfare and respect for the natural environment into account.
Both animal feed and feed supplements have to meet some strict criteria, without a simultaneous rise of animal breeding costs. In the past, antibiotics and other medicinal products had been broadly used, mainly in order to modify the alimentary microbiota and to boost productivity and animal growth. Long-term use of those substances has led to development of drug-resistant microorganisms, posing a threat to consumers’ health and exerting a negative effect on the environment [1, 2]. As a result, on 1 January 2006 the use of antibiotic-based growth stimulators was banned in the European Union. Therefore, alternative natural substances ensuring similar effects have been sought. The Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives used in animal nutrition, mentions probiotics and prebiotics among other substances, whereas in the Regulation (EC) No. 767/2009 on the placing on the market and use of feed, this aspect was not changed. High hopes are also evoked in relation to the synergistic combination of both these components, namely the so-called synbiotics.
Probiotics
The term “probiotic” comes from two Greek words (“pro” and “bios”) and it means “for life”. The first concept of probiotics was probably suggested in 1907 by Mechnikov [3], who noted that bacteria may have a beneficial influence on the natural intestinal microflora. The term of “probiotic” was probably invented by Ferdinand Vergin, who in his paper of 1954 entitled “Anti- und Probiotika” compared a harmful effect of antibiotics and other antimicrobial agents on the intestinal microbiota with a beneficial effect (“probiotica”) of selected bacteria [4]. With time, the definition of probiotic was largely modified (Table 1).
Table 1.
Definitions of probiotics
| Year | Definitions |
|---|---|
| 1965 | A substance secreted by one microorganism which stimulates the growth of another [5] |
| 1971 | Tissue extracts which stimulate microbial growth [6] |
| 1974 | Organisms and substances that contribute to intestinal microbial balance [7] |
| 1989 | Live microbial feed supplement which beneficially affects the host animal by improving microbial balance [8] |
| 1992 | Viable mono- or mixed culture of live microorganisms which, applied to animals or man, have a beneficial effect on the host by improving the properties of the indigenous microflora [9] |
| 1996 | A live microbial culture or cultured dairy product that beneficially influences the health and nutrition of the host [10] |
| 1996 | Living microorganisms which, upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition [11] |
| 1998 | Living microorganisms that on ingestion in certain numbers exert health benefits beyond inherent basic nutrition [12] |
| 1999 | A microbial dietary adjuvant that beneficially affects the host physiology by modulating mucosal and systemic immunity, as well as improving nutritional and microbial balance in the intestinal tract [13] |
| 2001 | A preparation of or a product containing viable, defined microorganisms in sufficient numbers, which alter the microflora (by implantation or colonization) in a compartment of the host and by that exert beneficial health effect in this host [14] |
| 2002 | Live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host [15] |
| 2004 | Preparation of viable microorganisms that is consumed by humans or other animals with the aim of inducing beneficial effects by qualitatively or quantitatively influencing their gut microflora and/or modifying their immune status [16, 17] |
| 2009 | Live microorganisms, which when administered in adequate amounts, confer a health benefit on the host [18] |
| 2013 | Live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host [19] |
The current definition formulated in 2002 by FAO and WHO working group experts states that probiotics are “live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [15]. The definition was in 2013 maintained by the International Scientific Association for Probiotics and Prebiotics (ISAPP). The term “probiotic’ is reserved for formulas or products that meet some strictly defined criteria. The most important of these criteria include: an appropriate count of viable cells, a beneficial effect on a host’s health (which may also involve stimulation of growth), and a beneficial effect on the function of the alimentary tract. Efficacy of probiotic preparations depends on numerous factors. For that reason proper selection of bacterial strains and application of a correct dose are highly important. Due to their beneficial effect on health and stimulation of growth, probiotics are broadly used in animal feeds, particularly for pigs and poultry. That type of formulas may contain one or more selected strains of microorganisms, and depending on the species and age of host animals they may be administered as a powder, suspension, capsules, pellet, gel or paste. They are used periodically or constantly, directly per os or as an additive to feed and premixes. Probiotic cultures used as feed additives must meet some specific criteria.
Selection criteria and requirements for probiotic strains
Assessment of the safety of probiotic strains is necessary for the optimization of their use. However, it is not an easy task [20]. The mode of action of probiotics as microbial additives to feed is not fully understood. By adhering to the alimentary tract probiotic organisms may survive difficult conditions, and offer a beneficial effect on the stability and protection of the intestinal ecosystem. They also influence the course of digestive and metabolic processes and the immunological response. Consequently, properties of probiotics lead to improved health of animals, increased productivity [21], and improved immunity of the host [22].
The immunomodulatory mechanism action of probiotics involved in animal health and diseases is particularly important and is based on innate or adaptive immune system. The gastro-intestinal lumen contains essential nutrients and beneficial microorganisms, but also pathogen microorganisms, toxins, and some foreign antigens [23, 24]. Epithelial cells in the GIT mucosa create a selectively permeable barrier between the lumen environment and the internal body tissues [25]. This barrier is the first line of host defense against harmful microbes in the GIT (gut innate immunity) but factors such as stress or disease conditions can disrupt this barrier [24, 26, 27]. Certain probiotic microorganisms can enhance the function of intestinal barrier through modulation of the phosphorylation of cytoskeletal and tight junction proteins and thereby influencing the intestinal mucosal cell–cell interactions and also cellular “stability” [24, 28]. Restitution of the GIT mucosa barrier function by probiotics has been observed in both in vitro and in vivo models [29, 30]. The mechanism can be related to the alterations in the secretion of mucus or chlorides, or the changes in the expression of tight junction proteins by epithelial cells, however the details of this mode of action are still not very clear [28, 31]. In other side, animals can adaptive immune system. Animal immune responses should be stimulated in some cases (for example, in infection and immune-deficiency situations) but be suppressed in others (for example, in allergy and autoimmune disease situations) [32]. Research has showed that the normal gut microbiota by stimulating gastrointestinal immune response (antibody production and increasing phagocytic activity) can support animal’s defense systems against invading pathogens [33]. Fuller [34] explained two ways in which the immune system is stimulated: they can either migrate through the gut wall as viable cells or multiply to a limited extent, and the antigens released by the dead organisms are absorbed and directly stimulate the host immune system. It is the product of this change that further induces the immune response [33].
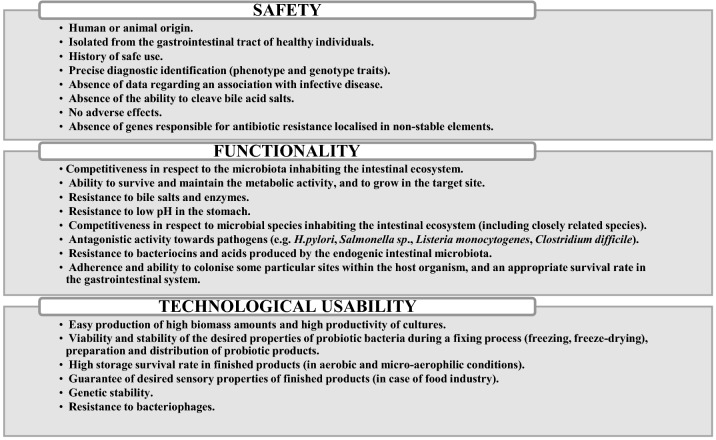
Selection of new probiotic organisms involves strains and even geni of microorganisms demonstrating the most beneficial or the most specific effects. The assessment focuses mostly on safety and the benefit-to-risk ratio associated with the use of a given probiotic strain. Microorganisms used for production of probiotic animal formulas should be isolated from individuals belonging to the species for which they are intended, because part of health beneficial effects is probably species specific. Due to that procedure, the obtained biological material is maximally adapted to the conditions present in the alimentary tract of the given species of animals [35]. Moreover, probiotic cultures added to feed should be resistant to temperatures and pressures used in the process of pelleting, and to humidity and the effect of adverse substances during feed handling and storage, such as heavy metals or mycotoxins. The period of high activity of probiotics in feed and premixes must not be shorter than 4 months [35]. To extend that period, formulas are encapsulated, which results in extended survival of strains [36]. According to the suggestions of the WHO, FAO and the European Food Safety Authority (EFSA), in their selection process probiotic strains must meet both safety and functionality criteria, and those related to their technological usefulness (Fig. 1).
Fig. 1.
Selection criteria and required properties of probiotic strains [15, 37]
Probiotic microorganisms
Probiotic products may contain one or more selected microbial strains. Microorganisms used as feed supplements in the EU are mostly bacteria. Most often they are Gram-positive bacteria belonging to the following geni: Bacillus, Enterococcus, Lactobacillus, Pediococcus, Streptococcus. Also some fungi and yeast strains of Saccharomyces cerevisiae and Kluyveromyces species are probiotics. Bacteria belonging to the geni Lactobacillus and Enterococcus are components of the natural microbiota of the animal alimentary tract, and are usually present in amounts of 107–108 and 105–106 CFU/g, respectively. On the other hand, yeast and Bacillus genus bacteria are not usually present in the gastrointestinal system. Majority of the abovementioned microorganisms should be potentially safe for the host. However, some of them may pose problems; e.g. Enterococcus genus bacteria may participate in transmission of antibiotic resistance, and Bacillus cereus strain are able to produce endotoxins and emetic toxins [38].
In 2005, only 13 of 21 probiotic products were approved as feed supplements in the EU to be used in piglets, and some in sows and porkers [39]. As many as seven of those products contained Enterococcus faecium strains (a natural environment of the gastrointestinal tract), two of them contained Bacillus genus spores (most commonly occurring in soil), another two contained Saccharomyces cerevisiae yeast strains, and only one contained Lactobacillus farciminis and Pediococcus acidilactici strains occurring in the gastrointestinal tract and in dairy products, respectively [39]. Therefore, the origin of strains that may potentially be used as feed supplements may be various. It is important, however, that probiotic organisms are present in appropriate amounts. The recommended dose for the majority of probiotic strains is 109 CFU/kg of feed [39].
Probiotics are subject to regulations contained in the general food law, and according to them, they should be safe for human and animal health. In the USA, microorganisms used for consumption purposes should have the Generally Regarded As Safe (GRAS) status, regulated by the FDA. In Europe, EFSA introduced the term of Qualified Presumption of Safety (QPS). The QPS concept involves some additional criteria of the safety assessment of bacterial supplements, including the history of safe usage and absence of the risk of acquired resistance to antibiotics [38, 40]. Importantly, GRAS status is applied to microorganisms and microbial-derived ingredients used in food products while QPS is applied to any biological agent in the form of bacteria, fungi, or virus, that is intentionally added at different stages into the food chain. Probiotic use may help decrease the rate of development of antibiotic-resistant strains secondary to widespread and rampant antibiotic use [41, 42]. On the other hand, some microorganisms used as probiotics are not exempted from acquiring antibiotic resistance genes. In views of their shared microbial environment in the gastrointestinal tract of animals, a risk of pathogenic microbes acquiring antibiotic resistance genes from probiotic microbes exists, and vice versa. If improperly cooked, livestock fed of probiotics that are consumed by humans as food may also pose as a possible source of antibiotic resistance genes for the human gut microbiota [43]. Therefore, given the emerging risk of spreading antibiotic resistance genes through probiotic strains, the QPS is considered by many as the more applicable and flexible probiotics criteria [44]. Table 2 presents probiotic microorganisms contained in animal feed supplements.
Table 2.
| Type Lactobacillus |
Type Bifidobacterium |
Other lactic acid bacteria | Other microorganisms |
|---|---|---|---|
| L. brevis a | B. animalis a | Enterococcus faecalis | Bacillus cereus |
| L. casei a | B. longum a | Enterococcus faecium | Bacillus licheniformis a |
| L. crispatus a | B. pseudolongum | Lactococcus lactis a | Bacillus subtilis a |
| L. farciminis a | B. thermophilum | Leuconostoc citreum a | Propionibact. Freudenreichi a |
| L. fermentum a | Leuconostoc lactis a | Saccharomyces cerevisiae (boulardi)a | |
| L. murinus | Leuconostoc mesenteroides a | Saccharomyces pastorianus a | |
| L. gallinarium a | Pediococcus acidilactici a | Kluyveromyces fragilis | |
| L. paracasei a | Pediococcus pentosaceus a | Kluyveromyces marxianus a | |
| L. pentosus a | Streptococcus infantarius | Aspergillus orizae | |
| L. plantarum a | Streptococcus salivarius | Aspergillus niger | |
| L. reuteri a | Streptococcus thermophilus a | ||
| L. rhamnosus a | Sporolactobacillus inulinus | ||
| L. salivarius a |
a QPS microorganisms
Ready-made probiotic formulas for animals usually contain one, two, or more strains of microorganisms [48]. Effectiveness of that type of formulas is affected by numerous factors, including: proper selection of strains, and unitary dose containing an appropriate count of viable cells. To preserve the properties of probiotic formulas, they have to be stored and used as recommended by their manufacturers. Due to the content of viable microorganisms, probiotic formulas are susceptible to unfavourable conditions, such as temperature and light. It is important that no other substances are used while probiotics are administered, and that water used for dilution contains no chlorine or other disinfectants. Water with the formula should be administered to animals within 6–12 h. An interval of 24–48 h between the end of antibiotic therapy or administration of any other antimicrobial agents and the onset of the probiotic therapy is also important. Formulas containing many ingredients (the highest number of microbial species) are usually the most effective [49]. Examples of probiotic formulas available in the market are presented in Table 3.
Table 3.
| Trade name of the preparation (producer) | Microorganisms | Destination |
|---|---|---|
| Acid-Pak-4-Way (Alltech) | Lactobacillus acidophilus, Enterococcus faecium | Poultry, pigs |
| Anta Pro EF (Dr. Eckel) | Enterococcus faecium | Pigs |
| Avian PAC (Soluble Loveland Industries) | Streptococcus faecium, Lactobacillus acidophilus, | Poultry |
| Biogen D (Bio-Gen) | Bifidobacterium bifidum, Lactobacillus acidophilus, Pediococcus faecium | Poultry |
| Biogen N (Bio-Gen) | Bifidobacterium bifidum, Lactobacillus acidophilus, Pediococcus faecium | Pigs |
| Biogen T (Bio-Gen) | Bifidobacterium bifidum, Lactobacillus acidophilus, Enterococcus faecium | Pigs |
| Bio Plus2B® (Chr. Hansen) | Bacillus subtilis, Bacillus licheniformis | Pigs, calves, poultry |
| BioPlus®YC (Evonik Industries) | Bacillus licheniformis, Bacillus subtilis | Pigs |
| B.I.O.Sol (Biochem) | Enterococcus faecium | Poultry |
| Bro-biofair (Vitality Co.) | Saccharomyces servisia | Pigs |
| Calsporin (ORFFA) | Bacillus subtilis | Poultry, pigs |
| Cerbiopor | Lactobacillus: acidophilus, brevis, casei, fermentum, lactis, plantarum; Bacillus: subtilis, megaterium, pumilus; Enterococcus faecium, Cellulomonas sp., Saccharomyces cerevisiae | Pigs |
| Cernivet LBC (Cerbios) | Enterococcus faecium | Calves, pigs |
| Cerbiogalli | Lactobacillus: acidophilus, casei, plantarum | Poultry |
| Cylactin (DSM) | Enterococcus faecium | Poultry, pigs, calves |
| Doctor Em® (Biotron) | Lactobacillus: paracasei, plantarum; Lactococcus lactis, Saccharomyces cerevisiae | Poultry, pigs, calves |
| Ecobiol (Norel Animal Nutrition) | Bacillus amyloliquefaciens | Poultry |
| Enviva™ Pro (DANISCO Animal Nutrition) | Bacillus subtilis | Poultry |
| Enviva®MPI (DANISCO Animal Nutrition) | Lactobacillus: farciminis, rhamnosus | Pigs |
| Farmaflore soluble (Farm’apro) | Lactobacillus: rhamnosus, farciminis | Poultry |
| FloraMax-B11 (Pacific Vet Group) | Lactobacillus salivarius, Pediococcus parvulus | Poultry |
| GalliPro® (Evonik Industries) | Bacillus subtilis | Poultry |
| Galvit Probiotyk (Galvit) | Enterococcus faecium | Poultry |
| Lactiferm | Enterococcus faecium | Pigs, poultry, calves |
| Lavipan® (JHJ) | Lactobacillus: plantarum, casei; Lactococcus lactis, Carnobacterium divergens, Saccharomyces cerevisiae | Poultry, pigs |
| LSP 122 (Alpharma) | Bacillus licheniformis | Pigs |
| Microguard (PeterLab Holdings) | Bacillus: licheniformis, megaterium, mesentricus, polymyxa, subtilis; Saccharomyces boulardii; Bididobacterium bifidum; Lactobacillus: acidophilus, bulgaricus, plantarum; Streptococcus faecium | Poultry, pigs |
| MicroSource S (Agtech Products Inc.) | Bacillus: subtilis, licheniformis | Pigs |
| Oralin® (Chevita GmbH) | Enterococcus faecium | Pigs, calves, poultry |
| PrimaLac (Star Labs, Inc.) | Bifidobacterium: bifidium, thermophilus; Enterococcus faecium; Lactobacillus: acidophilus, casei, | Pigs, beef, dairy, horses, poultry, deer |
| Probiomix | Bifidobacterium bifidum Lactobacillus amylovorus Enterococcus faecium | Calves, poultry |
| Probion (Woogene B&G Co. Ltd.) | Bacillus subtilis, Clostridium butyricum, Lactobacillus acidophilus | Pigs, poultry |
| Probios (Chr Hansen) | Lactobacillus: acidophilus, casei, plantarum, lactis; Enterococcus faecium; Bacillus subtilis | Poultry, pigs |
| Probiosacc C-I | Saccharomyces cerevisiae | Calves |
| Pro-Biotyk em15® (ProBiotics) |
Bacillus subtilis, Bifidobacterium: animalis, bifidum, longum, Lactobacillus: acidophilus, casei, delbrueckii subsp. bulgaricus, fermentum,.plantarum; Lactococcus lactis subsp. lactis; Saccharomyces cerevisiae; Streptococcus thermophilus | Poultry, pigs, calves, horses |
| Propoul (International Company s.r.o.) | Lactobacillus fermentum | Poultry |
| Protexin (Protexin Probiotics International Ltd.) | Lactobacillus: plantarum, delbruecki subsp. bulgaricus, acidophilus, rhamnosus; Bifidobacterium bifidum; Streptococcus salivarius subsp. thermophilus; Enterococcus faecium; Aspergilus oryzae; Candida pintolepesii | Poultry, pigs, sheep, cattle, |
| Provita LE (Schaumann) | Lactobacillus rhamnosus, Enterococcus faecium | Pigs, calves |
| Super-CyC (Choong Ang Biotech Co. Ltd.) | Bacillus subtilis, Saccharomyces cerevisiae | Poultry, cattle, horses, pigs |
| Toyocerin® (Rubinum S.A.) | Bacillus toyonensis | Pigs |
| UltraCruz (Santa Cruz Animal Health) | Enteroccus faecium, Lactobacillus: acidophilus, casei, plantarum | Cattle, calves, poultry |
| Yea Sacc (Alltech) | Lactobacillus rhamnosus, Enterococcus faecium | Cattle, calves |
Probiotics in animal breeding
Farm animals are exposed to the environment-related stress (e.g. rearing methods, diet, etc.). Various factors may cause disturbance of balance in the intestinal ecosystem and may become risk factors for pathogenic infections. Regardless of the species, animal health is crucial for the production chain. The use of probiotics in animal feeding is associated with their verified efficacy in modulation of the intestinal microbiota. Administration of probiotic strains, both individual and combined, may have a significant effect on absorption and utilisation of feed, daily increase of body weight and total body weight of various animals, including turkeys [53], chicken [54], piglets [55, 56], sheep, goats [57], cattle, and horses [58]. An addition of probiotic microorganisms to feed results in improved quantity and quality of milk, meat and eggs [59]. Moreover, probiotics reduce the effect of weak limbs in broiler chicken [60]. In the case of piglets, the main expected effect of probiotics is a reduction of frequency of diarrhoea, posing a problem in initial post-weaning weeks. The efficacy of probiotics in combating diarrhoea is one of the most commonly studied aspect. Recombined probiotics are one of the most novel biomedical applications of genetically modified organisms (GMO) [59]. The absence of clinical side effects is an important benefit of probiotics.
In the case of pig production, weaning is the critical moment, when animals are the most exposed to stress (nutrition changes from milk to the diet based on vegetable polysaccharides). Also the environment is changed, as a result of transfer to a production farm. All those factors may disturb immunological functions and have a negative effect on the balance of pigs’ intestinal microbiota [61]. Böhmer et al. [62] used a feed with an addition of a supplement of the Enterococcus faecium DSM 7134 probiotic strain in feeding of 33 sows between the 90th day of pregnancy and the 28th day of lactation. A significant improvement of feed consumption, offspring size and weight of studied animals was observed. Improved feed consumption and productivity may be helpful in prevention of the so called “starvation sterility” of young sows, caused by reduced feed consumption along with mobilisation of body tissue and insufficient energy during lactation [62]. Probiotics have a positive effect on various digestions processes, especially on the cellulolytic ones, and the synthesis of microbial proteins [63]. Mountzouris et al. [64] studied the efficacy of a probiotic formula containing two strains of Lactobacillus genus, and one strain of each geni: Bifidobacterium, Enterococcus, Pediococcus, compared to a product containing avilamycin. The experiment was conducted on 400 broiler chickens, for 6 weeks. It was found that administration of the probiotic caused stimulation of animal growth comparable to the effect of treatment with the avilamycin-containing product. Moreover, the addition of the formula to feed and/or water for chickens caused a significant probiotic effect by modulation of the composition and activity of the intestinal microbiota [64].
A favourable effect of feed supplemented with the YEA-SACC-1026 probiotic [65] and with bacterial strains Bacillus licheniformis and Bacillus subtilis [65] on the quality of milk (fat and protein content) and increased body weight of lambs was also confirmed. The probiotic was used during the late period of pregnancy and during milk feeding. Other studies indicated that the addition of the Bio Plus 2B® probiotic containing Bacillus subtilis and Bacillus licheniformis strains caused a significant improvement of sows’ blood parameters (higher cholesterol and total lipid levels) and milk parameters (higher content of milk fat and protein) during milk-feeding [66]. Yu et al. [67] determined the effect of steamed corn with the addition of Aspergillus oryzae culture in cows’ diet on their milk productivity. The experiment was carried out on 32 cows for 70 days. It was confirmed that the addition of A.oryzae to steamed corn resulted in an increased percentage of protein and dry fat-free solids (Solids-Not-Fat, SNF) in milk. Studies completed by Ceslovas et al. [68] focused on the effect of probiotics: YEASTURE, MICROBOND and of phytobiotics: YUCCA, QUILLAYA on the growth of pigs and quality of meat. It was found that the studied probiotics contributed to increased carcase production in the group of experimental animals. Moreover, those formulas had also an effect on improved culinary properties of pork, reduced loss on cooking and improved tenderness of meat. However, no significant improvement of daily body weight increase and carcase production was found in groups fed with phytobiotics compared to the control.
Moreover, probiotics contribute to increased production and improved quality of eggs [69, 70], and to reduced Salmonella contamination in eggs [71]. In the studies completed by Haddadin et al. [69] chickens were fed with a feed with a supplement of Lactobacillus acidophilus for 48 weeks. Based on obtained results, it was concluded that egg production and feed conversion levels were significantly higher in experimental animals compared to the control group of animals. A reduced cholesterol level was also noted in egg yolks from animals fed with the probiotic strain. The researchers suggested that the latter effect was a reflection of lower serum cholesterol levels in studied birds. Kurtoglu et al. [70] determined the effect of the commercial formula Bio Plus®2B on daily feed consumption, egg productivity and weight, specific gravity, feed conversion ratio, serum and egg yolk cholesterol and chicken serum triglycerides. The experiment was carried out on 480 chickens, using various doses of probiotic (depending on the study group) for 90 days. It was found that probiotic supplementation at the doses of 250, 500 and 750 mg/kg of feed caused increased production of eggs, and reduced egg damage ratio. Serum and egg yolk cholesterol levels also became reduced in probiotic-fed animals. Moreover, in the case of probiotic doses of 500 and 750 mg/kg of feed, a reduced triglyceride level was found in the serum of experimental animals, compared to the control group. On the other hand, the probiotic used in doses of 250 and 500 mg/kg of feed had a positive impact on the feed conversion ratio [70].
Studies also confirmed a favourable effect of probiotics on improved growth of farm animals, including cows [72], young calves, piglets [73] and broiler chickens [74]. Kyriakis et al. [73] demonstrated efficacy of the LSP 122 probiotic containing spores of Bacillus licheniformis in combating diarrhoea syndrome occurring in piglets in 3–10 days post weaning (post-weaning diarrhoea syndrome, PWDS) in relation to clinical symptoms, mortality, body weight gain and feed conversion. The principal cause of morbidity and mortality of newborn piglets and recently weaned pigs is infection with enterotoxic strains of Escherichia coli (ETEC). A lower frequency and intensity of diarrhoea was observed in animals receiving feed with an addition of a probiotic. Moreover, mortality of all pigs receiving supplementation with probiotics was significantly lower compared to the negative control (fed with non-modified feed).
A positive effect of probiotics compared to the negative control was determined based on data regarding the assessment of body weight increase and the feed conversion ratio. The summary of all results obtained in the study by Kyriakis et al. [73] indicated that the LSP 122 probiotic used at the dose of 107 viable spores of Bacillus licheniformis is useful in combating PWDS caused by ETEC.
An addition of probiotic microorganisms to animal feed plays a significant role in the fight against pathogens, including: Listeria monocytogenes, Salmonella Typhimurium, and in protection of piglets against diarrhoea [75]. In the case of chickens, the role of probiotics was demonstrated in protection against the following pathogens: Escherichia coli [76], Salmonella [77], Campylobacter [77], Clostridium and Eimeria [78]. Chateau et al. [76] studied the antagonistic properties of Lactobacillus ssp. strains isolated from commercial probiotic products, in relation to bacterial strains pathogenic for chickens (including the serotypes of Listeria monocytogenes, Escherichia coli and Salmonella). Growth inhibition of all pathogens was observed as a consequence of presence of one or a combination of several studied probiotic bacteria. The most pronounced inhibition was observed in relation to Listeria monocytogenes, but a satisfactory inhibition was also observed for Escherichia coli, Salmonella Typhimurium and Salmonella Enteritidis. Stern et al. [77] compared the efficacy of the CE culture used for elimination of Salmonella spp. infections (competitive exclusion) and of the MCE culture (mucosal competitive exclusion) used for combating of Campylobacter colonisations in broiler chickens. 210 chicks were studied. The results indicated that the microbiota of 90 birds treated with the CE culture was much more colonised by Salmonella Typhimurium than in 90 chicks treated with the MCE culture. Also in the case of colonisation with the Campylobacter genus bacteria, a superior effect of the MCE culture was found compared to the animals treated with the CE culture.
In summary, probiotics increase the control of pathogenic microorganisms in poultry, thanks to which they can prevent diseases such as salmonellosis, campylobacteriosis or coccidiosis [52, 79, 80]. In addition, diarrhea infections caused by enterotoxic E. coli strains is one of the major health problems in pigs in the post-weaning period. As a result, they cause significant economic losses by increasing mortality, decreasing the growth rate and related veterinary costs [81]. There is a positive effect of probiotics not only on reducing the frequency of diarrheas, but also on the alleviation of their course. Such effects are described, among others after the use of preparations containing Bacillus licheniformis [73] or B. toyonensis [82, 83]. Probiotic bacteria such as Lactobacillus sobrius [84] or Lactobacillus paracasei [85] have been shown to limit intestinal colonization by pathogenic E. coli.
There are reports indicating that the use of bacterial probiotics is more effective in the case of chickens, pigs and young calves, whereas administration of probiotic yeast strains (Saccharomyces cerevisiae) and fungi (Aspergillus oryzae) offers better results in adult ruminants [86].
Salmonella Enteritidis bacteria colonise the gastrointestinal tract of poultry and cause food-related diseases in humans. Reduced colonisation of Salmonella Enteritidis in the poultry alimentary tract causes reduction of the potential contamination of carcases, thus offering an improved quality of consumed meat. Tellez et al. [74] studied the effect of specific probiotics combined with specific antibodies against Salmonella Enteritidis, Salmonella Typhimurium and Salmonella Heidelberg on the colonisation of intestines and invasion of organs by Salmonella Enteritidis in broiler chickens, and also on the body weight of studied animals [73]. The results of the study indicate that the combination of probiotic strains: Lactobacillus acidophilus, Streptococcus faecium with bacterial strains Salmonella Enteritidis, Salmonella Typhimurium and specific antibodies against Salmonella Heidelberg exerts a favourable effect on reduced Salmonella Enteritidis colonisation in the bodies of broiler chickens at the productive age.
According to Simon [39], approximately 80% of experiments performed in order to combat diarrhoea in piglets, regardless of the applied probiotic microorganism (Bacillus cereus, Enterococcus faecium, Pediococcus acidilactici), confirmed a positive effect of those probiotics. Based on the experiment lasting for 6 weeks on three groups of piglets (two fed with a feed with an addition of a probiotic containing the Enterococcus faecium NCIMB 10415 genus bacteria and one with an addition of Bacillus cereus toyoi) the author concluded that modification of microbiota resulting from the activity of the probiotic Enterococcus faecium NCIMB 10415 bacteria caused a significant reduction in the frequency of diarrhoea, compared to the control group, with an overall positive effect on the health of sows and piglets. The author did not observe any significant effect on animal growth. The probiotic had also effect on the function of epithelial tissues and on immunological response (a significantly reduced level of cytotoxic T cells (CD8+) in piglets’ jejunal epithelium). Based on those observations, the author concluded that the applied bacterial strain may potentially replace antibiotic-based stimulants used in sow and piglet breeding [39].
When summing up the advantages of probiotic use, one should emphasise the role of probiotics in protection of animals against pathogens, enhancement of immunological response, reduced need for antibiotic-based growth stimulants, and high safety of those formulas. An increasing demand for meat products is currently observed, and consumers’ expectations are reflected in producers’ strive for the highest possible quality of meat. The use of feed supplementation with non-chemical formulas, such as probiotics, may meet that expectation. Table 4 lists the examples of results of studies on the effects of probiotic microorganisms in animal nutrition.
Table 4.
Examples of trials regarding the effect of probiotics on animal health
| Reference | Subjects | Microorganism | Time of administration | Main outcome |
|---|---|---|---|---|
| Absorption and utilisation of feed, diarrhoea, body weight gain | ||||
| [87] | 114 Piglets | E. faecium DSM 10,663 NCIMB 10415 | From birth to weaning (24 ± 3.2 days) | Reduced portion of subjects suffering from diarrhoea, improving performance as indicated by a higher daily weight gain |
| [53] | 118 Turkeys | Probiotic FM-B11 (Lactobacillus) | For 3 days post birth and after approx. 6 weeks of life | Use of the selected commercial probiotics resulted in increased market BW and reduced cost of production |
| [54] | 308 Broiler chickens | E. faecium NCIMB 10415 | 21 days | Confirmed efficacy of supplementation in relation to chicken body weight gain and FCR |
| [57] | 20 Growing maltese goat kids | L.acidophilus, L. salivarius, L.reuteri | 7 months | Improved metabolic activity, body weight and proportions in animals receiving a probiotic |
| [64] | 400 Broiler chickens | Lactobacillus (2 strains), Bifidobacterium, Enterococcus, Pediococcus | 6 weeks | Stimulated growth, comparable to the avilamycin-containing product (ASW) |
| [62] | 33 Sows | E. faecium DSM 7134 | From the 90th day of pregnancy to the 28th day of lactation | A significant improvement of feed consumption, offspring size and weight of studied animals |
| Intestinal ecosystem imbalance, pathogenic infections | ||||
| [88] | 153 Healthy piglets and 26 sows | E. faecium NCIMB 10415 | 17 weeks (sows), 6 weeks (piglets) | Reduced pathogenic bacterial (E. coli) load of healthy piglets and sows |
| [89] | 6 Piglets | L. plantarum Lq80 | 21 days | Increased total gut populations of lactobacilli in weaned pigs |
| [56] | 15 Pigs | 2 strains of L. murinus, and one of each: L. salivarius subsp. salivarius, L. pentosus, P. pentosaceous | 30 days | Animals treated with probiotics showed reduced incidence, severity, and duration of diarrhoea. The administered probiotic bacteria improved both the clinical and microbiological outcome of Salmonella infections |
| [77] | 210 Chickens | CE culture MCE culture | No data | Significantly lower colonisation of the intestinal microflora of experimental animals fed with CE by Salmonella Typhimurium and Campylobacter, compared to the group of animals fed with MCE |
| [39] | Sows and piglets | E. faecium NCIMB 10415, B. cereus toyoi | 6 weeks | Modification of microflora as a result of the action of the E. faecium strain caused a significant reduction of frequency of diarrhoea in comparison to the control group. The probiotic had also effect on the function of epithelial tissues and on immunological response (significantly reduced level of cytotoxic T cells (CD8+) in piglets’ jejunal epithelium) |
| Improved quality of meat, milk, eggs | ||||
| [90] | Lambs | Probiotic YEA-SACC-1026 | During pregnancy and milk-feeding | A positive effect on the quality of milk (fat and protein content) and increased body weight of lambs |
| [65] | Lambs | B. licheniformis, B. subtilis | During pregnancy and milk-feeding | A positive effect on the quality of milk (fat and protein content) and increased body weight of lambs |
| [66] | 109 Sows during milk-feeding | Probiotic Bio Plus 2B (B. licheniformis, B. subtilis) | From the day of allocation (14 days prior to the expected farrowing) up to the weaning day | A significant improvement of blood parameters (higher cholesterol and total lipid level) and of milk parameters (higher fat and protein content) during milk feeding in sows |
| [67] | 32 Cows | A. oryzae | 70 days | The effect on the increased ratio of protein and SNF in milk |
| [70] | 480 Chickens | Probiotic Bio Plus 2B (B. licheniformis, B. subtilis) | 90 days | Increased production of eggs and reduced ratio of damaged eggs in probiotic-fed animals. At appropriate doses: reduced level of serum and egg-yolk cholesterol. Reduced serum triglyceride levels compared to the control and a positive effect on FCR |
ASW antibiotic-based growth stimulator, CE competitive exclusion, FCR feed conversion ratio, MCE mucosal competitive exclusion
Prebiotics
Besides probiotics, also prebiotics are used as natural feed additives. Already in 1921 Rettger and Cheplin reported that after consumption of carbohydrates the human intestinal microbiota was enriched with lactic bacteria [91]. The prebiotic concept was first initiated in 1995 [92]. The concept has evolved since (Table 5). The currently used definition was created in December 2016 by the International Scientific Association of Probiotics and Prebiotics (ISAPP). The definition says that the group of prebiotics may involve other substances besides carbohydrates (such as polyphenols and polyunsaturated fatty acids transformed into corresponding conjugated fatty acids), and may act not only in the alimentary tract. Another important aspect is that they are no longer limited to human food, but may be also considered in other categories, such as animal nutrition. On the other hand, requirements concerning selective mechanisms of modulation of microbiota as well as the condition of documented beneficial effects on the health of the host have been maintained [93].
Table 5.
Definitions of prebiotics
| Year | Definitions |
|---|---|
| 1995 | “Non-digested food components that, through stimulation of growth and/or activity of a single type or a limited amount of microorganisms residing in the gastrointestinal tract, improve the health condition of a host” [92] |
| 2004 | “A selectively fermented component allowing specific changes in the composition and/or activity of microorganisms in the gastrointestinal tract, beneficial for host’s health and wellbeing” [96] |
| 2007 | “A nonviable food component that confers a health benefit on the host associated with modulation of the microbiota” [97] |
| 2010 | ‘Dietary prebiotics’ as “a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” [98] |
| 2015 | “A non-digestible compound that, through its metabolization by microorganisms in the gut, modulates the composition and/or activity of the gut microbiota, thus, conferring a beneficial physiological effect on the host” [99] |
| 2016 | “A substrate that is selectively utilized by host microorganisms conferring a health benefit” [93] |
Many different nutrients, such as pectins, cellulose and xylanes, favour development of various intestinal microorganisms. Prebiotics should not be extensively metabolised, but should induce targeted metabolic processes, thus bringing health benefits to the host’s ecosystem. The best documented benefits are associated with the use of indigestible oligosaccharides, such as fructans and galactans [94]. That phenomenon is explained by, among others, easy degradability of bonds present in the structure of fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) by certain enzymes, such as β-fructanosidase and β-galactosidase, commonly occurring in Bifidobacterium genus bacteria. Some types of nutritional fibre may be considered prebiotic. Prebiotics play a significant role in nutrition of both livestock and home pets. When assessing the effect of prebiotics on health, one has to take into account the fact that all groups of animals mentioned above differ in terms of anatomy, physiology, nutrition, intestinal microbiota and habitat [95].
Prebiotic selection criteria
In order to determine and demonstrate that a substance is a potential prebiotic, one has to indicate its source, origin, purity, chemical composition and structure. Prebiotics has to cover safe regulations required by all nations, such as posses Generally Recognized As Safe (GRAS) status, proper dose and side effects evaluation, no contaminants and impurities, do not alter intestinal microbiota to obtain negative effects on the host. It is emphasized that the term prebiotic may be used only when beneficial health effect related to the microbiota modulation in a specific site [97].
According to Wang [100], there are five basic criteria for classification of food components as prebiotics (Fig. 2). First of all, it is assumed that prebiotic substances must be resistant to digestion in the upper sections of the alimentary tract. As a result, prebiotics reach the large intestine, where they become selectively fermented by potentially beneficial intestinal bacteria (the second criterion). The fermentation may lead to changes in metabolic processes, and to improved operation of the immunological system, thus exerting a beneficial effect on the host’s health (the third criterion). Very important is selective stimulation of growth of the probiotic bacteria (another criterion). Also technological features of prebiotics, associated with their successful manufacturing and availability for bacterial metabolism in the intestine, are important (the last criterion).
Fig. 2.
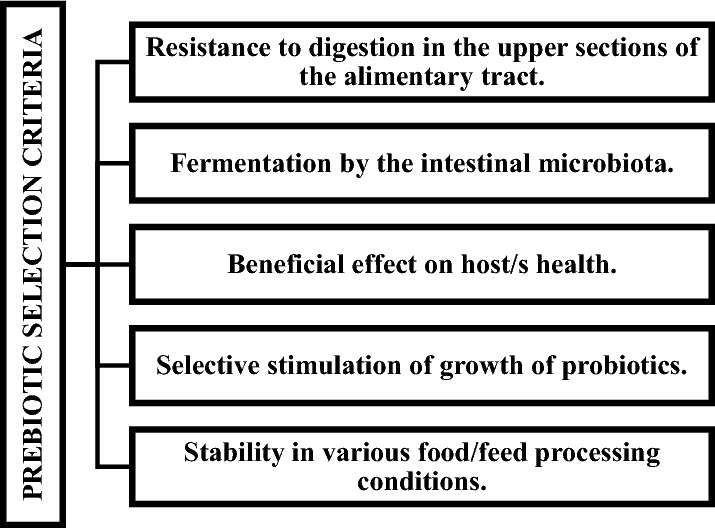
Prebiotic substances
Among prebiotic substances there are: non-absorbable carbohydrates (oligosaccharides and polysaccharides), peptides, proteins, and lipids. Legumes, fruit and cereals are natural sources of prebiotics. However, many similar substances are synthesized using industrial chemical and enzymatic methods [101]. Some commonly used prebiotics are: FOS, oligofructose, trans-galacto-oligosaccharides (TOS), gluco-oligosaccharides, glico-oligosccharides, lactulose, lactitol, malto-oligosaccharides, xylo-oligosaccharides, stachyose and raffinose [102–106]. When they reach the large intestine, those substances become nutritional substrates for beneficial intestinal bacteria [107]. In terms of properties that determine a favourable effect on the host’s health, prebiotics may be divided into following groups: not digested (or only partially digested), not absorbed in the small intestine, poorly fermented by bacteria in the oral cavity, well fermented by seemingly beneficial intestinal bacteria and poorly fermented by potential pathogens in the bowel [108]. Prebiotics most commonly used in livestock nutrition are [108, 109]: FOS, GOS, inulin, isomalto-oligosaccharides (IMO), xylo-oligosaccharides (XOS), lacticol, lactulose, cereal fibre. When designing the composition of prebiotic formulas, determination of an appropriate dosage is essential. Overdose of prebiotics may lead to flatulence and diarrhoea. On the other hand, a great advantage of that kind of formulas is that they may be used for a long time and preventively, having no adverse effects noted for antibiotics. Table 6 presents examples of formulas used in livestock nutrition and containing prebiotic substances.
Table 6.
Examples of prebiotic formulas available in the market and intended for livestock
| Trade name of preparation (producer) | Prebiotic substances | Destination |
|---|---|---|
| Bacto CS1000 | Polysaccharides, oligosaccharides | Poultry |
| BionatStart | MOS, β-glucans | Calves |
| DOLSORB DN (Dolfos) | MOS, β-glucans | Poultry |
| MetSac MOS (VITTRA) | MOS, β-glucans | Calves, pigs, poultry |
| Mycocyd forte (Herbiline) | β-glucans | Poultry |
| Mycostop (Extra-vit) | MOS, β-glucans | Poultry, pigs |
| PROFEED® (Beghin Meiji) | scFOS | Horses, pigs, poultry, calves |
FOS fructo-oligosacharides, MOS malto-oligosacharides, scFOS short chain fructo-oligosaccharides
Prebiotics for animals
Various feed additives are used in studies on the effect of prebiotics on the gastrointestinal microbiota and general health condition of pigs. Smiricky-Tjardes et al. [110] administered TOS at the dose of 35 g/kg feed to pigs for 6 weeks. A significant increase of stool Bifidobacterium and Lactobacillus count was found compared to the control group. Tzortzis et al. [111] used a novel blend of GOS produced as a result of activity of galactosyl transferase in Bifidobacterium bifidum 41171 bacteria. The administration of that prebiotic to pigs at the dose of 40 g/kg feed in a 3-step system of continuous culture caused a significant increase of Bifidobacterium count and of acetic acid level, with simultaneous reduction of intestinal pH, compared to the control group and the diet with an addition of inulin. Moreover, the studied blend of oligosaccharides caused a strong inhibition of adhesion of Escherichia coli (ETEC) and Salmonella enterica serotype Typhimurium to HT29 cells in in vitro studies [111]. An interesting study was also carried out on the effect of barley and oat varieties with different composition of carbohydrates on the intestinal microbiota of 72 weaned piglets, for 15 days. It was found, that the increased β-glucan levels and changes in the ratio of amylopectin and amylose led to a selective modulation of growth of butyric acid bacteria which is able to hydrolyse some complex carbohydrates, such as xylan or β-glucan. Therefore, differences between cereal varieties in form and amount of carbohydrates had an effect on piglets’ intestinal microbiota, and an appropriate selection of cereals had a positive effect on Bifidobacterium and Lactobacillus count.
Xu et al. [124] checked effects of FOS used in doses: 0, 2, 4 and 8 g/kg feed on the activity of digestive enzymes and on intestinal morphology and microbiota. It was found that the administration of FOS at the dose of 4 g/kg feed had a positive effect on the mean daily growth of studied animals, and on the growth of Bifidobacterium and Lactobacillus bacteria, with a simultaneous inhibition of growth of Escherichia coli in chickens’ gastrointestinal tract. On the other hand, in the study by Juśkiewicz et al. [112] carried on turkeys for 8 weeks, no effect of FOS used at concentrations of 0.5, 1 and 2% was found on animal growth and productivity. However, reduction of the intestinal pH was noted in case of FOS administration at the concentration of 2%. Supplementation of broiler chickens’ diet with prebiotics results in reduction of gastrointestinal pH and increased Lactobacillus and Bifidobacterium counts, caused by increased amount of volatile fatty acids [113]. In their study, Yusrizal and Chen [114] checked the effect of feeding broiler chickens with fructane (of chicory origin) containing feed on growth of birds and length and structure of the intestine of studied animals. The experiment was conducted on 96 broiler chickens, for 6 weeks. An improved body weight gain, feed turnover and reduced serum cholesterol were found. Moreover, feed supplementation with fructanes caused increase of Lactobacillus genus bacteria count and reduction of counts of potential pathogens, such as Salmonella and Campylobacter in the broiler chicken gastrointestinal tract [114]. In their study, Kleessen et al. [115] bred 380 chickens for 35 days, giving them drinking water with an addition of artichoke-based fructane-containing (0.5%) syrup. The effect of fructane supplementation on the animals’ intestinal microbiota was studied. It was observed that the addition of fructanes to drinking water caused a reduction of Clostridium perfringens count, and a decrease in the level of bacterial endotoxin. Stanczuk et al. [116] analysed the effect of addition of inulin and MOS administered to turkeys ad libitum in two different concentrations (0.1 and 0.4%) as a feed supplement, during the period of 8 weeks of rearing. No increased feed consumption or higher body weight of turkeys were observed. However, in prebiotic-fed groups a higher concentration of SCFA was observed compared to the control group. In other studies conducted by Sims et al. [117] on 180 turkeys bred for 18 weeks, a supplementation of feed with MOS resulted in better growth of study animals. Spring et al. [118] studied the effect of administration of Saccharomyces cerevisiae yeast containing MOS in their cellular wall on reduction of count of various intestinal pathogens in chickens. It was observed that the administration of MOS-containing yeast resulted in a reduced count of Salmonella in chicks’ intestines by 26%, compared to control animal receiving a non-modified diet. Studies completed by Thitaram et al. [119] verified the effect of isomalto-oligosaccharides (IMO) administered in the following concentrations: 1, 2 and 4% (by weight) on intestinal microbiota of broiler chickens infected with Salmonella Typhimurium. Supplementation of animal feed with IMO caused a significant reduction of Salmonella Typhimurium count. While chewing, digestion and effectiveness of the administered feed were not significantly different from the control group. It was also observed that the addition of IMO to feed caused an increase in Bifidobacterium genus bacteria count. Moreover a significant loss of weight was observed in the case of birds fed with 1% IMO compared to control animals fed with the non-modified feed [119]. In other studies, Biggs et al. [121] focused on the effect of feeding chicks with feed with addition of 5 different oligosaccharides (inulin, oligofructose, MOS, short-chain oligosaccharide and TOS) [120]. No significant increase in body weight was observed in any case. Moreover, the study demonstrated that excessively high prebiotic dose may have a negative impact on the gastrointestinal system and delay the process of growth of animals [120]. Similarly, other studies completed by Jung et al. [122] on broiler chickens demonstrated that administration of feed with an addition of GOS at various concentrations for 40 days of rearing had no effect on the feed conversion index, body weight and consumption of feed [121]. Nevertheless, the addition of the prebiotic had a positive effect on the increase of Bifidobacterium bacteria in intestines of study chickens. Summing up, the main effect of prebiotics on health of chickens consists in an increased count of Bifidobacterium and reduced intestinal colonisation by pathogenic bacteria [122, 123]. Results of studies on the effect of prebiotics on animal health are often contradictory, which is a result of high specificity of individual compounds, various doses and time of application. Table 7 presents the examples of studies on the effect of prebiotics on animal health.
Table 7.
Examples of trials regarding the effect of prebiotics on animal health
| Reference | Subjects | Prebiotic | Time | Main outcome |
|---|---|---|---|---|
| Absorption and utilisation of feed, diarrhoea, body weight gain | ||||
| [124] | 240 Broiler chickens | FOS | 49 days | Administration of fructooligosaccharides at the dose of 4 g/kg feed had a positive effect on the mean daily growth of studied animals, and on growth of Bifidobacterium and Lactobacillus bacteria, with a simultaneous inhibition of growth of Escherichia coli in experimental animals’’ gastrointestinal tract |
| [112] | 320 Turkeys | FOS | 8 weeks | No effect on growth and productivity of experimental animals. However, reduction of the intestinal pH was noted in case of FOS administration at the concentration of 2% |
| [125] | 96 Broiler chickens | Fructans from chicory | 6 weeks | An improved body weight gain, feed turnover and reduced serum cholesterol |
| [116] | 40 Turkeys | MOS, inulin | 8 weeks | No increased feed consumption or higher body weight of experimental animals were observed. A higher SCFA concentration was found in animals fed with prebiotics, compared to the control |
| [117] | 180 Turkeys | MOS | 18 weeks | Improved growth of experimental animals |
| [120] | 120 Chickens | Inulin, oligofructose, MOS, short-chain oligosaccharide, TOS | 21 days | No significant body weight gain. The study demonstrated that an excessively high prebiotic dose may have a negative impact on the gastrointestinal system and delay the process of growth of animals |
| Intestinal ecosystem imbalance, pathogenic infections | ||||
| [110] | 12 Pigs | TOS | 6 weeks | A significant increase of stool Bifidobacterium and Lactobacillus count compared to the control group |
| [111] | 40 Weaned male pigs | GOS | Mean of 34 days | A significant increase of Bifidobacterium genus bacteria count and of concentration of acetic acid, with simultaneous reduction of intestinal pH compared to the control group, and the diet with an addition of inulin. Moreover, the GOS supplementation caused a strong inhibition of adhesion of Escherichia coli (ETEC) and Salmonella enterica serotype Typhimurium to HT29 cells in in vitro studies |
| [114] | 98 Broiler chickens | Fructans from chicory | 6 weeks | The supplementation with fructans caused an increase Lactobacillus genus bacteria count and reduction of count of potential pathogens, such as Salmonella and Campylobacter |
| [115] | 380 Chickens | Fructans from artichoke | 35 days | Reduced Clostridium perfringens count and bacterial endotoxin level |
| [119] | 120 Broiler chickens infected with Salmonella Typhmiurium | IMO | 21 days | A significant reduction of Salmonella Typhimurium count. Chewing, digestion and effectiveness of the administered feed were not significantly different from the control. group. A significant loss of weight in case of animals fed with 1% IMO compared to the control group. The supplementation with IMO caused an increase of the Bifidobacterium count in the gastrointestinal system of experimental animals |
| Improved quality of meat, milk, eggs | ||||
| [126] | 350,560 Eggs from Ross 308 broiler | DiNovo (DN; laminarin and fucoidan), Bi2tos (BI; non-digestive TOS) | 42 days | No significant differences in the final count of chickens, mortality, breeding density (kg/m3), FCR, European Broiler Index between all experimental groups. The administration of DN and BI resulted in a minor increase (P > 0.05) of the mean BW and a minor improvement (P > 0.05) of FCR in the BI group. Chickens exposed to DN and BI demonstrated a significant increase of BW, carcase weight, weight of the myocardium and weight of the breast, compared to the control group. Summing up, the administration of prebiotics in ovo resulted in an improvement of many parameters significant for the commercial production of poultry |
BW body weight, FCR feed conversion ratio, FOS fructo-oligosaccharides, GOS galacto-oligosaccharides, IMO isomalto-oligosaccharides, MOS manno-oligosaccharides, TOS transgalacto-oligosaccharides
Synbiotics
Also formulas containing both probiotics and prebiotics are used in animal nutrition. In 1995, Gibson and Roberfroid introduced the term of “synbiotic” by specifying in this way “a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the GI tract, by selectively stimulating the growth and/or activating the metabolism of one or a limited number of health-promoting bacteria, and thus improving host welfare” [92]. As the word “synbiotic” implies synergy, the term should be reserved for those products in which a prebiotic component selectively favours a probiotic microorganism [127]. The principal purpose of that type of combination is improvement of survival of probiotic microorganisms in the gastrointestinal tract. Synbiotics have both probiotic and prebiotic properties and were created in order to overcome some possible difficulties in survival of probiotics in the gastrointestinal tract [128]. Probiotics beneficially influence the intestinal equilibrium, and constitute a protective barrier for the alimentary tract. Prebiotics, on the other hand, supply energy and nutrients for probiotic bacteria [129, 130]. Therefore, an appropriate combination of both components in a single product should ensure a superior effect, compared to the activity of the probiotic or prebiotic alone [131, 132]. The health effect of synbiotics is probably associated with the individual combination of a probiotic and prebiotic [133]. Considering a huge number of possible combinations, the application of synbiotics for modulation of intestinal microbiota in animals seems promising [134].
Synbiotic selection criteria
Most of all, probiotic strains and prebiotics considered in the process of designing a synbiotic formula should meet all the criteria presented in “Selection criteria and requirements for probiotic strains” and “Prebiotic selection criteria”. When composing the synbiotic formula, selection of probiotics and prebiotics that have a beneficial effect on the host’s health when used separately is crucial. When selecting probiotic substances, it is helpful to determine their potentially beneficial properties for the metabolism of a probiotic. A formula may be considered a synbiotic if a selective stimulation of growth of beneficial microorganisms is confirmed, along with no or limited stimulation of growth of other microbes. Also technological aspects should be considered. Determination of composition of a synbiotic formula is an extremely difficult task, requiring many studies.
Synbiotics in use
Previous sections discussed probiotic microorganisms and prebiotic substances most commonly used in animal nutrition. A combination of Bifidobacterium or Lactobacillus genus bacteria with FOS in synbiotic products seems to be the most popular. Table 8 presents examples of synbiotic formulas available in the market, and used for livestock nutrition.
Table 8.
Examples of commercial synbiotic formulas used in nutrition of livestock
| Trade name of the preparation (producer) | Microorganisms | Prebiotic substances | Destination |
|---|---|---|---|
| Biomin®IMBO (ME BIOMIN GmbH) | Enterococcus faecium | FOS | Poultry, pigs, calves |
| DigestAid™ | Pediococcus acidilactici, Saccharomyces: cerevisiae, boulardii | β-glucan, MOS | Horses |
| PoultryStar® (ME BIOMIN GmbH) | Bifidobacterium animalis, Enterococcus faecium, Lactobacillus: reuteri, salivarius, Pediococcus acidilactici, | Inulin | Poultry |
| Synbiotic poultry (Vetafarm) | Lactobacillus: acidophilus, casei, salivarius, plantarum, rhamnosus, brevis, Bifidobacterium: bifidum, lactis, Streptococcus thermophilus | Inulin | Poultry |
FOS fructo-oligosacharides, MOS mannano-oligosacharides
Synbiotics for animals
The animal gastrointestinal tract, besides being the environment for a huge number of microorganisms, plays also a significant immunological role and constitutes the most important barrier protecting the host from toxins, pathogens, and consequences of their action, namely inflammation. Currently available data regarding effects of synbiotic on animal health are insufficient and require further studies. However, they clearly indicate the effective synergistic action of probiotics and prebiotics in reduction of populations of bacterial gastrointestinal pathogens.
Recent years have seen a remarkable evolution in the development and applications of traditional and DNA-based molecular tools that are allowing the microbiologists to characterize and understand the microbial communities in unprecedented ways [135]. Metagenomic investigations, comprising isolation of entire microbial community genomes, construction and screening of clone libraries, enable the microbiologists to have a look at a more complete scenario of an environmental microbial communities, and thus, to better understand the microbes–environment interactions [136]. Metagenomics could be a promising strategy for appraising of the synbiotics effect of the intestinal microbiota of animals.
Nemcová et al. [137] confirmed the synergistic effect of Lactobacillus paracasei bacteria combined with FOS in the intestinal microbiota of piglets. The researchers observed an increase of total anaerobic and aerobic count, and increased number of beneficial Lactobacillus and Bifidobacterium genus bacteria in the group of animals fed with a synbiotic. At the same time, the Escherichia coli, Enterobacteriaceae and Clostridium genus bacteria count decreased in the stool of studied piglets [137].
Lee et al. [113] in a 16 day experiment studied the effect of synbiotics on growth, digestibility of nutrients, emission of harmful gases and composition of intestinal microbiota of 150 pigs during the weaning period. Supplementation with the synbiotic product containing a combination of a probiotic originating from anaerobic microbiota (bacteria—109 CFU/ml, yeast—105 CFU/ml, moulds—103 CFU/ml) and a prebiotic (MOS, sodium acetate, ammonia citrate) results in improved digestion of nutrients, reduced emission of harmful gases and prevents bacterial infections during the weaning period [138].
Mohnl et al. [139] observed that the synbiotic product (Biomin® PoultryStar) had a comparable growth stimulating potential to avilamycin (an antibiotic-based growth stimulant) in broiler chicken. Vicente et al. [140] verified the effect of a synbiotic product containing Lactobacillus spp. with the addition of lactose. 320 turkeys infected with Salmonella were bred, and a positive effect of the synbiotic on feed conversion and body weight gain of study animals was demonstrated. Li et al. [141] assessed the effect of administration of FOS and Bacillus subtilis bacteria to broiler chickens. 720 broiler chickens were bred and improvement of the average daily growth and of the feed conversion ratio, as well as reduced incidence of diarrhoea and mortality of animals in comparison to animals treated with aureomycin (tetracycline antibiotic) were observed. During the administration of a combination of GOS and Bifidobacterium lactis bacteria to broiler chickens during a 40 day rearing period, a significant increase of Bifidobacterium and Lactobacillus count and in overall population of anaerobic bacteria was observed in the intestinal microbiota of the study animals. However, no effect on feed consumption and conversion, and on body weight was observed. Awad et al. [142] studied the effect of the synbiotic product containing Enterococcus faecium bacteria and FOS as a prebiotic, and immunomodulating substances from marine algae (ficophytic substances) on health of broiler chickens. 600 broiler chickens bred for 5 weeks were studied. A significant increase of the average daily body weight gain, carcase ratio and the feed conversion ratio was found in comparison to control animals. However, no effect of the synbiotic on body weight gain was observed, except for the small intestine, in which a significant growth of intestinal villi was observed within the duodenum and the ileum. Based on the study on 240 broiler chickens, it was found that probiotics and prebiotics have a favourable effect on performance parameters, during some terms even superior to antibiotics used for the comparison. Moreover, it was observed that prebiotic supplementation may be helpful in reduction of abdominal fat following 42 days of breeding. It was observed that probiotics and prebiotics may be possibly used as substitutes of antibiotic-based growth stimulants [143].
Summing up, researchers agree that synbiotic products provide a better efficacy compared to the separate application of probiotics and prebiotics [121, 142, 144, 145]. Table 9 lists the examples of results of studies focusing on the effect of synbiotics on animal health.
Table 9.
Examples of trials regarding the effect of synbiotics on animal health
| Reference | Subjects | Composition of synbiotic | Time | Main outcome |
|---|---|---|---|---|
| Absorption and utilisation of feed, diarrhoea, body weight gain | ||||
| [140] | 320 Turkeys infected with Salmonella | Lactobacillus spp., lactose | 14 days (trial 1–3), 18 days (trial 4) | The effect of a synbiotic on improved feed conversion and increased body weight of experimental animals |
| [141] | 720 Broiler chickens | B. subtilis, FOS | 6 weeks | Improved average daily growth, FCR, reduced incidence of diarrhoea and mortality, compared to animals treated with aureomycin |
| [142] | 600 Broiler chickens | E. faecium, FOS | 5 weeks | A significant increase of the average daily body weight gain, carcase ratio and FCR compared to the control |
| Intestinal ecosystem imbalance, pathogenic infections | ||||
| [146] | 33 Conventional healthy sucking piglets | L. plantarum, maltodextrin and/or FOS | 7 days | Reduced counts of E. coli O8:K88 in the jejunum and colon of piglets, and it was associated with increased acetate concentrations in the ileum and colon |
| [138] | 150 Pigs during weaning | A probiotic of anaerobic microflora (bacteria/yeast/moulds), MOS, sodium acetate, ammonia citrate | 16 days | Improved digestion of nutrients, reduced emission of harmful gases and prevention of bacterial infections during the weaning period |
| Improved quality of meat, milk, eggs | ||||
| [147] | 58 Holsten dairy cows | L. casei, dextran | 1 year | Significant increase in Holstein cow milk production; including total milk, fat, protein and solids-non-fat production |
FCR feed conversion ratio, FOS fructo-oligosaccharides, MOS mannano-oligosaccharides
Conclusions
Despite numerous difficulties associated with the registration of feed additives, particularly in the category of zootechnical feed additives, modern global economy and strong market competition result in the need to introduce new technologies to animal nutrition. Numerous scientific reports confirm a beneficial effect of probiotics on animal health, particularly in terms of protection against pathogens, stimulation of immunological response and increased production capacity. Prebiotics may be used alternatively or support the effect of probiotics. Interestingly, the use of combination of those components demonstrating a synergistic effect may be even more efficient in the stimulation of intestinal microbiota and protection of animal health. The greatest problem encountered by the scientists who attempt to create synbiotic formulas is selection of appropriate probiotic and prebiotic (high selectivity of action). Feeds containing probiotic organisms are a great hope for that field of the food industry. The hope is even greater considering the fact that consumers do not accept animal products originating from animals in which antibacterial substances had been used. Meeting all expectations requires much work in the field of scientific research, development of innovative technologies and convincing breeders that the spending on prebiotic-containing feed will translate to better production effects and higher quality of animal products, and thus it will guarantee an expected economic profit. It should be underlined that the use of feed additives, such as probiotics, prebiotics and synbiotics is safe, does not have a negative impact on the natural environment, and reduces the demand for antibiotic-based growth stimulators. However, the mechanisms of action of probiotic organisms, prebiotics, as well as their combinations in synbiotics, require further studies.
Authors’ contributions
PM and KŚ wrote the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
We would like to thank the National Centre for Research and Development for the financial support of publication of this paper within the project PBS3/A8/32/2015 realised within the framework of the Program of Applied Studies.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data analysed are publicly available in source articles and data citations was included in the reference list.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The publication of this paper is realised with the financial support of the project PBS3/A8/32/2015 (The Program of Applied Studies).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paulina Markowiak, Email: paulina.markowiak@dokt.p.lodz.pl.
Katarzyna Śliżewska, Email: katarzyna.slizewska@p.lodz.pl.
References
- 1.Truszczyński M, Pejsak Z. Wpływ stosowania u zwierząt antybiotyków na lekooporność bakterii chorobotwórczych dla człowieka. Med Weter. 2006;62:1339–1343. [Google Scholar]
- 2.Biernasiak J, Śliżewska K, Libudzisz Z. Negatywne skutki stosowania antybiotyków. Postęp Nauk Rol. 2010;3:105–117. [Google Scholar]
- 3..Miecznikow E. O Naturze Ludzkiej – Zarys Filozofii Optymistycznej (translation F. Wermiński). Wydawnictwo Biblioteka Naukowa. Warszawa; 1907.
- 4.Vergin F. Anti- und Probiotica. Hipokrates. 1954;25:116–119. [PubMed] [Google Scholar]
- 5.Lilly DM, Stillwell RH. Probiotics: growth promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 6.Sperti GS. Probiotics. West Point (CT): AVI Publishing Co; 1971. [Google Scholar]
- 7.Parker RB. Probiotics, the other half of the antibiotic story. Anim Nutr Health. 1974;29:4–8. [Google Scholar]
- 8.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 9.Havenaar R, Huis In’t Veld JHJ. Probiotics: a general view. In: Wood BJB, editor. Lactic acid bacteria in health and disease. London: Elsevier Applied Science Publishers; 1992. pp. 151–170. [Google Scholar]
- 10.Salminen S. Uniqueness of probiotic strains. Int Dairy Fed Nutr Newsl. 1996;5:16–18. [Google Scholar]
- 11.Schaafsama G. State-of-the-art concerning probiotic strains in milk products. Int Dairy Fed Nutr Newsl. 1996;5:23–24. [Google Scholar]
- 12.Guarner F, Schaafsma GJ. Probiotics. Int. J Food Microbiol. 1998;39:237–238. doi: 10.1016/S0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 13.Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB) Crit Rev Food Sci Nutr. 1999;39:13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 14.Schrezenmeir J, dr Vrese M. Probiotics, prebiotics and synbiotics—approaching a definition. Am Soc Clin Nutr. 2001;73:361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 15.FAO. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Gidelines for the evaluation of probiotics in food. 2002;30.04–01.05.2002, London, Ontario, Kanada.
- 16.Fuller R. What is a probiotic? Biologist. 2004;51:232. [Google Scholar]
- 17.Sanders ME. How do we know when something called “probiotic” is really a probiotic? A guideline for consumers and health professionals. Funct Food Rev. 2009;1:3–12. [Google Scholar]
- 18.FAO. Food and Agriculture Organization of the United Nations. Guidelines for the evaluation of probiotics in food. 2009;27.01.2009.
- 19.Hill C, Guarner F, Reid G, et al. Sanders expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 20.Anadón A, Castellano V, Martínez-Larrañaga MR. Regulation and guidelines of probiotics and prebiotics. In: Ötles S, editor. Probiotics and prebiotics in food, nutrition and health. Boca Raton, FL: CRC Press, LLC Taylor & Francis Group; 2014. pp. 91–113. [Google Scholar]
- 21.Isolauri E, Salminen S, Ouwehand AC. Probiotics. Best Pract Res Clin Gastroenterol. 2004;18:299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Shukla R, Goyal A. Probiotics in valorization of innate immunity across various animal models. J Funct Foods. 2015;14:549–561. doi: 10.1016/j.jff.2015.02.022. [DOI] [Google Scholar]
- 23.Takahashi T, Nakagawa E, Nara T, Yajima T, Kuwata T. Effects of orally ingested Bifidobacterium longum on the mucosal IgA response of mice to dietary antigens. Biosci Biotechnol Biochem. 1998;62:10–15. doi: 10.1271/bbb.62.10. [DOI] [PubMed] [Google Scholar]
- 24.Willing BP, Malik G, van Kessel AG. Nutrition and gut health in swine. In: Chiba LI, editor. Sustainable swine nutrition. Chichester: Wiley; 2012. pp. 197–213. [Google Scholar]
- 25.Liao SF, Nyachoti M. Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr. 2017;3(4):331–343. doi: 10.1016/j.aninu.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajagai YS, Klieve AV, Dart PJ, Bryden WL. Probiotics in animal nutrition—production, impact and regulation. In: Makkar HPS, editor. FAO animal production and health paper No. 179. Food and Agriculture Organization of the United Nation, Rome, Italy; 2016.
- 27.Lee IK, Kye YC, Kim G, Kim HW, Gu MJ, Umboh J, et al. Stress, nutrition, and intestinal immune responses in pigs—a review. Asian Australas J Anim Sci. 2016;29:1075–1082. doi: 10.5713/ajas.16.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 29.García-Lafuente A, Antolín M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001;48:503–507. doi: 10.1136/gut.48.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Wang A, Zeng X, Hou C, Liu HS, Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. doi: 10.1186/s12866-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 33.Yirga H. The use of probiotics in animal nutrition. J Prob Health. 2015;3:132. doi: 10.4172/2329-8901.1000132. [DOI] [Google Scholar]
- 34.Fuller R. Probiotics: the scientific basis. 1. New York: Chapman & Hall; 1992. [Google Scholar]
- 35.Mizak L, Gryko R, Kwiatek M, et al. Probiotyki w żywieniu zwierząt. Życie Weterynaryjne. 2012;87(9):736–741. [Google Scholar]
- 36.Hollister A, Cheeke P, Robinson A, et al. Effects of water administrated probiotics and acidifiers on growth, feed conversion and enteritis mortality of weanling rabbits. J Appl Rabbit Res. 1989;12:143–147. [Google Scholar]
- 37.EFSA Opinion of the Scientific Committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 2005;226:1–12. [Google Scholar]
- 38.Anadón A, Martínez-Larrańaga MR, Martínez MA. Probiotics for animal nutrition in the European Union, regulation and safety assessment. Regul Toxicol Pharmacol. 2006;45:91–95. doi: 10.1016/j.yrtph.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Simon O. Microorganisms as feed additives—probiotics. Adv Pork Prod. 2005;16:161–167. [Google Scholar]
- 40.Gaggia F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz-Atienza E, Gómez-Sala B, Araújo C, Campanero C, Del Campo R, Hernández PE, et al. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013;13:15. doi: 10.1186/1471-2180-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varankovich NV, Nickerson MT, Korber DR. Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front Microbiol. 2015;6:685. doi: 10.3389/fmicb.2015.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imperial ICVJ, Ibana JA. Addressing the antibiotic resistance problem with probiotics: reducing the risk of its double-edged sword effect. Front Microbiol. 2016;7:1983. doi: 10.3389/fmicb.2016.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach JT, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.EFSA The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J. 2013;3129:1–250. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.EFSA Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update) EFSA J. 2013;2013(3449):1–108. [Google Scholar]
- 47.EFSA Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017 update) EFSA J. 2017;15(3):1–177. doi: 10.2903/j.efsa.2017.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rachwał A. Naturalne promotory wzrostu. Hodowca drobiu. 2003;8:31–32. [Google Scholar]
- 49.Szeleszczuk P. Weterynaryjne aspekty stosowania żywych kultur mikroorganizmów w praktyce drobiarskiej. Cz I Praktyka kliniczna. 2005;11(99):56–58. [Google Scholar]
- 50.Holzapfel WH, Schillinger U. Introduction to pre- and probiotics. Food Res Int. 2002;35:109–116. doi: 10.1016/S0963-9969(01)00171-5. [DOI] [Google Scholar]
- 51.Śliżewska K, Biernasiak J, Libudzisz Z. Probiotyki jako alternatywa dla antybiotyków. Zeszyty Naukowe Politechniki Łódzkiej. 2006;984:79–81. [Google Scholar]
- 52.FAO, Bajagai YS, Klieve AV, Dart PJ, Bryden WL. Probiotics in animal nutrition—production, impact and regulation. In: Makkar HPS, editor. Paper No. 179. Rome: FAO Animal Production and Health; 2016.
- 53.Torres-Rodriquez A, Donoghue AM, Donoghue DJ, et al. Performance and condemnation rate analysis of commercial turkey flocks treated with a Lactobacillus sp.—based probiotic. Poult Sci. 2007;86:444–446. doi: 10.1093/ps/86.3.444. [DOI] [PubMed] [Google Scholar]
- 54.Samli HE, Senkoylu N, Koc F, et al. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch Anim Nutr. 2007;61:42–49. doi: 10.1080/17450390601106655. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Yin J, Li D, et al. Dietary supplementation with zinc oxide increases igf-I and igf-I receptor gene expression in the small intestine of weanling piglets. J Nutr. 2006;136:1786–1791. doi: 10.1093/jn/136.7.1786. [DOI] [PubMed] [Google Scholar]
- 56.Casey PG, Gardiner GE, Casey G, et al. A 5-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl Environ Microb. 2007;73:1858–1863. doi: 10.1128/AEM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiofalo V, Liotta L, Chiofalo B. Effects of the administration of Lactobacilli on body growth and on the metabolic profile in growing Maltese goat kids. Reprod Nutr Dev. 2004;44:449–457. doi: 10.1051/rnd:2004051. [DOI] [PubMed] [Google Scholar]
- 58.de Rezende ASC, Trigo P, Lana AMQ, Santiago JM, Silva VP, Montijano FC. East as a feed additive for training horses. Cienc Agrotecnol. 2012;36:354–362. doi: 10.1590/S1413-70542012000300012. [DOI] [Google Scholar]
- 59.Musa HH, Wu SL, Zhu CH, et al. The potential benefits of probiotics in animal production and health. J Anim Vet Adv. 2009;8(2):313–321. [Google Scholar]
- 60.Plavnik I, Scott ML. Effects of additional vitamins, minerals or brewers yeast upon leg weaknesses in broiler chickens. Poult Sci. 1980;59:459–464. doi: 10.3382/ps.0590459. [DOI] [PubMed] [Google Scholar]
- 61.Modesto M, D’Aimmo MR, Stefanini I, et al. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest Sci. 2009;122:248–258. doi: 10.1016/j.livsci.2008.08.017. [DOI] [Google Scholar]
- 62.Böhmer BM, Kramer W, Roth-Maier DA. Dietary probiotic supplementation and resulting effects on performance, health status and microbial characteristics of primiparous sows. J Anim Physiol Anim Nutr. 2006;90:309–315. doi: 10.1111/j.1439-0396.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 63.Yoon IK, Stern MD. Influence of direct-fed microbials on ruminant microbial fermentation and performance of ruminants: a review. Asian Australas J Anim Sci. 1995;8:533–555. doi: 10.5713/ajas.1995.553. [DOI] [Google Scholar]
- 64.Mountzouris KC, Tsirtsikos P, Kalamara E, et al. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci. 2007;86(2):309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- 65.Kritas SK, Govaris A, Christodoulopouls G, et al. Effect of Bacillus licheniformis and Bacillus subtilis supplementation of Ewe’s feed on sheep milk production and young lamb mortality. J Vet Med Ser. 2006;53:170–173. doi: 10.1111/j.1439-0442.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 66.Alexopoulos C, Georgoulakis IE, Tzivara A, et al. Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J Anim Physiol Anim Nutr. 2004;88:381–392. doi: 10.1111/j.1439-0396.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 67.Yu P, Huber JT, Theurer CB, et al. Effect of steam-flaked or steam-rolled corn with or without Aspergillus oryzae in the diet on performance of dairy cows fed during hot weather. J Dairy Sci. 1997;80(12):3293–3297. doi: 10.3168/jds.S0022-0302(97)76304-5. [DOI] [PubMed] [Google Scholar]
- 68.Ceslovas J, Vigilijus J, Almantas S. The effect of probiotic and phytobiotics on meat properties and quality in pigs. Vet Zootech. 2005;29:80–84. [Google Scholar]
- 69.Haddadin MSY, Abdulrahim SM, Hashlamoun EAR, et al. The effects of Lactobacillus acidophilus on the production and chemical composition of hen’s eggs. Poult Sci. 1996;75:491–494. doi: 10.3382/ps.0750491. [DOI] [PubMed] [Google Scholar]
- 70.Kurtoglu V, Kurtoglu F, Seker E, et al. Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol. Food Addit Contam. 2004;21:817–823. doi: 10.1080/02652030310001639530. [DOI] [PubMed] [Google Scholar]
- 71.Van Immerseel F, Russell JB, Flythe MD, et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 72.Doreau M, Jouany JP. Effect of a Saccharomyces cerevisiae culture on nutrient digestion in lactating dairy cows. J Dairy Sci. 1998;81:3214–3221. doi: 10.3168/jds.S0022-0302(98)75885-0. [DOI] [PubMed] [Google Scholar]
- 73.Kyriakis SC, Tsiloyiannis VK, Vlemmas J, et al. The effect of probiotic LSP 122 on the control of post-weaning diarrhea syndrome of piglets. Res Vet Sci. 1999;67:223–228. doi: 10.1053/rvsc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 74.Tellez G, Petrone VM, Escorcia M, et al. Evaluation of avian-specific probiotic and Salmonella Enteritidis, Salmonella Typhimurium and Salmonella Heidelberg-specific antibodies on cecal colonization and organ invasion of Salmonella Enteritidis in broilers. J Food Prot. 2001;64:287–291. doi: 10.4315/0362-028X-64.3.287. [DOI] [PubMed] [Google Scholar]
- 75.Corr SC, Li Y, Riedel CU, et al. Bacteriocin production as mechanism for the anti-infective activity of Lactobacillus salivarius UCC118. PNAS. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chateau N, Castellanos I, Deschamps AM. Distribution of pathogen inhibition in the Lactobacillus isolates of a commercial probiotic consortium. J Appl Bacteriol. 1993;74:36–40. doi: 10.1111/j.1365-2672.1993.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 77.Stern NJ, Cox NA, Bailey JS, et al. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter sp. colonization in broiler chickens. Poult Sci. 2001;80:156–160. doi: 10.1093/ps/80.2.156. [DOI] [PubMed] [Google Scholar]
- 78.Dalloul RA, Lillehoj HS. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49:1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- 79.Zhang ZF, Kim IH. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. 2014;93(2):364–370. doi: 10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]
- 80.Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian Austral J Anim. 2015;28(2):239–246. doi: 10.5713/ajas.14.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fairbrother JM, Nadeau É, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6(1):17–39. doi: 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 82.Taras D, Vahjen W, Macha M, Simon O. Response of performance characteristics and faecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch Anim Nutr. 2005;59(6):405–417. doi: 10.1080/17450390500353168. [DOI] [PubMed] [Google Scholar]
- 83.Kantas D, Papatsiros V, Tassis P, Giavasis I, Bouki P, Tzika E. A feed additive containing Bacillus toyonensis (Toyocerin®) protects against enteric pathogens in postweaning piglets. J Appl Microbiol. 2015;118(3):727–738. doi: 10.1111/jam.12729. [DOI] [PubMed] [Google Scholar]
- 84.Konstantinov SR, Smidt H, Akkermans ADL, Casini L, Trevisi P, Mazzoni M, De Filippi S, Bosi P, de Vos W. Feeding of Lactobacillus sobrius reduces Escherichia coli F4 levels in the gut and promotes growth of infected piglets. FEMS Microbiol Ecol. 2008;66:599–607. doi: 10.1111/j.1574-6941.2008.00517.x. [DOI] [PubMed] [Google Scholar]
- 85.Bomba A, Nemcova R, Gancarcikova S, Herich R, Guba P, Mudronova D. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructo-oligosaccharides and polyunsaturated fatty acids. Br J Nutr. 2002;88(1):95–99. doi: 10.1079/BJN2002634. [DOI] [PubMed] [Google Scholar]
- 86.Fuller R. Probiotics for farm animals. Probiotics: A Crit Rev; 1999. pp. 15–22. [Google Scholar]
- 87.Zeyner A, Boldt E. Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhoea patterns and performance of piglets. J Anim Physiol Anim Nutr. 2006;90:25–31. doi: 10.1111/j.1439-0396.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 88.Taras D, Vahjen W, Macha M, et al. Performance, diarrhoea incidence, and occurrence of Escherichia coli virulence genes during long-term administration of a probiotic Enterococcus faecium strain to sows and piglets. J Anim Sci. 2006;84:608–617. doi: 10.2527/2006.843608x. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi S, Egawa Y, Simojo N, et al. Oral administration of Lactobacillus plantarum strain Lq80 to weaning piglets stimulates the growth of indigenous lactobacilli to modify the lactobacillal population. J Gen Appl Microbiol. 2007;53:325–332. doi: 10.2323/jgam.53.325. [DOI] [PubMed] [Google Scholar]
- 90.Sara A, Cighi V, Odagiu A, et al. Research concerning the effect of the probiotic YEA-SACC-1026 on productive performances in sheep. Ser Zooteh si Biotechnol. 2002;57:254–258. [Google Scholar]
- 91.Rettger LF, Cheplin HAA. Treatise on the Transformation of the Intestinal Flora: with Special Reference to the Implantation of Bacillus acidophilus. New Haven: Yale University Press; 1921. p. 13. [Google Scholar]
- 92.Gibson RG, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Appl Bacteriol. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 93.Gibson GR, Hutkins R, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 94.Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–46. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev. 1998;78:393–427. doi: 10.1152/physrev.1998.78.2.393. [DOI] [PubMed] [Google Scholar]
- 96.Gibson GR, Probert HM, van Loo J, et al. Dietary modulation of the human colonic microbiota: updating the concept of the prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 97.FAO. Technical Meeting on Prebiotics: Food Quality and Standards Service (AGNS), Food and Agriculture Organization of the United Nations (FAO) 15-16.09.2007. FAO Technical meeting Report. 2007.
- 98.Gibson GR, Scott KP, Rastall RA, et al. Dietary prebiotics: current status and new definition. Food Sci Tech Bull Funct Food. 2010;7:1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 99.Bindels LB, Delzenne NM, Cani PD, et al. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y. Prebiotics: present and future in food science and technology. Food Res Int. 2009;42:8–12. doi: 10.1016/j.foodres.2008.09.001. [DOI] [Google Scholar]
- 101.Śliżewska K, Nowak A, Barczyńska R, et al. Prebiotyki - definicja, właściwości i zastosowanie w przemyśle. ŻYWNOŚĆ Nauka Technolog Jakość. 2013;1(86):5–20. [Google Scholar]
- 102.Monsan P, Paul F. Oligosaccharide feed additives. In: Wallace RJ, Chesson A, editors. VHC biotechnology in animal feeds and animal feeding New York. Weinheim: Wiley; 1995. pp. 233–245. [Google Scholar]
- 103.Orban JI, Patterson JA, Sutton AL, et al. Effect of sucrose thermal oligosaccharide caramel, dietary vitamin-mineral level, and brooding temperature on growth and intestinal bacterial populations in broiler chickens. Poult Sci. 1997;76:482–490. doi: 10.1093/ps/76.3.482. [DOI] [PubMed] [Google Scholar]
- 104.Patterson JA, Orban JI, Sutton AL, et al. Selective enrichment of bifidobacteria in the intestinal tract of broilers by thermally produced kestoses and effect on broiler performance. Poult Sci. 1997;76:497–500. doi: 10.1093/ps/76.3.497. [DOI] [PubMed] [Google Scholar]
- 105.Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1042S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 106.Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 107.Grajek W, Olejnik A, Sip A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochim Pol. 2005;52(3):665–671. [PubMed] [Google Scholar]
- 108.Crittenden R, Playne MJ. Prebiotics. In: Lee YK, Salminen S, editors. Handbook of probiotics and prebiotics. Hoboken, New Jersey: Wiley; 2009. pp. 535–561. [Google Scholar]
- 109.Olveira G, González-Molero I. An update on probiotics, prebiotics and synbiotics in clinical nutrition. Endocrinol Nutr. 2016;63(9):482–494. doi: 10.1016/j.endonu.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Smiricky-Tjardes MR, Grieshop CM, Flickinger EA, et al. Dietary galactooligosaccharides affect ileal and total-tract nutrient digestibility, ileal and fecal bacterial concentrations, and ileal fermentative characteristics of growing pigs. J Anim Sci. 2003;81:2535–2545. doi: 10.2527/2003.81102535x. [DOI] [PubMed] [Google Scholar]
- 111.Tzortzis G, Goulas AK, Gee JM, et al. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J Nutr. 2005;135(7):1726–1731. doi: 10.1093/jn/135.7.1726. [DOI] [PubMed] [Google Scholar]
- 112.Juśkiewicz J, Jankowski J, Zduńczyk Z, et al. Performance and gastrointestinal tract metabolism of turkeys fed diets with different contents of fructooligosaccharides. Poult Sci. 2006;85:886–891. doi: 10.1093/ps/85.5.886. [DOI] [PubMed] [Google Scholar]
- 113.Ziggers D. Tos, a new prebiotic derived from whey. Anim Feed Sci Technol. 2000;5:34–36. [Google Scholar]
- 114.Yusrizal X, Chen TC. Effect of adding chicory fructans in feed on fecal and intestinal microflora and excreta volatile ammonia. Int J Poult Sci. 2003;2:188–194. doi: 10.3923/ijps.2003.188.194. [DOI] [Google Scholar]
- 115.Kleessen B, Elsayed NA, Loehren U, et al. Jerusalem artichokes stimulate growth of boiler chickens and protect them against endotoxins and potential cecal pathogens. J Food Prot. 2003;11:2171–2175. doi: 10.4315/0362-028X-66.11.2171. [DOI] [PubMed] [Google Scholar]
- 116.Stanczuk J, Zdunczyk Z, Juskiewicz J, et al. Indices of response of young turkeys to diets containing mannanoligosaccharide or inulin. Vet Zootech. 2005;31:98–101. [Google Scholar]
- 117.Sims MD, Dawson KA, Newman KE, et al. Effects of dietary mannanoligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poult Sci. 2004;83:1148–1154. doi: 10.1093/ps/83.7.1148. [DOI] [PubMed] [Google Scholar]
- 118.Spring P, Wenk C, Dawson KA, et al. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult Sci. 2000;79:205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- 119.Thitaram SN, Chung CH, Day DF, et al. Siragusa, “Isomaltooligosaccharide increases cecal Bifidobacterium population in young broiler chickens. Poult Sci. 2005;84:998–1003. doi: 10.1093/ps/84.7.998. [DOI] [PubMed] [Google Scholar]
- 120.Baurhoo B, Letellier A, Zhao X, et al. Cecal populations of Lactobacilli and Bifidobacteria and Escherichia coli after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult Sci. 2007;86:2509–2516. doi: 10.3382/ps.2007-00136. [DOI] [PubMed] [Google Scholar]
- 121.Biggs P, Parsons CM, Fahey GC. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult Sci. 2007;86(11):2327–2336. doi: 10.3382/ps.2007-00427. [DOI] [PubMed] [Google Scholar]
- 122.Jung SJ, Houde R, Baurhoo B, et al. Effects of galactooligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult Sci. 2008;87:1694–1699. doi: 10.3382/ps.2007-00489. [DOI] [PubMed] [Google Scholar]
- 123.Biggs P, Parsons CM. The effects of probiotic on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult Sci. 2008;87:1796–1803. doi: 10.3382/ps.2007-00450. [DOI] [PubMed] [Google Scholar]
- 124.Xu ZR, Hu CH, Xia MS, et al. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 125.Yusrizal X, Chen TC. Effect of adding chicory fructans in feed on broiler growth performance serum cholesterol and intestinal length. Int J Poult Sci. 2003;2:214–219. doi: 10.3923/ijps.2003.214.219. [DOI] [Google Scholar]
- 126.Maiorano G, Stadnicka K, Tavaniello S, et al. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult Sci. 2017;96(2):511–518. doi: 10.3382/ps/pew311. [DOI] [PubMed] [Google Scholar]
- 127.Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2(6):611–625. doi: 10.3390/nu2060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rioux KP, Madsen KL, Fedorak RN. The role of enteric microflora in inflammatory bowel disease: human and animal studies with probiotics and prebiotics. Gastroenterol Clin North Am. 2005;34:465–482. doi: 10.1016/j.gtc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 129.Blottiere H, Cherbut C, Le G, et al. Prolonged intake of fructoligosaccharides inductes a short-term elevation of lactic lacid—producing bacteria and a persistent increase in cecal butyrate in rats. Am Soc Nutr Sc. 1999;129:2231–2235. doi: 10.1093/jn/129.12.2231. [DOI] [PubMed] [Google Scholar]
- 130.Gibson GR. Prebiotics. Best Pract Res Clin Gastroenterol. 2003;18:287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Bengmark S. Bioecological control of the gastrointestinal tract: the role of flora and supplemented probiotics and synbiotics. Gastroenterol Clin North Am. 2005;34:413–436. doi: 10.1016/j.gtc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 132.Panesar PS, Kaur G, Panesar R, et al. Synbiotics: potential dietary supplements in functional foods. Cent: Food Sci; 2009. [Google Scholar]
- 133.De Vrese M, Schrezenmeir J. Probiotics, prebiotics and synbiotics. In: Stahl U, Donalies UEB, Nevoigt E, editors. Food biotechnology, advances in biochemical engineering/biotechnology. Berlin: Springer; 2008. pp. 1–66. [DOI] [PubMed] [Google Scholar]
- 134.Scavuzzi BM, Henrique FC, Miglioranza LHS, et al. Impact of prebiotics, probiotics and synbiotics on components of the metabolic syndrome. Ann Nutr Disord Ther. 2014;1:1009. [Google Scholar]
- 135.Pontes DS, Lima-Bittencourt CI, Chartone-Souza E, Amaral Nascimento AM. Molecular approaches: advantages and artifacts in assessing bacterial diversity. J Ind Microbiol Biotechnol. 2007;34:463–473. doi: 10.1007/s10295-007-0219-3. [DOI] [PubMed] [Google Scholar]
- 136.Singh B, Gautam SK, Verma V, Kumar M, Singh B. Metagenomics in animal gastrointestinal ecosystem: potential biotechnological prospects. Anaerobe. 2008;14:138–144. doi: 10.1016/j.anaerobe.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Nemcová R, Bomba A, Gancarčíková S, et al. Study of the effect of Lactobacillus paracasei and fructooligosaccharides on the faecal microflora in weanling piglets. Berl Munchener Tierärztliche Wochenschr. 1999;112:225–228. [PubMed] [Google Scholar]
- 138.Lee YK. Selection and maintenance of probiotic microorganisms. In: Lee YK, Salminen S, editors. Handbook of probiotics and prebiotics. New Jersey: Wiley; 2009. pp. 177–187. [Google Scholar]
- 139.Mohnl M, Acosta Aragon Y, Acosta Ojeda A, et al. Effect of synbiotic feed additive in comparison to antibiotic growth promoter on performance and health status of broilers. Poult Sci. 2007;86(1):217. [Google Scholar]
- 140.Vicente J, Wolfenden A, Torres-Rodriguez A, et al. Effect of a Lactobacillus species-based probiotic and dietary lactose prebiotic on turkey poultry performance with or without Salmonella Enteritidis challenge. J Appl Poult Res. 2007;16:361–364. doi: 10.1093/japr/16.3.361. [DOI] [Google Scholar]
- 141.Li X, Liu LQ, Xu CL. Effects of supplementation of fructooligosaccharide and/or Bacillus subtilis to diets on performance and intestinal microflora in broilers. Archiv für Tierzucht. 2008;51:64–70. [Google Scholar]
- 142.Awad WA, Ghareeb K, Abdel-Raheem S, et al. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 2009;88:49–55. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- 143.Toghyani M, Toghyani M, Tabeidian SA. Effect of probiotic and prebiotic as antibiotic growth promoter substitutions on productive and carcass traits of broiler chicks. Int Conf Food Eng Biotechnol. 2011;9:82–86. [Google Scholar]
- 144.Revolledo L, Ferreira CSA, Ferreira AJP. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- 145.Vandeplas S, Dubois Dauphin R, Thiry C, et al. Efficiency of a Lactobacillus plantarum-xylanase combination on growth performances, microflora populations, and nutrient digestibilities of broilers infected with Salmonella Typhimurium. Poult Sci. 2009;88:1643–1654. doi: 10.3382/ps.2008-00479. [DOI] [PubMed] [Google Scholar]
- 146.Nemcová R, Bomba A, Gancarčíková S, et al. Effects of the administration of lactobacilli, maltodextrins and fructooligosaccharides upon the adhesion of E. coli O8:K88 to the intestinal mucosa and organic acid levels in the gut contents of piglets. Vet Res Commun. 2007;31:791–800. doi: 10.1007/s11259-007-0048-x. [DOI] [PubMed] [Google Scholar]
- 147.Yasuda K, Hashikawa S, Sakamoto H, et al. A new synbiotic consisting of Lactobacillus casei subsp. casei and dextran improves milk production in Holstein dairy cows. J Vet Med Sci. 2007;69:205–208. doi: 10.1292/jvms.69.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed are publicly available in source articles and data citations was included in the reference list.