Abstract
We present a case of tube endothelial touch where a suture technique for repositioning of the Ahmed glaucoma valve was performed. Advantage of this technique is that it is minimally invasive and anterior chamber stability is maintained during the procedure.
Keywords: Ahmed glaucoma valve, glaucoma drainage device, tube-corneal touch
When a glaucoma drainage device (GDD) is not positioned properly, it can cause more complications other than tube occlusion. An anteriorly malpositioned tube can cause corneal-endothelial damage and posteriorly placed tube can cause chronic iritis and cataract formation.[1] In these cases, removal and reinsertion of the tube through a new pathway is usually the treatment of choice,[2] but if the conjunctiva is fibrosed it can be difficult to reopen the conjunctiva for the reinsertion of the tube. In this report, we describe the use of an innovative approach of suturing back the tube away from the cornea in a case of tube endothelial touch.
Case Report
A 57-year-old male with diabetes, hypertension, and renal impairment came for glaucoma opinion. He gave a history of using antiglaucoma medication (timolol brimonidine combination, latanoprost, and dorzolamide) in his left eye (LE) for 3 years. He had a family history of glaucoma. On examination, his right eye (RE) was absolute stage of neovascular glaucoma (NVG). His LE best-corrected visual acuity was 6/12 and intraocular pressure (IOP) was 29 mmHg with pseudophakia and NVG. His fundus in LE showed near total cupping. The LE had undergone laser for the proliferative diabetic retinopathy.
Since his IOP was not controlled with maximum tolerable medication, he underwent LE, Ahmed glaucoma valve (AGV) implantation which was performed in 2015. He came 1 year later after surgery with a complaint of progressive diminution of vision in his LE. His visual acuity in LE was 6/36 and IOP in LE was 12 mmHg on timolol eye drops.
On slit-lamp examination, it was seen that the AGV tube was touching the endothelium causing localized corneal edema in LE [Fig. 1].
Figure 1.
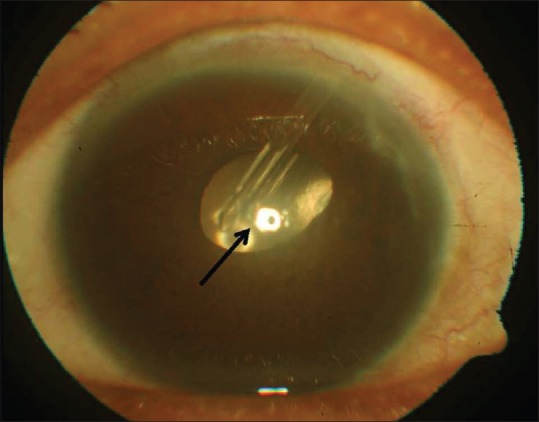
Tube-corneal touch. Localized central corneal edema is shown by the black arrow
Repositioning of the tube was performed using the following technique:
First, the position of the tube was determined. Since it was placed in superotemporal quadrant, then rough position of the suture was planned over the cornea, and accordingly, insertion and removal of a 9-0 prolene (Ethicon; Johnson and Johnson, USA) straight transchamber needle was planned so that the suture lies over the body of the AGV tube. On both sides, fornix-based conjunctival peritomies were done and partial-thickness scleral flaps created. Then, the straight needle of the prolene suture was inserted into the eye from 10 o'clock limbus under the scleral flap and exited from 2 o'clock limbus.
Care was taken that the needle is above the AGV tube and the second end of the needle is then passed 2 mm posterior to the first suture. Then, the suture was cut and tightened with a 3-2-1 knot. Tightening of the suture displaces the tube away from the cornea. The scleral flaps and conjunctival peritomies on both sides were closed such that the suture knot is not exposed.
After tube repositioning, corneal edema resolved and his visual acuity improved to 6/18. A panretinal photocoagulation was augmented for the persistent iris neovascularization. This modified McCannel suture prevented further tube endothelial touch. The patient's cornea remains clear even after 12 months of the procedure [Fig. 2].
Figure 2.

After tube repositioning using 9–0 Prolene suture
Discussion
Glaucoma drainage devices (GDDs) are the treatment of choice for refractory glaucomas. Even after successful implantation of a GDD, many complications can occur over the course of follow-up. Tube-corneal touch leading to corneal decompensation is one of the complications after AGV implantation.[3] Patients who have a shallow anterior chamber, corneal guttata, corneal edema, or eyes with corneal transplant may be at greater risk. Apart from mechanical touch which may be constant or intermittent,[4] endothelial loss may also be due to the jet stream of aqueous humor through the silicon tube during heartbeat.[5]
Many different approaches to address the problem of corneal touch have been advocated.[6,7] Conventional treatment of corneal touch after AGV implantation through the anterior chamber involves cutting the long tube or repositioning the tube through a new scleral pathway, ciliary sulcus, or pars plana.[2]
Repositioning a GDD tube requires one to redissect the scarred conjunctiva and the patch graft and then remove and reinsert the tube. This dissection carries a significant risk of inadvertent tube damage during the surgery and wound healing problems postoperatively which may lead to tube or patch graft exposure. Moreover the chances of intra operative bleeding could be more in eyes with iris neovascualrisation such as ours. Hence, an alternative minimal approach was planned by placing a transcameral double armed 9-0 prolene suture from limbus to limbus. It did not require any incision; hence, we did not have any wound-related complications such as wound leakage, infection, and scarring.[8,9] This technique also avoids intraoperative hypotony which in eyes with advanced glaucomatous optic neuropathy could lead to wipe-out phenomenon causing complete loss of vision which was avoidable with this approach.
Conclusion
The closed chamber technique to depress the tube away from endothelium with double-armed suture is quick, safe, minimally invasive procedure to correct the tube endothelial touch.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: A systematic literature review and current controversies. Surv Ophthalmol. 2005;50:48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Weiner A, Cohn AD, Balasubramaniam M, Weiner AJ. Glaucoma tube shunt implantation through the ciliary sulcus in pseudophakic eyes with high risk of corneal decompensation. J Glaucoma. 2010;19:405–11. doi: 10.1097/IJG.0b013e3181bdb52d. [DOI] [PubMed] [Google Scholar]
- 3.Sarkisian SR., Jr Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20:126–30. doi: 10.1097/ICU.0b013e328323d519. [DOI] [PubMed] [Google Scholar]
- 4.Law SK, Coleman AL, Caprioli J. Dynamic tube movement of Ahmed glaucoma valve. J Glaucoma. 2009;18:628–31. doi: 10.1097/IJG.0b013e3181996f33. [DOI] [PubMed] [Google Scholar]
- 5.McDermott ML, Swendris RP, Shin DH, Juzych MS, Cowden JW. Corneal endothelial cell counts after Molteno implantation. Am J Ophthalmol. 1993;115:93–6. doi: 10.1016/s0002-9394(14)73530-5. [DOI] [PubMed] [Google Scholar]
- 6.Tello C, Espana EM, Mora R, Dorairaj S, Liebmann JM, Ritch R, et al. Baerveldt glaucoma implant insertion in the posterior chamber sulcus. Br J Ophthalmol. 2007;91:739–42. doi: 10.1136/bjo.2006.107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar H, Gupta A, Gupta V. Achieving an ideal position of the tube in anterior chamber: A new approach. Ophthalmol Point Care. 2017;1:e56–7. [Google Scholar]
- 8.Kataria P, Kaushik S, Singh SR, Pandav SS. A novel technique of a transcorneal suture to manage an iris tuck into the tube of a glaucoma drainage device. J Glaucoma. 2016;25:e731–3. doi: 10.1097/IJG.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 9.Dada T, Gupta R, Tinwala SI, Sobti A, Panda A. Repositioning of Ahmed glaucoma valve tube in the anterior chamber with prolene sutures to manage tube-endothelial touch. Nepal J Ophthalmol. 2012;4:309–11. doi: 10.3126/nepjoph.v4i2.6549. [DOI] [PubMed] [Google Scholar]


