Abstract
Background:
Elevated mean pulmonary artery pressure (mPAP) and right heart failure increase mortality in patients with chronic kidney disease (CKD).
Objectives:
The objective of this study is to determine the prevalence of elevated mPAP in children with CKD compared with matched controls and to ascertain the relationship between elevated mPAP with right ventricular dysfunction and history of hemodialysis.
Materials and Methods:
A cross-sectional comparative study of mPAP and tricuspid annular plane systolic excursion of 21 children with CKD and age- and sex-matched controls asymptomatic for cardiac disease was conducted.
Results:
Median mPAP was 27.69 (18.3–36.1) mmHg in CKD patients compared with 14.55 (13.5–17.1) mmHg in controls (P = 0.002). Elevated mPAP was present in 42.9% of CKD group and 0% in controls (P < 0.001). The prevalence of right ventricle (RV) dysfunction in CKD was 9.5% and 0% in controls (P = 0.49). Right ventricular dysfunction was significantly more common in patients with elevated mPAP compared with those with normal mPAP (P < 0.001). Children with CKD who had a history of having been dialyzed were less likely to have elevated mPAP (P < 0.001).
Conclusion:
Elevated mPAP is significantly more common in children with CKD compared with controls. CKD population with mPAP elevation is more likely to have impaired RV function. The occurrence of elevated mPAP was more common in those who were never dialyzed.
Keywords: Chronic kidney disease, mean pulmonary artery pressure, right ventricular dysfunction, tricuspid annular plane systolic excursion
INTRODUCTION
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure ≥25 mmHg.[1] Unclear or multifactorial mechanisms such as hormonal and metabolic derangements associated with chronic kidney disease (CKD) might lead to pulmonary arterial vasoconstriction and increase in pulmonary vascular resistance with resultant PH in these patients.[2,3] The gold standard for diagnosing PH is right heart catheterization (RHC),[4,5] but this is invasive, costly, and not readily available, so transthoracic echocardiography, which has a good correlation with RHC, is recommended for PH screening using derived mean pulmonary artery pressure (mPAP).[6,7,8] Similarly, tricuspid annular plane systolic excursion (TAPSE) on M-mode echocardiography provides a simple but specific method for systolic functional assessment of the right ventricle (RV) and correlates excellently with right ventricular ejection fraction as assessed by radionuclide ventriculography.[9]
PH occurs frequently in patients with CKD as several studies based on echocardiographic evaluation of mPAP have reported a prevalence of 30%–60%.[10] It is usually asymptomatic until right ventricular dysfunction begins to manifest by worsening fatigue, dyspnea, and syncope.[4] Di Lullo et al.[11] reported that about 20% of a cohort of adult hemodialysis patients had right ventricular dysfunction as shown by low TAPSE values (<15 mm). These reports are mainly from the adult population as there is a paucity of data on the prevalence of PH through mPAP elevation in children with CKD despite the fact that children are not spared as Sharma et al.[12] reported PH in a 15-year-old boy with end-stage renal disease on chronic hemodialysis. Adiele et al.[13] reported echocardiographic findings in children from southeast Nigeria but neither reported PH nor right ventricular dysfunction in them.
The current report aims to determine the prevalence of elevated mPAP in children with CKD compared with matched controls and to ascertain the relationship between mPAP elevation with right ventricular dysfunction and history of hemodialysis.
MATERIALS AND METHODS
This study was carried out at the Aminu Kano Teaching Hospital (AKTH), Kano. Eligible children with CKD hospitalized or attending the pediatric nephrology clinic were recruited after due written informed parental consent and verbal assent from children aged 7 years and above.
Study design
This was a cross-sectional/comparative study.
Inclusion criteria
Cases – Children with CKD defined as either kidney damage or estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 for ≥3 months. Kidney damage is defined as structural or functional abnormalities of the kidney, manifested by pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies.[14]
Controls
Apparently healthy children with no obvious renal disease of the same number were matched for age and gender. They were obtained from the Pediatric Outpatient Department of AKTH.
Exclusion criteria
Children with preexisting acquired or congenital heart disease were excluded from the study.
Ethical considerations
Informed consent was obtained from the parents/guardians and assent from study participants aged 7 years or older. Approval of the Ethics and Research Committee of the AKTH, Kano, was obtained before commencement of this study.
Sample size
Sample size was determined using the prevalence of PH of 8% in CKD patients reported by Pabst et al.[15] in Germany and a population of 30 children with CKD seen at the study center over the study period. Minimum sample size obtained was 23.5. Consecutive participants who met the inclusion criteria were recruited for this study.
Echocardiography
Two-dimensional, M-mode, and Doppler echocardiograms were performed on all patients and controls using a SonoScape SSI-8000 cardiac ultrasound system with 3.5 MHz and 7.5 MHz transducers for older and younger children, respectively.
With each participant in steep left lateral decubitus position, tricuspid regurgitation was assessed in apical 4-chamber, parasternal short axis and parasternal long axis views, and the highest velocity was determined. Color flow Doppler was applied to visualize tricuspid regurgitation and to facilitate the alignment of the continuous wave Doppler beam. The peak velocity of tricuspid regurgitant Doppler flow signal was recorded. The transtricuspid peak pressure gradient was also automatically generated by the echo machine, and this corresponds to the pressure obtained from the modified Bernoulli equation.[16] Pulmonary artery systolic pressure was quantified by adding the generated peak pressure gradient to the estimated mean right atrial pressure. By applying the Chemla et al.'s[17] regression equation: (0.61 × PASP) +2 mmHg, the mPAP was derived for each participant. Cutoff value for elevated mPAP was set at ≥25 mmHg.[1,18]
TAPSE was measured from standard apical 4-chamber view by placing an M-mode cursor through the lateral tricuspid annulus and measuring the length of longitudinal motion of the annulus at peak systole.[9] TAPSE was indexed to the body surface area in m2, compared with published normal values for age, and adjudged to be impaired if below the normal range for age.[9]
Laboratory samples
Serum creatinine levels of study participants were determined. The eGFR was calculated for each of the patients using the modified Schwartz formula.[19]
Statistical analysis
The data collected were analyzed using the Statistical Package for Social Sciences (SPSS Inc., Chicago Illinois, USA) version 16. Continuous variables were tested for normality using the Shapiro–Wilk test. Student's t-test or Mann–Whitney U-test was used to compare the means or median, respectively, of measurements between groups depending on the normality of the data. Frequencies were compared between groups using Chi-squared or Fisher's test where necessary. Level of significance was regarded as < 0.05 at 95% confidence interval.
RESULTS
A total of 21 patients with the same number of controls met the inclusion criteria and were studied, making a response rate of 89.4%. There were 15 males and 6 females in the CKD and control group, respectively, with M:F ratio 2.5:1. Three children (14.3%) with CKD had undergone at least 1 session of hemodialysis. The respondents' median age (interquartile range [IQR]) is 10 (7.0–12.0) years. Although controls were taller – 135.0 (IQR: 122.0–144.8) cm and heavier – 27.40 ± 9.9 Kg than patients – 130.0 (IQR: 113.5–137.5) cm and 25.40 ± 6.2 Kg, respectively, these differences were not significant (P = 0.11 and P = 0.45, respectively).
The median mPAP of the patients, 27.69 (IQR: 18.3–36.1) mmHg, was significantly higher than those of the controls, 14.55 (IQR: 13.5–17.1) mmHg (P = 0.002) [Table 1 and Figure 1]. Of the CKD patients, estimated right atrial pressure of 5, 10, and 15 mmHg was present in 10, 8, and 3 patients, respectively, while 17 of the controls had 5 mmHg and 4 of them had 10 mmHg. The patients had less TAPSE than the controls (2.22 ± 0.5 cm vs. 2.42 ± 0.3 cm), but this difference was not statistically significant (P = 0.11).
Table 1.
Mean pulmonary artery pressure and tricuspid annular plane systolic excursion compared between children with chronic kidney disease and matched controls
| Parameter | CKD (n=21) | Controls (n=21) | Test statistic | P |
|---|---|---|---|---|
| mPAP mmHg, median (IQR) |
27.69 (18.3-36.1) |
14.55 (13.5-17.1) |
−3.11## | 0.002* |
| TAPSE cm, mean±SD | 2.22±0.5 | 2.42±0.3 | −1.64# | 0.11 |
##Wilcoxon rank-sum test statistic, #Independent t-test statistic, *Statistically significant. mPAP=Mean pulmonary arterial pressure, TAPSE=Tricuspid annular plane systolic excursion, CKD=Chronic kidney disease, IQR=Interquartile range, SD=Standard deviation
Figure 1.
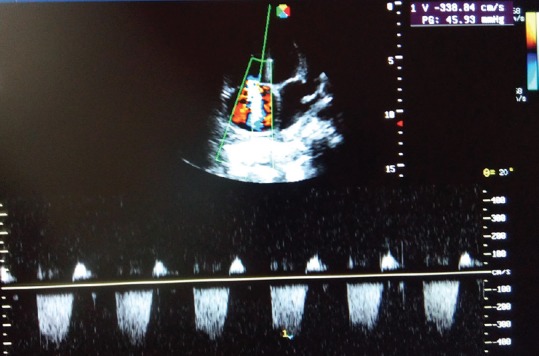
Elevated tricuspid regurgitant jet velocity
Patients with elevated mPAP had a lower eGFR (85.40 ± 59.0 ml/1.73 m2/min) compared with the controls (102.15 ± 57.9 ml/1.73 m2/min), but this difference was not significant (0.52). Although declining eGFR and lower TAPSE values were related with rising mPAP (r = −0.29 and − 0.45, respectively), these relationships were not statistically significant (P = 0.28 and 0.08, respectively).
Elevated mPAP was found to be more common in girls (66.7%) with CKD when compared with their male counterparts (33.3%) P < 0.001 [Table 2]. Participants with mPAP elevation did not differ with respect to duration since diagnosis of CKD (P = 1.00), but those with a history of having been dialyzed were less likely to have elevated mPAP (P < 0.001).
Table 2.
Participant characteristics and the presence or absence of pulmonary hypertension
| PH (n=9) | No PH (n=12) | P | |
|---|---|---|---|
| Gender (%) | <0.001* | ||
| Male | 5 (33.3) | 10 (66.7) | |
| Female | 4 (66.7) | 2 (33.3) | |
| Duration CKD (%) | 1.00 | ||
| <1 | 4 (44.4) | 5 (55.6) | |
| 1-2 | 3 (37.5) | 5 (62.5) | |
| >2 | 2 (50.0) | 2 (50.0) | |
| Ever dialyzed (%) | <0.001* | ||
| Yes | 0 (0.0) | 3 (100.0) | |
| No | 9 (50.0) | 9 (50.0) |
*Statistically significant. PH=Pulmonary hypertension, CKD=Chronic kidney disease
The prevalence of RV dysfunction in CKD was 9.5% and 0% in controls (P = 0.49). Right ventricular dysfunction was significantly more common in patients with elevated mPAP compared with those with normal mPAP (P < 0.001) [Table 3].
Table 3.
Relationship between impaired right ventricle function pulmonary hypertension among children with chronic kidney disease
| Impaired RV function (n=2) (%) | Normal RV function (n=19) (%) | P | |
|---|---|---|---|
| PH | <0.001* | ||
| Present | 1 (11.1) | 8 (88.9) | |
| Absent | 1 (8.3) | 11 (91.7) |
*Statistically significant. RV=Right ventricle, PH=Pulmonary hypertension
DISCUSSION
Children with CKD were found to have a significantly higher mPAP than controls, and the prevalence of elevated mPAP in the CKD patients in this study is 42.9% compared to 0% in the control group. Hence, mPAP elevation is present in more than two in every five children with CKD in the cohort studied and absent in the controls. It can be said that the prevalence of elevated mPAP in the present study falls within the reported range of 30%–60% from previous reports[10] and so reiterating the fact that mPAP elevation is common in children with CKD. This is alarming as significant PH is not only a relative contraindication to renal transplantation in patients with CKD but also has been associated with increased early allograft dysfunction and reduced patient survival in them.[20,21] PH could have multifactorial reasons; pulmonary artery blood flow, pulmonary vascular resistance, and pulmonary venous pressure which are determinants of pulmonary artery pressure are variably altered in CKD patients as a result of their hyperdynamic circulation and volume overload.[22,23,24,25,26] In these patients, their uremic state predisposes them to endothelial dysfunction and synthesis of vasoactive molecules such as endothelin-1 and asymmetric dimethylarginine which is an endogenous inhibitor of vasodilator effect of nitric oxide culminating in elevated pulmonary arterial pressure.[27]
We observed that mPAP elevation was found to be more common in girls with CKD than their male counterparts (P < 0.001). To the best of our knowledge, this is the first study suggesting a gender-specific risk for mPAP elevation in pediatric CKD patients as Kawar et al.[10] had earlier observed this unaddressed risk.
PH in children with CKD may remain asymptomatic and sometimes even misdiagnosed over a period of time until right ventricular dysfunction begins to manifest by worsening fatigue, dyspnea, and syncope.[4] We found that RV dysfunction as assessed by TAPSE was more common in patients with elevated mPAP compared with those with normal mPAP (P < 0.001). Raina[28] in Pittsburgh reported right ventricular dysfunction with TAPSE of 1.6 cm in an adult CKD patient. There is a dearth of reports on the occurrence of RV dysfunction in pediatric CKD patients with elevated mPAP; however, this unfortunate duo of PH and RV dysfunction would portend a dismal outcome in children with CKD.
We observed that children with CKD who had no history of hemodialysis were more likely to have elevated mPAP (P < 0.001). We note this observation with some caution as only three of our patients had hemodialysis, and two of these had only one session of it. Significantly, none of these had elevated mPAP. Di Lullo et al.[11] had earlier observed that single hemodialysis treatment does not have a pathological effect on pulmonary artery pressure values. Other researchers have, however, observed an alarmingly high occurrence of PH in patients with a history of hemodialysis with prevalence ranging from 30% to 58%.[29,30,31,32] Different criteria for PH definition, means of vascular access for hemodialysis, age, and sample size could account for this glaring disparity. Fabbian et al.[29] reported that 58.6% of their 29 adult CKD patients on hemodialysis all using arteriovenous fistula as vascular access had PH defined as systolic pulmonary arterial pressure >35 mmHg. Furthermore, Yigla et al.[33] who defined PH as systolic pulmonary arterial pressure >45 mmHg reported that of their adult CKD patients, 13.4% had PH before initiation of hemodialysis and 29% after initiation of hemodialysis using arteriovenous shunt as vascular access. All of our CKD patients who had hemodialysis had central venous catheter placed in the femoral vein as means of vascular access instead of an arteriovenous fistula. Beigi et al.[34] reported a positive correlation between mean fistula flow and PAP. It has been reported that PH improved after short arteriovenous fistula compression, indicating that both end-stage renal disease and arteriovenous fistula compression contribute to its pathogenesis.[35]
In summary, the prevalence of elevated mPAP in children with CKD in our center is 42.9%. Females were more likely to have mPAP elevation than the males, and those who had received hemodialysis treatment were more likely to have normal mPAP. Right ventricular dysfunction was more common in patients with mPAP than those with normal mPAP.
CONCLUSION
Our findings bring to the fore, a need for routine echocardiographic evaluation for elevated pulmonary pressure in pediatric CKD patients as this may remain asymptomatic till RV dysfunction supervenes. Larger collaborative studies comparing the prevalence of PH in CKD patients (in end-stage renal disease) on hemodialysis with various modes of vascular access with suggest the preferred mode.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Dento C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Rubin LJ. Pathology and pathophysiology of primary pulmonary hypertension. Am J Cardiol. 1995;75:51A–4A. doi: 10.1016/s0002-9149(99)80383-x. [DOI] [PubMed] [Google Scholar]
- 3.Pastan S, Bailey J. Dialysis therapy. N Engl J Med. 1998;338:1428–37. doi: 10.1056/NEJM199805143382006. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, et al. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 6.Forfia PR, Vachiéry JL. Echocardiography in pulmonary arterial hypertension. Am J Cardiol. 2012;110:16S–24S. doi: 10.1016/j.amjcard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, et al. Continuous wave Doppler determination of right ventricular pressure: A simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 8.Lanzarini L, Fontana A, Lucca E, Campana C, Klersy C. Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am Heart J. 2002;144:1087–94. doi: 10.1067/mhj.2002.126350. [DOI] [PubMed] [Google Scholar]
- 9.Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children, and adolescents: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22:715–9. doi: 10.1016/j.echo.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: Epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37:281–90. doi: 10.1159/000348804. [DOI] [PubMed] [Google Scholar]
- 11.Di Lullo L, Floccari F, Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c257–61. doi: 10.1159/000321867. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Kirpalani AL, Kulkarni A. Severe pulmonary hypertension in a young patient with end-stage renal disease on chronic haemodialysis. Ann Pediatr Cardiol. 2010;3:184–6. doi: 10.4103/0974-2069.74055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adiele DK, Okafor HU, Ojinnaka NC, Onwubere BJ, Odetunde OI, Uwaezuoke SN. Echocardiographic findings in children with chronic kidney disease as seen in the resource -limited setting. J Nephrol Ther. 2014;4:158–61. [Google Scholar]
- 14.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–153. [PubMed] [Google Scholar]
- 15.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohé C, Nickenig G, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: Results of the PEPPER-study. PLoS One. 2012;7:e35310. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feigenbaum H, Armstrong WF, Ryan T, editors. Hemodynamics. 6th ed. Lippincott Williams and Wilkins: Philadelphia; 2005. Feigenbaum's Echocardiography; pp. 215–46. [Google Scholar]
- 17.Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Hervé P, et al. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest. 2009;135:760–8. doi: 10.1378/chest.08-0904. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlotnick DM, Axelrod DA, Chobanian MC, Friedman S, Brown J, Catherwood E, et al. Non-invasive detection of pulmonary hypertension prior to renal transplantation is a predictor of increased risk for early graft dysfunction. Nephrol Dial Transplant. 2010;25:3090–6. doi: 10.1093/ndt/gfq141. [DOI] [PubMed] [Google Scholar]
- 21.Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG, et al. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation. 2008;86:1384–8. doi: 10.1097/TP.0b013e318188d640. [DOI] [PubMed] [Google Scholar]
- 22.McGoon MD. Murphy JG, Lloyd MA. 3rd ed. USA: Mayo Clinic Scientific Press and Informa Healthcare, Inc; 2007. Pulmonary hypertension; pp. 929–50. [Google Scholar]
- 23.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, et al. Pulmonary hypertension: Prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98:1805–11. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. 2013;84:682–92. doi: 10.1038/ki.2013.186. [DOI] [PubMed] [Google Scholar]
- 25.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai YC, Tsai JC, Chen SC, Chiu YW, Hwang SJ, Hung CC, et al. Association of fluid overload with kidney disease progression in advanced CKD: A prospective cohort study. Am J Kidney Dis. 2014;63:68–75. doi: 10.1053/j.ajkd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Reque J, Garcia-Prieto A, Linares T, Vega A, Abad S, Panizo N, et al. Pulmonary hypertension is associated with mortality and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2017;45:107–14. doi: 10.1159/000453047. [DOI] [PubMed] [Google Scholar]
- 28.Raina A. Pulmonary hypertension in patients with chronic kidney disease: Noninvasive strategies for patient phenotyping and risk assessment. Adv Pulm Hypertens. 2013;12:76–81. [Google Scholar]
- 29.Fabbian F, Cantelli S, Molino C, Pala M, Longhini C, Portaluppi F, et al. Pulmonary hypertension in dialysis patients: A cross-sectional Italian study. Int J Nephrol. 2010;2011:283475. doi: 10.4061/2011/283475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–82. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 31.Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: Prevalence and clinical significance. Int J Gen Med. 2010;3:279–86. doi: 10.2147/IJGM.S12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unal A, Tasdemir K, Oymak S, Duran M, Kocyigit I, Oguz F, et al. The long-term effects of arteriovenous fistula creation on the development of pulmonary hypertension in hemodialysis patients. Hemodial Int. 2010;14:398–402. doi: 10.1111/j.1542-4758.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 33.Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–75. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 34.Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–6. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 35.Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20:1686–92. doi: 10.1093/ndt/gfh840. [DOI] [PubMed] [Google Scholar]


