Abstract
Background:
Bicuspid aortic valve (BAV) is the most common congenital heart disease, affecting 0.5%–2% of the general population. It is associated not only with notable valvular risk (aortic stenosis and/or regurgitation, endocarditis) but also with aortopathy with a wide spectrum of unpredictable clinical presentations, including aneurysmal dilation of the aortic root and/or ascending thoracic aorta, isthmic coarctation, aortic dissection, or wall rupture.
Methods:
The REgistro della Valvola Aortica Bicuspide della Società Italiana di ECocardiografia e CArdiovascular Imaging is a retrospective (from January 1, 2010)/prospective, multicenter, observational registry, expected to enroll 3000 patients with definitive diagnosis of BAV made by transthoracic and/or transesophageal echocardiography, computed tomography, cardiovascular magnetic resonance, or at surgery. Inclusion criteria were definitive diagnosis of BAV. Patients will be enrolled regardless of the presence and severity of aortic valve dysfunction or aortic vessel disease and the coexistence of other congenital cardiovascular malformations. Exclusion criteria were uncertain BAV diagnosis, impossibility of obtaining informed consent, inability to carry out the follow-up. Anamnestic, demographic, clinical, and instrumental data collected both at first evaluation and during follow-up will be integrated into dedicated software. The aim is to derive a data set of unselected BAV patients with the main purpose of assessing the current clinical presentation, management, and outcomes of BAV.
Conclusions:
A multicenter registry covering a large population of BAV patients could have a profound impact on the understanding of the natural history of this disease and could influence its management.
Keywords: Aortic dilation, aortopathy, ascending thoracic aorta, bicuspid aortic valve, cardiovascular magnetic resonance, computed tomography, echocardiography
INTRODUCTION
Bicuspid aortic valve (BAV) is the most common congenital heart disease, affecting 0.5%–2% of the general population. It can be clinically silent, and it is often identified as an incidental finding in otherwise healthy and asymptomatic patients. However, it is often considered a serious condition with notable valvular risk, particularly of aortic valve endocarditis, frequent progression to aortic valve stenosis, especially in men, and frequent aortic regurgitation requiring aortic valve replacement. Furthermore, BAV is not only a peculiar valve morphology, but it also features a more complex disease defined as “bicuspid aortopathy,” characterized at an early stage by asymptomatic dilation of the different portions of the aorta, particularly of the ascending aorta, and later by frequent susceptibility to aortic aneurysm formation and to the most dreaded complication, aortic dissection.[1,2,3,4,5,6,7]
Early recognition and monitoring of an enlarged ascending thoracic aorta are crucial as it entails a high risk of dissection. BAV patients have a 25% risk of developing ascending aortic aneurysms 25 years after BAV diagnosis, with a risk of aortic dissection ranging from 0.03% to 0.1% per year. This risk may increase to 0.5% per patient-year in those with an aortic diameter of >45 mm, and the ascending aorta is the most common site of involvement (60%–70% of cases).[8]
In addition, BAV is frequently associated with other congenital heart defects and can present in BAV families as an autosomal dominant trait with variable penetrance. Noteworthy, valve anomalies and aortopathy may be inherited separately. BAV is also more common in the first-degree relatives of patients with congenital diseases of the left ventricular outflow tract (LVOT). Owing to genetic transmission, screening of the first-degree relatives is mandatory.[7]
It is estimated that about 25% and 5% of patients with BAV require aortic valve and aortic vessel surgery, respectively, in their lifetime. Of note, up to 50% of surgical interventions for aortic valve disease in adulthood are performed in patients with BAV.[9]
Valvular and vascular complications of BAV can virtually occur at any age during lifetime. Clinical presentation is extremely variable, including incidentally detected heart murmurs or incidental echocardiographic findings, thoracic aortic aneurysm, hospitalization for heart failure, as well as other life-threatening conditions (e.g., aortic dissection or endocarditis), and sudden death.
Distinctly different from the tricuspid aortic valve (TAV), the anatomy of BAV usually includes two unequal-sized cusps (due to fusion of two cusps resulting in one larger cusp) and the presence of a raphe at the site of fusion of the conjoined cusps. A minority of BAVs show two equally sized cusps without raphe (“pure” BAV). There are different classification schemes of BAVs with raphe, based either on the morphotype of the fused cusps (right and left coronary cusp fusion [type 1, the most common], right and noncoronary cusp fusion [type 2], left and noncoronary cusp fusion [type 3, the rarest variant]) or on the cusp orientation related to the position of the raphe. BAVs without raphe are classified according to the cusp orientation.[10,11]
Although the presence of two Valsalva sinuses has been recognized in the past as a hallmark of BAV, three Valsalva sinuses are frequently observed in BAV patients. Therefore, the true anatomical marker of BAV is currently considered the presence of a rudimentary intercuspal triangle or the absence of one of the three intercuspal triangles.[10,11,12,13,14]
Although up to date the clinical and prognostic significance of valve phenotype remains elusive, various data suggest a possible relationship between type of BAV morphology, valve dysfunction, and features of aortic dilation; hence, accurate phenotype valve characterization is strongly recommended.[15]
Diagnosis of BAV by common imaging techniques relies on a multimodality approach to assess valve morphology and function. The most widely used imaging technique is transthoracic echocardiography (TTE) with a sensitivity and specificity of >90% in BAV diagnosis; these values drop significantly in case of suboptimal image quality and/or calcification. In some patients, transesophageal echocardiography (TEE), cardiovascular magnetic resonance (CMR), or computed tomography (CT) may be necessary for precise evaluation of valve morphology and accurate assessment of each of the aortic vessel segments.[14,16,17]
Valve morphology, signs of degeneration, and valve function must be carefully evaluated during long-term follow-up.
An essential step in the morphologic characterization of the aortic valve is to assess the degree of valve degeneration.[18,19] An echocardiographic aortic valve degeneration score including variables such as calcification, thickening, and mobility reduction (0–3 to each, ranging 0–9 overall) was found to be a predictor of aortic valve surgery and cardiovascular events (cardiac death, heart failure, new cardiovascular symptoms, stroke, and endocarditis) in asymptomatic patients with normally functioning or slightly dysfunctional BAV during a 15-year follow-up.[20]
The current guidelines recommend the assessment of the entire thoracic aorta by multimodality imaging both at diagnosis and during follow-up, especially in patients with aortic diameters of ≥40 mm, aortic coarctation, Turner syndrome, or Loeys–Dietz syndrome.[21]
Although normal values of ascending aortic diameter according to age, body size, and gender have been reported in the literature, in clinical practice, a tubular ascending aorta of ≥37 mm or an aortic root of ≥40 mm is considered cut-off points for aortic dilation in adults.[21,22,23,24] Alternatively, an aortic diameter of >27.5 mm/m2 (aortic diameter in mm indexed to body surface area in m2) can be considered as cut-off point in patients of small size.[21,22,23,24]
If an aortic diameter of ≥ 40 mm is detected by echocardiography, aortic measurement should be confirmed by CT or CMR, and BAV patients should undergo annual follow-up regardless of the coexistence of valve disease.[21,22,23,24]
Both CT and CMR have superior spatial resolution compared with echocardiography, allowing assessment of aortic vessel size and morphology, regardless of its course in the thorax. Moreover, through “double-oblique” measurements, both these imaging modalities provide a cross-sectional diameter perpendicular to the longitudinal axis of the ascending aorta.[21,22,23,24]
Global cardiovascular risk should be estimated in every patient to implement a tailored treatment plan (i.e., beta-blockers).[21,22,23,24] Prophylactic surgery of isolated aneurysms seems reasonable when the aortic diameter reaches ≥55 mm or grows faster than 3 mm per year.[21,22,23,24] According to the 2017 European Society of Cardiology guidelines, surgery of the aortic root or tubular ascending aorta should be considered for BAV patients with a maximal ascending aortic diameter of ≥50 mm in the presence of additional risk factors (i.e., family history, systemic hypertension, or coarctation of the aorta), irrespective of the severity of aortic valve disease.[24]
BAV patients usually undergo aortic valve surgery at an earlier age than TAV patients, raising questions regarding the most appropriate prosthesis choice, need for anticoagulation, desire of pregnancy, type of job, and patient's lifestyle.
BAV syndrome is characterized by a wide clinical and functional spectrum with different prognoses, probably related to genetic and biomolecular heterogeneity.[25,26,27] Future studies should aim at identifying predictors of increased risk for developing aortic valvulopathy and vessel disease and integrating demographic, clinical, imaging, genetic, and biomolecular findings in large populations of BAV patients.
METHODS
Study design
The BAV registry of the Italian Society of Echocardiography and Cardiovascular Imaging (SIECVI) (REgistro della Valvola Aortica Bicuspide della Società Italiana di ECocardiografia e CArdiovascular Imaging – REBECCA) is a retrospective/prospective, multicenter, observational study that will enroll patients with a definitive diagnosis of BAV made by TTE and/or TEE, CT, CMR, or at surgery. For the retrospective enrollment, all patients with BAV identified by reviewing hospital discharge diagnosis records, and surgical, echocardiographic, CMR, or CT databases from January 1, 2010, will be included.
Study participation will neither interfere with the current guideline recommendations nor influence the common patient management or the therapeutic strategy. The study protocol was approved by the Ethics Committee of the Coordinating Center as well as by each enrolling site. Informed consent will be obtained from all patients.
Aims
The REBECCA registry aims to derive a clinical, echocardiographic, radiologic, and anatomic (intraoperative) data set of unselected patients with BAV, reaching the largest population ever reported on a national scale. The main purpose is to assess the current clinical presentation, management, and outcomes of BAV. Primary and secondary endpoints are as follows:
Primary endpoints
Cardiovascular and noncardiovascular mortality
Aortic valve and/or aortic surgery
Onset or worsening of cardiovascular symptoms
Hospitalization for heart failure or arrhythmias
Stroke
Endocarditis.
Related to:
Clinical features
Personal and family risk factors
BAV phenotype and valvular degeneration
Aortic vessel phenotype
Aortic valve function
Associated heart diseases.
Secondary endpoints
To assess the incidence of valvular and vascular (aortic) complications in different age groups (≤35, 36–65, 66–75, and ≥76 years)
To determine the most appropriate imaging technique according to the patient's clinical condition
To evaluate the efficacy of different imaging techniques in diagnosing BAV and detecting/monitoring associated complications
To compare the data obtained with those reported in the literature
To establish the incidence and progression of aortic dilation (maximum diameter of ≥1 segments of the proximal thoracic aorta, including aortic root and ascending aorta diameters >40 mm) and its complications both in patients with BAV and TAV to assess aortopathy evolution in these two populations.
Definition of bicuspid aortic valve
A congenital BAV has two functional cusps that are conjoined or failed to separate completely during embryonic development. The two cusps are arranged in either an anterior-posterior orientation with right-left commissures or a right-left orientation with anterior-posterior commissures, with the former arrangement reported in 50% of cases. Features including cusp-free edge length, cusp surface area, presence or absence of aortic sinuses, and presence or absence of the intercuspal triangle are helpful in distinguishing congenital from acquired BAVs and conjoint from nonconjoint cusps. In addition, the classical type of BAV is characterized by the presence of a raphe along the aortic aspect of the conjoined cusp. Usually, a right-left BAV has a raphe in the right cusp, with the left and right coronary arteries originating from each cusp separately. In anterior-posterior BAV, the raphe is in the anterior cusp with both the left and right coronary arteries originating from the front of the anterior cusp. The raphe may be so prominent that its free margin reaches the free edge of the cusp or may be fenestrated or partially developed (miniraphe).[28]
Inclusion criteria
Definitive diagnosis of BAV made at any age by TTE or TEE, CMR, CT, and/or intraoperatively in surgical patients. Patients will be enrolled regardless of the presence and severity of aortic valve dysfunction or aortic vessel disease and the coexistence of other congenital cardiovascular malformations.
Exclusion criteria
Uncertain BAV diagnosis
Impossibility of obtaining informed consent
Inability to carry out the follow-up.
Statistical analysis
Data will be described as mean and standard deviation (for normally distributed variables), median and interquartile range (for nonnormally distributed variables), or prevalence and percentage frequency (for categorical data). Survival and event rates will be determined with the Kaplan–Meier method and compared with log-rank test. For evaluation of prognostic factors, univariate and multivariate Cox regression models will be used to determine the impact of confounding variables (e.g. ejection fraction, comorbidities). Confidence intervals for each variable will be reported as appropriate. For each continuous variable (such as valvular degeneration score), receiver operating characteristic (ROC) analysis will be performed to identify retrospectively the optimal sensitivity and specificity cut-off values toward the prognostic standard. A P < 0.05 will be considered statistically significant. Statistical analysis will be performed with the software Statistical Package for the Social Sciences, v. 16 (SPSS Inc., Chicago, Illinois, USA).
Sample size calculation
According to the primary endpoints, sample size calculation was based on a previously published pilot study in over 200 patients with identical inclusion criteria, which demonstrated that patients with high aortic valve degeneration scores (valve fibrosis or calcification) have at least a two-fold increased risk of cardiovascular events at 5 years compared to patients with low or intermediate degeneration scores (7.5% vs. 3.0%).[20] A sample size of approximately 3000 patients (i.e., approximately 100 patients from 30 centers) was planned to achieve 80% power with an alpha error of 4% and a relative risk of 7.5% at 5-year follow-up in case of a high valve degeneration score.
Data collection
The diagnosis of BAV is established in the presence of an aortic valve with two cusps and two commissures. Echocardiography is the first-line imaging diagnostic tool for BAV detection. In patients with inadequate acoustic window, CMR or CT will be used to confirm the diagnosis or to provide a more comprehensive evaluation of the thoracic aorta. In patients undergoing aortic valve and/or aortic surgery, intraoperative diagnosis will be considered if provided.
Clinical and instrumental data will be entered into dedicated data collection software called REBECCA (1.0, 2016, designed in Italy under Windows XP environment) [Figure 1]. A central database will be managed under the direct responsibility of the SIECVI.
Figure 1.

Screenshot from REBECCA software with the classification of bicuspid aortic valve as reported by Schaefer et al.
Patient demographics and clinical variables (i.e. sex, age, weight, height, systolic and diastolic blood pressure, heart rate, and rhythm) will be obtained both at first evaluation and during follow-up.
Anamnestic data collection will include as follows: risk factors (hypertension, diabetes mellitus, dyslipidemia, and smoking), personal and family history of previous or current cardiovascular disease, congenital cardiovascular anomalies, and ongoing pharmacological therapy.
To detect the presence of valve malformations or thoracic aortic aneurysms/dilation, anamnestic and echocardiographic data will be obtained in at least one of the first-degree relatives of BAV patients, whenever possible.
Echocardiography
The diagnosis of BAV will be based on TTE images obtained in parasternal long- and short-axis views. In patients with nondiagnostic TTE, a TEE will be performed, with the assessment of aortic valve morphology in the mid-esophageal short-axis view (at 45°–60°).
Different valve phenotypes will be distinguished according to the classification proposed by Schaefer et al.:[11]
Type 1 with raphe: Right and left coronary cusp fusion (anterior-posterior cusp orientation) with the raphe in the anterior position
Type 1 without raphe: Anterior-posterior cusp orientation without evidence of a raphe
Type 2 with raphe: Right and noncoronary cusp fusion (right-left cusp orientation) with the raphe in the right position
Type 2 without raphe: Right-left cusp orientation without evidence of a raphe
Type 3 with raphe: Left and noncoronary cusp fusion (right-left cusp orientation) with the raphe in the left position
Type 3 without raphe: This is an extremely rare phenotype indistinguishable from type 2 without raphe and is not included in this classification [Figures 2-4].
Figure 2.
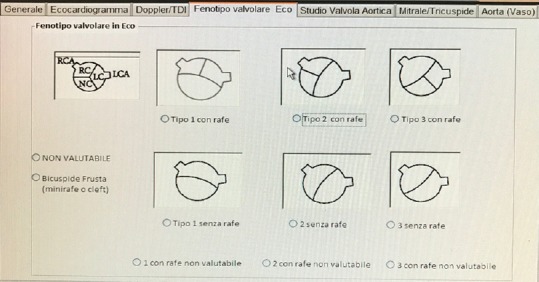
Screenshot from REBECCA software showing a window that includes data on morphologic and functional echocardiographic parameters of the aortic valve
Figure 4.
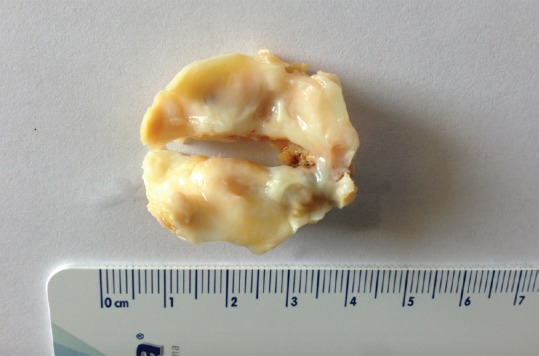
Surgically specimen of excised bicuspid aortic valve type 1
Figure 3.
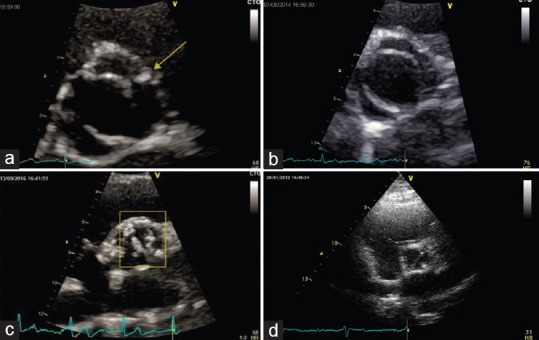
Echocardiographic images of different bicuspid aortic valve phenotypes. Panel A, type 1 (right and left coronary cusp fusion) with raphe; Panel B, type 1 (right and left coronary cusp fusion) without raphe; Panel C, type 2 (right and noncoronary cusp fusion) with raphe; Panel D, type 3 (left and noncoronary cusp fusion)
For data analysis, type 2 without raphe and type 3 without raphe will be considered as a single group.
Cusp orientation – either anterior-posterior or right-left – will be defined even if the presence of a raphe and its position cannot be ascertained.
Estimation of the presence and severity of aortic valve disease (stenosis and/or regurgitation) will be performed in accordance with the European Society of Cardiovascular Imaging (EACVI) recommendations.[29]
Valve function will be assessed using the Doppler technique. Aortic stenosis severity will be estimated based on the maximum and mean aortic gradient obtained by aortic transvalvular flow measurement by continuous wave (CW) Doppler recording in the apical five-chamber, apical long-axis, subcostal, and suprasternal views and in the modified right parasternal view for imaging the ascending aorta.
The aortic valve area will be calculated applying the continuity equation according to the standard criteria. The LVOT diameter will be measured in parasternal long-axis view at mid-systole using the inner edge-to-inner edge convention. The LVOT velocity–time integral will be measured by positioning the pulsed wave (PW) sample volume close to the aortic valve plane in the apical long-axis and apical five-chamber views. Mean and peak aortic valve gradients will be derived from the aortic velocity–time integral using CW Doppler. The criteria used for the definition of severe aortic stenosis include as follows: aortic valve area (AVA) <1 cm2 or AVA indexed for body surface area <0.6 cm2/m2; mean aortic gradient >40 mmHg in patients with normal cardiac output and normal transvalvular flow; maximum transvalvular velocity >4 m/s (to be interpreted according to heart rate, valid in patients in sinus rhythm); velocity ratio <0.25.
Aortic regurgitation severity will be assessed using color Doppler (mainly for vena contracta measurement), CW Doppler (for evaluation of pressure half-time and slope of the regurgitant velocity curve), and PW Doppler (diastolic flow reversal in the proximal descending aorta).
Although the proximal isovelocity surface area is a well-established method for assessing aortic regurgitation, this parameter is influenced by loading conditions and is not routinely used.
The definition of severe aortic regurgitation will be based on qualitative criteria, including valve morphology (abnormal/flail/coaptation defect), regurgitant flow at color Doppler (wide in central jets and variable in eccentric jets), strong signal of regurgitant jet at CW Doppler, and holodiastolic flow reversal in the descending aorta (end-diastolic volume >20 cm/s).
Other semi-quantitative parameters (i.e., vena contracta width >6 mm and pressure half-time <200 ms) will also be assessed according to the EACVI recommendations.[29]
The thoracic aorta will be assessed through the measurement of the anteroposterior diameters at aortic annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta in the longitudinal parasternal long-axis view, perpendicular to the longitudinal axis of the aortic lumen at end-diastole. In case of suboptimal visualization of the thoracic aorta with the standard approach, unconventional sections (i.e., modified off-axis left parasternal, right parasternal, apical, suprasternal, supraclavear, substernal, and subcostal views) will be used for the measurement of maximum vessel diameter.
The aortic diameter at the sinuses of Valsalva will be measured not only in a long-axis parasternal view but also in a short-axis orientation (with TEE) with cusp-to-cusp measurement, as recommended for radiological imaging.
The aortic arch diameter will be measured in the suprasternal view under clear visualization of the vessel segment between the origin of the innominate artery and the left subclavian artery.
The descending thoracic aorta diameter will be measured with TTE posteriorly to the left atrium in the modified apical two-chamber view or in dedicated modified views.
The abdominal aortic diameter will be measured distally to the origin of the renal arteries.
The aortic diameter will be obtained perpendicular to the long axis of the vessel, avoiding measurements in the oblique planes that would generate an overestimation of aortic dimensions. Measurements will be accomplished at end-diastole (or, alternatively, in the second half of diastole) using the leading edge-to-leading edge approach, in accordance with the latest guidelines.[30,31] The aortic annulus will be measured at mid-systole using the inner edge-to-inner edge convention.
A degeneration score will also be calculated according to the semi-quantitative estimation of:
Valve thickening
Valve calcification
Reduction in cusp movement.
Each of these three variables will be assigned a score of 0 (normal), 1 (mild), 2 (moderate), or 3 (severe). In particular, the distribution of valvular calcification will be classified as: 0 (absent); 1 (mild, 1 calcified nodule in a cusp); 2 (moderate, ≥2 nodules); and 3 (severe, diffuse calcification extended to almost all cusps).
A global score of aortic valve degeneration (0–9) will result from the sum of the scores of each component.
Cardiovascular magnetic resonance
CMR is a useful imaging tool for assessing aortic valve and aortic valve disease. This imaging modality can be used to define BAV morphology and motion, presence of a raphe, number and symmetry of the sinus of Valsalva, and functional data, including quantification of forward and reverse aortic flow and aortic valve planimetry. CMR allows measurement of all aortic segments and is useful during the follow-up of patients with aortic dilation as well as in both preoperative and postoperative evaluation. CMR can also be used to assess aortic complications (e.g., aneurysm, ulceration, and dissection) and to detect areas of wall thickening related to aortitis or intramural hematoma.
As for the CMR technique, a stack of contiguous, retrospectively gated, cine-steady-state free precession (SSFP) acquisitions in cross-section across the aortic root will be obtained following a plane derived from two complimentary views, the coronal localizer and the standard three-chamber view. Cine-SSFP images will be obtained during an end-expiratory breath-hold with an in-plane spatial resolution of 1.3 mm × 2.6 mm, a slice thickness of 5–6 mm, and a temporal resolution adjusted at <50 ms. Aortic valve morphology will be determined according to the classification system described by Schaefer et al.[11]
For each acquisition, the frames demonstrating maximal systolic and diastolic distension at the level of the sinuses of Valsalva will be visually selected and measured; in aortic roots with three distinct cusps, measurements will be drawn from one blood–wall interface to another, from cusp-to-opposite commissure (three cusp-commissure measurements: from right, left, and noncoronary cusps to respective opposite commissures), and from cusp-to-cusp (three cusp–cusp measurements: right-left coronary cusp, right-noncoronary cusp, and left-noncoronary cusp). In aortic roots with only two distinct cusps (“pure” BAVs), measurements will be limited to cusp–cusp and commissure–commissure dimensions only. The sequence “phase-contrast” imaging of the aortic valve, with an in-plane and through plane direction at the sinus of Valsalva, will be used to assess forward flow and to define stenotic and/or regurgitant valve. In addition, contrast-enhanced CMR angiography will be performed to provide a three-dimensional (3D) data set of the aorta and branch vessels.
Computed tomography
CT imaging of the chest will be performed using multislice dual-energy CT scanners. Automated detection of peak enhancement in the aortic root will be used to time the scan. All images will be acquired during an inspiratory breath-hold, with simultaneous recording of the patient's electrocardiogram. Acquisition will be centered at the 75% phase of the R-R cardiac cycle to ensure minimal motion artifacts. Axial data sets will be transferred to a workstation for subsequent evaluation. Maximal aortic diameter measurements at the sinuses of Valsalva, sinotubular junction, and ascending aorta will be obtained using the double-oblique short-axis technique. The three cusp-to-commissure and the three cusp-to-cusp diameters will be measured at the level of the aortic root and the two diameters (anteroposterior and laterolateral) at the rest of the aortic levels. Aortic diameters will be measured using the inner edge-to-inner edge and outer edge-to-outer edge conventions. To better assess the ascending aorta caliber, a second measurement 90° perpendicular to the measurement of maximum diameter will be made on the short-axis image. Therefore, two measurements of the ascending aorta will be obtained: maximum identified diameter and its perpendicular diameter. Measurements will be made according to the current American College of Cardiology/American Heart Association and American Society of Echocardiography recommendations.[21,31]
To evaluate interobserver agreement, measurements will be repeated by a second expert reader on a randomly selected subset of patients.
Aortic elasticity
In BAV patients, the assessment of aortic elasticity could be useful although the clinical relevance of this parameter is still controversial. For this reason, aortic stiffness by multimodality imaging (CT, MRI, M-mode echo, and 2D strain) should be optionally performed.[32]
Quality control of enrolling centers
Quality control of diagnostic performance among the enrolling centers is of critical importance to acquire meaningful information into the data bank and to reduce interobserver variability. Quality control will be performed based upon two criteria, each one having to be met to fulfill the quality control requirements. The first criterion is passing the test prepared by the steering committee and available on https://www.siec.it/ricerca/test-per-laccreditamento-allo-studio-rebecca/, which includes 12 questions concerning BAV phenotype definition and methods of aorta measurement, to be completed by the principal local investigator of each participating center providing at least 10 correct answers. The second criterion consists, in random sampling, of 10 consecutive studies from each contributing center. These 10 studies will be examined in a blinded fashion by two experienced cardiologists–echocardiographists – members of the steering committee – whose reading is arbitrarily assumed to be the “gold standard.” It is assumed a priori that the minimum threshold of concordance to pass the quality control should be 80%.
DISCUSSION
Owing to the high incidence of valve dysfunction and thoracic aorta complications often requiring surgical intervention, BAV should be considered as a valvulo-aortopathy with a wide spectrum of unpredictable clinical presentations.[33] The issues that need to be clarified concern both phenotypical and genetic aspects.
The high incidence of aortic valve dysfunction in BAV patients compared with TAV patients has been well demonstrated. However, the genetic and mechanical mechanisms underlying the progression of BAV stenosis and/or regurgitation are still unknown. In addition, BAV is frequently associated with aneurysmal dilation of the aortic root and/or ascending thoracic aorta or aortic isthmic coarctation. Other serious complications such as aortic dissection or wall rupture can also occur. These associations are likely to be framed in a complex disorder of cardiac development, but not limited to the aortic valve and probably related to genetic susceptibility. As for the genetic aspects, mutations in NOTCH1 and GATA5 have been identified in different clusters of BAV patients, but the genetic causes and their potential clinical implications remain largely unknown and need to be further investigated.[33]
The initial diagnosis of bicuspid aortopathy is likely to be made using TTE. Visualization of the mid-distal ascending aorta and aortic arch may be difficult in adults, in whom either CT or CMR should be considered. If CT or CMR is contraindicated, then a TEE study should be obtained. CMR is preferable to CT for serial surveillance as it is not associated with radiation exposure.
Yearly echocardiographic surveillance is recommended in patients with an aortic root or ascending aorta diameter of >40 mm, as assessed by TTE, and no concomitant indication for valve replacement or repair. In patients with an aortic root or ascending aorta diameter of 40–44 mm and no concomitant valvular indication for intervention, a baseline CT or CMR scan may be obtained. If ongoing imaging surveillance, by means of echocardiography, CT, or CMR, reveals a growth rate exceeding 5 mm per year, surgical management may be considered. In patients with an aortic root or ascending aorta diameter of 45–49 mm, a CT or CMR scan should be obtained.[34] A predominant sinus of Valsalva dilation phenotype has been demonstrated to be mainly associated with type 1 (right-left cusp fusion) BAV morphology[11] and male sex.[35] This root phenotype has been linked with faster ascending aorta dilation and aortic regurgitation,[36] implicating a higher risk for acute aortic syndromes in this limited BAV subgroup.[37]
Only a few published studies have estimated the rate of progressive aortic dilation in adults with BAV with a long follow-up period [Table 1].[8,20,38,39,40,41,42,43,44,45,46,47,48,49,50,51] Hence, further evidence is needed to confirm the relation between BAV and aortic phenotypes, with the aim to predict disease progression, to refine patient stratification, and to offer an appropriately timed surgical approach.
Table 1.
Published studies with a long follow-up period that evaluated the rates of progressive aortic dilation in adults with bicuspid aortic valve
| Authors | Endpoint | Number of patients | Follow-up (years) | Age (years) | Mortality | AS (%) | AR (%) | Ao dilation (>45 mm) | Aortic dissection (%) | Surgery (%) | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Morosin et al.[38] | Predictive model of outcome of patients with BAV | 337 | 8-21 | 29.2±19.8 | 0.1% per patient per year | 7.4 | 21.6 | 18.4 | 1.2 | 45.2 | Hypertension, wider Ao diameter, moderate-to-severe AR and AS were independently correlated with AVR, aortic surgery and death |
| Michelena et al.[39] | Sex-related differences in morbidity and survival in BAV | 2242 | 4-14 | 51±16 | 16% at 9 years | 12 | 8 | 27 | 24 | BAV-related morbidity, AR, and endocarditis are more prevalent in men in the community, women exhibit a significantly higher relative risk of death in the surgical group | |
| Kinoshita et al.[40] | Assessment of risk factors for Ao dilation over time in BAV patients undergoing AVR, focusing on valve phenotype | 167 | 5 | 64±12 | 4.1% at 5 years | 29 | 24 | 39 | 24 | The presence of AR and ascending Ao diameter >40 mm at the time of surgery emerged as significant predictors of Ao dilation after AVR but valve fusion phenotype was not | |
| Rodrigues et al.[41] | Incidence and predictors of cardiac events in adults with BAV | 227 | 13 | 28±14 | 3.1% at 13 years | 34 | 35 | 12.3 | 0.9 | 33 | Long-term survival was excellent. Baseline AV calcification and dysfunction were the only independent predictors of frequent cardiovascular events |
| Masri et al.[42] | Impact of surgical intervention on long-term outcome in BAV with complications | 1890 | 4 | 50±14 | 9% at years | 17 | 31 | 35 | 0.4 | 49 | Patients with BAV have a high prevalence of AV dysfunction and concomitant aortopathy. Undergoing surgery was associated with a significantly lower incidence of death and/or dissection |
| Matsuyama et al.[43] | Long-term results after treatment of the ascending Ao for BAV | 145 | 1975-2016 | 59.3±1.5 | 20% at 10 years 54% at 20 years | 0.7 | 25 | No surgical treatment for ascending Ao 40 mm, an artificial graft if 40-50 mm, AVR if >50 mm. Hospital mortality was 1.4%. There were no significant differences among groups | |||
| Itagaki et al.[44] | Long-term risk for aortic complications after AVR in patients with BAV versus Marfan syndrome undergoing AVR | 2079 with BAV, 73 with Marfan syndrome | 1995-2010 | 69.5±13.0 | 28% at 15 years | 0.5 | 2.5 for only aortic surgery | Patients with Marfan syndrome were significantly more likely to undergo thoracic aortic surgery in late follow-up | |||
| Svensson et al.[45] | Long-term durability of BAV repair | 728 | 1985-2011 | 42±12 | 18% at 20 years | 9 | 85 | 38 | 42 | Freedom from AV reoperation at 10 years was 78%. Risk of reoperation 2.6% per year up to 15 years. Primary reasons for reoperation were cusp prolapse (38%), AS or AR (17%) and AR from root aneurysm (15%) | |
| Girdauskas et al.[46] | Aortic events after isolated AVR for BAV versus TAV stenosis with concomitant ascending Ao dilation | 325 | 15 | 59.5±10 | 22% at 15 years | 47 | 39 | 0 | 3 for only aortic surgery | Patients with BAV and TAV stenosis with concomitant ascending Ao dilation are at comparably low risk of adverse aortic events 15 years after isolated AVR | |
| Michelena et al.[8] | Incidence of aortic complications in BAV patients | 416 | 16±7 | 35±21 | 20% at 25 years | 61 | 29 | 26 | 0.5 | 53 | In BAV patients, the incidence of aortic dissection over a mean follow-up of 16 years was low but significantly higher than in the general population |
| McKellar et al.[47] | Long-term risk of aortic events following AVR in BAV patients | 1286 | 12±7 | 58±14 | 48% at 15 years | 10 | 1 | 1 | Despite a true risk for aortic events after AVR for BAV, the occurrence of aortic dissection was low | ||
| Tzemos et al.[48] | Frequency and predictors of cardiac outcomes in a large consecutive series of adults with BAV | 642 | 9±5 | 35±16 | 4% at 10 years | 61 | 27 | 45 | 1 | 28 | Age, severity of AS, and severity of AR were independently associated with primary cardiac events. Over a mean follow-up of 9 years, survival rates were not lower than for the general population |
| Michelena et al.[39] | Clinical outcome of patients diagnosed with normal or mildly dysfunctional BAV | 212 | 15±6 | 32±20 | 10% at 20 years | 67 | 15 | 39 | 0 | 29 | In the community, asymptomatic patients with BAV and no or minimal hemodynamic abnormality, enjoy excellent long-term survival but incur frequent cardiovascular events |
| Davies et al.[49] | Natural history of ascending Ao aneurysms in the setting of an unreplaced BAV | 79 | 5 | 48 | 9% at 5 years | 9 | 68 | AS presents a significant added risk for patients with aneurysmal disease in the face of BAV. Despite faster rates of growth, patients with BAV have similar rates of aortic rupture, dissection, and death and improved long-term survival | |||
| Borger et al.[50] | Optimal diameter at which replacement of the ascending Ao should be performed in BAV patients | 201 | 10±4 | 56±15 | 33% at 15 years | 9 | 0 | 9 | Patients undergoing operations for BAV disease should be considered for concomitant replacement of the ascending Ao if the diameter is ≥45 mm | ||
| Russo et al.[51] | Long-term changes in the ascending Ao after AVR in patients with BAV and TAV | 50 | 20±2 | 51±12 | 60% at 15 years | 10 at 20 years |
6 for only aortic surgery | Prophylactic replacement of mildly enlarged ascending Ao is recommended in patients with BAV at the time of AVR |
Ao=Aorta, AR=Aortic regurgitation, AS=Aortic stenosis, AV=Aortic valve, AVR=Aortic valve replacement, BAV=Bicuspid aortic valve, TAV=Tricuspid aortic valve
A multicenter registry covering a large population of BAV patients could have a profound impact on the understanding of this disease, filling the knowledge gaps regarding the natural history of BAV aortopathy according to valve morphology, prognostic significance of aortic phenotypes, and indications for aortic valve and/or aortic surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 2.Braverman AC. Aortic involvement in patients with a bicuspid aortic valve. Heart. 2011;97:506–13. doi: 10.1136/hrt.2009.183871. [DOI] [PubMed] [Google Scholar]
- 3.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–5. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: Pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–90. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 5.Cecconi M, Nistri S, Quarti A, Manfrin M, Colonna PL, Molini E, et al. Aortic dilatation in patients with bicuspid aortic valve. J Cardiovasc Med (Hagerstown) 2006;7:11–20. doi: 10.2459/01.JCM.0000199777.85343.ec. [DOI] [PubMed] [Google Scholar]
- 6.Lewin MB, Otto CM. The bicuspid aortic valve: Adverse outcomes from infancy to old age. Circulation. 2005;111:832–4. doi: 10.1161/01.CIR.0000157137.59691.0B. [DOI] [PubMed] [Google Scholar]
- 7.Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC, et al. Familial thoracic aortic dilation and bicommissural aortic valve: A prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143A:1960–7. doi: 10.1002/ajmg.a.31872. [DOI] [PubMed] [Google Scholar]
- 8.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 9.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: A surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74:14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: An integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–8. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 12.Angelini A, Ho SY, Anderson RH, Devine WA, Zuberbuhler JR, Becker AE, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J Thorac Cardiovasc Surg. 1989;98:362–7. [PubMed] [Google Scholar]
- 13.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 14.Brandenburg RO, Jr, Tajik AJ, Edwards WD, Reeder GS, Shub C, Seward JB. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: Echocardiographic-anatomic correlation in 115 patients. Am J Cardiol. 1983;51:1469–73. doi: 10.1016/0002-9149(83)90659-8. [DOI] [PubMed] [Google Scholar]
- 15.Conti CA, Della Corte A, Votta E, Del Viscovo L, Bancone C, De Santo LS, et al. Biomechanical implications of the congenital bicuspid aortic valve: A finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. 2010;140:890. doi: 10.1016/j.jtcvs.2010.01.016. 896e1-2. [DOI] [PubMed] [Google Scholar]
- 16.Alkadhi H, Leschka S, Trindade PT, Feuchtner G, Stolzmann P, Plass A, et al. Cardiac CT for the differentiation of bicuspid and tricuspid aortic valves: Comparison with echocardiography and surgery. AJR Am J Roentgenol. 2010;195:900–8. doi: 10.2214/AJR.09.3813. [DOI] [PubMed] [Google Scholar]
- 17.Buchner S, Hülsmann M, Poschenrieder F, Hamer OW, Fellner C, Kobuch R, et al. Variable phenotypes of bicuspid aortic valve disease: Classification by cardiovascular magnetic resonance. Heart. 2010;96:1233–40. doi: 10.1136/hrt.2009.186254. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu P, Bossé Y, Huggins GS, Della Corte A, Pibarot P, Michelena HI, et al. The pathology and pathobiology of bicuspid aortic valve: State of the art and novel research perspectives. J Pathol Clin Res. 2015;1:195–206. doi: 10.1002/cjp2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Corte A, Body SC, Booher AM, Schaefers HJ, Milewski RK, Michelena HI, et al. Surgical treatment of bicuspid aortic valve disease: Knowledge gaps and research perspectives. J Thorac Cardiovasc Surg. 2014;147:1749. doi: 10.1016/j.jtcvs.2014.01.021. 1757.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–84. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE., Jr 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/AMERICAN Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–88. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 25.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G, et al. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nistri S, Sorbo MD, Basso C, Thiene G. Bicuspid aortic valve: Abnormal aortic elastic properties. J Heart Valve Dis. 2002;11:369–73. [PubMed] [Google Scholar]
- 27.Fedak PW. Bicuspid aortic valve syndrome: Heterogeneous but predictable? Eur Heart J. 2008;29:432–3. doi: 10.1093/eurheartj/ehm609. [DOI] [PubMed] [Google Scholar]
- 28.Fyler DC. Philadelphia: Hanley & Belfus, Inc; 1992. Nada'pediatric cardiology. In: Aortic Outflow Abnormalitis; pp. 493–512. [Google Scholar]
- 29.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–44. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, et al. Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–82. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Longobardo L, Carerj ML, Pizzino G, Bitto A, Piccione MC, Zucco M, et al. Impairment of elastic properties of the aorta in bicuspid aortic valve: Relationship between biomolecular and aortic strain patterns. Eur Heart J Cardiovasc Imaging. 2017 doi: 10.1093/ehjci/jex224. [DOI] [PubMed] [Google Scholar]
- 33.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (BAVCon) Circulation. 2014;129:2691–704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–9. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 35.Detaint D, Michelena HI, Nkomo VT, Vahanian A, Jondeau G, Sarano ME, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: A comparative study with Marfan syndrome and degenerative aortopathy. Heart. 2014;100:126–34. doi: 10.1136/heartjnl-2013-304920. [DOI] [PubMed] [Google Scholar]
- 36.Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1301–10. doi: 10.1016/j.jcmg.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T, et al. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–7. doi: 10.1093/ejcts/ezs137. [DOI] [PubMed] [Google Scholar]
- 38.Morosin M, Leonelli V, Piazza R, Cassin M, Neglia L, Leiballi E, et al. Clinical and echocardiographic predictors of long-term outcome of a large cohort of patients with bicuspid aortic valve. J Cardiovasc Med (Hagerstown) 2017;18:74–82. doi: 10.2459/JCM.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 39.Michelena HI, Suri RM, Katan O, Eleid MF, Clavel MA, Maurer MJ, et al. Sex differences and survival in adults with bicuspid aortic valves: Verification in 3 contemporary echocardiographic cohorts. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004211. pii: e004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinoshita T, Naito S, Suzuki T, Asai T. Valve phenotype and risk factors of aortic dilatation after aortic valve replacement in Japanese patients with bicuspid aortic valve. Circ J. 2016;80:1356–61. doi: 10.1253/circj.CJ-15-1378. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues I, Agapito AF, de Sousa L, Oliveira JA, Branco LM, Galrinho A, et al. Bicuspid aortic valve outcomes. Cardiol Young. 2017;27:518–29. doi: 10.1017/S1047951116002560. [DOI] [PubMed] [Google Scholar]
- 42.Masri A, Kalahasti V, Alkharabsheh S, Svensson LG, Sabik JF, Roselli EE, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2016;151:1650–90. doi: 10.1016/j.jtcvs.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama S, Nishida T, Ushijima T, Tominaga R. Long-term results after treatment of the ascending aorta for bicuspid aortic valve patients. Surg Today. 2016;46:729–34. doi: 10.1007/s00595-015-1274-4. [DOI] [PubMed] [Google Scholar]
- 44.Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus Marfan syndrome. J Am Coll Cardiol. 2015;65:2363–9. doi: 10.1016/j.jacc.2015.03.575. [DOI] [PubMed] [Google Scholar]
- 45.Svensson LG, Al Kindi AH, Vivacqua A, Pettersson GB, Gillinov AM, Mihaljevic T, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg. 2014;97:1539–47. doi: 10.1016/j.athoracsur.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 46.Girdauskas E, Disha K, Borger MA, Kuntze T. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2014;147:276–82. doi: 10.1016/j.jtcvs.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 47.McKellar SH, Michelena HI, Li Z, Schaff HV, Sundt TM., 3rd Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol. 2010;106:1626–33. doi: 10.1016/j.amjcard.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 48.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 49.Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM, Jr, Elefteriades JA. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–44. doi: 10.1016/j.athoracsur.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 50.Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–83. doi: 10.1016/j.jtcvs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Russo CF, Mazzetti S, Garatti A, Ribera E, Milazzo A, Bruschi G, et al. Aortic complications after bicuspid aortic valve replacement: Long-term results. Ann Thorac Surg. 2002;74:S1773–6. doi: 10.1016/s0003-4975(02)04261-3. [DOI] [PubMed] [Google Scholar]


