Abstract
Aims
To investigate cellular changes in the spinal trigeminal nucleus (STN) and trigeminal ganglion associated with trigeminal nociception mediated by inflammation in the temporomandibular joint (TMJ) capsule.
Methods
Sprague Dawley rats (n=86) were utilized to investigate cellular and behavioral responses to prolonged TMJ inflammation caused by bilateral injection of complete Freund’s adjuvant (CFA) in the capsule. To investigate the cellular effects of protein kinase A (PKA) in the STN, rats were injected intrathecally with the selective PKA inhibitor KT5720 prior to injection of CFA into both TMJ capsules. Levels of calcitonin gene-related peptide (CGRP), active PKA, and ionized calcium-binding adapter molecule 1(Iba1) in the STN and expression of phosphorylated ERK (p-ERK) in the trigeminal ganglion were determined by immunohistochemistry (n ≥ 3). Nocifensive head withdrawal responses to mechanical stimulation of the cutaneous tissue over the TMJ were monitored following CFA injection in the absence or presence of KT5720 (n = 7). Statistical analysis was performed using parametric ANOVA tests.
Results
Intrathecal injection of KT5720 significantly inhibited the stimulatory effect of CFA on levels of CGRP, PKA, and the microglial protein Iba1 in the STN. In addition, administration of KT5720 decreased the average number of CFA-induced nocifensive withdrawal responses to mechanical stimulation and the CFA-mediated increase in p-ERK expression in the ganglion.
Conclusion
These findings provide evidence that elevated PKA activity in the STN promotes cellular events temporally associated with trigeminal nociception caused by prolonged TMJ inflammation.
Keywords: TMJ, calcitonin gene-related peptide, protein kinase A, trigeminal ganglion, nociception
Temporomandibular disorders (TMD), which affect between 5–12% of the adult population, are characterized by pain or tenderness in temporomandibular joint (TMJ) and muscles after or during mastication, jaw clicking, limited movement, tinnitus, and headache/migraine (1–4). TMD is at least twice as prevalent in women as in men with the incidence of pain highest during adolescence (5), and individuals often exhibit increased sensitivity to other experimentally induced pains (6, 7). The pathological pain associated with TMD involves activation of trigeminal ganglion nerves, which provide sensory innervation of the head and face and relay nociceptive signals to the spinal trigeminal nucleus (STN) (8–10).
Peripheral and central sensitization of trigeminal nociceptive neurons is associated with the pathology of prevalent and debilitating orofacial pain conditions including TMD (10). Following peripheral activation of trigeminal nerves in response to tissue injury, the neuropeptide calcitonin gene-related peptide (CGRP) and other inflammatory mediators can cellular changes in second order neurons and glial cells involved in the initiation and maintenance of central sensitization and persistent pain (11, 12). Elevated CGRP levels in the spinal cord are implicated in the development of central sensitization by mediating changes in the expression of ion channels, receptors, and inflammatory genes in second order neurons and glial cells including astrocytes and microglia. Activation of astrocytes and microglia, which results in a prolonged inflammatory response, facilitates sustained central sensitization and promotes a pathological pain state (13–15).
CGRP is involved in the initiation and maintenance of central sensitization via activation of CGRP receptors that are localized on secondary neurons and glial cells within the spinal cord (16–18). Based on prior studies, activation of CGRP receptors in neurons and glial cells would likely lead to an increase in intracellular levels of the secondary messenger cAMP that binds to and stimulates activation of protein kinase A (PKA) (19–22). The signaling protein PKA induces expression of pro-inflammatory genes including cytokines that are involved in sustaining a sensitized state of second order neurons (23). Elevated PKA activity in the cytosol is correlated with sensitization and activation of nociceptive neurons and glial cells via modulation of receptor expression and ion channel activity (11, 24). CGRP is also known to cause activation of the mitogen activated protein (MAP) kinases including p38, c-Jun kinase (JNK), and extracellular regulated kinase (ERK) in trigeminal neurons and glia (25, 26), that facilitate an inflammatory response in the ganglion associated with sensitization of trigeminal neurons (27). Similar to PKA, increased expression of these signaling proteins leads to a prolonged state of sensitization via modulation of ion channels, receptors, and transcription factors. Given the importance of the CGRP/PKA pathway in promoting increased neuron-glia communication, a major goal of this study was to better understand the role of these cellular events implicated in peripheral and central sensitization of trigeminal nociceptive neurons in TMD pathology.
Several animal models of orofacial pain have been developed that mimic certain aspects of TMD to study the mechanisms involved in promoting prolonged trigeminal nociceptor sensitization and inflammation by injection of inflammatory agents into the joint capsule. Capsaicin is often used to induce a transient state of sensitization characterized by hyperalgesia that last about 24 hours and fully resolves within 2 to 3 days. A more chronic sensitization state can be established using complete Freund’s adjuvant (CFA), which is an emulsion of heat-killed Mycobacterium tuberculosis. Findings from previous studies have shown that CFA can cause sustained induction and maintenance of peripheral and central sensitization for more than 14 days post-injection (28–30). The aim of this study was to investigate the role of CGRP and PKA in promoting cellular changes in the STN and trigeminal ganglion, and nociceptive response to mechanical stimulation in the CFA model of TMD. Results provide evidence that PKA signaling in the STN is involved in initiating and maintaining an elevated state trigeminal nociceptor sensitivity, which is characteristic of human chronic TMD.
Materials and Methods
Animals
A total of 86 adult, male Sprague-Dawley rats (350–500 g) were obtained from Charles River Laboratories Inc. (Wilmington, MA) or purchased from Missouri State University (internal breeding colonies). Upon arrival, animals were acclimated to the environment for a minimum of 1 week prior to use. Animals were housed individually in clean, standard plastic rat cages (VWR, Radnor, PA) with unrestricted access to both food and water in a room with 12 hour/light dark cycles. All protocols were approved by Missouri State University’s Institutional Animal Care and Use Committee and conducted in compliance with all established guidelines in the Animal Welfare Act of 2007, National Institutes of Health, and ARRIVE Guidelines. Concerted efforts were made to minimize suffering, as well as the number of animals used in this study.
Reagents
Complete Freund’s adjuvant (CFA, Sigma-Aldrich, St. Louis, MO) was prepared as a 1:1 emulsion in 0.9% saline solution immediately prior to use. The selective signaling inhibitor of PKA, KT5720 (Tocris, Bristol, UK) was prepared at a stock concentration of 1 mM in DMSO (Sigma-Aldrich) and frozen in 5 μl aliquots at −20°C. On the day of the experiment, a fresh 500 nM solution of KT5720 was prepared via dilution in 0.9% saline.
Immunohistochemistry
To investigate changes in protein expression, immunohistochemical analysis of spinal cord and trigeminal ganglion tissue was performed as described previously (28, 31). Animals were anesthetized by inhalation of 5% isoflurane. To promote inflammation in the TMJ, 50 μL of CFA (1:1 CFA/0.9% saline emulsion,) was injected into each capsule using a 26 ½ gauge needle (Becton Dickinson, Franklin Lakes, NJ) and a 50 μL Hamilton syringe (Hamilton Company, Reno, NV). Some animals were injected intrathecally between the occipital bone and the first cervical vertebrae (C1) with 20 μl of KT5720 (500 nM) or a 0.9% saline solution immediately prior to bilateral TMJ injections. The naïve controls received no treatment. Immunohistochemical data were obtained from 13 CFA only animals, 9 CFA and KT5720 animals, 10 saline animals, 9 KT5720 animals, and 15 naïve control animals.
The brain stem and upper spinal cord (6 mm posterior to the obex) were removed at 2 hours, 7 days, or 21 days after injection. Right and left trigeminal ganglion tissues were also acquired through cranial dissection 2 hours post CFA injection. Immediately after removal, tissues were incubated in 4% paraformaldehyde at 4°C overnight and then washed in 1X Phosphate Buffered Saline (PBS) before being placed in 12.5% sucrose for one hour at 4°C and then in 25% sucrose at 4°C overnight. At this point they were removed from sucrose and stored at −20°C. Before sectioning, tissues were embedded in Optimal Cutting Temperature compound (OCT; Sakura Finetek, Torrance, CA). Transverse sections 14 μm thick were taken between 4 and 5 mm caudal to the obex of the STN with a cryostat set to −24°C. Transverse sections 14 μm thick were taken from the middle of the trigeminal ganglion tissue which contains all three branches of the ganglion. Sections were placed on Superfrost Plus microscope slides (Fisher Scientific, Pittsburg, PA) with the caudal side facing down and stored at −20°C.
Slides with sectioned tissues were rehydrated by incubation in PBS for 5 minutes, then blocked and permeabilized in a solution of 0.1% Triton X-100 in 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) for 20 minutes at room temperature. Primary antibodies were prepared according the manufacturer’s recommended dilutions (Table 1) in 5% donkey serum. Slides were thoroughly rinsed with PBS and the primary antibodies were prepared and incubated for either 3 hours at room temperature or overnight at 4°C in a humidified chamber. Next, slides were incubated for one hour at room temperature with Donkey Alexa-fluor conjugated secondary antibodies diluted in PBS. Vectashield medium (H-1200, Vector Laboratories Inc, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole was used to mount the tissue sections and visualize cell nuclei using fluorescent microscopy. A Zeiss Axiocam mRm camera (Carl Zeiss Microscopy, Thornwood, NY) mounted on a Zeiss Imager Z2 fluorescent microscope equipped with an ApoTome was used to collect 100× images of the outer lamina of the STN or the V3 branch of the trigeminal ganglia. Image acquisition was performed using Zeiss Zen 2012 software. No specific immunostaining pattern was observed when the protocol was conducted in the absence of primary antibodies, thus providing evidence of the specificity of the fluorescently-conjugated antibodies.
Table 1.
Summary of antibodies and conditions used for immunohistochemistry.
| Protein | Dilution | Incubation time | Incubation Temperature | Company | Location |
|---|---|---|---|---|---|
| CGRP | 1:1000 | 3 Hours | 20–22°C | Sigma-Aldrich, Inc. | St. Louis, MO |
| Iba-1 | 1:400 | 3 Hours | 20–22°C | Wako Chemicals USA, Inc. | Richmond, VA |
| NeuN | 1:1000 | 3 Hours | 20–22°C | EMD Millipore, Corp. | Temecula, CA |
| p-ERK | 1:500 | OVN | 4°C | Bioworld Technology, Inc. | St. Louis Park, MN |
| Active PKA | 1:500 | 3 Hours | 20–22°C | Abcam, Inc. | Cambridge, MA |
| Alexa-Fluor 488 | 1:200 | 1 Hour | 20–22°C | Jackson Immuno Research, Inc. | West Grove, PA |
| Alexa-Fluor 568 | 1:200 | 1 Hour | 20–22°C | Life Technologies | Grand Island, NY |
| Alexa-Fluor 647 | 1:200 | 1 Hour | 20–22°C | Life Technologies | Grand Island, NY |
Nocifensive Response to Mechanical Stimulation
Mechanical nociception was evaluated using calibrated von Frey filaments (Ugo Basile, Varese Lakes, Italy). All behavioral procedures were conducted between the hours of 7 A.M. and 11 A.M. Behavioral assessments were performed as described in previously published studies in the laboratory using the Durham Animal Holder (Ugo Basile) (28, 31, 32). The animals were gently guided into the Durham holding device and secured using a plastic blockade inserted behind the hind paws. To minimize false responses during von Frey filament testing, a pipette tip was used to touch the animal’s head and face to acclimate the rats to having the cutaneous tissue over the TMJ capsule touched with a filament. This was done for the three consecutive days prior to testing with von Frey filaments.
Following the acclimation period, basal response levels to a series of calibrated von Frey filaments (North Coast Medical, Inc., Gilroy, CA; 26, 60, 100, 180, grams) were determined 24 hours prior to TMD procedures through application to the cutaneous tissue over the TMJ capsule. A scientist blinded to the experimental conditions applied the filaments bilaterally in order of increasing pressure with 5 applications per filament. A positive response was recorded and verified by another scientist when the animal visibly flinched away from the filament prior to it bending. The day after establishing mechanical sensitivity baselines, the animals were anesthetized and treated as described for the immunohistochemistry studies. The nocifensive head withdrawal response to mechanical stimulation was determined at 2 hours, 1 day, 5 days, 7 days, 14 days, and 21 days following treatments. Behavioral data were obtained from 7 CFA only animals, 7 CFA and KT5720 animals, 6 saline animals, 4 KT5720 animals, and 6 naïve control animals.
Statistical analysis
Statistical analysis was performed as described in previous published studies (28, 31, 33). Immunohistochemical analyses were performed and confirmed by at least two scientists blinded to experimental conditions. For immunohistochemical analysis of the spinal cord (n ≥ 3 independent experiments per condition), relative levels of the proteins of interest were analyzed using NIH image J software. Fluorescent intensity was measured in ten non-overlapping rectangular regions in laminas I–III of the STN. To normalize intensity measurements within each image, background intensity values were obtained from ten non-overlapping regions in the acellular area of the outer lamina as determined by DAPI, and average values were subtracted from region of interest staining intensity values. All data are presented as mean fold-change from the average naïve value ± the standard error of the mean (S.E.M.). Analysis was performed by a one-way ANOVA with a Games-Howell post-hoc due to unequal variances or a Tukey’s post-hoc test in the case of equal variances, as determined by Levene’s test. To quantify p-ERK expression in the trigeminal ganglion, the number of neuronal cells exhibiting nuclear localization of p-ERK was divided by the total number of visible neuronal nuclei as identified by DAPI and NeuN staining. Results are reported as the average percent ± S.E.M of neurons with p-ERK nuclear staining. Statistical significance was set at P < .05. For the mechanical stimulation studies, the data are reported as the mean number of withdrawal responses ± S.E.M. to 100 g of force at each condition and time point. Subsequent analysis was then performed on data with n ≥ 6 for each experimental condition using a mixed design repeated measures ANOVA to test for general statistical significance, followed by a paired-samples t-test to find changes within subjects from basal, and an independent samples t-test to test for differences between groups. Statistical significance was set at P < .05.
Results
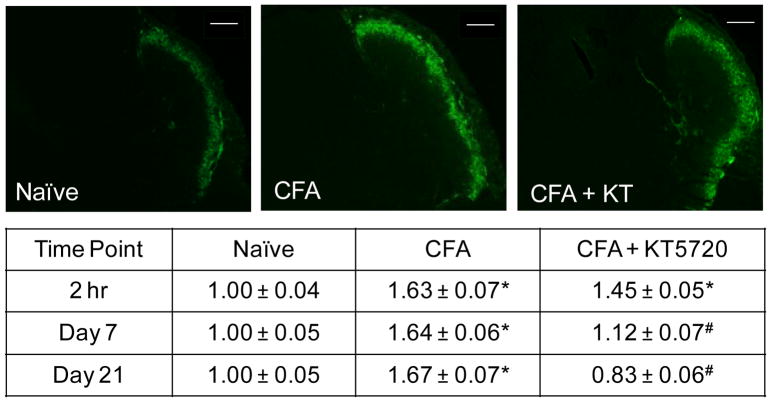
The inhibitory molecule KT5720 was used to determine if the stimulatory effect of CFA-mediated inflammation in the TMJ involved upregulation of CGRP and activation of the PKA pathway. Tissues from naïve, unstimulated animals exhibited low levels of CGRP in the outer lamina of the STN (Fig. 1). The saline control showed no detectable differences in staining intensity when compared to the levels in the naïve controls (0.96 ± 0.06 fold, P = .958) two hours after injections. However, levels were significantly different than naïve levels at day 7 (1.27 ± 0.13 fold, P < .001), but were again similar to naïve levels 21 days after injections (1.14 ± 0.08 fold, P = .447). The intrathecal delivery of the selective PKA inhibitor KT5720 exhibited no detectable differences in CGRP levels at 2 hours or 7 days post-treatment (1.04 ± 0.05, P = .192, 1.00 ± 0.05, P = .101), but levels were significantly lower at 21 days compared to naïve (0.74 ± 0.05 fold, P < .001) and saline (P < .001) controls. The CFA-injected animals exhibited elevated CGRP levels compared to both the naïve and saline controls at 2 hours (1.63 ± 0.07 fold, P < .001), 7 days (1.64 ± 0.06 fold, P < .001, P < .001), and 21 days (1.67 ± 0.07 fold, P < .001, P < .001) after injections. CFA + KT5720 treatment displayed lower levels of CGRP immunostaining compared to the CFA-treated animals at 2 hours (1.45 ± 0.05 fold, P < .001). At day 7, the CFA + KT5720 (1.12 ± 0.07 fold, P = .501) animals exhibited CGRP levels similar to naïve controls and significantly lower than CFA levels (P < .001). At 21 days after treatment, CFA + KT5720 animals continued to express levels of CGRP similar to those observed in naïve controls (0.83 ± 0.06 fold, P = .053) but less than those in CFA animals (P < .001).
Fig. 1.
CFA stimulation of CGRP expression in the STN is repressed by inhibition of PKA activity. Representative images of sections from the STN obtained at the 2 hour time point from naïve, CFA injected, or CFA injection and intrathecal administration of KT5720 animals are shown. The average change in relative staining intensity from naïve levels for CGRP at 2 hours, 7 days, and 21 days after injections is reported. Data are reported as a fold-change ± SEM from levels in naïve animals, whose mean was made equal to one. * P < .05 when compared to naïve control levels and # P < .05 when compared to CFA levels. Scale bar = 200 μm.
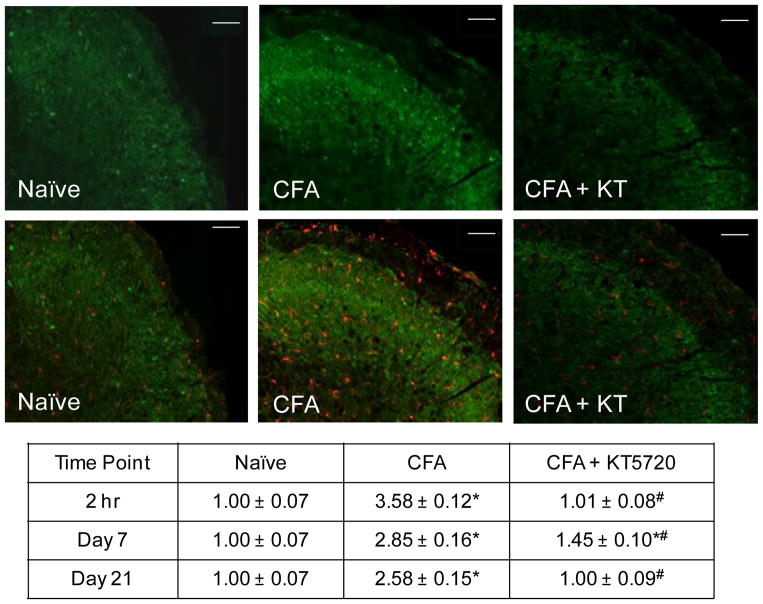
Levels of the active form of PKA were evaluated to determine the amount of intracellular signaling occurring that promotes neuron-glia interactions and maintains a hyperexcitable state in nociceptive neurons. In naïve animals, low levels of PKA immunostaining were detected within the STN (Fig. 2). The saline controls showed no significant difference from the naïve animals at 2 hours (1.15 ± 0.08 fold, P = .331), 7 days (1.02 ± 0.10 fold, P = .999), or 21 days (0.98 ± 0.09 fold, P =.981) post-injection. The KT5720 alone treated animals did not exhibit detectable differences from naïve PKA levels at 2 hours (1.17 ± 0.04 fold, P = .068), 7 days (0.82 ± 0.09 fold, P = .075), or 21 days post-treatment (1.05 ± 0.09 fold, P = .847). CFA-injected animals showed an increase in PKA immunostaining intensity at 2 hours post-treatment compared to both naïve (3.58 ± 0.12 fold, P < .001) and saline controls (P < .001) which persisted through day 7 (2.85 ± 0.16 fold, P < .001) and day 21 (2.58 ± 0.15 fold, P < .001). Based on co-staining with Iba1 and PKA was detectable in microglial cells (Fig. 2) as well as NeuN positive neurons (data not shown). At 2 hours, the CFA + KT5720 animals had PKA levels similar to naïve controls (1.01 ± 0.08 fold, P = .435) and were significantly lower than CFA. This changed on day 7 when PKA levels were significantly increased compared to naïve (1.45 ± 0.10 fold, P < .001) and saline controls (P = .001), but remained significantly lower than CFA levels (P < .001) . The CFA + KT5720 animals again displayed PKA levels similar to naïve (1.00 ± 0.09 fold, P = 1.00) and saline (P = 1.00) levels 21 days after injections that were significantly lower than the elevated levels mediated by CFA (P < .001).
Fig. 2.
Inhibition of PKA signaling in the STN repressed CFA-induced increase in PKA immunoreactive levels. Representative images of sections from the STN obtained at the 2 hour time point from naïve, CFA injected, or CFA injection and intrathecal administration of KT5720 animals stained for the active form of PKA (green) are shown in the top panels. The same images co-stained for the expression of Iba1(red) are presented in the lower panels. The average change in relative staining intensity from naïve levels for PKA at 2 hours, 7 days, and 21 days after injections is reported. Data are reported as a fold-change ± SEM from levels in naïve animals, whose mean was made equal to one. * P < .05 when compared to naïve control levels and # P < .05 when compared to CFA levels. Scale bar = 100 μm.
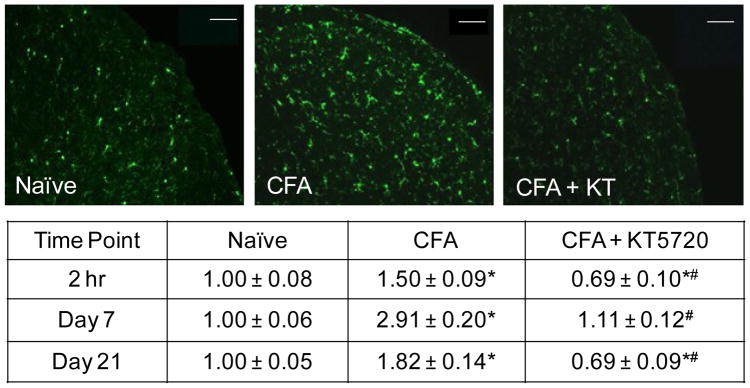
The activation of microglia within the STN was assessed by immunohistochemistry for the microglial marker, ionized calcium-binding adaptor molecule (Iba1). Saline control animals exhibited low levels of Iba1 staining that was not significantly different from naive levels 2 hours (1.15 ± 0.13 fold, P = .778), 7 days (1.07 ± 0.14 fold, P = 1.00) and 21 days (1.05 ± 0.09 fold, P = .99) after treatment. Animals treated with KT5720 alone displayed no detectable differences from naïve controls (1.03 ± 0.11 fold, P = .993) 2 hours after injections, but on day 7, animals treated with KT5720 alone displayed significantly less Iba1 staining compared to naïve and saline controls (0.72 ± 0.08 fold, P = .001, P = .002, respectively). However, this decrease in Iba1 expression was no longer significant 21 days post-treatment (0.76 ± 0.10 fold, P = .10). As seen in Figure 3, CFA-injected animals showed an increase in Iba1 immunostaining intensity relative to both naïve (1.50 ± 0.09 fold, P = .017) and saline controls (P = .017) at 2 hours. Iba1 levels remained significantly increased in CFA animals on day 7 (2.91 ± 0.20 fold, P < .001) and day 21 (1.82 ± 0.14 fold, P < .001). The CFA + KT5720 treatment animals had relative intensity levels significantly lower than naïve (0.69 ± 0.10 fold, P = .037) and saline (P = .003) control levels, and CFA 2 hours post injections. However, at 7 days, Iba1 levels were increased slightly in animals receiving this treatment to levels similar to that of naïve and saline controls (1.11 ± 0.12 fold, P = .894). In animals receiving both CFA + KT5720, Iba1 expression on day 21 was significantly reduced to levels below naïve controls (0.69 ± 0.09 fold, P = .025).
Fig. 3.
CFA-mediated increase in Iba1 in microglia is inhibited by intrathecal PKA inhibitor. Representative images of sections of the STN obtained at the 2 hour time point from naïve, CFA injected, or CFA injection + KT5720 animals are shown. The average change in relative staining intensity from naïve levels for Iba1 at 2 hr, 7 days, and 21 days after injections is reported. Data are reported as a fold-change ± SEM from levels in naïve animals, whose mean was made equal to one. * P < .05 when compared to naïve control levels and # P < .05 when compared to CFA levels. Scale bar = 100 μm.
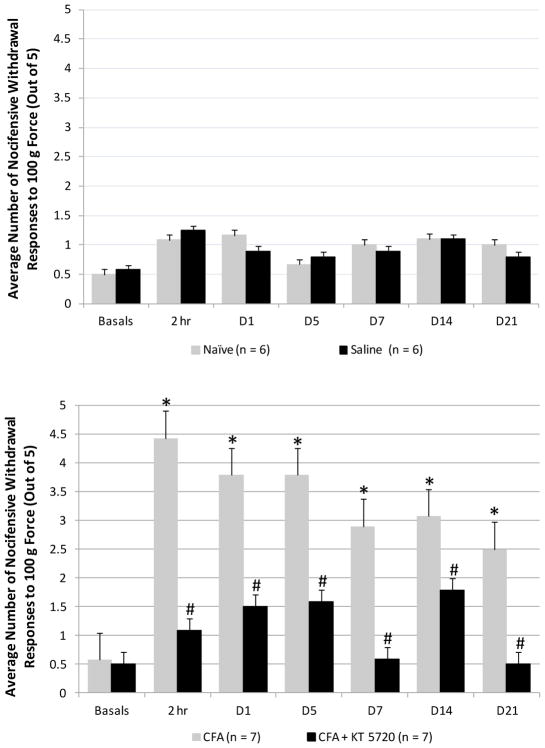
Behavioral testing was performed to determine if cellular changes in the STN mediated by inhibition of PKA signaling would correlate with an analogous reduction in nocifensive behavior. Responses to the 100 g von Frey filament were reported since animals rarely responded to this force at baseline readings, but consistently responded to the subsequent 180 g filament. The average number of nocifensive head withdrawals by control animals for both the left and right side to the 100 g filament 2 hours, 1 day, 5 days, 7 days, 14 days, and 21 days in naïve, intrathecal saline, and intrathecal KT5720 animals is shown in Figure 4. All naïve animals withdrew from the 100 g filament on average 0.5 ± 0.1 times at baseline, 1.1 ± 0.4 at 2 hours, 1.2 ± 0.8 on day 1, 0.7 ± 0.2 on day 5, 1.0 ± 0.3 on day 7, 1.1 ± 0.2 on day 14, and 1.0 ± 0.4 on day 21. Animals injected intrathecally with saline withdrew on average 0.6 ± 0.2 times at baseline, 1.3 ± 0.3 at 2 hours post-injection, 0.9 ± 0.2 on day 1, 0.8 ± 0.3 on day 5, 0.9 ± 0.3 on day 7, 1.1 ± 0.3 on day 14, and 0.8 ± 0.3 on day 21. The KT5720 animals withdrew on average 0.5 ± 0.4 times at baseline, 1.5 ± 0.0 at 2 hours, 0.9 ± 0.4 at day 1, 0.5 ± 0.2 at day 5, 0.5 ± 0.4 at day 7, 0.4 ± 0.2 at day 14, and 1.0 ± 0.4 at day 21 (data not shown). No animals from any of the control conditions at any time point exhibited sufficient nocifensive response to reach statistical significance from baseline values. In contrast, CFA-injection into the TMJ capsule resulted in a significantly increase in the average number of nocifensive responses compared to naïve and saline controls at 2 hours, respectively (P < .001, P < .001), day 1 (P = .007, P < .001), day 5 (P < .001, P < .001), day 7 (P = .001, P = .002), and day 14 (P = .013, P = .024). CFA animals withdrew on average 0.6 ± 0.1 times at baseline, 4.4 ± 0.4 at 2 hours post-injection, 3.8 ± 0.4 on day 1, 3.8 ± 0.4 on day 5, 2.9 ± 0.3 on day 7, 3.1 ± 0.7 on day 14, and 2.5 ± 0.7 on day 21. In addition, CFA animals displayed a significant increase in the average number of withdrawal responses compared to their own baseline levels at every time point post-injection: 2 hours, P < .001; day 1, P < .001; day 5, P < .001; day 7, P = .001; day 14, P = .003; day 21, P = .034. CFA treated animals injected intrathecally with KT5720 exhibited a significant inhibition of nocifensive behavior across all time points post-treatment. CFA + KT5720 animals responded to the 100 g filament on average 0.5 ± 0.1 at baseline, 1.1 ± 0.3 2 hours post-injection, 1.5 ± 0.6 on day 1, 1.6 ± 0.6 on day 5, 0.6 ± 0.3 on day 7, 1.8 ± 0.6 on day 14, and 0.5 ± 0.4 on day 21. At every time point, the average number of withdrawals responses for the CFA + KT5720 animals were similar to values reported for the naïve or saline control animals.
Fig. 4.
Inhibition of PKA signaling is sufficient to repress nocifensive head-withdrawal responses to mechanical stimulation mediated by CFA. Average nocifensive withdrawal responses ± SEM to 100g force applied to the cutaneous area over the TMJ capsule at 2 hours, and days 1, 5, 7, 14, and 21 after intrathecal injection of sterile saline (top panel) or CFA injection and intrathecal KT5720 (bottom panel) when compared to naïve control levels. * P < .05 when compared to basal levels and # P < .05 when compared to CFA values.
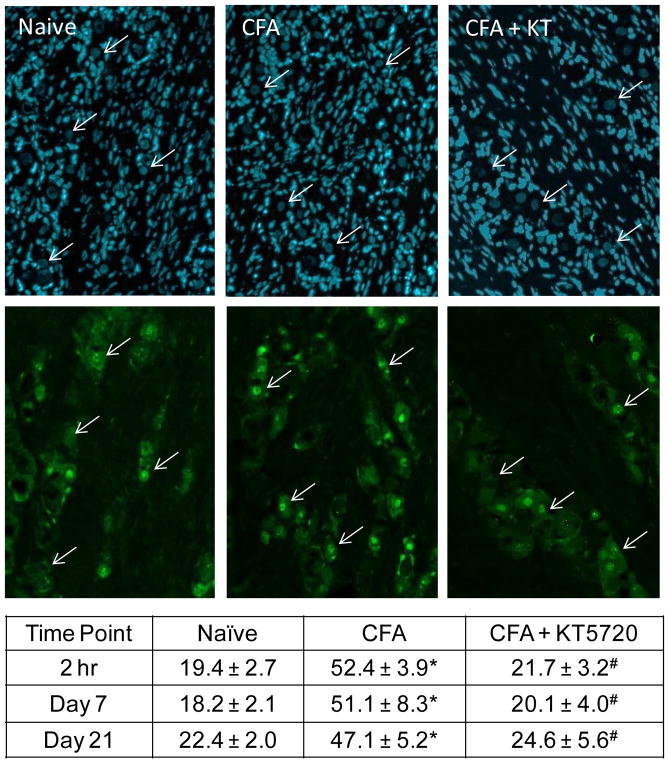
The significant reduction in nocifensive behavior in response mediated by the PKA inhibitor prompted investigation of cellular changes in the peripheral trigeminal ganglion neurons. Activation of peripheral nociceptors was evaluated via immunohistochemical analysis of p-ERK expression in the nucleus of DAPI positive neuronal cell bodies in the ganglion. At 2 hours in naïve tissue, the average number of neurons exhibiting elevated nuclear levels of p-ERK was 19.4 ± 2.7% of neurons (Fig. 5). This trend remained consistent at both subsequent timepoints, with values of 18.2 ± 2.1% and 22.4 ± 2.0% exhibiting nuclear p-ERK staining at days 7 and 21, respectively. Saline controls were similar to naïve levels at 2 hours (19.2 ± 5.6%, P = 1.00), day 7 (19.0 ± 6.9 %, P = 1.00) and day 21 (18.0 ± 3.4 %, P = .871). Tissues from KT5720 control animals also expressed low p-ERK levels at 2 hours (11.7 ± 1.26 %, P = 0.574), day 7 (20.9 ± 1.61 %, P = .763), and day 21 (20.3 ± 4.8 %, P = .990). In contrast, CFA injected animals displayed significantly elevated expression from naïve and saline control animals at 2 hours (52.4 ± 3.9 %, P < .001, P < .001), day 7 (51.1 ± 8.3 %, P = .001 P = .002), and day 21 (47.1 ± 5.2 %, P = .002, P = .001). P-ERK levels in CFA + KT5720 tissues were significantly repressed compared to CFA levels at 2 hours (21.7 ± 3.2%, P < .001), day 7 (20.1 ± 4.0 %, P = .002), and day 21 (24.64 ± 5.6 %, P = .006). Nuclear p-ERK levels in ganglia from animals receiving CFA + KT5720 were not significantly different when compared to naïve at any time point (P = .989, P = .997, P = .988).
Fig. 5.
Increased nuclear p-ERK expression in trigeminal ganglion neurons in response to CFA was inhibited by KT5720. Representative images of sections from the V3 region of trigeminal ganglia obtained from naïve, CFA, and CFA + KT5720 treated animals at 2 hr, 7 days, and 21 days after injections are shown. All cell nuclei are identified by the nuclear dye DAPI (top panels). The same images were co-stained for p-ERK (bottom panels). Arrows indicate neuronal cell body nuclei identified by DAPI. The average percent ± SEM of p-ERK positive neuronal nuclei, as identified by DAPI staining, for each condition is reported in the table. * P < .05 when compared to naïve levels and #P < .05 when compared to saline control levels.
Discussion
The main goal of this study was to investigate the role of PKA in the STN in promoting cellular changes in trigeminal neurons associated with nociception in an established in vivo model of TMD. The inflammatory mediator CFA was injected into both joint capsules to mimic prolonged TMD pathology mediated by sustained inflammation that promotes a persistent state of trigeminal nociceptor sensitization (28, 34–38). The emulsion of heat-killed Mycobacterium tuberculosis elicits a robust innate immune response characterized by the recruitment of pro-inflammatory mediators including cytokines, chemokines, and CGRP that promote prolonged edema and pain localized to the joint (37, 39). In addition to a local inflammatory response, TMD is characterized by the development of peripheral and central sensitization of trigeminal nociceptive neurons (10). The neuropeptide CGRP is thought to play a primary role in the initiation and maintenance of central sensitization of nociceptive neurons (11, 40, 41). In agreement with this notion, CFA-induced inflammation in the TMJ resulted in an increase in CGRP expression in the outer lamina of the STN at 2 hours that was sustained at days 7 and 21, the longest timepoint included in this study. CGRP receptors, which are present on trigeminal ganglion neurons that provide sensory innervation of the joint capsule, are also expressed on second order spinal neurons and on the associated glial cells, astrocytes and microglia (42). To determine if PKA was also involved in mediating the stimulatory effects of CFA, the PKA kinase activity inhibitory molecule KT5720 (500 nM) was injected intrathecally immediately prior to bilateral CFA injection into the capsules. The concentration of 500 nM is lower than that used in other studies investigating the role of PKA in the central nervous system (43, 44) and is not likely to cause inhibition of protein kinase G or protein kinase C (Ki > 2 μM). Administration of KT5720 significantly inhibited the sustained stimulatory effect of CFA on CGRP levels observed on days 7 and 21. Although KT5720 decreased the stimulatory effect of CFA at 2 hours, the level of repression did not reach significance. The coupling of CGRP receptor activation to PKA signal transduction in the STN is in agreement with a previous study reporting a similar relationship in the dorsal horn of the lower spinal cord (24). Taken together, results from these cellular studies support the notion that CFA-induced inflammation in the TMJ capsule involves a sustained increase in the expression of CGRP and the active form of PKA in the STN.
Activation of the PKA signaling pathway in spinal nociceptive neurons and glial cells is implicated in the development of a persistent state of central sensitization (45–47). In response to CFA injection into the TMJ capsule, significantly elevated levels of PKA were observed for up to 21 days in the neurons and microglia in the STN based on co-localization with NeuN and Iba1. Upon binding of CGRP to its receptor on primary neurons, adenylyl cyclase is activated and increases intracellular cAMP concentration that leads to activation of PKA and stimulation of CGRP synthesis via an autocrine mechanism (42). It was found that intrathecal administration of KT5720 inhibited CFA-mediated stimulation of PKA activation at each time point. PKA signaling leads to phosphorylation of the transcription factor CREB and its translocation into the nucleus where it can bind to the cAMP response element DNA regulatory site located in the promoter region of the CGRP and numerous cytokine genes. Increased expression of these genes is implicated in promoting and sustaining a hyperexcitable state of spinal nociceptive neurons that involves glial activation (11). Activation of astrocytes and microglia is known to contribute to prolonged sensitization of nociceptive neurons within the spinal cord, leading to development of chronic pain conditions (48, 49). Results from immunohistochemical studies of Iba1, which is a protein implicated in microglia activation (49), provided evidence that CFA-mediated inflammation in the TMJ promotes a sustained increase in Iba1 expression in the STN for up to 21 days post injection. The CFA-mediated increase in Iba1 expression was greatly inhibited in response to intrathecal administration of the PKA inhibitor KT5720 at all time points. These results in conjunction with the observed co-localization of activated PKA with Iba1 in the STN of CFA-stimulated animals provide evidence of a key role of PKA in the initial and sustained activation of microglia in response to CFA inflammation in the TMJ.
TMD pathology is characterized by the development of peripheral and central sensitization of trigeminal nociceptive neurons (10). Having demonstrated an involvement of PKA in cellular events associated with central sensitization, the potential role of PKA signaling in the STN to promote nociception of trigeminal neurons was investigated. Intrathecal administration of KT5720 immediately prior to CFA injection into the capsules significantly reduced the average number of nocifensive head withdrawal responses to mechanical stimulation of trigeminal neurons at each time point. Thus, inhibition of PKA signaling within the STN was sufficient to greatly reduce the nocifensive response associated with persistent inflammation within the TMJ capsule. This finding is in agreement with results from a previous study that utilized KT5720 to demonstrate the involvement of PKA in mediating spinal nociception (50). Intrathecal administration of KT5720 also inhibited CFA-induced increases in the nuclear expression of the signaling protein p-ERK in trigeminal ganglion neurons. Translocation of p-ERK into neuronal nuclei is associated with increased expression of pro-inflammatory proteins including CGRP and cytokines that are known to promote peripheral nociceptor sensitization (51–53). Recent findings provide evidence of bidirectional signaling in the trigeminal system in which intrathecal administration of CGRP caused increased nuclear localization of p-ERK in the ganglion and increased neuron-satellite glial cell coupling (54). Thus, blocking CFA-induced PKA activation in the STN, which resulted in lower CGRP levels in the spinal cord, likely mediates in part the observed decrease in p-ERK, and hence the inhibition of trigeminal nociception in response to mechanical stimulation.
In conclusion, results from this study provide evidence of the involvement of CGRP-PKA signaling in the STN that promotes cellular changes associated with an increase in trigeminal nociception in a model of TMD. Inhibition of PKA signaling in the STN was shown to decrease the average number of nocifensive withdrawals to mechanical stimuli and correlated with decreased levels of nuclear p-ERK in trigeminal ganglion neurons. These findings support the notion that inhibiting the PKA-mediated neuronal and glial changes would be beneficial in the treatment of TMD by reducing nociception. A potential limitation of this study is that only male rats were utilized in a model of TMD, which is a condition reported to be more prevalent in females (55, 56). Future animal studies are planned to investigate sex differences in multiple models of TMD.
Footnotes
Conflict of interest: The authors of this paper do not have any conflict of interests to report.
Contributor Information
Lindsey K. Koop, Graduate Student, Center for Biomedical & Life Sciences, Missouri State University, Springfield, MO USA.
Jordan L. Hawkins, Senior Research Scientist, Center for Biomedical & Life Sciences, Missouri State University, Springfield, MO USA.
Lauren E. Cornelison, Junior Research Scientist, Center for Biomedical & Life Sciences, Missouri State University, Springfield, MO USA.
Paul L. Durham, Distinguished Professor, Director, Center for Biomedical & Life Sciences, Missouri State University, Springfield, MO USA.
References
- 1.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furquim BD, Flamengui LM, Conti PC. TMD and chronic pain: a current view. Dental Press J Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12:T61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poveda Roda R, Bagan JV, Diaz Fernandez JM, Hernandez Bazan S, Jimenez Soriano Y. Review of temporomandibular joint pathology. Part I: classification, epidemiology and risk factors. Medicina oral, patologia oral y cirugia bucal. 2007;12:E292–298. [PubMed] [Google Scholar]
- 5.Bonjardim LR, Lopes-Filho RJ, Amado G, Albuquerque RL, Jr, Goncalves SR. Association between symptoms of temporomandibular disorders and gender, morphological occlusion, and psychological factors in a group of university students. Indian J Dent Res. 2009;20:190–194. doi: 10.4103/0970-9290.52901. [DOI] [PubMed] [Google Scholar]
- 6.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 7.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 8.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, et al. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 11.Seybold VS. The role of peptides in central sensitization. Handbook of experimental pharmacology. 2009:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 12.Durham PL, Garrett FG. Emerging importance of neuron-satellite glia interactions within trigeminal ganglia in craniofacial pain. TOPAINJ. 2010;3:3–13. [Google Scholar]
- 13.Xie YF. Glial involvement in trigeminal central sensitization. Acta Pharmacol Sin. 2008;29:641–645. doi: 10.1111/j.1745-7254.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 14.Davies AJ, Kim YH, Oh SB. Painful Neuron-Microglia Interactions in the Trigeminal Sensory System. TOPAINJ. 2010:14–28. [Google Scholar]
- 15.Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol Pain. 2012;8:43. doi: 10.1186/1744-8069-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno MJ, Terron JA, Stanimirovic DB, Doods H, Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 17.Marvizon JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience. 2007;148:250–265. doi: 10.1016/j.neuroscience.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 19.Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Y, Jiang L, Chen H, Zou J, Liu Z, Shi Y. The Neuroprotective Effect of Liraglutide is Mediated by Glucagon-Like Peptide 1 Receptor-Mediated Activation of cAMP/PKA/CREB Pathway. Cell Physiol Biochem. 2015;36:2366–2378. doi: 10.1159/000430199. [DOI] [PubMed] [Google Scholar]
- 22.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 23.Staud R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr Rheumatol Rev. 2015;11:109–115. doi: 10.2174/1573397111666150619094819. [DOI] [PubMed] [Google Scholar]
- 24.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 25.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cady RJ, Denson JE, Sullivan LQ, Durham PL. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience. 2014;269:79–92. doi: 10.1016/j.neuroscience.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Villa G, Ceruti S, Zanardelli M, Magni G, Jasmin L, Ohara PT, et al. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain. 2010;6:89. doi: 10.1186/1744-8069-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Ren K, Shimada M, Iwata K. Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp Neurol. 2008;214:209–218. doi: 10.1016/j.expneurol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins JL, Denson JE, Miley DR, Durham PL. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience. 2015;290:115–125. doi: 10.1016/j.neuroscience.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrett FG, Hawkins JL, Overmyer AE, Hayden JB, Durham PL. Validation of a novel rat-holding device for studying heat- and mechanical-evoked trigeminal nocifensive behavioral responses. J Orofac Pain. 2012;26:337–344. [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins JL, Durham PL. Prolonged Jaw Opening Promotes Nociception and Enhanced Cytokine Expression. Journal of oral & facial pain and headache. 2016;30:34–41. doi: 10.11607/ofph.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper RP, Kerins CA, McIntosh JE, Spears R, Bellinger LL. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund’s adjuvant. Osteoarthritis Cartilage. 2001;9:619–624. doi: 10.1053/joca.2001.0461. [DOI] [PubMed] [Google Scholar]
- 35.Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Kitagawa J, Ren K, Tanaka H, Tanabe A, Watanabe T, et al. Activation of trigeminal intranuclear pathway in rats with temporomandibular joint inflammation. J Oral Sci. 2005;47:65–69. doi: 10.2334/josnusd.47.65. [DOI] [PubMed] [Google Scholar]
- 37.Spears R, Dees LA, Sapozhnikov M, Bellinger LL, Hutchins B. Temporal changes in inflammatory mediator concentrations in an adjuvant model of temporomandibular joint inflammation. J Orofac Pain. 2005;19:34–40. [PubMed] [Google Scholar]
- 38.Romero-Reyes M, Pardi V, Akerman S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: Role of CGRP in orofacial pain. Exp Neurol. 2015;271:95–103. doi: 10.1016/j.expneurol.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Hutchins B, Patel H, Spears R. Attenuation of pro-inflammatory neuropeptide levels produced by a cyclooxygenase-2 inhibitor in an animal model of chronic temporomandibular joint inflammation. J Orofac Pain. 2002;16:312–316. [PubMed] [Google Scholar]
- 40.Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V. Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP. Mol Pain. 2006;2:31. doi: 10.1186/1744-8069-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun RQ, Lawand NB, Lin Q, Willis WD. Role of calcitonin gene-related peptide in the sensitization of dorsal horn neurons to mechanical stimulation after intradermal injection of capsaicin. J Neurophysiol. 2004;92:320–326. doi: 10.1152/jn.00086.2004. [DOI] [PubMed] [Google Scholar]
- 42.Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170:1293–1307. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience. 2013;250:632–643. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, et al. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, et al. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu HJ, Glauner KS, Gereau RWt. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol. 2003;90:1671–1679. doi: 10.1152/jn.00340.2003. [DOI] [PubMed] [Google Scholar]
- 47.Levy D, Strassman AM. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J Physiol. 2002;538:483–493. doi: 10.1113/jphysiol.2001.013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu HY, Lin HH, Lai CC. Potentiation of spinal NMDA-mediated nociception by cocaine- and amphetamine-regulated transcript peptide via PKA and PKC signaling pathways in rats. Regul Pept. 2009;158:77–85. doi: 10.1016/j.regpep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RWt. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci. 2010;30:11537–11547. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durham PL, Russo AF. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Mol Endocrinol. 1998;12:1002–1009. doi: 10.1210/mend.12.7.0135. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- 54.Cornelison LE, Hawkins JL, Durham PL. Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons. Neuroscience. 2016;339:491–501. doi: 10.1016/j.neuroscience.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagis B, Ayaz EA, Turgut S, Durkan R, Ozcan M. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int J Med Sci. 2012;9:539–544. doi: 10.7150/ijms.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14:T20–32. e21–23. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]