Abstract
Background
Intensive glycemic control in type 2 diabetes (glycated hemoglobin [HbA1c] level <7%) is an established, cost-effective standard of care. However, guidelines recommend individualizing goals on the basis of age, comorbidity, diabetes duration, and complications.
Objective
To estimate the cost-effectiveness of individualized control versus uniform intensive control (HbA1c level <7%) for the U.S. population with type 2 diabetes.
Design
Patient-level Monte Carlo–based Markov model.
Data Sources
National Health and Nutrition Examination Survey 2011–2012.
Target Population
The approximately 17.3 million persons in the United States with diabetes diagnosed at age 30 years or older.
Time Horizon
Lifetime.
Perspective
Health care sector.
Intervention
Individualized versus uniform intensive glycemic control.
Outcome Measures
Average lifetime costs, life-years, and quality-adjusted life-years (QALYs).
Results of Base-Case Analysis
Individualized control saved $13 547 per patient compared with uniform intensive control ($105 307 vs. $118 854), primarily due to lower medication costs ($34 521 vs. $48 763). Individualized control decreased life expectancy (20.63 vs. 20.73 years) due to an increase in complications but produced more QALYs (16.68 vs. 16.58) due to fewer hypoglycemic events and fewer medications.
Results of Sensitivity Analysis
Individualized control was cost-saving and generated more QALYs compared with uniform intensive control, except in analyses where the disutility associated with receiving diabetes medications was decreased by at least 60%.
Limitation
The model did not account for effects of early versus later intensive glycemic control.
Conclusion
Health policies and clinical programs that encourage an individualized approach to glycemic control for U.S. adults with type 2 diabetes reduce costs and increase quality of life compared with uniform intensive control. Additional research is needed to confirm the risks and benefits of this strategy.
Primary Funding Source
National Institute of Diabetes and Digestive and Kidney Diseases.
Diabetes affects 9% of the U.S. population and costs the United States an estimated $245 billion annually (1). Because of the substantial financial and public health burden of diabetes, understanding the cost-effectiveness of treatments is important. More than a decade ago, a cost-effectiveness analysis found that intensive glycemic control (plasma glucose concentration <6 mmol/L [<108 mg/dL]) with glucose-lowering medications was cost-effective, with an incremental cost-effectiveness ratio of $41 384 per quality-adjusted life-year (QALY) compared with conventional control (2). This study provided evidence for the cost-effectiveness of the American Diabetes Association (ADA) recommendations to pursue glycated hemoglobin (HbA1c) values less than 7.0% using glucose-lowering medications.
The standards of diabetes care have changed over the past decade. Results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial showed that patients who had or were at high risk for cardiovascular disease had an increased mortality risk with very intensive glycemic control (achieved HbA1c level of 6.4% vs. 7.5%) (3). In addition, several observational studies have revealed a high incidence of severe hypoglycemia (an adverse effect of glucose-lowering agents) in older Medicare and managed care beneficiaries (4–6). As a result of accumulating evidence for the potential harms of intensive glycemic treatment, in 2012, the ADA and the European Association for the Study of Diabetes (EASD) published a position statement recommending that providers individualize glycemic goals on the basis of several factors, including age, life expectancy, comorbidity, diabetic complication history, diabetes duration, and hypoglycemia risk (7).
These guidelines could have major implications for the U.S. population with type 2 diabetes. It is estimated that more than half of U.S. adults with diabetes have advanced age, high comorbidity, preexisting diabetic complications, or long disease duration (8). However, to date, the clinical implications and economic value of individualizing glycemic goals have not been examined. Also, it is highly unlikely that diabetes trials will be designed to compare the lifetime effects of individualized versus uniform intensive glycemic control. Without these analyses, the need for policymakers to prioritize individualized diabetes care is uncertain. We sought to examine the cost-effectiveness of individualized glycemic control compared with uniformly applied intensive control (HbA1c level <7.0%) for the U.S. population with type 2 diabetes.
Methods
Study Model
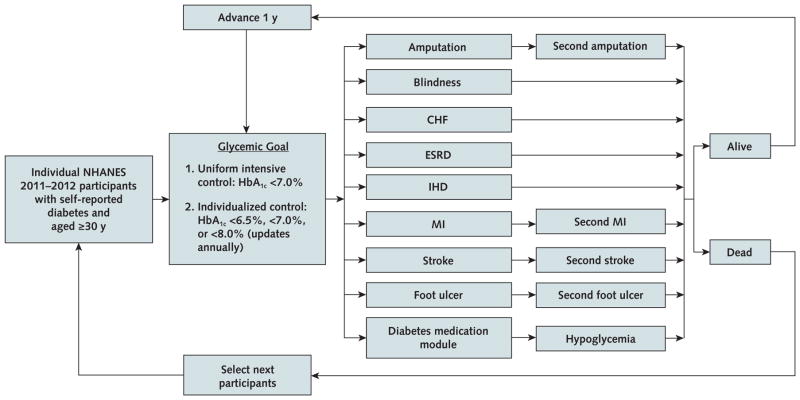
The U.S. Type 2 Diabetes Policy Model is an individual patient–level, Monte Carlo–based Markov model of the incidence, prevalence, mortality, and costs related to type 2 diabetes among U.S. adults with self-reported disease (Appendix Figure 1, available at Annals.org). We integrated the diabetes-related complication and mortality modules from the UKPDS (United Kingdom Prospective Diabetes Study) Outcomes Model version 2 (OM2) (9) with a hypoglycemic event module (10), an ADA/EASD-based diabetes medication algorithm (7), and a module for utility and 2015 U.S. costs.
The equations from the UKPDS OM2 and its predecessor (UKPDS OM1) (11) are used by all major type 2 diabetes simulation models worldwide (12–14). The UKPDS OM2 has been shown to accurately predict results for the population in which it was developed (internally validated) (9) and for other diabetes populations (externally validated) (see the Appendix, available at Annals.org, for details) (13). We internally validated our version of the UKPDS OM2 by comparing our results with predicted results for the same UKPDS population. Our model results were within 1% of published results for all complications and within 3% for composite death. The UKPDS OM1 risk equations were used in the prior cost-effectiveness analysis of intensive versus standard glycemic control (2). No other risk equations for diabetic complications and mortality meet these standards, which necessitated our use of the UKPDS OM2 equations. However, it is important to note their limitations. Because the UKPDS OM2 equations predict annual risk for events, they do not account for long-term effects of early intensive glucose control (legacy effect) on cardiovascular and microvascular events and mortality seen in the follow-up of the UKPDS (15), the ACCORD trial (16), and the VADT (Veterans Affairs Diabetes Trial) (17, 18). They also do not include the excess mortality risk observed in the ACCORD trial.
Using 26 individual patient–level characteristics, the UKPDS OM2 predicts lifetime risk for diabetes-related complications (foot ulcer, blindness, renal failure, first and subsequent amputation, first and subsequent myocardial infarction, ischemic heart disease, and first and subsequent stroke) using 13 risk equations and mortality using 4 risk equations. For mortality, the model assumes that all diabetic complications (except foot ulcer and blindness) increase the probability of death. The model predicts both non–diabetes-related and diabetes-related death.
We simulated risk for hypoglycemia based on use of diabetes medications (see the Appendix for details) (19). We assumed that severe hypoglycemia was associated with a greater decrease in quality of life (utility) than mild or moderate events and that only severe hypoglycemic events increased the probability of health care use.
We started patients in the model with their self-reported combination of diabetes medications and their measured HbA1c value from NHANES (National Health and Nutrition Examination Survey) 2011–2012 (Appendix Table 1, available at Annals.org). We followed the 2015 ADA/EASD algorithm for initiating use of diabetes medications (7). The algorithm includes all medication classes except α-glucosidase inhibitors and meglitinides because neither is recommended by ADA/EASD. The choice of medication was based on the proportion of its use in the United States as a second-line agent from reported literature (20). We assumed that the decision to start or stop use of a diabetes medication depended on the HbA1c value.
We assumed that HbA1c values would increase annually, based on an equation from UKPDS OM1 (11). We also assumed that patients’ medications would change in the year after their HbA1c value increased above (or decreased >1% below) their goal and that each medication reduced the HbA1c level by 1% (21–23). Under the individualized strategy, as patients developed complications and aged, their goals increased (for example, from an HbA1c level <6.5% to a level <7.0% to a level <8.0%). When the individualized HbA1c goal decreased and this resulted in an HbA1c level that was more than 1% below the goal, the last medication added would be removed the next year (see the Appendix for details).
Model Inputs
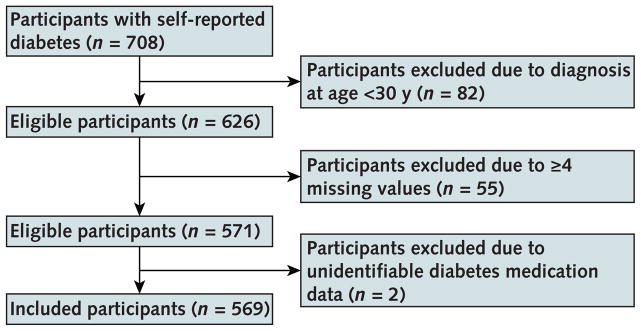
We used data from individual participants with self-reported diabetes in NHANES 2011–2012 (Appendix Table 1). We included adults who answered “yes” to the question, “[Other than during pregnancy,] have you ever been told by a doctor or other health professional that you have diabetes or sugar diabetes?” To identify patients with likely type 2 diabetes, we included only those who were aged 30 years or older at diagnosis. For missing values that were necessary for the model, we used the average of 5 values generated using Markov-chain Monte Carlo multiple imputation (see the Appendix and Appendix Table 2 [available at Annals.org] for details). We excluded participants with 4 or more missing model inputs or unidentifiable diabetes medication data (Appendix Figure 2, available at Annals.org). Characteristics were similar between included and excluded participants (Appendix Table 1).
Individual characteristics were used as baseline data. Baseline variables included age, sex, race/ethnicity, smoking status, diabetes duration, and self-reported medical conditions (coronary heart disease, angina, heart failure, myocardial infarction, stroke, dialysis in the previous year, retinopathy, lung disease, rheumatoid arthritis, liver disease, and cancer [excluding skin cancer]). Measured values included HbA1c level, body mass index, estimated glomerular filtration rate, low-density lipoprotein and high-density lipoprotein cholesterol levels, hemoglobin level, leukocyte count, heart rate, systolic blood pressure, and albuminuria. History of peripheral vascular disease, atrial fibril-lation, amputation, blindness, and neuropathy were not available in NHANES. The age- and sex-based probabilities of having peripheral vascular disease or atrial fibrillation were imputed on the basis of published literature (24, 25). We assumed that no patients had amputations, blindness, or neuropathy at baseline.
Costs and Health Utility
Costs associated with complications, hospital use, medication use, and self-monitoring (testing and supplies) were included. Medication costs were calculated using the average wholesale price across drug classes (26). Generic prices were used when available. All costs were from a health care sector perspective and were in 2015 U.S. dollars (Appendix Table 3, available at Annals.org) (27). The Impact Inventory is provided in Appendix Table 4 (available at Annals.org).
An annual health utility was calculated to estimate QALYs. Health utilities are used to value health states, with 0 equal to death and 1 equal to perfect health. Using established utilities in the literature, we accounted for the independent quality-of-life effects of each diabetic complication, hypoglycemia, and routine use of glucose-lowering medications (oral agents and insulin) (Appendix Table 3) (28, 29). The multiplicative method, which entails multiplying all utility values within a patient cycle, was used to combine utilities (30).
Statistical Analysis
For NHANES participants with self-reported diabetes in 2011 to 2012, we compared the strategies suggested by the ADA: individualized glycemic goals and a uniform intensive goal (HbA1c level <7.0%). For individualized goals, the guidelines specify more and less stringent goals than an HbA1c level less than 7.0%. We assumed that the more stringent HbA1c goal was less than 6.5% and the less stringent goal was less than 8.0%. To operationalize these goals, we used age, complication history, comorbidity status, and diabetes duration because these are objective measures included in recommendations from the ADA and the EASD (see the footnote to Table 1) (7, 31). The individualized goals were at the patient level and were updated in the model every year. To define complication history, we considered a patient-reported history of any of the following: angina pectoris, myocardial infarction, stroke, coronary heart disease, congestive heart failure, retinopathy, dialysis in the past year, an albumin–creatinine ratio greater than 300 mg/g, or development of a diabetic complication during the simulation. Comorbidity status was calculated using a weighted combined Charlson Comorbidity Index score based on the patient’s baseline self-report of myocardial infarction, heart failure, stroke, lung disease, rheumatoid arthritis, liver disease, diabetes, or cancer or development of these complications during the simulation. A score less than 5 (vs. ≥5) indicated low (vs. high) comorbidity status (32).
Table 1.
Incremental Cost-Effectiveness of a Strategy of Individualized Versus Uniform Intensive Glycemic Control for U.S. Adults With Diabetes Diagnosed at Age ≥30 Years (n = 569)*
| Variable | Mean (95% CI) | Incremental Difference† | |
|---|---|---|---|
|
| |||
| Uniform Intensive Control | Individualized Control | ||
| Lifetime costs, $‡ | |||
|
| |||
| Medications | 48 763 (41 660 to 55 867) | 34 521 (28 908 to 40 133) | −14 242 |
|
| |||
| Complications | 55 459 (35 302 to 75 616) | 56 920 (37 642 to 76 198) | 1461 |
|
| |||
| Hypoglycemia | 1040 (529 to 1550) | 760 (331 to 1190) | −280 |
|
| |||
| Office visits | 10 307 (9349 to 11 264) | 10 174 (9225 to 11 123) | −133 |
|
| |||
| Self-management | 3285 (2897 to 3672) | 2932 (2597 to 3267) | −353 |
|
| |||
| Total | 118 854 (96 312 to 141 395) | 105 307 (84 443 to 126 172) | −13 547 |
|
| |||
| Life expectancy, y§ | 20.73 (18.77 to 22.69) | 20.63 (18.67 to 22.58) | −0.10 |
|
| |||
| Change in QALYs|| | |||
| Medications | −0.62 (−0.55 to −0.70) | −0.45 (−0.38 to −0.52) | 0.17 |
|
| |||
| Complications | −0.08 (−0.06 to −0.10) | −0.09 (−0.07 to −0.11) | −0.01 |
|
| |||
| Hypoglycemia | −0.20 (−0.17 to −0.23) | −0.15 (−0.12 to −0.18) | 0.05 |
|
| |||
| Total QALYs | 16.58 (14.99 to 18.18) | 16.68 (15.08 to 18.29) | 0.10 |
HbA1c = glycated hemoglobin; QALY = quality-adjusted life-year.
These participants represent about 17.3 million U.S. adults aged ≥30 y with self-reported diabetes. Individualized glycemic goals were assigned annually to patients on the basis of their age, history of complications, comorbidity level, and diabetes duration. An HbA1c goal <6.5% was assigned to patients aged 30–44 y who did not have a history of diabetic complications or high comorbidity. An HbA1c goal <7.0% was assigned to patients aged 30–44 y with a history of diabetic complications and low comorbidity, those aged 45–64 y without a history of complications or high comorbidity, and those aged 65–75 y without a history of complications or high comorbidity and diabetes duration <10 y. All other patients were assigned an HbA1c goal <8.0%.
P < 0.001 for all values.
Expressed in 2015 U.S. dollars and discounted at 3% per year. Negative incremental costs indicate cost savings from individualized vs. uniform intensive control.
Not discounted.
Quality-adjusted life expectancy was discounted at 3% per year. The changes in QALYs due to medications, complications, and hypoglycemia do not sum to the total incremental difference in quality-adjusted life expectancy because QALYs were combined annually using the multiplicative method (30).
We ran 2500 simulations for the lifetime of each NHANES participant for each strategy (see the Appendix for details). To obtain national population estimates, we applied medical examination subsample weights to the model results, which include average per-person lifetime costs, life expectancy, QALYs, and complication and hypoglycemia rates. Differences in results by strategy were compared using general linear models. Costs and QALYs were discounted at 3% annually. We combined annual costs and QALYs by applying the Simpson 1/3 rule (33). The models were run using Microsoft Excel–based @Risk 7.0 software (Palisade Corporation). Analyses were conducted using SAS, version 9.4 (SAS Institute).
Subgroup and Sensitivity Analyses
We analyzed subpopulations defined by age (30 to 44, 45 to 64, 65 to 75, and >75 years), diabetes duration (<10 or ≥10 years), and complication history (present or absent). We also performed 1-way sensitivity analyses on model assumptions. The effectiveness of diabetes medications at decreasing HbA1c level was changed from 1% to 0.8% and 1.2%. We examined our assumptions in the hypoglycemia model by increasing and decreasing the rates of hypoglycemia due to medication use and the rates of severe hypoglycemia–related health care use by 20% each. We also analyzed how results were affected by 20% increases and decreases in costs and utilities related to microvascular and macrovascular complications, hypoglycemia, and diabetes medications. Furthermore, we examined how results changed when receiving diabetes medications was associated with no decrease in utility. Finally, we varied the discount rate to 1% and 5% from its original value of 3% and modeled the additive approach for combining utilities in calculating QALYs (34).
Role of the Funding Source
The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
In NHANES 2011–2012, there were 569 participants who met inclusion criteria, representing about 17.3 million U.S. adults aged 30 years or older with self-reported diabetes (Appendix Table 1). The average age was 61 years, and 50% were female. Participants had diabetes for an average of 10 years; 36% had a history of diabetic complications, and 42% had high comorbidity scores. The most common diabetes medications were metformin (51%), sulfonylureas (33%), and basal insulin (18%).
Individualized glycemic control dominated uniform intensive control (HbA1c level <7%) (Table 1). The individualized strategy cost $13 547 less per person ($105 307 vs. $118 854), mainly due to differences in medication costs ($34 521 vs. $48 763). This strategy was associated with fewer remaining life-years than uniform intensive control (20.63 vs. 20.73; difference, −0.10 [36 days]) because of a higher rate of diabetic complications (7854 vs. 7456 events per 10 000 patients). However, the individualized strategy was also associated with less medication use (−0.6 medication per year) and fewer hypoglycemic events (−2.1 events). These differences translated into an increase in QALYs due to medications (0.17 QALY) and hypoglycemia (0.05 QALY), which led to slightly improved QALYs relative to uniform intensive control (16.68 vs. 16.58; difference, 0.10 [36 quality-adjusted life-days]).
Complications and Hypoglycemia
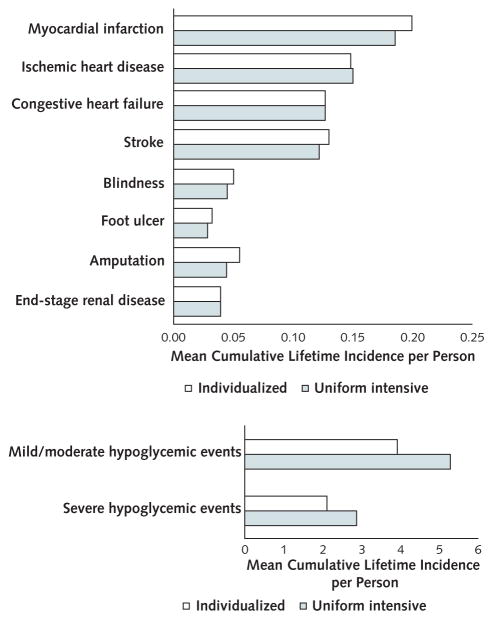
Individualized control increased the absolute lifetime risk for myocardial infarction by 1.39%, amputation by 1.05%, and stroke by 0.85% versus uniform intensive control (Figure 1). However, individualized control decreased the absolute lifetime risk for mild or moderate (−1.4%) and severe (−0.8%) hypoglycemia compared with uniform glycemic control (5.3 mild or moderate and 2.9 severe hypoglycemic events).
Figure 1.
Diabetes-related complications (top) and hypoglycemic events (bottom), by glycemic control strategy.
Subpopulations
Among all subgroups analyzed, individualized glycemic control dominated uniform intensive control (Table 2). Individualized control reduced costs the most and yielded the highest increase in QALYs for adults in the youngest age group (30 to 44 years) (−$16 365 and 0.20 QALY) and those with low comorbidity (−$16 540 and 0.11 QALY). The individualized strategy remained cost-saving for the oldest age group (>75 years) but, as expected, reduced costs and increased QALYs by less (−$5401 and 0.05 QALY), with similar findings for adults with high comorbidity (−$8863 and 0.04 QALY). The greatest savings and increase in QALYs occurred for young patients with low comorbidity (−$18 191 and 0.25 QALY), and the smallest savings and increase in QALYs occurred for young patients with complications and low comorbidity (−$7901 and 0.02 QALY) (Table 2).
Table 2.
Incremental Cost-Effectiveness of Individualized Versus Uniform Intensive Glycemic Control for Subpopulations of U.S. Adults With Diabetes Diagnosed at Age ≥30 Years
| Patient Characteristic | NHANES Population (95% CI), % | Incremental Lifetime Costs, $* | Incremental Life-Years | Incremental QALYs |
|---|---|---|---|---|
| Age | ||||
|
| ||||
| 30–44 y | 9 (6 to 13) | −16 365 | −0.10 | 0.20 |
|
| ||||
| 45–64 y | 48 (44 to 52) | −16 315 | −0.17 | 0.09 |
|
| ||||
| 65–75 y | 26 (21 to 31) | −11 692 | −0.10 | 0.04 |
|
| ||||
| >75 y | 17 (14 to 19) | −5401 | −0.09 | 0.05 |
| Complications† | ||||
|
| ||||
| No | 64 (58 to 70) | −14 885 | −0.14 | 0.10 |
|
| ||||
| Yes | 36 (30 to 42) | −10 452 | −0.11 | 0.04 |
| Comorbidity status‡ | ||||
|
| ||||
| Low | 58 (53 to 63) | −16 540 | −0.13 | 0.11 |
|
| ||||
| High | 42 (37 to 47) | −8863 | −0.13 | 0.04 |
| Diabetes duration | ||||
|
| ||||
| <10 y | 44 (38 to 50) | −14 508 | −0.14 | 0.10 |
|
| ||||
| ≥10 y | 56 (50 to 62) | −11 735 | −0.11 | 0.05 |
| Age 30–44 y | ||||
|
| ||||
| Without complications, with low comorbidity | 7 (3 to 11) | −18 191 | −0.08 | 0.25 |
|
| ||||
| With complications and low comorbidity | 2 (0 to 4) | −7901 | −0.11 | 0.02 |
|
| ||||
| Age 45–64 y, without complications, with low comorbidity | 35 (32 to 39) | −14 961 | −0.16 | 0.09 |
|
| ||||
| Age 65–75 y, without complications, with low comorbidity and diabetes for <10 y | 5 (3 to 7) | −10 131 | −0.10 | 0.08 |
|
| ||||
| All other patients§ | 50 (48 to 53) | −11 982 | −0.12 | 0.05 |
NHANES = National Health and Nutrition Examination Survey; QALY = quality-adjusted life-year.
Expressed in 2015 U.S. dollars. Negative values indicate cost savings from individualized vs. uniform intensive control.
Self-reported history of angina pectoris, myocardial infarction, stroke, coronary heart disease, congestive heart failure, or retinopathy; receipt of dialysis in previous year; or measured albumin–creatinine ratio >300 mg/g.
Calculated by using a weighted combined Charlson Comorbidity Index score based on the patient’s self-report of myocardial infarction, heart failure, stroke, lung disease, rheumatoid arthritis, liver disease, diabetes, and cancer (excluding skin cancer). A score <5 (vs. ≥5) indicated low (vs. high) comorbidity status.
Those aged 30–44 y with complications and high comorbidity, those aged 45–75 y with complications, all patients aged >75 y, and all patients with high comorbidity.
Sensitivity Analyses
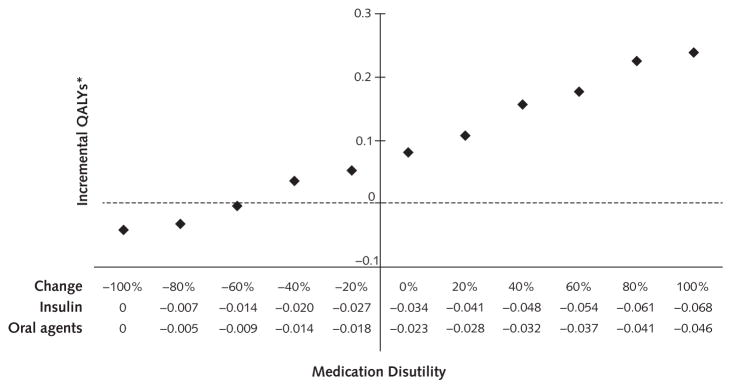
Individualized control was cost-saving in all sensitivity analyses compared with uniform intensive control (savings range, $10 084 to $15 504 per person) (Appendix Table 5, available at Annals.org). The individualized strategy increased QALYs compared with the uniform intensive strategy in all sensitivity analyses except those that varied medication disutility (the quality-of-life burden from taking medications) (Figure 2). Individualized control remained preferred over uniform intensive control unless the disutility was reduced by at least 60% from our baseline estimate.
Figure 2.
Sensitivity analysis of medication disutility.
QALY = quality-adjusted life-year.
*Represents difference between individualized vs. uniform intensive glycemic goals.
Discussion
Because of the large economic burden of diabetes in the United States, the need to reduce treatment costs is a public health and health policy priority. Our study found that individualized glycemic control for U.S. adults with type 2 diabetes was cost-saving (−$13 547 per person), mainly due to reductions in medication costs (−$14 242 per person). Overall, individualized glycemic control resulted in a slight improvement in quality of life (0.10 year) that more than offset a slight decrease in length of life (−0.10 year) due to an expected decrease in quality of life from adverse effects of taking more diabetes medications, including hypoglycemia.
Our major finding was that individualized glycemic control in the United States would be cost-saving compared with uniform intensive glycemic control. Across the remaining life of the U.S. diabetes population, the lifetime cost savings ($13 547 per person) would total $234 billion. In contrast, a previous cost-effectiveness analysis of standard versus intensive glycemic control showed that intensive control was cost-effective but not cost-saving, mostly because of an increase in treatment costs (2). Because individualized control shifts patients from very intensive to less intensive control over their lifetime, our finding that the major source of cost savings was decreased use of diabetes medications rather than complication-related costs is not surprising. The prospect of reducing costs by using fewer medications (−0.6 per person per year) and not substantially worsening patient outcomes is appealing, especially given that many patients prefer to avoid diabetes medications if they can do so safely (35). Our finding agrees with a study in low- and middle-income countries, which found that treatment based on risk for diabetic complications was cost-effective compared with a uniform goal of an HbA1c level less than 7% (36).
Our finding that individualized control is cost-saving and increases quality of life compared with uniform intensive control is conservative because we made model assumptions that favored uniform control. Findings of observational studies (37) and the ACCORD trial (38) have suggested a relationship between severe hypoglycemia and mortality that seemed to be independent of treatment intensity. For example, in the ACCORD trial, a relationship between severe hypoglycemia and mortality was found among patients in the control group but not among those in the intensive treatment group. On the basis of this evidence, we linked the risk for hypoglycemia to the intensification of medication use but assumed no association between severe hypoglycemia and mortality. These assumptions may have resulted in underestimation of the harms of hypoglycemia. In addition, we used the UKPDS OM2 equations because they have been validated (39) and are widely used in analyses for diverse populations throughout the world (12, 14). However, the equations are based on epidemiologic associations and have limitations, as noted in the Methods. As a result of these limitations, the long-term effects of early, very intensive glycemic control (HbA1c level <6.5%), which is possible with the individualized strategy, may have been underestimated. Also, the UKPDS OM2 is unlikely to account for harms associated with intensive glycemic control in patients with cardiovascular disease (16), which explains why we found decreased life expectancy overall and decreased quality of life among the subgroup with preexisting diabetic complications in our model.
Patient-centered care involves accounting for patient preferences and quality of life, and such individualization has been found to be more valuable than uniform care (40). Likewise, we found that the superiority of individualized glycemic control depended on the decrement in quality of life associated with taking diabetes medications. Previous studies have also found that decrements in quality of life due to diabetes treatments can be large (41), with significant individual-level variation (42). In our study, we assumed that the routine task of receiving diabetes medications was associated with a decrement in quality of life above and beyond their relationship with hypoglycemia risk. We know that diabetes agents have adverse effects apart from hypoglycemia and that the route of delivery (injectable or oral) affects patient quality of life (43). Another decision analysis also found that the net benefit of diabetes treatment may be especially sensitive to decrements in quality of life due to diabetes treatment in patients with an HbA1c level less than 9% (44). The collective findings of our analyses along with prior literature suggest that intensive glycemic control may still be preferred for individual patients who do not object to receiving diabetes medications. This consideration is especially important for adults aged 30 to 44 years with diabetic complications because for this population, individualized glycemic control was less cost-saving and had marginal quality-of-life benefits.
Our study had additional limitations. First, we assumed that all diabetes medications included in the ADA/EASD algorithm decreased HbA1c levels by 1%. This assumption is based on a systematic review of the effects of first- and second-line diabetes medications (21). However, evidence suggests that the effectiveness of diabetes medications at decreasing HbA1c levels depends on the HbA1c level. We did not examine this scenario because this evidence is based on meta-regressions that include results from trials of α-glucosidase inhibitors and meglitinide (45, 46), and our model excluded these drug classes because they are not recommended by the ADA/EASD. Future diabetes care guidelines may prioritize sodium–glucose cotransporter-2 inhibitors and glucagon-like peptide-1 (GLP-1)–receptor agonists because they have been found to reduce cardiovascular outcomes (47–49). These benefits have been identified in patients with high risk for or preexisting cardiovascular disease, and the mean trial HbA1c values were greater than 8.0%. If future diabetes medication algorithms prioritize these new drug classes, costs of diabetes care will increase dramatically, but the question of the cost-effectiveness of different glycemic control strategies will remain. Second, our analysis did not include harmful but more distal effects of hypoglycemia, such as falls and cognitive impairment (50, 51). Third, we did not account for lifestyle interventions and patient adherence, but the degree to which these differ across treatment groups is unclear. Fourth, we did not model treatment inertia, which was examined in a previous study. That study compared the cost-effectiveness of intensifying treatments at guideline-recommended HbA1c levels (range, <6.5% to ≤8.5%) versus actual levels seen in clinical practice (range, <8.75% to <9.0%) (52) and found that adhering to the guideline-recommended levels was more cost-effective than current clinical practice. However, we did conduct sensitivity analyses on all major assumptions, including rates of hypoglycemia due to medication use and hypoglycemia-related health care use, costs, and utilities, and found minimal effects on our main conclusions.
In summary, we found that individualized glycemic control for U.S. adults with diabetes diagnosed at age 30 years or older would be cost-saving due to decreased medication costs and would slightly improve quality of life compared with uniform intensive control. The dominance of individualized glycemic control over uniform intensive control was sensitive to patient preferences against receiving diabetes medications. Additional research is needed to understand how glycemic control affects outcomes differently over the disease course and the pleiotropic effects of newer diabetes agents in order to develop diabetes simulation models that can better inform future strategies in diabetes management and health care policy.
Acknowledgments
Financial Support: Dr. Laiteerapong was supported by grant K23 DK092783 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Huang is supported by grant K24 DK105340 from the NIDDK. Drs. Laiteerapong and Huang are members of the NIDDK Chicago Center for Diabetes Translation Research at the University of Chicago (P30 DK092949). Dr. Winn was supported by the Royster Society of Fellows at the University of North Carolina at Chapel Hill.
Appendix: Methods
UKPDS OM2 Internal and External Validation
The UKPDS OM2 has been internally and externally validated (9, 13). Internal validation is the performance of a predictive model in a population similar to that from which the sample originated. The UKPDS OM2 was developed based on patient-level data from 5102 UKPDS participants with newly diagnosed type 2 diabetes mellitus who were aged 25 to 65 years and were recruited between 1977 and 1991 (9). The internal validation of the model compared its predictive accuracy for patient-level outcomes at 25 years for the same UKPDS participants. The predicted failure curves for diabetes outcomes and mortality were within the 95% CI of the actual cumulative failure curves for all events and death.
The UKPDS OM2 was externally validated in a study of the Swedish Institute for Health Economics Cohort Model of Type 2 Diabetes (13). This study included data from 12 clinical trials and observational studies. The UKPDS OM2 slightly underestimated diabetes outcomes, especially macrovascular events, compared with the UKPDS OM1 (slope, 0.899 vs. 0.996, where 1 indicates perfect prediction). The correlation between predicted and actual events was high (>0.96).
Hypoglycemia Module
We assumed that diabetes medications were associated with an increased annual risk for mild or moderate and severe hypoglycemia events, based on published literature (19). Patients receiving insulin (medium, combination, short, or long-acting) were assumed to have a 52% probability of a mild or moderate hypoglycemic event and a 21% risk for a severe hypoglycemic event each year. Patients receiving sulfonylureas were assumed to have a 33% risk for a mild or moderate hypoglycemic event and a 5% risk for a severe hypoglycemic event each year. Patients receiving other diabetes medications were assumed to have a 5% risk for a severe hypoglycemic event each year. Patients were assumed to have no more than 1 mild or moderate hypoglycemic event and 1 severe hypoglycemic event per year and could have multiple hypoglycemic events during their lifetime.
On the basis of published literature, we assumed that a severe hypoglycemic event would result in physician visits 96.5% of the time, emergency department visits 2.6% of the time, and hospitalizations 0.9% of the time (10). The costs associated with these visits are provided in Appendix Table 3.
Appendix Table 1.
Characteristics of U.S. Adults Aged ≥30 Years With Self-reported Diabetes, NHANES 2011–2012 (n = 569)
| Characteristic | Included Participants (n = 569) | Included Participants (n = 17.3 million) | Excluded Participants (n = 57) | P Value | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean (SD) | Number (Percentage) | WeightedMean (SD) | Percentage (95% CI) | Mean (SD) | Number (Percentage) | ||
| Age, y | 63.1 (11.6) | – | 61.0 (0.6) | – | 65.9 (12.0) | – | 0.09 |
|
| |||||||
| Age group | |||||||
| 30–44 y | – | 45 (8) | – | 9 (6–13) | – | 3 (5) | – |
|
| |||||||
| 45–64 y | – | 265 (56) | – | 48 (44–52) | – | 21 (37) | 0.14 |
|
| |||||||
| 65–75 y | – | 153 (27) | – | 26 (21–31) | – | 15 (26) | – |
|
| |||||||
| 75 y | – | 106 (19) | – | 17 (14–19) | – | 19 (32) | – |
|
| |||||||
| Female | – | 265 (47) | – | 50 (45–55) | – | 34 (60) | 0.06 |
|
| |||||||
| Black race | – | 199 (35) | – | 17 (8–25) | – | 25 (44) | 0.18 |
|
| |||||||
| HbA1c level, % | 7.5 (1.8) | – | 7.4 (0.1) | – | 7.2 (1.6) | – | 0.71 |
|
| |||||||
| Duration of diabetes, y | 10.4 (9.1) | – | 9.7 (0.4) | – | 14.2 (10.1) | – | 0.004 |
|
| |||||||
| Duration of diabetes <10 y | – | 261 (46) | – | 44 (38–50) | – | 38 (67) | 0.003 |
|
| |||||||
| Current smoker | – | 93 (16) | – | 17 (13–21) | – | 5 (9) | 0.18 |
|
| |||||||
| Body mass index, kg/m2 | 31.9 (7.4) | – | 32.8 (0.5) | – | 32.0 (9.5) | – | 0.95 |
|
| |||||||
| Low-density lipoprotein cholesterol level, mmol/L | 2.6 (1.0) | – | 2.6 (0.1) | – | 4.0 (0) | – | 0.16 |
|
| |||||||
| High-density lipoprotein cholesterol level, mmol/L | 1.2 (0.3) | – | 1.2 (0.02) | – | 1.6 (0.4) | – | 0.16 |
|
| |||||||
| Hemoglobin level, g/dL | 13.5 (1.6) | – | 13.7 (0.01) | – | 12.9 (1.4) | – | 0.29 |
|
| |||||||
| Leukocyte count, × 1000 cells/μL | 7.2 (2.0) | – | 7.5 (0.1) | – | 7.8 (2.1) | – | 0.34 |
|
| |||||||
| Heart rate, beats/min | 73.1 (13.2) | – | 73.4 (1.0) | – | 70.9 (12.0) | – | 0.38 |
|
| |||||||
| Systolic blood pressure, mm Hg | 131.3 (18.9) | – | 129.9 (0.9) | – | 131.5 (23.8) | – | 0.97 |
|
| |||||||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 78.6 (28.1) | – | 78.8 (1.1) | – | 88.5 (35.4) | – | 0.62 |
|
| |||||||
| Albuminuria | – | 139 (24) | – | 19 (13–24) | – | 7 (12) | 0.047 |
|
| |||||||
| History of diabetic complications | – | 225 (40) | – | 36 (30–42) | – | 26 (46) | 0.37 |
|
| |||||||
| Macrovascular events | – | 157 (28) | – | 28 (23–33) | – | 22 (39) | 0.09 |
|
| |||||||
| Microvascular events | – | 110 (19) | – | 14 (10–18) | – | 14 (25) | 0.35 |
|
| |||||||
| High comorbidity* | – | 269 (47) | – | 42 (37–47) | – | 36 (63) | 0.02 |
| Medications | |||||||
|
| |||||||
| Metformin | – | 275 (44) | – | 51 (42–60) | – | 18 (32) | 0.02 |
|
| |||||||
| Sulfonylurea | – | 168 (29) | – | 33 (25–42) | – | 11 (19) | 0.26 |
|
| |||||||
| Thiazolidinedione | – | 37 (6) | – | 7 (4–11) | – | 7 (12) | 0.10 |
|
| |||||||
| Dipeptidyl peptidase-4 inhibitor | – | 31 (5) | – | 7 (4–10) | – | 1 (2) | 0.23 |
|
| |||||||
| Glucagon-like peptide-1–receptor agonist | – | 5 (1) | – | 0.4 (0–1) | – | 0 (0) | 0.48 |
|
| |||||||
| Meglitinide | – | 7 (1) | – | 2 (0–4) | – | 1 (2) | 0.74 |
|
| |||||||
| α-Glucosidase inhibitor | – | 5 (1) | – | 1 (0–2) | – | 0 (0) | 0.47 |
|
| |||||||
| Insulin | |||||||
| Basal | – | 91 (16) | – | 18 (12–23) | – | 7 (12) | 0.74 |
|
| |||||||
| Short-acting bolus | – | 14 (2) | – | 2 (0.3–3) | – | 2 (4) | 0.63 |
|
| |||||||
| Medium-acting bolus | – | 66 (12) | – | 11 (8–13) | – | 8 (14) | 0.64 |
HbA1c = glycated hemoglobin; NHANES = National Health and Nutrition Examination Survey.
Comorbidity status was calculated by using a weighted combined Charlson Comorbidity Index score based on the patient’s self-report of myocardial infarction, heart failure, stroke, lung disease, rheumatoid arthritis, liver disease, diabetes, and cancer (excluding skin cancer). A score <5 (vs. ≥5) indicated low (vs. high) comorbidity status (28).
Appendix Table 2.
Comparison of Patient Characteristics From Multiple Imputation Data Sets
| Variable | Imputation 1 | Imputation 2 | Imputation 3 | Imputation 4 | Imputation 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Age, y | 61.99 | 0.63 | 61.99 | 0.63 | 61.99 | 0.63 | 61.99 | 0.63 | 61.99 | 0.63 |
|
| ||||||||||
| Female, % | 0.50 | 0.02 | 0.50 | 0.02 | 0.50 | 0.02 | 0.50 | 0.02 | 0.50 | 0.02 |
|
| ||||||||||
| Black race, % | 0.17 | 0.04 | 0.17 | 0.04 | 0.17 | 0.04 | 0.17 | 0.04 | 0.17 | 0.04 |
|
| ||||||||||
| HbA1c level, % | 7.39 | 0.10 | 7.38 | 0.10 | 7.38 | 0.10 | 7.39 | 0.10 | 7.39 | 0.10 |
|
| ||||||||||
| Duration of diabetes, y | 9.71 | 0.42 | 9.71 | 0.42 | 9.71 | 0.42 | 9.71 | 0.42 | 9.71 | 0.42 |
|
| ||||||||||
| Current smoker, % | 0.17 | 0.02 | 0.17 | 0.02 | 0.17 | 0.02 | 0.17 | 0.02 | 0.17 | 0.02 |
|
| ||||||||||
| Body mass index, kg/m2 | 32.78 | 0.54 | 32.77 | 0.53 | 32.77 | 0.54 | 32.81 | 0.55 | 32.82 | 0.53 |
|
| ||||||||||
| Low-density lipoprotein cholesterol, mmol/L | 2.59 | 0.06 | 2.53 | 0.06 | 2.63 | 0.05 | 2.59 | 0.05 | 2.58 | 0.05 |
|
| ||||||||||
| High-density lipoprotein cholesterol, mmol/L | 1.20 | 0.01 | 1.20 | 0.01 | 1.19 | 0.02 | 1.19 | 0.01 | 1.19 | 0.02 |
|
| ||||||||||
| Hemoglobin level, g/dL | 13.72 | 0.12 | 13.72 | 0.12 | 13.72 | 0.12 | 13.72 | 0.12 | 13.72 | 0.12 |
|
| ||||||||||
| Leukocyte count, × 1000 cells/μL | 7.45 | 0.14 | 7.45 | 0.14 | 7.45 | 0.14 | 7.45 | 0.14 | 7.45 | 0.14 |
|
| ||||||||||
| Heart rate, beats/min | 73.15 | 0.94 | 73.11 | 0.96 | 73.27 | 0.96 | 73.38 | 0.97 | 73.19 | 0.96 |
|
| ||||||||||
| Systolic blood pressure, mm Hg | 130.12 | 0.89 | 129.82 | 0.89 | 129.76 | 0.92 | 129.60 | 0.89 | 129.72 | 0.93 |
|
| ||||||||||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 78.51 | 1.02 | 78.84 | 1.09 | 78.71 | 1.08 | 79.05 | 1.30 | 78.55 | 1.03 |
|
| ||||||||||
| Albuminuria, % | 0.19 | 0.03 | 0.19 | 0.03 | 0.19 | 0.03 | 0.19 | 0.03 | 0.19 | 0.03 |
|
| ||||||||||
| Metformin, % | 0.56 | 0.04 | 0.56 | 0.04 | 0.56 | 0.04 | 0.56 | 0.04 | 0.56 | 0.04 |
|
| ||||||||||
| Sulfonylurea, % | 0.35 | 0.04 | 0.35 | 0.04 | 0.35 | 0.04 | 0.35 | 0.04 | 0.35 | 0.04 |
|
| ||||||||||
| Thiazolidinedione, % | 0.08 | 0.02 | 0.08 | 0.02 | 0.08 | 0.02 | 0.08 | 0.02 | 0.08 | 0.02 |
|
| ||||||||||
| Dipeptidyl peptidase-4 inhibitor, % | 0.09 | 0.02 | 0.09 | 0.02 | 0.09 | 0.02 | 0.09 | 0.02 | 0.09 | 0.02 |
|
| ||||||||||
| Glucagon-like peptide-1 receptor agonist, % | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
|
| ||||||||||
| Meglitinide, % | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 |
|
| ||||||||||
| α-Glucosidase inhibitor, % | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 |
|
| ||||||||||
| Insulin, % | ||||||||||
|
| ||||||||||
| Basal | 0.18 | 0.03 | 0.18 | 0.03 | 0.18 | 0.03 | 0.18 | 0.03 | 0.18 | 0.03 |
|
| ||||||||||
| Short-acting bolus | 0.11 | 0.01 | 0.11 | 0.01 | 0.11 | 0.01 | 0.11 | 0.01 | 0.11 | 0.01 |
|
| ||||||||||
| Medium-acting bolus | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 |
HbA1c = glycated hemoglobin.
Appendix Figure 2.
Participant flow chart.
Appendix Table 3.
Model Cost and Utility Parameters
| Definition (Reference) | Value |
|---|---|
| Medication costs (26)* | |
|
| |
| Metformin | $835.18 per year |
|
| |
| Sulfonylurea | $526.81 per year |
|
| |
| Meglitinide | $3623.41 per year |
|
| |
| Thiazolidinedione | $3237.94 per year |
|
| |
| Dipeptidyl peptidase-4 inhibitor | $2891.02 per year |
|
| |
| Glucagon-like peptide-1 receptor agonist | $4047.43 per year |
|
| |
| α-Glucosidase inhibitor | $1124.29 per year |
|
| |
| Bolus insulin† | $110.90 per 1000 units (vial) (rapid) $48.18 per 1000 units (vial) (short) |
|
| |
| Basal insulin† | $48.18 per 1000 units (vial) (intermediate) $106.86 per 1000 units (vial) (long) |
|
| |
| Self-monitoring costs | |
| Noninsulin (55) | $94 per year |
|
| |
| Insulin (26) | $289 per year |
|
| |
| Health care use costs | |
| Outpatient visit: noninsulin (55) | $500.39 per year |
|
| |
| Outpatient visit: insulin (56) | $547.60 per year |
|
| |
| Complication and hypoglycemia costs | |
| Nonfatal cardiac arrest or myocardial infarction (27) | $59 212.99 per event |
|
| |
| History of cardiac arrest or myocardial infarction (27) | $1997.37 per year |
|
| |
| Ischemic heart disease (27) | $22 455.72 per event |
|
| |
| History of ischemic heart disease (27) | $1997.37 per year |
|
| |
| Congestive heart failure (27) | $24 923.06 per event |
|
| |
| History of congestive heart failure (27) | $1997.37 per year |
|
| |
| Stroke (27) | $44 184.46 per year |
|
| |
| History of stroke (27) | $16 302.06 per year |
|
| |
| Amputation (27) | $9484.36 per event |
|
| |
| Foot ulcer (27) | $2252.29 per event |
|
| |
| Blindness (27) | $3002.35 per year |
|
| |
| End-stage renal disease (27) | $82 659.01 per year |
|
| |
| Severe hypoglycemic episode requiring emergency department visit (27) | $1375.29 per event |
|
| |
| Severe hypoglycemic episode requiring hospitalization (27) | $17 286.06 per event |
|
| |
| Severe hypoglycemic episode requiring physician visit (27) | $189.88 per event |
| Utility | |
|
| |
| Diabetes (57) | 0.863 |
|
| |
| Myocardial infarction (29) | 0.945 |
|
| |
| Ischemic heart disease (29) | 0.910 |
|
| |
| Congestive heart failure (29) | 0.892 |
|
| |
| Stroke (29) | 0.836 |
|
| |
| Amputation (29) | 0.720 |
|
| |
| Foot ulcer (58) | 0.830 |
|
| |
| Blindness (29) | 0.926 |
|
| |
| End-stage renal disease with hemodialysis (59) | 0.836 |
|
| |
| End-stage renal disease with peritoneal dialysis (59) | 0.796 |
|
| |
| Mild/moderate hypoglycemia (60) | 0.986 |
|
| |
| Severe hypoglycemia (60) | 0.953 |
|
| |
| Oral diabetes medication (61)‡ | 0.977 |
|
| |
| Insulin (61)‡ | 0.966 |
Average wholesale prices inflated to 2015 U.S. dollars (www.bls.gov/data/inflation_calculator.htm). Generic drug prices were used if available; otherwise, brand-name prices were used.
Basal insulin was assumed to be for partial β-cell replacement at a rate of 0.3 unit per kilogram of body weight per day. Bolus insulin was assumed to be for complete β-cell replacement at a rate of an additional 0.3 unit per kilogram of body weight per day.
A single utility value was used to indicate taking an oral diabetes medication or insulin, regardless of the actual number (or type) of medication being taken.
Appendix Table 4.
Impact Inventory
| Type of Impact | Included in This Reference Case Analysis From Each Perspective | Notes on Sources of Evidence | |
|---|---|---|---|
|
| |||
| Health Care Sector | Societal | ||
| Formal health care sector | |||
| Health | |||
| Health outcomes (effects) | |||
|
| |||
| Longevity effects | Yes | UKPDS OM2, ADA/EASD guidelines | |
|
| |||
| Health-related quality-of-life effects | Yes | Utilities from published literature | |
|
| |||
| Other health effects | No | ||
|
| |||
| Medical costs | |||
| Paid for by third-party payers | Yes | Costs from published literature | |
|
| |||
| Paid for by patients out of pocket | No | ||
|
| |||
| Future related medical costs | Yes | Costs from published literature | |
|
| |||
| Future unrelated medical costs | No | ||
|
| |||
| Informal health care sector | |||
| Health | |||
| Patient-time costs | No | ||
|
| |||
| Unpaid caregiver-time costs | No | ||
|
| |||
| Transportation costs | No | ||
| Non–health care sectors | |||
|
| |||
| Productivity | No | ||
|
| |||
| Consumption | No | ||
|
| |||
| Social services | No | ||
|
| |||
| Legal or criminal justice | No | ||
|
| |||
| Education | No | ||
|
| |||
| Housing | No | ||
|
| |||
| Environment | No | ||
ADA = American Diabetes Association; EASD = European Association for the Study of Diabetes; UKPDS OM2 = United Kingdom Prospective Diabetes Study Outcomes Model 2.
Appendix Table 5.
One-Way Sensitivity Analyses: Individualized Versus Uniform Intensive Control
| Scenario | Incremental Lifetime Costs, $* | Incremental Life-Years | Incremental QALYs |
|---|---|---|---|
| Medication changes HbA1c level | |||
|
| |||
| By 1.2% | −12 574 | −0.17 | 0.06 |
|
| |||
| By 0.8% | −12 520 | −0.08 | 0.14 |
| Medication increases hypoglycemic event rates | |||
|
| |||
| By 20% more | −13 443 | −0.11 | 0.10 |
|
| |||
| By 20% less | −13 376 | −0.09 | 0.10 |
| Severe hypoglycemia increases health care use | |||
|
| |||
| By 20% more | −13 983 | −0.16 | 0.06 |
|
| |||
| By 20% less | −12 911 | −0.16 | 0.06 |
| Costs of microvascular complications | |||
|
| |||
| 20% more | −12 993 | −0.13 | 0.07 |
|
| |||
| 20% less | −13 228 | −0.09 | 0.11 |
| Costs of macrovascular complications | |||
|
| |||
| 20% more | −13 739 | −0.14 | 0.08 |
|
| |||
| 20% less | −13 443 | −0.10 | 0.11 |
| Costs of hypoglycemic events | |||
|
| |||
| 20% more | −13 675 | −0.13 | 0.08 |
|
| |||
| 20% less | −13 182 | −0.10 | 0.10 |
| Costs of diabetes medications | |||
|
| |||
| 20% more | −15 504 | −0.08 | 0.12 |
|
| |||
| 20% less | −10 084 | −0.12 | 0.09 |
| Disutility due to microvascular complications | |||
|
| |||
| 20% more | −13 373 | −0.10 | 0.10 |
|
| |||
| 20% less | −13 404 | −0.08 | 0.04 |
| Disutility due to macrovascular complications | |||
|
| |||
| 20% more | −13 560 | −0.11 | 0.11 |
|
| |||
| 20% less | −13 600 | −0.16 | 0.08 |
| Disutility due to hypoglycemic events | |||
|
| |||
| 20% more | −13 295 | −0.10 | 0.10 |
|
| |||
| 20% less | −13 134 | −0.12 | 0.10 |
| Discount rate | |||
|
| |||
| 1% annually | −13 826 | −0.12 | 0.09 |
|
| |||
| 5% annually | −12 563 | −0.09 | 0.11 |
| Combination of utilities: additive method | Not applicable | Not applicable | 0.12 |
HbA1c = glycated hemoglobin; QALY = quality-adjusted life-year.
Expressed in 2015 U.S. dollars. Negative values indicate cost savings from individualized vs. uniform intensive control.
Diabetes Medication Algorithm
We assumed that HbA1c values would drift upward over time to create a need for additional medications over the patient’s lifetime and in keeping with the known natural history of diabetes (53). We used a risk equation from the UKPDS OM1 to model the change in HbA1c level over time (11). For patients who had HbA1c values above their glycemic goal, medications were added sequentially. Each addition of a medication class was assumed to decrease HbA1c level by 1.0% (21–23). Only 1 medication class was added per year. Medication classes were added on the basis of guidelines from ADA/EASD (7). These guidelines include metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, GLP-1–receptor agonists, basal insulin, and bolus insulin as medication options. We followed all recommended combinations and restrictions. Insulin was dosed at 0.3 unit per kilogram of the patient’s measured weight; if patients were receiving combination basal and bolus insulin, it was assumed that they required an additional 0.3 unit per kilogram and that 50% of their dose was basal insulin and 50% was bolus insulin. It was also assumed that patients would be started on analogue insulins with syringes (not pens, which would have increased uniform intensive control costs more than the individualized control strategy). If patients developed end-stage renal disease or had it at baseline, use of all noninsulin medications was discontinued.
Second- and third-line medication classes were added in proportions equivalent to those reported in a national study of diabetes medication use that was based on data from NAMCS (National Ambulatory Medical Care Survey) 2012 (20). The NAMCS paper includes the number and percentage of visits with 2 or more diabetes medication classes used in the United States. We used these percentages as the basis for the probability that use of each medication class could be started. To use an example from NAMCS, among visits where 2 or more medications were used, metformin was used in combination with sulfonylureas in 30.6% of visits, thiazolidinediones in 10.1% of visits, DPP-4 inhibitors in 20.4% of visits, GLP-1–receptor agonists in 3.3% of visits, and basal insulin in 11.0% of visits. To determine the second medication that would be started, we adjusted the percentages to total 100% for each medication class (for example, for metformin, sulfonylureas would be started 40.6% of the time, thiazolidinediones would be started 13.4% of the time, DPP-4 inhibitors would be started 27.0% of the time, GLP-1–receptor agonists would be started 4.4% of the time, and basal insulin would be started 14.6% of the time).
As an example, if a hypothetical patient in the model was receiving metformin in year 1 and their HbA1c level increased above the goal in year 5, use of a second medication would be started in year 6. According to the 2015 ADA/EASD guidelines, the medication class options for this patient should be sulfonylureas, thiazolidinediones, DPP-4 inhibitors, sodium–glucose cotransporter-2 inhibitors, GLP-1–receptor agonists, or basal insulin. Given the prevalence of medication use, this hypothetical patient would have a 40.6% probability of starting a sulfonylurea, a 13.4% probability of starting a thiazolidinedione, a 27.0% probability of starting a DPP-4 inhibitor, a 4.4% probability of starting a GLP-1–receptor agonist, and a 14.6% probability of starting basal insulin.
Imputation of Missing Data
To include NHANES participants with missing data, we used multiple imputation using multivariate normal distribution (Markov-chain Monte Carlo) to impute missing data. We chose this method because almost all of our variables of interest were continuous (54). We used the variables that were necessary for the simulation model as the variables for the imputation models (age, sex, race/ethnicity, age at diabetes diagnosis, duration of diabetes, body mass index, estimated glomerular filtration rate, hemoglobin level, HbA1c level, leukocyte count, high-density lipoprotein and low-density lipoprotein cholesterol level, heart rate, systolic blood pressure, and smoking status). The proportion of missing observations for each variable was as follows: body mass index, 2% (n = 12); HbA1c level, 0.3% (n = 2); high-density lipoprotein cholesterol level, 3% (n = 19); low-density lipoprotein cholesterol level, 52% (n = 294); heart rate, 3% (n = 15); systolic blood pressure, 3% (n = 18); and smoking status, 49% (n = 276). The following variables had no missing data: age, sex, race/ethnicity, age at diabetes diagnosis, duration of diabetes, hemoglobin level, leukocyte count, and estimated glomerular filtration rate. We used the average of 5 imputed values to estimate missing data (Appendix Table 2). The imputation was conducted using SAS, version 9.4.
For peripheral vascular disease and atrial fibrillation, we used the prevalence of these conditions to determine their event probabilities by age and sex. Then, for each model iteration, we compared the age- and sex-based probability to a random number from a uniform distribution between 0 and 1, and if the number was less than the probability, the iteration was assumed to have the condition (Monte Carlo simulation).
Number of Model Iterations
To determine the number of iterations per participant, we assessed the number of iterations needed for the major outcomes of total costs, life-years, and QALYs. We used the convergence feature in @Risk to assess the number of iterations needed for our outcome. We set parameters of a 3% convergence tolerance and a 95% confidence level. Convergence was achieved at 2400 iterations for total costs and 1000 iterations for life-years and QALYs. We chose to model 2500 iterations per participant.
Appendix Figure 1.
U.S. Type 2 Diabetes Policy Model.
All individual NHANES participants were simulated to receive both glycemic interventions, and 2500 independent simulation replications were performed for each patient. CHF = congestive heart failure; ESRD = end-stage renal disease; HbA1c = glycated hemoglobin; IHD = ischemic heart disease; MI = myocardial infarction; NHANES = National Health and Nutrition Examination Survey.
Footnotes
Note: Dr. Laiteerapong and Ms. Cooper had access to the data in the study. Dr. Laiteerapong takes full responsibility for the integrity of the data and the accuracy of the data analysis. The authors completed the CHEERS (Consolidated Health Economic Evaluation Reporting Standards) checklist (Supplement, available at Annals.org).
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17-0537.
Reproducible Research Statement: Study protocol and data set: Not available. Statistical code: Readers with questions about the simulation model used in this analysis may contact Dr. Laiteerapong (nlaiteer@medicine.bsd.uchicago.edu). The model is not available without written agreement with the authors.
Author Contributions: Conception and design: N. Laiteerapong, M.R. Skandari, R.N. Naylor.
Analysis and interpretation of the data: N. Laiteerapong, J.M. Cooper, P.M. Clarke, A.N. Winn.
Drafting of the article: N. Laiteerapong, M.R. Skandari, A.N. Winn.
Critical revision of the article for important intellectual content: N. Laiteerapong, M.R. Skandari, P.M. Clarke, A.N. Winn, R.N. Naylor, E.S. Huang.
Final approval of the article: N. Laiteerapong, J.M. Cooper, M.R. Skandari, P.M. Clarke, A.N. Winn, R.N. Naylor, E.S. Huang.
Statistical expertise: N. Laiteerapong, M.R. Skandari, A.N. Winn.
Obtaining of funding: N. Laiteerapong, E.S. Huang.
Administrative, technical, or logistic support: N. Laiteerapong, J.M. Cooper, A.N. Winn, E.S. Huang.
Collection and assembly of data: N. Laiteerapong, J.M. Cooper, A.N. Winn.
Current author addresses and author contributions are available at Annals.org.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287:2542–51. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, et al. ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174:1116–24. doi: 10.1001/jamainternmed.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the Diabetes and Aging Study. JAMA Intern Med. 2014;174:251–8. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathak RD, Schroeder EB, Seaquist ER, Zeng C, Lafata JE, Thomas A, et al. SUPREME-DM Study Group. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005–2011. Diabetes Care. 2016;39:363–70. doi: 10.2337/dc15-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Laiteerapong N, John PM, Nathan AG, Huang ES. Public health implications of recommendations to individualize glycemic targets in adults with diabetes. Diabetes Care. 2013;36:84–9. doi: 10.2337/dc11-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 10.Ginde AA, Espinola JA, Camargo CA., Jr Trends and disparities in U.S. emergency department visits for hypoglycemia, 1993–2005. Diabetes Care. 2008;31:511–3. doi: 10.2337/dc07-1790. [DOI] [PubMed] [Google Scholar]
- 11.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. UK Prospective Diabetes Study (UKDPS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–59. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 12.Palmer AJ, Clarke P, Gray A, Leal J, Lloyd A, Grant D, et al. Mount Hood 5 Modeling Group. Computer modeling of diabetes and its complications: a report on the Fifth Mount Hood Challenge Meeting. Value Health. 2013;16:670–85. doi: 10.1016/j.jval.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Lundqvist A, Steen Carlsson K, Johansen P, Andersson E, Willis M. Validation of the IHE Cohort Model of Type 2 Diabetes and the impact of choice of macrovascular risk equations. PLoS One. 2014;9:e110235. doi: 10.1371/journal.pone.0110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17:714–24. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 16.Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care. 2016;39:1089–100. doi: 10.2337/dc16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edridge CL, Dunkley AJ, Bodicoat DH, Rose TC, Gray LJ, Davies MJ, et al. Prevalence and incidence of hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PLoS One. 2015;10:e0126427. doi: 10.1371/journal.pone.0126427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997–2012. Diabetes Care. 2014;37:985–92. doi: 10.2337/dc13-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35–48. [PMC free article] [PubMed] [Google Scholar]
- 23.Mearns ES, Saulsberry WJ, White CM, Kohn CG, Lemieux S, Sihabout A, et al. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in type 2 diabetes: a network meta-analysis. Diabet Med. 2015;32:1530–40. doi: 10.1111/dme.12837. [DOI] [PubMed] [Google Scholar]
- 24.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 26.Red Book. Pharmacy’s Fundamental Reference. New York: Thomson Reuters; 2011. [Google Scholar]
- 27.Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012) J Med Econ. 2014;17:176–83. doi: 10.3111/13696998.2014.882843. [DOI] [PubMed] [Google Scholar]
- 28.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17:462–70. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Making. 2002;22:340–9. doi: 10.1177/0272989X0202200412. [DOI] [PubMed] [Google Scholar]
- 30.Hanmer J, Vanness D, Gangnon R, Palta M, Fryback DG. Three methods tested to model SF-6D health utilities for health states involving comorbidity/co-occurring conditions. J Clin Epidemiol. 2010;63:331–41. doi: 10.1016/j.jclinepi.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 33.Elbasha EH, Chhatwal J. Myths and misconceptions of within-cycle correction: a guide for modelers and decision makers. Pharmacoeconomics. 2016;34:13–22. doi: 10.1007/s40273-015-0337-0. [DOI] [PubMed] [Google Scholar]
- 34.Fu AZ, Kattan MW. Utilities should not be multiplied: evidence from the preference-based scores in the United States. Med Care. 2008;46:984–90. doi: 10.1097/MLR.0b013e3181791a9c. [DOI] [PubMed] [Google Scholar]
- 35.Fairchild PC, Nathan AG, Quinn M, Huang ES, Laiteerapong N. Patients’ future expectations for diabetes and hypertension treatments: “Through the diet … I think this is going to go away”. J Gen Intern Med. 2017;32:49–55. doi: 10.1007/s11606-016-3871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu S, Shankar V, Yudkin JS. Comparative effectiveness and cost-effectiveness of treat-to-target versus benefit-based tailored treatment of type 2 diabetes in low-income and middle-income countries: a modelling analysis. Lancet Diabetes Endocrinol. 2016;4:922–32. doi: 10.1016/S2213-8587(16)30270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 38.Yakubovich N, Gerstein HC. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia. Circulation. 2011;123:342–8. doi: 10.1161/CIRCULATIONAHA.110.948489. [DOI] [PubMed] [Google Scholar]
- 39.McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the Cardiff Diabetes Model. Cost Eff Resour Alloc. 2015;13:12. doi: 10.1186/s12962-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu A, Meltzer D. Value of information on preference heterogeneity and individualized care. Med Decis Making. 2007;27:112–27. doi: 10.1177/0272989X06297393. [DOI] [PubMed] [Google Scholar]
- 41.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes Care. 2006;29:259–64. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc. 2008;56:1183–90. doi: 10.1111/j.1532-5415.2008.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care. 2008;46:275–86. doi: 10.1097/MLR.0b013e318158af40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–34. doi: 10.1001/jamainternmed.2014.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33:1859–64. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006;29:2137–9. doi: 10.2337/dc06-1120. [DOI] [PubMed] [Google Scholar]
- 47.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 48.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. LEADER Steering Committee. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 50.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–72. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, Strotmeyer ES, et al. Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300–6. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McEwan P, Gordon J, Evans M, Ward T, Bennett H, Bergenheim K. Estimating cost-effectiveness in type 2 diabetes: the impact of treatment guidelines and therapy duration. Med Decis Making. 2015;35:660–70. doi: 10.1177/0272989X14565821. [DOI] [PubMed] [Google Scholar]
Web-Only References
- 53.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 54.Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171:624–32. doi: 10.1093/aje/kwp425. [DOI] [PubMed] [Google Scholar]
- 55.National Center for Health Statistics. National Health Interview Survey Diabetes Supplement. Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 56.Centers for Medicare & Medicaid Services. Physician Fee Schedule - January 2012 release. Baltimore: Centers for Medicare & Medicaid Services; 2012. [on 10 November 2017]. Accessed at www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files-Items/CMS1254038.html. [Google Scholar]
- 57.Harris S, Mamdani M, Galbo-Jørgensen CB, Bøgelund M, Gundgaard J, Groleau D. The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes. 2014;38:45–52. doi: 10.1016/j.jcjd.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14:217–30. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 59.Wasserfallen JB, Halabi G, Saudan P, Perneger T, Feldman HI, Martin PY, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1594–9. doi: 10.1093/ndt/gfh175. [DOI] [PubMed] [Google Scholar]
- 60.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, Mc-Ewan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22:1523–34. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 61.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25:2238–43. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]