Highlights
-
•
Characteristics of free and immobilized catalase.
-
•
The kinetic parameters and stability of free and immobilized catalase were studied.
-
•
FTIR spectra of free and immobilized catalase were studied.
Keywords: Catalase, Immobilization, Chitosan, Chitosan–bentonite, Hydrogen peroxide
Abstract
The immobilization of catalase onto chitosan and chitosan–bentonite was investigated and immobilization yield of 95.91 and 95.26 was obtained respectively. The optimum pH and temperature were found as 7.5 and 8.0 at 40 °C for free and immobilized enzyme. The value of Vmax decreased by 33,000–26,300, 24,500 μmol (min mg protein)−1 and Km increased by 12.5–25 and 20 mM for free and immobilized on chitosan and chitosan–bentonite respectively. The thermal stability, half life, FTIR analyses of the beads was also performed in order to characterise the structural differences. The remaining immobilized catalase onto chitosan and chitosan–bentonite activity was 50% and 70% after 20 cycles respectively. The storage stability were found as 22%, 60%, and 70% from its original activity in case of free enzyme and immobilization of chitosan, chitosan–bentonite beads respectively after 60 days.
1. Introduction
Catalase (EC 1.11.1.6) enzyme is an oxidoreductase enzyme as it plays a crucial role in quenching the reactive oxygen species (ROS), i.e. hydrogen peroxide, often produced as a by-product of aerobic respiration [1], by converting it into oxygen and water. Hydrogen peroxide metabolism is mainly regulated by this enzyme. Catalase is a common enzyme found in nearly all living organisms aerobic as well as anaerobic. It has one of the highest turnover of all enzymes as it has the capacity to decompose more than one million molecules of hydrogen peroxide per molecule of enzyme [[2], [3], [4], [5]]. Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long. It contains four porphyrin haem (iron) groups that allow the enzyme to react with the hydrogen peroxide. Catalase has a fairly broad range of working from optimum pH between 4–11 [[6], [7], [8]]. Catalase is usually located in a cellular, bipolar environment organelle called the peroxisome. As this enzyme is found in mainly all organisms (aerobic and anaerobic), it has been exploited in many applications including food processing, textile, paper, pharmaceutical industry, medical areas, and also in the field of bioremediation as one of the upcoming areas of its application [[9], [10], [11], [12], [13]].
Catalase has been immobilized on various supports for use in the numerous industrial prospects. The immobilization of catalase onto chitosan beads is one of the mainly used techniques because of its being simple and cheap [14]. Chitosan proves to be attractive for the purpose of enzyme immobilization because of its inert nature; moreover it is an inexpensive and hydrophilic support material and is also biocompatible, biodegradable and non toxic, making it one of the main immobilization methods [[15], [16], [17]]. Amino groups that are present on chitosan facilitate the binding of the enzyme covalently to the support [18,19]. The literature suggests that the immobilization can be either by the entrapment of the enzyme in the chitosan beads, or by the covalent binding to the chitosan transparent films [[20], [21], [22], [23]]. The use of glutaraldehyde as a cross linked for chitosan beads are more applicable in biochemical engineering due to their good biocompatibility and mechanical strength. However their minor operational defect like density (close to water) causing, it floats easily and surface is also soft. To overcome this problem chitosan was mixed with some inactive materials like clays [24].
Bentonite is one of the clay minerals that have been proven to be of great importance for the immobilization of catalase. Bentonite is a sedimentary rock with a three layered structure (smectites) like nontronite, montmorillonite, etc. and the presence of quartz, zeolites, feldspars are also frequently found in it. Bentonite is an inexpensive matrix and in addition to its low cost, it has several other advantages that include its high chemical reactivity, low toxicity, and availability of functional groups that allow the easy fixation of the enzyme [25,26].
The main aim and objective of this research paper was to form a cost effective immobilization using natural clay ingredient i.e. bentonite for the formation of the chitosan beads instead of glutaraldehyde. The chitosan-bentonite beads were formed for the purpose of catalase immobilization and the stability and working of the catalase immobilized chitosan-bentonite complex beads was compared to the glutaraldehyde reinforced chitosan beads, so that these can replace the commonly used catalase beads formulations requiring glutaraldehyde as a cross-linker. Therefore, the activity, pH, temperature dependence, kinetics and other physical parameters of both free and immobilized enzyme were studied.
2. Materials and methods
2.1. Chemicals used
Hydrogen peroxide was used as a substrate and obtained from Fisher Scientific, Bovine liver catalase (15,200 U/mg), Glutaraldehyde, Acetic acid, Chitosan, Bentonite, NaOH were obtained from HIMEDIA.
2.2. Immobilization of catalase on chitosan beads
The chitosan (2.5 g) was dissolved in 1% acetic acid (100 ml) solution prepared by mixing one hour at room temperature. After the completion of the mixing, the beads were formed by pouring the solution drop wise into 2% of NaOH. Chitosan beads were formed in the precipitating solution [16,17]. To prepare the crosslinking solution, 200 μl of glutaraldehyde was added to 100 ml of distilled water and mixed thoroughly. The beads prepared were added into this crosslinking solution and kept at room temperature for 3 h. The reinforced beads were then collected in a sieve and stored at room temperature before immobilization of the enzymes onto the beads. The immobilization of the enzyme onto chitosan beads were immersed in 10 ml of catalase solution (2.0 mg ml−1) in 0.5 mM Phosphate buffer (pH 7.5). The beads were kept in this solution for 5 h at 25 °C in a rotating incubator. The immobilized beads were washed with cold phosphate buffer (pH 7.5) thoroughly and stored for further study [4,16,20].
2.3. Immobilization of catalase on chitosan–bentonite complex beads
The chitosan (2.5 g) solution was prepared by dissolving 1% acetic acid (100 ml). In this 50 mg bentonite was separately activated by mixing it with 10 ml catalase (2.0 mg ml−1) in 0.5 mM phosphate buffer (pH 7.5). These two solutions prepared separately were then mixed together in a beaker and subjected to magnetic stirring for 4 h at 4 °C to form a uniform mixture. After the completion of the mixing, the beads were formed by pouring the solution drop wise into 2 M NaOH precipitating solution. Thereafter the beads were washed with cold phosphate buffer (pH 7.5) [2,28].
2.4. Enzyme activity of free and immobilized catalase
The catalase activity was determined by spectrophotometrically by measuring the decrease in absorbance of H2O2 at 240 nm in a reaction mixture containing 3.0 ml of substrate (10 mM H2O2 in pH 7.5 phosphate buffer) and 1.0 ml of the Catalase (2 mg ml−1) solution. The reaction mixture was kept at 40 °C temperature for 15 min and stop by adding 0.5 ml of 1 M HCL. For activity of immobilized enzyme (approximate 25 mg) were mixed substrate as above prepared at 40 °C temperature for 15 min and stop by adding 0.5 ml of 1 M HCL after removal of the beads. The activity of free and immobilized catalase were expressed as μmol min−1 and μmol (min mg protein)−1 respectively [16,17]. One unit of enzyme activity was defined as the amount of enzyme which converts 1 μmol H2O2 in to product per minute at pH 7.5 and 40 °C.
2.5. Determination of total protein and immobilization yield
Total protein concentration was determined according to Lowry’s method using Bovine serum albumin (BSA) as a standard [29]. The amount of bound protein was determined by subtracting the amount of protein in supernatant after immobilization from the total amount of protein used for immobilization. The protein loading and immobilization efficiency of immobilization onto chitosan and chitosan bentonite was calculated using formula [30]
2.6. Effect of pH and temperature on enzyme activity
The effect of pH on free and immobilized catalase activity was carried out at different pH values ranging from 3 to 9 using 0.5 M citric acid (pH 3.0–4.0), 0.5 M sodium acetate (pH 4.0–6.0), 0.5 M sodium phosphate (pH 6.0–8.0), 0.5 M Tris−HCl (pH 8.0–9.0), 0.5 M glycine–NaOH (pH 9.0). The highest value of enzyme activity in each set was assigned as 100% activity [31,32]. The effect of temperature on free and immobilized catalase was tested by performing the activity in the various temperatures ranging from 5 to 60 °C with the interval of 10 °C.
2.7. Thermal stability and half life of enzyme
The thermal stability and half life of the free enzyme and the enzyme immobilized on the two beads were determined by monitoring the activity of catalase within temperature range of 20–60 °C at time intervals of 0, 30, 45 and 60 min respectively against H2O2 as a substrate [20]. The results were obtained by plotting a graph of −ln E/E0 (E0: Initial enzyme activity and E: enzyme activity at time t) on Y axis against time (t) on the X axis. The slope of the graph gives Kd of the enzyme and t1/2 is calculated as:
2.8. Determination of kinetic parameters
The kinetic parameters of the immobilized catalase and free catalase were determined by measuring the rates of the reaction at various substrate concentrations ranging from 2 to 10 mM at optimum temperature and pH. The kinetic parameters Km and Vmax were calculated from the lineweaver–burk plot [20].
2.9. FT-IR spectroscopy
The characteristic of a molecule can be well determined by the vibrational spectrum of that molecule. It is considered as one of the most fundamental tool for characterisation of a molecule [17]. It serves as an impression for the identification by comparing the spectrum of our unknown sample with previously recorded reference spectra. In the case of chitosan beads prepared for immobilization as well as chitosan-bentonite complex beads, Fourier Transform Infrared Spectroscopy (FTIR), is a popular tool for the identification and characterization of various bonds and linkages involved in their preparation. The complexity of infrared spectra in the 1500–500 cm−1 region makes it difficult to assign all the absorption bands as it contains a very complicated series of adsorption because of the manner of all the bending vibrations within the molecule, and because of the unique patterns found there, it is often called the fingerprint region. It is found in the right hand side of the FTIR graph. Absorption bands in the 4000–1500 cm−1 region are usually due to stretching vibrations of the diatomic units, and this is called the group frequency region [22].
2.10. Operational stability (reusability)
The retention of the immobilized catalase activity was tested as described in activity assays of catalase. After each reaction run, the chitosan beads were removed and washed with 0.5 mM phosphate buffer (pH 7.5) to remove any residual substrate within the chitosan beads. After that the chitosan beads were put into fresh reaction medium and enzyme activities were tested at optimal conditions. The process was repeated with twenty times [33].
2.11. Storage stability
The storage stabilities of free and immobilized catalase were stored at 4 °C and the relative activities were measured during 60 days of their storages. The catalase activity of fresh enzyme was taken to be 100% [34].
3. Results and discussions
3.1. Immobilization of catalase onto chitosan and chitosan bentonite beads
As chitosan is considered to be soluble in acidic solutions, the continuous and prolonged exposure of the chitosan beads formed, made via counter-ion precipitation in NaOH solution, may result in gel softening and bead disintegration. The chitosan beads formed in the precipitating solution are therefore subjected to treatment with cross linker glutaraldehyde in many immobilization studies [16,17]. The beads prepared without the use of any cross linker was prepared by forming chitosan–bentonite complex beads. The beads formed were comparable to the cross-linked beads with high mechanical strength and rigidity. The Table 1 shows that protein loading of immobilized catalase onto chitosan and chitosan– bentonite beads were 78.4 and 74.4% and immobilization yield 95.91 and 95.26 respectively. On comparing the two types of the beads formed, it was found that both the beads were approximately same in physical parameters like rigidity, strength and appearance [26,27].
Table 1.
Immobilization of catalase onto chitosan and chitosan bentonite beads.
| Enzyme type | Enzyme activity (Unit) | Protein (mg) | Specific enzyme activity (Unit/mg) | Protein loading (%) | Immobilization yield (%) |
|---|---|---|---|---|---|
| Free enzyme | 16225 | 12.5 | 1298.00 | – | – |
| Catalase immobilized on chitosan beads | 12200 | 9.8 | 1244.90 | 78.4 | 95.91 |
| Catalase immobilized on chitosan–bentonite complex beads | 11500 | 9.3 | 1236.56 | 74.4 | 95.26 |
3.2. Effect of pH on catalase activity
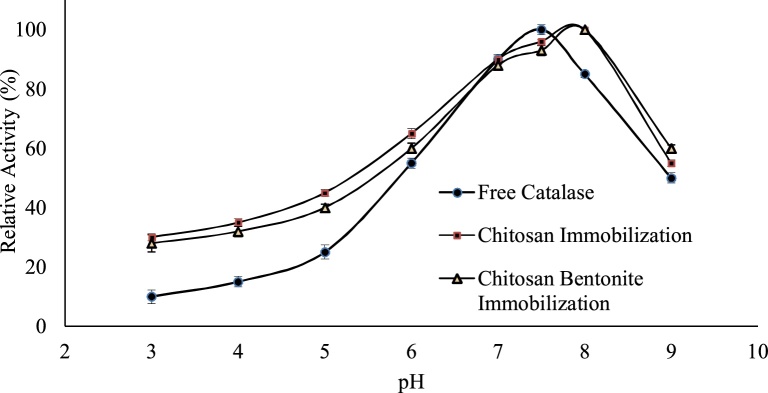
The effect of change in pH concentration on free and immobilized catalase was studied in this work in order to compare the difference in the chemical characterisation of catalase after immobilization [[16], [17], [18], [19]]. The procedure of immobilization of the enzymes commonly has a range of effects on the state of ionisation of the enzyme, its dissociation, and also on the conformation of the enzyme. These changes results in the difference of relationship between the pH, stability and the catalytic activity of the immobilized enzymes. The effect of pH on enzymes activity for free and immobilized forms was studied with 3–9 pH range using various buffers. The results obtained are depicted in the graph further shown by Fig. 1. The optimum activity of catalase is represented as 100% and other activities are articulated relative to this optimum activity of the enzyme. Free enzyme exhibits maximum activity in the pH range of (7–8) with optimum activity at pH of 7.5. Decrease in the enzymatic activity at pH 3–5 and 8.5–9.0 and observed for both free and immobilized enzyme; however, maximum immobilized enzyme activity at pH 8. This therefore shows that the process of immobilization provides a structural stability to catalase, thus preventing an irreversible unfolding of the enzymatic protein molecules resulting in pH stability of the immobilized catalase when compared to free catalase. The inhibition of the activity of catalase enzyme in the lower pH ranges 3–4, may be because of two reasons, i.e., firstly a lower loading and a possible change in the enzyme’s conformation because of an unfavourable distribution of charge on the amino acid residues of the enzyme. Secondly a change in the pH environment of the enzyme can affect the intra-molecular hydrogen bonding therefore leading to a inaccurate conformation of the enzyme that can reduce the activity of the enzyme [30].
Fig. 1.
Effect of pH on free catalase, catalase immobilized onto chitosan beads and chitosan–bentonite complex beads.
3.3. Effect of temperature on catalase activity
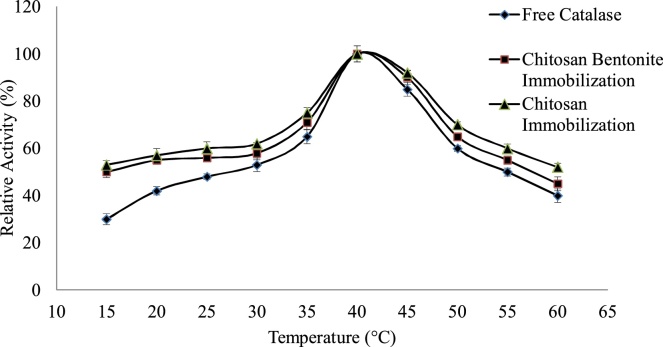
The effect of temperature on the activity of free and immobilized catalase enzyme was studied in this work by carrying out the reaction of catalase enzyme both free and immobilized onto the beads by changing the incubation temperature of the enzyme in the range of 15–60 °C. The temperature was maintained in a water bath [[20], [21], [22]]. The results are shown in the Fig. 2. The optimum temperature for both free and immobilized enzyme is around 40 °C. It is clear that at lower temperature that is between 15–30 °C the immobilized enzyme shows more stability as compared to free catalase. The increases the temperature enzyme activity increases and after 40 °C decreases for both cases. The temperature dependence of the two beads is approximately the same and comparable which is our required result, thus making the use of chitosan–bentonite complex beads are also suitable option for immobilization.
Fig. 2.
Effect of Temperature on free catalase, catalase immobilized onto chitosan beads and chitosan–bentonite complex beads.
3.4. Study of kinetic parameters
The velocity of any enzyme reaction is conclusively influenced by the concentration of the substrate of that particular enzyme. This relationship obeys the Michaelis–Menten kinetics, given by the following equation,
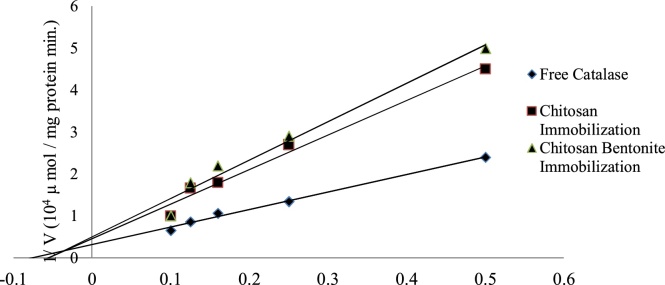
Where Km represents the Michaelis–Menten constant and Vmax gives the maximum reaction rate. The Km is calculated through the Lineweaver–Burk plot which shows a linear relationship between 1/V (on y axis) and 1/S (on x axis) respectively. The kinetics of our study were analysed by changing the substrate concentration (s), i.e. the concentration of hydrogen peroxide ranging from 2 to 10 mM H2O2 for both free and immobilized catalase. The Km calculated for free catalase, catalase immobilized on glutaraldehyde treated chitosan beads and a chitosan-bentonite complex bead that is studied in this work were 12.5, 25 and 20 mM respectively. These results indicate that the Km value of free enzyme is lower than that of the immobilized enzyme systems formulated. This result indicates that free enzyme catalase, has a higher affinity for its substrate i.e. H2O2 as compared to the catalase enzyme that is immobilized on the beads. Further, Vmax of free catalase, immobilized catalase on glutaraldehyde reinforced chitosan beads and catalase immobilized on chitosan-bentonite complex beads were calculated as 33,000, 26,300, and 24,500 μmol (min mg protein)−1 respectively from the graphs (Fig. 3). The reason for Vmax of free enzyme being higher than the immobilized systems can be explained on the basis of conformational change of the immobilized enzyme when supported on a support, (the two types of chitosan beads in this case). The Km and Vmax values obtained are depicted in the Table 2.
Fig. 3.
Lineweaver-Burk plots of free Catalase, Catalase immobilized onto chitosan beads and chitosan–bentonite complex beads.
Table 2.
Km and Vmax values for free and immobilized systems.
| Enzyme type | Km (mM) | Vmax μmol (min mg protein)−1 |
|---|---|---|
| Free enzyme | 12.5 | 33,000 |
| Catalase immobilized on chitosan beads | 25 | 26,300 |
| Catalase immobilized on chitosan-bentonite complex beads | 20 | 24,500 |
The results also show that the Km and Vmax values of both the beads are comparable and almost nearly the same with a slight difference. This shows that both the beads show almost the same enzyme kinetic characteristic which is a desirable result. As compared to the results of reported paper [20], where the Km was reported as 35 mM and Vmax as 32,000 μmol (min mg protein)−1 for free enzyme and Km of 77.5 mM, Vmax 122 μmol (min mg protein)−1 for catalase immobilized on chemically crosslinked chitosan beads, the results above show an improved working of the catalase enzyme in both free and immobilized forms.
3.5. Thermal stability and half life
The thermal stability and half life for free and immobilized catalase were determined at different temperatures, between 20–60 °C and at time intervals of 0, 30, 45, and 60 min respectively. The results are shown in the Fig. S1 for temperatures of 20 °C–60 °C. Table 3 gives the value of Kd as calculated from the slope of the graphs and t1/2 for all the temperature profiles.
Table 3.
Deactivation constant (Kd) and half life (t1/2) of enzyme for free and immobilized.
| Temperature (°C) | Kd (Free catalase) | t1/2 (Free catalase) in minutes | Kd (Glutaraldehyde reinforced chitosan beads) | t1/2 (Glutaraldehyde reinforced chitosan beads) in minutes | Kd (Chitosan-bentonite complex beads) | t1/2 (Chitosan-bentonite complex beads) in minutes |
|---|---|---|---|---|---|---|
| 20 | 0.01 | 69.3 | 0.0076 | 91.1 | 0.0078 | 88.8 |
| 30 | 0.009 | 77 | 0.0067 | 102 | 0.007 | 98.6 |
| 40 | 0.007 | 99 | 0.0057 | 121.6 | 0.0055 | 126 |
| 50 | 0.01 | 69.3 | 0.007 | 99 | 0.008 | 86.6 |
| 60 | 0.013 | 53.3 | 0.008 | 86.5 | 0.0092 | 75.3 |
According to the results depicted in the Table 3, it is clear that immobilization of the catalase onto both the beads provides more stability as compared to free catalase as the half life of enzyme (t1/2). The t1/2 values at temperature of 40 °C is highest for both free and catalase immobilized onto the beads i.e. glutaraldehyde reinforced chitosan beads, and the chitosan-bentonite complex beads are 99, 12.6 and 126 min respectively. Even at 30 °C, the t1/2 values are quite high in compare to 50 °C, so that suggesting range of temperature between 30–40 °C is quite suitable for working of this enzyme with minimal damage. At higher temperatures, the enzyme is degraded easily thus showing lower values of t1/2, due to thermal degradation and denaturation of the enzyme.
3.6. FTIR spectra
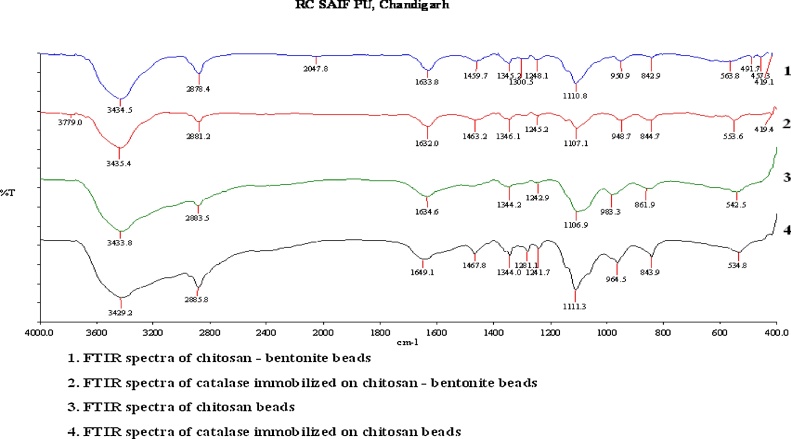
The FTIR analysis was conducted to characterize the functional groups present in both blank and catalase immobilized beads in order to compare their characteristics. The FTIR Spectrophotometer used was Perkin Elmer, USA having model as Spectrum 400 FT-IR / FIR Spectrometer available in the CIL laboratories in Panjab university chandigarh. The Fig. 4-3, 4 shows the comparison between the FTIR spectra of the glutaraldehyde reinforced chitosan blank beads and catalase immobilized beads respectively. The Fig. 4-1, 2 shows the comparison between the FTIR spectra of the chitosan–bentonite blank beads and catalase immobilized beads respectively. The FTIR spectra of the chitosan beads reinforced with glutaraldehyde (Fig. 4-3, 4) show adsorption bands at 935 and 1106 cm−1. These bands are attributed to the monosaccharide structure configuration found in the polymer i.e. chitosan. The presence of a band at 1344.95 cm−1 indicates the characteristic bending vibrations of the N—H groups present in chitosan. Weak adsorption band between the frequencies of 3100 and 2400 cm−1 indicates that the beads have a chelate structure [22]. In the FTIR spectra of the catalase immobilized beads, the band at the frequency of 1649 cm−1 indicates the presence of catalase in the beads. Bovine catalase (CAT Lyophilized powder) is mainly in the β-sheet form and this enzyme has particularly several number of tryptophan and histidine residues in its structural conformation [17]. Previous studies in the literature showed that β sheet structure was observed between the ranges of 1615–1650 cm−1. The parallel β-sheet structure that is not common in synthetic polypeptides leads to an amide adsorption near 1650 cm−1 frequency. As shown in the FTIR spectra of the enzyme immobilized chitosan beads, the band appears around 1649 cm−1, thus indicating the presence of catalase in the beads. This band therefore indicates the binding of the protein to the matrix of the beads. The adsorption bands between 1500–600 cm−1 in both the FTIR graphs indicate the presence of C—N single bond formed as a result of the crosslinking reaction taking place between chitosan-glutaraldehyde, which is comparatively more stronger on the introduction of the enzyme i.e. catalase into the beads indicating more robust and strong immobilization of the enzyme and stability of the beads.
Fig. 4.
FTIR spectra of chitosan–bentonite beads 1, Catalase immobilized on chitosan–bentonite beads 2, chitosan beads 3, Catalase immobilized on chitosan beads 4.
From the FTIR analysis of the chitosan-bentonite beads (Fig. 4-1, 2) formed the bands at frequency 1459 cm−1 for the blank beads and 1463 cm−1 for catalase immobilized beads indicate the presence of bending vibrations of amine chitosan present in the beads. The main bands of the complex chitosan-bentonite, were observed at the frequencies of 3434.60 cm−1 for blank beads and 3485 cm−1 for catalase immobilized beads which represent the deformation vibration of the OH group. The high intensity bands at the frequency of 1110 cm−1 for blank beads, and 1107 cm−1 for the catalase immobilized beads are due to the Si—O stretching vibrations in the beads. The band at 1632 cm−1 in the spectra of the immobilized beads, shows the presence of protein with β-sheet configuration which is attributed to the presence of the enzyme catalase on the beads. This band suggests the immobilization of the enzyme on the beads and attachment of the enzyme on the support matrix [35,36].
3.7. Operational stability (reusability)
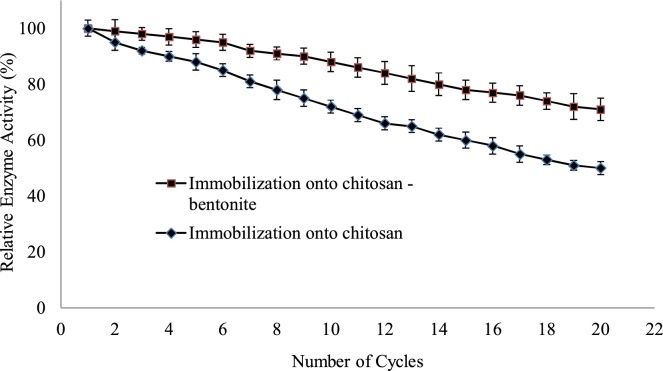
The reusability of immobilized enzymes is the most important features for economical as well as industrial application point of view. The reusability tests were performed as optimum conditions and investigated enzyme activity for twenty times (Fig. 5). The result showed excellent reusability and activity retain after 20 cycles 70% and 50% loss of enzyme activity of the original activity for catalase immobilization of chitosan–bentonite and chitosan beads respectively. Thus the immobilized enzyme activities gradually decreased while reused number increased. These results could be clarified by the inactivation of enzyme caused by the leakage and denaturation of enzyme.
Fig. 5.
Operational stability (reusability) of immobilized enzymes.
3.8. Storage stability
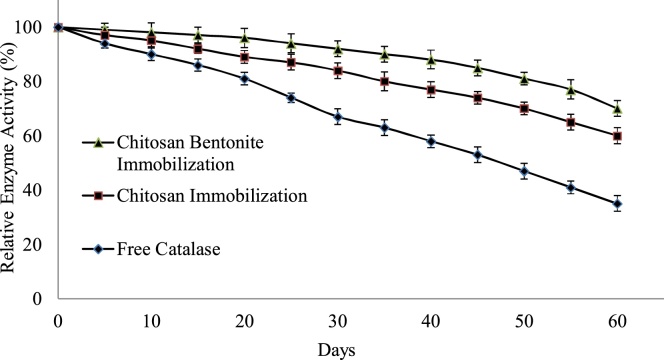
Storage stability for the immobilized enzyme was one of the major key to estimate the properties of enzyme, which can make the immobilized enzyme more applicable than that of the free one. Free and immobilized beads were stored at 4 °C in phosphate buffer (0.5 mM, pH 7.5) and enzyme activity were performed for a period of 60 days. In general storage of enzymes is not suitable for long time in free form in compare to immobilized system. Fig. 6 shows that immobilized enzyme activity slower decrease in compare to free form. After 60 days enzyme activity was calculated and found remaining 22% 60%, and 70% from initial enzyme activity in case of free enzyme and immobilization of chitosan, chitosan–bentonite beads respectively. It is also showing that chitosan–bentonite immobilized beads are more stable and advantageous in compare to chitosan beads.
Fig. 6.
Storage Stability at 4 °C in phosphate buffer (0.5 mM, pH 7.5).
Conflicts of interest
The authors declare no conflict of interest to disclose.
Acknowledgments
The authors with listed names are immediately certified that they have no affiliations with or involvement in any organization or entity with any financial interest or non- financial interest in the subject matter mentioned above.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2018.e00258.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Sooch B.S., Kauldhar B.S., Puri M. Recent insights into microbial catalases: isolation, production and purification. Biotechnol. Adv. 2014;32:1429–1447. doi: 10.1016/j.biotechadv.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Abbott D.A., Suir E., Duong G.H., Hulster E.D., Pronk J.T., Maris A.J. Catalase overexpression reduces lactic acid induced oxidative stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009;75(8):2320–2325. doi: 10.1128/AEM.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamocky M., Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999;72:19–66. doi: 10.1016/s0079-6107(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 4.Alptekin O., Tukel S.S., Yildirim D. Immobilization and characterization of bovine liver catalase on eggshell. J. Serb. Chem. Soc. 2008;73(6):609–618. [Google Scholar]
- 5.Singh Rahul, Wiseman Ben, Deemagarn Taweewat, Jha Vikash, Switala Jacek, Loewen Peter C. Comparative study of catalase-peroxidases (KatGs) Arch. Biochem. Biophys. 2008;471:207–214. doi: 10.1016/j.abb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Chelikani P., Fita I., Loewena P.C. Review: diversity of structures and properties among catalases. CMLS Cell. Mol. Life Sci. 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barynin V.V., Whittaker M.M., Antonyuk S.V., Lamzin V.S., Harrison P.M., Artymiuk P.J. Crystal structure of manganese catalase from Lactobacillus plantarum. Structure. 2001;9:725–738. doi: 10.1016/s0969-2126(01)00628-1. [DOI] [PubMed] [Google Scholar]
- 8.Diaz A., Loewen P.C., Fita I., Carpena X. Thirty years of haem catalases structural biology. Arch. Biochem. Biophys. 2012;525:102–110. doi: 10.1016/j.abb.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Fita I., Rossmann M.G. The active center of catalase. J. Mol. Biol. 1985;185:21–27. doi: 10.1016/0022-2836(85)90180-9. [DOI] [PubMed] [Google Scholar]
- 10.Gromada A., Fiedurek J. Optimization of catalase biosynthesis in submerged cultures of Aspergillus niger mutant. J. Basic Microbiol. 1997;37(2):85–91. doi: 10.1002/jobm.3620370203. [DOI] [PubMed] [Google Scholar]
- 11.Hussein A.A. Purification and characterization of thermo-alkali stable catalase from Bacillus sp. Int. Res. J. Biotechnol. 2012;3(10):207–214. [Google Scholar]
- 12.Northrop J.H. The kinetics of the decomposition of peroxide by catalase. J. Gen. Physiol. 1923;vi:429. doi: 10.1085/jgp.7.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dı´az A., Rangel P., Montes De Oca Y., Lledı´as F., Hansberg W. Molecular and kinetic study of catalase-1, a durable large catalase of Neurospora crassa. Free Radic. Biol. Med. 2001;31(No. 11):1323–1333. doi: 10.1016/s0891-5849(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 14.Grigoras A.G. Catalase immobilization—a review. Biochem. Eng. J. 2017;117:1–20. [Google Scholar]
- 15.Tukel S.S., Alptekin O. Immobilization and kinetics of catalase onto magnesium silicate. Process Biochem. 2004;39:2149–2155. [Google Scholar]
- 16.Arabaci G., Usluoglu A. Catalytic properties and immobilization studies of catalase from Malva sylvestris L. J. Chem. 2012 6. [Google Scholar]
- 17.Cetinus S.A., Sahin E., Saraydin D. Preparation of Cu (II) adsorbed chitosan beads for catalase immobilization. Food Chem. 2009;114:962–969. [Google Scholar]
- 18.Eberhardt A.M., Pedroni V., Volpe M., Ferreira M.L. Immobilization of catalase from Aspergillus niger on inorganic and biopolymeric supports for H2O2 decomposition. Appl. Catal. B: Environ. 2004;47:153–163. [Google Scholar]
- 19.Cetinus S.A., Oztop H.N. Immobilization of catalase on chitosan film. Enzyme Microb. Technol. 2000;26:497–501. doi: 10.1016/s0141-0229(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 20.Cetinus S.A., Oztop H.N. Immobilization of catalase into chemically crosslinked chitosan beads. Enzyme Microb. Technol. 2003;32:889–894. [Google Scholar]
- 21.Shentu J., Wu J., Song W., Jia Z. Chitosan microspheres as immobilized dye affinity support for catalase adsorption. Int. J. Biol. Macromol. 2005;3:42–46. doi: 10.1016/j.ijbiomac.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Cetinus S.A., Oztop H.N., Saraydın D. Immobilization of catalase onto chitosan and cibacron blue F3GA attached chitosan beads. Enzyme Microb.Technol. 2007;41:447–454. [Google Scholar]
- 23.Zang J., Jia S., Liu Y., Wu S., Zhang Y. A facile method to prepare chemically crosslinked and efficient polyvinyl alcohol/chitosan beads for catalase immobilization. Catal. Commun. 2012;27:73–77. [Google Scholar]
- 24.Cengiz S., Çavaş L., Yurdakoç K. Bentonite and Sepiolite as supporting media: immobilization of catalase. Appl. Clay Sci. 2012;65–66:114–120. [Google Scholar]
- 25.Chang M.Y., Juang R.S. Use of chitosan–clay composite as immobilization support for improved activity and stability of β-glucosidase. Biochem. Eng. J. 2007;35:93–98. [Google Scholar]
- 26.Ozturk N., Tabak A., Akgo S., Denizli A. Reversible immobilization of catalase by using a novel bentonite–cysteine (Bent–Cys) microcomposite affinity sorbents. Colloids Surf. A: Physicochem. Eng. Asp. 2008;322(1–3):148–154. [Google Scholar]
- 27.Dong H., Li Y., Li J., Sheng G., Chen H. Comparative study on lipases immobilized onto bentonite and modified bentonites and their catalytic properties. Ind. Eng. Chem. Res. 2013;52(26):9030–9037. [Google Scholar]
- 28.Baysal Z., Bukut Y., Yavuz M., Aytekin C. Immobilization of α-amylase via adsorption onto bentonite/chitosan composite: determination of equilibrium, kinetics and thermodynamic parameters. Starch. 2014;66:484–490. [Google Scholar]
- 29.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 30.Kumari A., Kayastha A.M. Immobilization of soybean (Glycine max) α-amylase onto Chitosan and Amberlite MB-150 beads: optimization and characterization. J. Mol. Catal. B Enzym. 2011;69:8–14. [Google Scholar]
- 31.Jiang B., Zhang Y. Immobilization of catalase on crosslinked polymeric hydrogels effect of anion on the activity of immobilized. Eur. Polym. J. 1993;29:1251–1254. [Google Scholar]
- 32.Divakar K., Suryia M.P., Pennathur G. Purification, immobilization and kinetic characterization of G-x-S-x-G esterase with short chain fatty acid specificity from Lysinibacillus fusiformis AU01. Biocatal. Agric. Biotechnol. 2017;12:131–141. [Google Scholar]
- 33.Wahba M.I. Chitosan-glutaraldehyde activated calcium pectinate beads as a covalent immobilization support. Biocatal. Agric. Biotechnol. 2017;12:266–274. [Google Scholar]
- 34.Okutucu B., Çelem E.B., Önal S. Immobilization of α-galactosidase on galactose-containing polymeric beads. Enzyme Microb. Technol. 2010;46:200–205. [Google Scholar]
- 35.Joshi S., Srivastava R.K. Characterisation and synthesis of chitosan-silica gel and chitosan-bentonite composites for adsorption of heavy metals. Nat. Environ. Pollut. Technol. 2016;15:1237–1240. [Google Scholar]
- 36.Hou C., Wang Y., Zhu H., Wei H. Construction of enzyme immobilization system through metal-polyphenol assisted Fe3O4/chitosan hybrid microcapsules. Chem. Eng. J. 2016;283:397–403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.