Graphical abstract
Size distribution of tellurium nanoparticles produced by Aspergillus welwitschiae F5k.
Keywords: Tellurium, Gamma irradiation, Nano-particles, Biogenic production, Aspergillus welwitschiae
Highlights
-
•
Six fungal isolates have been screened for their ability to produce elemental tellurium nanoparticles (TeNPs).
-
•
The most promising fungal isolate was identified based on molecular basis.
-
•
The most promising fungal isolate has been used for biogenic (enzymatic) production of TeNPs.
-
•
The produced TeNPs have been characterized using DLS, TEM and FTIR.
-
•
The produced TeNPs have been evaluated for antimicrobial activity against pathogenic microorganisms.
Abstract
Tellurium has attracted the attention of many researchers and manufacturers due to its unique properties. Through the current work, six fungal isolates have been screened for their ability to reduce potassium tellurite (K2TeO3) into elemental tellurium nanoparticles (TeNPs). The most promising fungal isolate was identified as Aspergillus welwitschiae and given the accession number (KY766958) based on molecular basis and has been used for biogenic (enzymatic) production of TeNPs. The produced TeNPs have been characterized using DLS, TEM and FTIR. Data showed that, the particle size is 60.80 d.nm with oval to spherical shape. The produced TeNPs have been evaluated for antimicrobial activity at 25 mg/ml. Data revealed antibacterial activity against E. coli and Staphylococcus aureus (MRSA). Evaluation of the effect of γ-irradiation on TeNPs production showed that, the productivity was improved at 1 kGy and suppressed gradually at higher doses.
1. Introduction
Nanotechnology is a fast growing discipline of the high tech economy [[1], [41]]. Products of nanoparticles or nanomaterials (substances with particle sizes less than 100 nm) became a part of everyday life and the focus of many industries including cosmetics, clothing, foods and drug products [[18], [43], [44], [46]]. Although may cause adverse health effects on human, animal and environment, the different properties of nanoparticles compared to their respective bulk materials such as a high surface area to volume ratio, new mechanical, chemical, electrical, optical, magnetic, electro-optical, and magneto-optical [17] made them useful for many applications. Consequently, careful environmental, human and animal health safety assessment [[10], [12]] should be considered during the speedy commercialization of nanotechnology.
Baesman et al. [5] indicated that, tellurium (Te) is a toxic metalloid present in the earth crust at very low levels (0.002 ppm) [5], in seawater at a range from 0.5 pmol/l at depth to 1 to 2 pmol/l in open surface waters and in coastal (up to 7 pmol/l) and fresh (26 pmol/l) rain waters [4].
Tellurium is a semiconductor usually added to copper, tin, gold or silver to produce a certain desired electrical characteristics. Tellurium is used as a secondary vulcanizing agent for rubber, in alloying agents for copper and stainless steel to make them easier to machine and mill and in color glass and ceramics [21]. The relatively low melting points of tellurium (Te) nanosturctures, along with catalytic activity in hydration and oxidation reactions, high thermoelectric along with piezoelectric responses, photoconductivity and nonlinear optical responses have attracted wide attention [[19], [25], [26], [27], [31], [45]]. Nanostuructures of tellurium may find many possible future uses including cancer drug development, antibiotics and in medicine [[8], [23]].
The aim of the current work is to prepare stable-dispersed and pure tellurium nano-particles (TeNPs) in easy, fast and simple method, studying the effect of radiation on productivity and testing for antimicrobial activity.
2. Materials and methods
2.1. Microorganisms and growth
Six Egyptian fungal isolates; coded F1G, F3k, F4Sew, F5k, F7k and F8k (Table 1) have been grown and preserved on Czapek Dox agar. Czapek Dox medium has the following composition [13] (g/l): 30, sucrose, 2, sodium nitrate, 1, dipotassium phosphate, 0.5, magnesium sulphate, 0.5, potassium chloride and 0.01, ferrous sulphate. The final pH was adjusted at 7.3 ± 0.2. Medium was autoclaved at 121 °C for 15 min before inoculation.
Table 1.
Sources of fungal isolation and growth on Czapek's Dox medium supplemented with sodium tellurite.
| Isolate code | Source of isolation | Growth on Czapek's Dox agar medium supplemented with sodium tellurite at*: |
|
|---|---|---|---|
| 3% | 4% | ||
| F1G | Spoiled garlic | +++ | ++ |
| F3k | Abo Zaabal waste composting plant (soil sample) | ++ | + |
| F4Sew | Agricultural sewage water | ++ | + |
| F5k | Kitchen drainage tube | +++ | ++ |
| F7k | Kitchen drainage tube | +++ | ++ |
| F8k | Kitchen drainage tube | +++ | ++ |
Where: +: good growth, ++: very good growth, +++: excellent growth.
2.2. Screening for tellurium nanoparticles-producing fungi
All fungal isolates have been grown on Czapek Dox broth medium at 30 °C for 7 days under 150 rpm shaking condition. At the end of the incubation period, media were filtered through microbial filter (0.45 nm pore size) to get a clear broth. For each organism, 1 ml of the broth medium was added to 1 ml of 4 mmol potassium tellurite (K2TeO3) to get a final concentration of 2 mmol and incubated for 48 h at 30 °C. The presence of black suspended particles indicates elemental tellurium [[3], [42]]. Equal volumes of fungal extracts and distilled water were used as blanks. The most promising tellurium nanoparticles-producing fungal isolate(s) was selected based on the intensity of blackening.
2.3. Molecular based identification of the most promising fungal isolate
The most promising fungal isolate was identified based on 18s-rRNA. Ribosomal RNA was extracted using GeneJet Plant genomic DNA purification Kit (Thermo) according to the manufacturer protocol. Primers ITS1-F (5′TCC GTA GGT GAA CCT TGC GG 3′) & ITS4-B (5′TCC TCC GCT TAT TGA TAT GC 3′) have been used for isolation of the 18s-rRNA. PCR product has been cleaned up using GeneJET™ PCR Purification Kit (Thermo). Sequencing of the PCR product was performed at GATC Company using ABI 3730xl DNA sequencer. The sequence results were processed by using the web-based blasting program, basic local alignment search tool (BLAST), at the NCBI site (http://www.ncbi.nlm.nih.gov/BLAST), and the data were compared with the NCBI/Genebank database [2].
2.4. Characterization of the produced elemental tellurium
The promising fungal isolate (F5k) selected based on production of black suspension have been tested for optical properties using Spectro UV–vis Double Beam UVD 3500, Labomed, Inc USA.
Tellurium nanoparticles produced by the most active fungal isolate, based on spectral analysis, has been analyzed for particle size, morphology and FT-IR. Particles nano-size was measured by dynamic light scattering (DLS) using Zetasizer NANO-ZS (Ver. 7.04, Serial Number: MAL 1074157, Malvern Instruments Ltd., United Kingdom) at the wavelength of 633 nm and a power of 4.0 mW as a light source collecting data at a fixed scattering angle of 173°. TeNPs morphology was determined using JEOL Transmission Electron Microscope (JEM-1230, Japan, with 500.000 x magnification power, 100 KV acceleration voltages and 0.5 nm resolving power)
For Fourier transform infrared (FT-IR) spectra, TeNPs were dried under reduced pressure and the dried sample was packed in KBr-form pellets and spectrum was recorded using FT-IR JASCO 6100, Japan in the range of 400–4000 cm−1.
2.5. Effect of γ-irradiation of potassium tellurite/broth culture mixture
For studying the effect of γ-irradiation on the mixture of potassium tellurite/F5k broth culture, one ml of the broth medium was added to 1 ml of 4 mmol potassium tellurite (K2TeO3) to get a final concentration of 2 mmol. The mixtures were subjected to γ-radiation (60Co) at dose rate of 3.2 KGy/h using Russian γ-radiation cell at Nuclear Research Center, Inshas, Egypt. The mixtures were subjected to nine doses; 1, 2, 5, 10, 15, 25, 30, 35 and 40 kGy and then incubated for 48 h at 30 °C. The irradiated mixtures were tested for optical properties and compared with non-irradiated positive control and radiated negative controls.
2.6. Evaluation of TeNPs for antimicrobial activity
Luria-Bertani (LB) agar medium was used in this respect. It was poured in Petri dishes and allowed to dry. Colonies of E. coli, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella, Candida albicans and Aspergillus niger were spread on the surface of LB medium. Only 100 μl of a suspension of – washed, dried and pre-weighed – TeNPs in distilled water (25 mg/ml) was added to a well of 1 cm diameter in plates cultured with the test strains under study. The plates were incubated for 48 h at 30 °C and then inspected for growth inhibition zones.
3. Results
3.1. Screening for tellurium nanoparticles-production
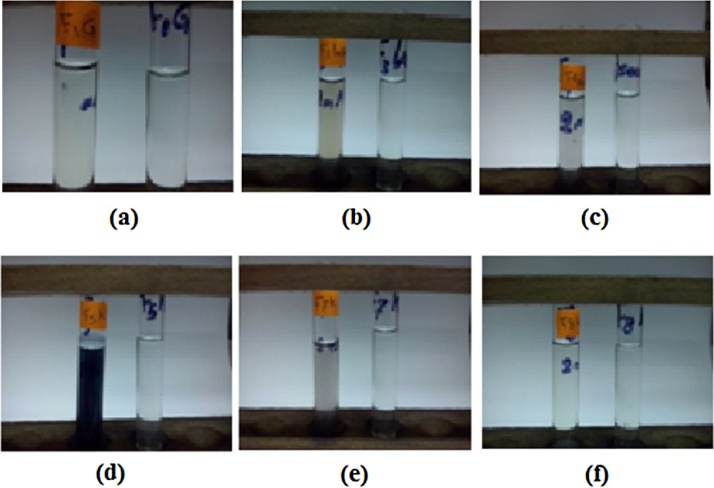
The extracts of the six fugal isolates showed different activities for production of elemental tellurium. Fig. 1(a–g) showed only three tubes with black turbidity; namely F3k, F5k and F7k, while the rest fungal isolates didn't give any blackening. The presence of black turbidity is an indicator of elemental tellurium.
Fig. 1.
Screening for blackening by tellurium nanoparticles production; tubes on the right hand are controls and those on the left hand are tests; (a): F1G, (b): F3k, (c): F4 sew, (d): F5k, (e): F7k and (f): F8k.
Ultra violet/visible light spectral analysis of the tellurium nanoparticles produced from potassium tellurite/broth culture medium of the isolates F3 K, F5k and F7k are shown in Fig. 2. In addition to visual observation as provided in Fig. 1, Fig. 2, shows that F5k is the most active fungus for production of tellurium nanoparticles (TeNPs) with maximum absorption at wavelength (∼700 nm) and therefore it was the focus for further studies. The absorbance at 700 nm was 0.426, 0.529 and 0.311 for F3 K, F5k and F7k, respectively.
Fig. 2.
UV/Vis Spectral analysis of the most active fungal isolates (diluted to 1:10). Molecular identification of F5 K based on 18s-rRNA.
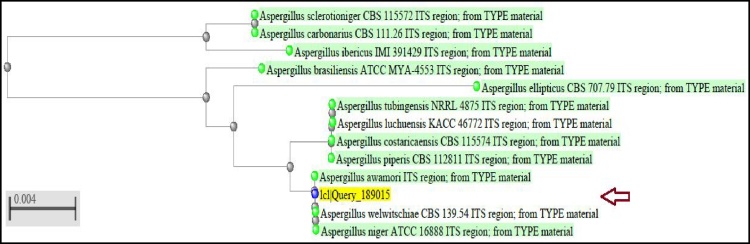
The most promising fungus (Fig. 3) was identified as Aspergillus welwitschiae based on 18s-rRNA and was given the accession number of (KY766958) by the Gene bank. Phylogenetic analysis of 18s-rRNA identification is presented in (Fig. 4).
Fig. 3.
Growth of Aspergillus welwitschiae KY766958 on Czapek's Dox agar medium (a) and under light microscope (b) at power 400×.
Fig. 4.
Phylogenetic tree based on F5 K 18s-rRNA gene sequences.
Size distribution report by number using dynamic light scattering (DLS) and Transmission Electron Microscopy (TEM) analysis of tellurium nanoparticles (TeNP) produced by F5k are shown in Fig. 5, Fig. 6, respectively. For size distribution (Fig. 5), a good quality results have been shown with particle size of 60.80 d.nm, Z-average of 114.8 d.nm and a polydispersity index (PDI) of 0.22. TEM (Fig. 6) showed oval to spherical-shaped TeNPs.
Fig. 5.
Size distribution of tellurium nanoparticles produced by Aspergillus welwitschiae KY766958.
Fig. 6.
TEM of tellurium nanoparticles produced by A. welwitschiae KY766958.
Fourier transform infrared (FTIR) spectroscopic analysis of the produced TeNPs (Table 2 and Fig. 7) indicates the presence of amino acid molecules which are the building blocks of the protein structure. Bonds represented by peaks number 4 (3428.81 cm−1) and number 11 (1599.66 cm−1) may correspond to the primary amine or amide owing to bond N—H and the bond —NH2, respectively. While bonds represented by peak number 9 at 1737.55 cm−1 and peaks 5, 6 and 7 at 2925.48, 2856.06 and 2515.69 cm−1, respectively, may correspond to the C O and O—H of the carboxylic acid. The peak number 17 (1078.01 cm−1) may correspond to the bond of C—O.
Table 2.
FTIR spectra of the biogenic TeNPs synthesized using Aspergillus welwitschiae KY766958.
| No | Position | Intensity | No | Position | Intensity | No | Position | Intensity | No | Position | Intensity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3744.12 | 95.6689 | 2 | 3734.48 | 95.6053 | 3 | 3710.37 | 95.2484 | 4 | 3428.81 | 57.4692 |
| 5 | 2925.48 | 81.8676 | 6 | 2856.06 | 87.4928 | 7 | 2515.69 | 98.0415 | 8 | 2228.34 | 97.9764 |
| 9 | 1737.55 | 91.7427 | 10 | 1627.63 | 76.5812 | 11 | 1599.66 | 76.9506 | 12 | 1455.03 | 74.4186 |
| 13 | 1385.6 | 77.4897 | 14 | 1319.07 | 90.2641 | 15 | 1263.15 | 91.2372 | 16 | 1188.9 | 92.4491 |
| 17 | 1078.01 | 88.6119 | 18 | 1038.48 | 87.9958 | 19 | 872.631 | 94.2795 | 20 | 773.315 | 92.9797 |
| 21 | 624.823 | 86.0347 | 22 | 543.828 | 88.9867 | 23 | 450.297 | 95.5862 |
Fig. 7.
FTIR spectra of the biogenic TeNPs synthesized using Aspergillus welwitschiae KY766958.
3.2. Effect of irradiated of potassium tellurite/broth culture medium mixture
The effect of different doses of γ-irradiation on productivity of TeNPs (Fig. 8) showed that the dose 1 kGy increased TeNPs production compared to the non-irradiated control. On the other hand, the increase in the dose of radiation resulted in gradual decrease in TeNPs production up to 30 kGy. Increase of γ-radiation over 30 kGy inhibits TeNPs production.
Fig. 8.
UV/Vis Spectral analysis of irradiated potassium tellurite/broth culture medium mixture at different doses.
3.3. Evaluation of the antimicrobial effect of TeNPs
Screening for antimicrobial activity of TeNPs against different microbial isolates was provided in Table 3 and Fig. 9. Results revealed inhibition of E. coli and MRSA growth with 29 mm and 31 mm inhibition zone diameter, respectively. Alternatively, the growth of Staph aureus, klebsiella, C. albicans and A. niger were not inhibited.
Table 3.
Evaluation of TeNPs antimicrobial activity at (25 mg/ml).
| Microbes | E. coli | S. aureus | MRSA | klebsiella | C. albicans | A. niger |
|---|---|---|---|---|---|---|
| Inhibition zone (mm) | 29 | Nil | 31 | Nil | Nil | Nil |
Fig. 9.
Antimicrobial activity if TeNP against E. coli, MRSA, Staphylococcus aureus, Klebsiella, C. albicans and A. niger.
4. Discussion
Microbial nano-biotechnology had attracted the attention of scientists and lead to progress and development in many fields including engineering, chemistry and biological sciences [[24], [35]].
The biogenic production of nanoparticles became far superior over that of chemical methods. Despite chemical methods are able to, rapidly, produce large quantities of nanoparticles with a well-defined shapes and sizes; they are inefficient, costly and produce toxic and harmful wastes. Alternatively, the biogenic production of nanoparticles is a “green” and environmentally friendly process and requires less energy compared with chemical methods [[7], [38]]. Microbial biosynthesis of nanoparticles may be intra- or extra-cellular process that is, highly, sensitive to changes in temperature, pH and nanoparticles concentration [24]. Tellurium nanoparticles (TeNPs) are often produced inside the microbial cells and hence the isolation of these particles from these cells requires additional downstream processing steps in order to release the metal, through ultrasound treatments and the use of detergents [11]. In the current study, TeNPs have been produced through cell-free enzymatic process because the enzymatic production of nanoparticles is more advantageous as it less sensitive to environmental changes compared to cellular processes and easier to obtain pure nanoparticles.
In the present investigation, screening for the most active fungal isolate(s) for biogenic production of TeNPs was done, using supernatant aliquots of six fungal isolates, in two steps; the formation of black suspension indicating reduction of potassium tellurite into elemental tellurium (Te0) [[3], [5], [42]] and spectral analysis at wavelength range (300–900 nm). The first step in screening resulted in three fungal isolates with high TeNPs yield, namely; F3 K, F5k and F7k which have been subjected to the second step. The isolate F5k exhibited the highest TeNPs production.
Spectral analysis of TeNPs suspensions showed that maximum absorption was at wavelength (∼700 nm) and the absorbance was 0.426, 0.529 and 0.311 for F3 K, F5k and F7k, respectively, at this wavelength. Alternatively, Zare et al. [47] used wavelength of 500 nm to measure the turbidity produced by elemental tellurium. The wavelength in the present study, 700 nm, is the correct one as it measure in black range color, while wavelength 500 nm measures in green color range as it was reported by [9].
In the present study, F5k represented the most potent fungal isolate for TeNPs production, so it has been subjected to characterization using molecular techniques. The molecular methods used for identification of fungi are highly sensitive and selective compared to morphological and physiological characterization that is sensitive to environmental conditions and hence turned out to be more difficult [48]. The phylogenetic tree in Fig. 4 shows phylogenetic analysis of the isolate F5k using 18 s rRNA sequence data with highest similarity of 100% to Aspergillus welwitschiae and was taken the accession number of (KY766958) by the Gene bank.
The shape and size of the produced TeNPs has been characterized using dynamic light scattering (DLS) and transmission electron microscopy (TEM). TeNPs was oval to spherical-shaped particles with size distribution of 60.80 d.nm. The nanoparticles shape is similar to that demonstrated by [11]. On the other hand, Zare et al. [47] obtained rod-shaped tellurium nanoparticles by Bacillus sp. BZ. In addition, nano-size tellurium particles (68 nm) have been produced enzymatically as it was reported by Pugin et al. [35].
FT-IR analysis of the produced TeNPs indicated the presence of many peaks that may correspond to primary amine or amide and carboxylic acid. These bonds confirm the presence of amide or amine and carboxylic acid groups of the protein amino acids. Most likely, this protein is the enzyme(s) catalyzing the reduction of tellurite group into elemental tellurium (Te0).
Gamma rays are short wave, high-energy electromagnetic radiations capable of ionizing other atoms [15]. Gamma irradiation of potassium tellurite/broth culture mixture improved TeNPs production at 1 kGy. This increase in productivity may be attributed to the activation of the enzyme(s) responsible for reduction of tellurite group. Gamma radiation has also been reported to increase the level of activity of many enzymes at law levels [[16], [29]]. At higher levels of γ-radiation, the enzyme(s) responsible for reduction of potassium tellurite is disrupted and subsequently, reduction in TeNPs productivity. Maity et al. [28] and Kiong et al. [22] reported that, γ-radiation is considered to produce amino acids by breaking down of proteins. In addition, Stoeva and co-workers [40] reported the disruption of enzyme activity by high dose of γ-radiation.
The produced TeNPs have also been studied for their potential antimicrobial activity. Evaluation of antimicrobial activity of the produced TeNPs was performed at concentration of (25 mg/ml) on six microbial isolates including bacteria, yeast and fungi; E. coli, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella, Candida albicans and Aspergillus niger. Of these microbial isolates, only E. coli and Methicillin-resistant Staphylococcus aureus (MRSA) have been inhibited with inhibition zone diameters of 29 mm and 31 mm, respectively.
The antimicrobial activity of nanoparticles has been studied and applied by many researchers [[14], [20], [36], [37]] and showed promising results. Although, the mechanism responsible for the antimicrobial activity of TeNPs is not yet completely understood, there are possible modes of action that has been proposed to explain the toxicity of these nanoparticles. One possible mode of action is the production of reactive oxygen species (ROS) (Manke et al.). Other possible mechanisms include the functional damages of cell membrane or wall by disrupting their integrity [33] and the surface features of the nanoparticles may, also, be involved in conferring toxicity to NPs [6].
In a similar result to the current, it has been reported by Pérez et al. [32] that the damage of E. coli may be attributed to reactive oxygen species (ROS) released by TeNPs that is probably tellurite. This suggestion was confirmed when tellurium nano-rods were observed inside E. coli after being exposed to TeNPs. Alternatively, it has been reported by Pugin et al. [34] that S. aureus exhibits much more resistance to tellurite and generates lower amounts of ROS which explains the resistance of S. aureus to TeNPs in the present study.
Although it may be surprising to obtain a resistant Staph aureus and sensitive MRSA, S. aureus resistance of methicillin has a different mechanism from that of ROS [39] and therefore the resistance of S. aureus to methicillin does not influence or interfere with its sensitivity to TeNPs. Furthermore, Pugin et al. [34] showed no growth inhibition of S. aureus ATCC 6538 in presence of tellurium nano-particles.
In similar studies, tellurite NPs were reported to inhibit the growth of S. aureus by Gupta et al. [49] and had no inhibitory effect in a study by Molina-Quiroz et al. [30]. In addition, Zare et al. [47] demonstrated the antimicrobial activity of tellurium nanoparticles produced by Bacillus sp., isolated from the Caspian Sea against different clinical isolates (S. aureus, P. aeruginosa, S. typhi and K. pneumonia) between 125 and 500 mg/l. This conflict of the results may be attributed to that, TeNPs have a strain-specific antimicrobial activity. Conclusively, the use of TNPs as antimicrobial agents is a novel and feasible strategy to face the antibacterial multi-resistance problem.
5. Conclusion
Tellurium nanoparticles have been synthesized using the fungal isolate Aspergillus welwitschiae KY766958. The produced TeNPs was oval to spherical shaped and have particle size of 60.80 d.nm. The use of γ-radiation showed that the used of 1 kGy increased TeNPs yield compared with the non-irradiated sample. The increase of the γ-radiation dose negatively affects the yield of TeNPs. Studying the antimicrobial effect of tellurium nanoparticles on bacterial and fungal strains revealed a good activity against Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA).
Funding
This work was not funded by any organization.
Conflict of interest
All authors declare that there is no conflict of interest and that this article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgement
The molecular identification has been accomplished at Sigma Scientific Services Co., Egypt (http://www.sigmaeg-co.com).
References
- 1.Allahverdiyev A.M., Abamor E.S., Bagirova M., Rafailovich M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011;6(8):933–940. doi: 10.2217/fmb.11.78. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amoozegar M.A., Ashengroph M., Malekzadeh F., Razavi M.R., Naddaf S., Kabiri M. Isolation and initial characterization of the tellurite reducing moderately halophilic bacterium, Salinicoccus sp. strain QW6. Microbiol. Res. 2008;163:456–465. doi: 10.1016/j.micres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Andreae M.O. Determination of inorganic tellurium species in natural waters. Anal. Chem. 1984;56:2064–2066. doi: 10.1021/ac00276a019. [DOI] [PubMed] [Google Scholar]
- 5.Baesman S.M., Bullen T.D., Dewald J., Zhang D., Curran S., Islam F.S., Beveridge T.J., Oremland R.S. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl. Environ. Microbiol. 2007;7(Apr. (73)):2135–2143. doi: 10.1128/AEM.02558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao P., Chen Z., Tai R.Z., Shen H.M., Martin F.L., Zhu Y.G. Selenite-induced toxicity in cancer cells is mediated by metabolic generation of endogenous selenium nanoparticles. J. Proteome Res. 2015;14:1127–1136. doi: 10.1021/pr501086e. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya R., Mukherjee P. Biological properties of naked metal nanoparticles. Adv. Drug Deliv. Rev. 2008;60(11):1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Bijan Z., Mohammad A.F., Zargham S., Mojtaba S., Sassan R., Ahmad R.S. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012;47:3719–3725. [Google Scholar]
- 9.Bohren C.F., Clothiaux E.E. Wiley-VCH Verlag GmbH & Co; 2006. Fundamentals of Atmospheric Radiation: An Introduction with 400 Problems. [Google Scholar]
- 10.Bondarenko O., Juganson K., Ivask A., Kasemets K., Mortimer M., Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 2013;87(7):1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghese R., Baccolini C., Francia F., Sabatino P., Turner R.J., Zannoni D. Reduction of chalcogen oxyanions and generation of nanoprecipitatesby the photosynthetic bacterium Rhodobacter capsulatus. J. Hazard. Mater. 2014;269:24–30. doi: 10.1016/j.jhazmat.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 12.De Lima R., Seabra A.B., Duran N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012;32(11):867–879. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- 13.Eaton A.D., Clesceri L.S., Greenberg A.E., editors. Standard Methods for the Examination of Water and Waste Water. 20th ed. American Public Health Association; Washington, D.C: 1998. [Google Scholar]
- 14.El-Batal A.I., Sidkey N.M., Ismail A.A., Arafa R.A., Fathy R.M. Impact of silver and selenium nanoparticles synthesized by gamma irradiation and their physiological response on early blight disease of potato. J. Chem. Pharm. Res. 2016;8(4):934–951. [Google Scholar]
- 15.Fawzi E.M., Hamdy H.S. Improvement of carboxymethyl cellulase production from chaetomium cellulolyticum nrrl 18756 by mutation and optimization of solid state fermentation. Bangladesh J. Bot. 2011;40(2):139–147. [Google Scholar]
- 16.Hameed A., Shah T.M., Atta B.M., Haq M.A., Sayed H. Gamma irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in desi and kabuli chickpea. Pak. J. Bot. 2008;40(3):1033–1041. [Google Scholar]
- 17.Hajipour M.J., Fromm K.M., Ashkarran A.A., Jimenez de Aberasturi D., De Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Hoff D., Sheikh L., Bhattacharya S., Nayar S., Webster T.J. Comparison study of ferrofluid and powder iron oxide nanoparticle permeability across the blood–brain barrier. Int. J. Nanomed. 2013;8:703–710. doi: 10.2147/IJN.S35614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikari T., Berger H., Levy F. Electrical properties of vapour grown tellurium single crystals. Mater. Res. Bull. 1986;21:99–105. [Google Scholar]
- 20.Ismail A.A., Sidkey N.M., Arafa R.A., Fathy R.M., El-Batal A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against alternaria solani caused early blight disease on potato. Br. Biotechnol. J. 2016;12(3):1–11. [Google Scholar]
- 21.Jassim A.M.N., Mohammed Al-Kazazz F.F., Khalaf Ali A. Biochemical study for bismuth oxide and tellurium nanoparticles on thyroid hormone levels in serum and saliva of patients with chronic renal failure. Int. J. Chem. Sci. 2013;11(3):1299–1313. [Google Scholar]
- 22.Kiong P., Ling A., Lai A.G., Hussein S., Harun A.R. Physiological responses of Orthosiphon stamineus plantles to gamma irradiation. Am-Eur. J. Sust. Agric. 2008;2(2) [Google Scholar]
- 23.Lalla A.B., Mandy D., Vincent J., Claus J. Tellurium: an element with great biological potency and potential. Org. Biomol. Chem. 2010;8:4203–4216. doi: 10.1039/c0Ob00086h. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Xu H., Chen Z., Chen G. Biosynthesis of nanoparticles by microorganisms and their applications (Review article) J. Nanomater. 2011;2011 [Google Scholar]
- 25.Liang H.W., Liu J.W., Qian H.S., Yu S.H. Multiplex templating process in one-dimensional nanoscale: controllable synthesis, macroscopic assemblies, and applications. Acc. Chem. Res. 2013;46:1450–1461. doi: 10.1021/ar300272m. [DOI] [PubMed] [Google Scholar]
- 26.Liu J.W., Xu J., Liang H.W., Wang K., Yu S.H. Macroscale ordered ultrathin telluride nanowire films, and tellurium/telluride hetero-nanowire films. Angew. Chem. 2012;124:7538–7543. doi: 10.1002/anie.201201608. [DOI] [PubMed] [Google Scholar]
- 27.Liu J.W., Zhu J.H., Zhang C.L., Liang H.W., Yu S.H. Mesostructured assemblies of ultrathin superlong tellurium nanowires and their photoconductivity. J. Am. Chem. Soc. 2010;132:8945–8952. doi: 10.1021/ja910871s. [DOI] [PubMed] [Google Scholar]
- 28.Maity J.P., Chakraborty A., Saha A., Santra S.C., Chanda S. Radiation induced effects on some common storage edible seeds in India infested with surface microflora. Radiat. Phys. Chem. 2004;71:1065–1072. [Google Scholar]
- 29.Marchenko M.M., Bloshko M.M., Kostyshin S.S. The action of low doses of gamma irradiation on the function of the glutathione system in corn (Zea mays L.) Ukr. Biokhim. Zh. 1996;68:94–98. [PubMed] [Google Scholar]
- 30.Molina-Quiroz R.C., Muñoz-Villagrán C.M., de la Torre E., Tantaleán J.C., Vásquez C.C., Pérez-Donoso J.M. Enhancing the antibiotic antibacterial effect by sub lethal tellurite concentrations: tellurite and cefotaxime act synergistically in Escherichia coli. PLoS One. 2012;7:e35452. doi: 10.1371/journal.pone.0035452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon G.D., Ko S., Xia Y., Jeong U. Chemical transformations in ultrathin chalcogenide nanowires. ACS Nano. 2010;4:2307–2319. doi: 10.1021/nn9018575. [DOI] [PubMed] [Google Scholar]
- 32.Pérez J.M., Calderón I.L., Arenas F.A., Fuentes D.E., Pradenas G.A., Fuentes E.L., Sandoval J.M., Castro M.E., Elías A.O., Vásquez C.C. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One. 2007;2:e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pi J., Yang F., Jin H., Huang X., Liu R., Yang P., et al. Selenium nanoparticles induced membrane bio-mechanical property changes in MCF-7 cells by disturbing membrane molecules and F-actin. Bioorg. Med. Chem. Lett. 2013;23:6296–6303. doi: 10.1016/j.bmcl.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 34.Pugin B., Cornejo F.A., García J.A., Díaz-Vásquez W.A., Arenas F.A., Vásquez C.C. Thiol-mediated reduction of Staphylococcus aureus tellurite tesistance. Adv. Microbiol. 2014;4:183–190. [Google Scholar]
- 35.Pugin B., Cornejo F.A., Muñoz-Díaz P., Muñoz-Villagrán C.M., Vargas-Pérez J.I., Arenas F.A., Vásquez C.C. Glutathione reductase-mediated synthesis of tellurium-containing nanostructures exhibiting antibacterial properties. Appl. Environ. Microbiol. 2014;80:7061–7070. doi: 10.1128/AEM.02207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidkey N.M., Arafa R.A., Moustafa Y.M., Morsi R.E., Elhateir M.M. Biosynthesis of mg and mn intracellular nanoparticles via extremo-Metallotolerant Pseudomonas stutzeri, B4 Mg/W and Fusarium nygamai, F4 Mn/S. J. Microbiol. Biotech. Food Sci. 2017;6(5):1181–1187. [Google Scholar]
- 37.Sidkey N.M., Moustafa Y.M., Arafa R.A., Morsi R.E., Elhateir M.M. Corrosion resistance and antimicrobial activity of extra- and intracellular Fe(II) nanoparticles biosynthesized via Aspergillus foetidus ATCC 14916. Am. Chem. Sci. J. 2016;17(1):1–10. [Google Scholar]
- 38.Simkiss K., Wilbur K.M. Academic; New York, NY, USA: 1989. Biomineralization. [Google Scholar]
- 39.Stapleton P.D., Taylor P.W. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 2002;85:57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoeva N., Zlatev Z., Bineva Z. Physiological response of beans (Phaseolus vulgaris L.) to gamma-radiation contamination, II. Water-exchange, respiration and peroxidase activity. J. Env. Prot. Eco. 2001;2:304–308. [Google Scholar]
- 41.Suresh A.K., Pelletier D.A., Doktycz M.J. Relating nanomaterial properties and microbial toxicity. Nanoscale. 2013;5(2):463–474. doi: 10.1039/c2nr32447d. [DOI] [PubMed] [Google Scholar]
- 42.Taylor D.E. Bacterial tellurite resistance. Trends Microbiol. 1999;7(3):111–115. doi: 10.1016/s0966-842x(99)01454-7. [DOI] [PubMed] [Google Scholar]
- 43.Thorley A.J., Tetley T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013;140(2):176–185. doi: 10.1016/j.pharmthera.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Valdiglesias V., Costa C., Kilic G., Costa S., Pasaro E., Laffon B., Teixeira J.P. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ. Int. 2013;55:92–100. doi: 10.1016/j.envint.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Tang Z.Y., Podsiadlo P., Elkasabi Y., Lahann J., Kotov N.A. Mirror-like photoconductive layer-by-layer thin films of Te nanowires: the fusion of semiconductor, metal, and insulator properties. Adv. Mater. 2006;18:518–522. [Google Scholar]
- 46.Warheit D.B. How to measure hazards/risks following exposures to nanoscale or pigment-grade titanium dioxide particles. Toxicol. Lett. 2013;220(2):193–204. doi: 10.1016/j.toxlet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Zare B., Faramarzi M.A., Sepehrizadeh Z., Shakibaie M., Rezaie S., Shahverdi A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012;47:3719–3725. [Google Scholar]
- 48.Bakri Y., Masson M., Thonart P. Isolation and identification of two new fungal strains for xylanase production. Appl. Biochem. Biotechnol. 2010;162:1626–1634. doi: 10.1007/s12010-010-8944-x. [DOI] [PubMed] [Google Scholar]
- 49.Gupta P.K., Sharma P.P., Sharma A., Khan Z.H., Solank P.R. Electrochemical and antimicrobial activity of tellurium oxide nanoparticles. Mater. Sci. Eng. B. 2016;211:166–172. [Google Scholar]