The risk of human immunodeficiency virus type 1 (HIV-1) acquisition by women per condomless sex act was 3-fold and 4-fold higher during late pregnancy and the postpartum period, respectively, relative to the nonpregnant period. Enhanced HIV-1 prevention and testing strategies are needed during pregnancy and the postpartum period.
Keywords: HIV, pregnancy, postpartum, infectivity, per coital act, HIV serodiscordant couples
Abstract
Background
Understanding the absolute and relative risk of human immunodeficiency virus type 1 (HIV) acquisition during pregnancy and the postpartum period can inform HIV prevention strategies for women.
Methods
We used a complementary log-log model and data from 2751 HIV-serodiscordant couples to compare the probability of HIV acquisition among women per sex act during early pregnancy, late pregnancy, the postpartum period, and the nonpregnant period.
Results
At total of 686 pregnancies were identified, and 82 incident HIV infections occurred. After adjustment for condom use, age, preexposure prophylaxis (PrEP) use, and HIV viral load, the per-act probability of HIV acquisition was higher in late pregnancy (adjusted relative risk [aRR], 2.82; P = .01) and the postpartum period (aRR, 3.97; P = .01) as compared to that during nonpregnant period. For a 25-year-old woman not taking PrEP, the HIV acquisition probability per condomless sex act with an HIV-infected male partner with a viral load of 10 000 copies/mL was 0.0011 (95% confidence interval [CI] 0.005–0.0019), 0.0022 (95% CI, 0.0004–0.0093), 0.0030 (95% CI, 0.0007–0.0108), and 0.0042 (95% CI, 0.0007–0.0177) during the nonpregnant period, early pregnancy, late pregnancy, and the postpartum period, respectively.
Conclusion
The HIV acquisition probability per condomless sex act steadily increased during pregnancy and was highest during the postpartum period, suggesting that biological changes during pregnancy and the postpartum period increase HIV susceptibility among women.
(See the Editorial commentary by Mofenson, on pages 1–4.)
Human immunodeficiency virus type 1 (HIV) is the leading cause of death worldwide among women of reproductive age [1]. In countries of sub-Saharan Africa with a high HIV burden, where women represent 56% of new adult HIV infections [2], fertility rates are also high, and women spend a significant proportion of their reproductive years pregnant or breastfeeding. A recent meta-analysis calculated a pooled HIV incidence rate among African women of 4.7 cases per 100 person-years during pregnancy and 2.9 cases per 100 person-years during the postpartum period, both of which exceed the HIV incidence among female sex workers and HIV-serodiscordant couples [3]. One hypothesis for the high HIV incidence among women of reproductive age is increased susceptibility during and after pregnancy.
Some, but not all, studies have observed increased risk of HIV acquisition during pregnancy and the postpartum period, relative to the nonpregnant period [3]. Physiological changes that accompany pregnancy, including immune and hormonal alterations and shifts in the vaginal microbiome, offer mechanistic hypotheses to support increased HIV risk [4–12]. Analyses of pregnancy as a risk factor are challenging because data on sexual behavior require frequent measurement so that changes in sexual frequency do not obscure the potential increased biological risk of HIV due to pregnancy itself. A per-coital-act analysis quantifies the probability of HIV acquisition per sex act, given exposure to HIV during condomless sex. A commonly cited estimate for the per-coital-act probability of heterosexual HIV transmission is 0.001 (1 infection per 1000 sex acts) [13], although this does not account for behavioral or biological differences, such as pregnancy or partner HIV viral load.
Quantifying the absolute and relative risks (RRs) of female HIV acquisition during pregnancy and the postpartum period, as well as further insight into whether any increased risk is attributable to biological or behavioral changes, is critical to inform efforts to prevent HIV. We assessed differential risk in HIV acquisition across reproductive stages by calculating the per-coital-act probability of female HIV acquisition in early pregnancy, late pregnancy, and the postpartum period and comparing these estimates to that during the nonpregnant period.
METHODS
Study Population
The current analysis includes data from HIV-uninfected women in 7 African countries (Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, and Zambia) followed for up to 48 months in 2 randomized clinical trials between 2004 and 2013. The Partners in Prevention HSV/HIV Transmission Study (clinical trials registration NCT00194519) evaluated the efficacy of daily herpes simplex virus type 2–suppressive acyclovir therapy for the prevention of HIV transmission; there was no significant difference between the intervention and placebo groups (hazard ratio, 0.92; P = .69) [14]. The Partners PrEP Study (clinical trials registration NCT00557245) evaluated the efficacy of daily oral preexposure prophylaxis (PrEP) to prevent HIV acquisition; the reduction in HIV acquisition was 67% (P < .001) and 75% (P < .001) in the tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC)/TDF arms, relative to placebo [15]. In both studies, women were ≥18 years of age and in stable partnerships with men known to be HIV infected and not eligible for antiretroviral therapy (ART) at enrollment.
Data Collection
Demographic data and 30-day recall of sexual behavior were collected in both studies via interviewer-administered forms in either English or local languages. In the Partners PrEP Study, HIV-uninfected women attended monthly visits and HIV-infected male partners attended quarterly visits. In the Partners in Prevention HSV/HIV Transmission Study, HIV-uninfected women attended quarterly visits, and HIV-infected male partners attended monthly visits. HIV RNA viral load was measured in HIV-infected partners at enrollment and every 6 months in the Partners in Prevention HSV/HIV Transmission Study and annually in the Partners PrEP Study.
Assessment of HIV Status
Women underwent monthly HIV testing in the Partners PrEP Study and quarterly in the Partners in Prevention HSV/HIV Transmission Study, using 2 parallel HIV antibody tests, and positive results were confirmed by an HIV-specific enzyme-linked immunoassay. HIV acquisition events were classified as genetically linked (ie, likely transmitted from the male study partner) on the basis of HIV env and gag or pol sequencing assays [16, 17]. Archived plasma specimens, collected at enrollment and every quarterly visit, were tested for HIV RNA to determine the first evidence of infection for all HIV seroconversions.
Pregnancy Procedures
HIV-uninfected women could be pregnant at enrollment in the Partners in Prevention HSV/HIV Transmission Study, and women provided urine specimens for pregnancy testing when clinically indicated during follow-up (eg, at the time of missed menses). Pregnancy was an enrollment exclusion criterion for HIV-uninfected women in the Partners PrEP Study, and women provided urine specimens monthly for pregnancy testing; study drug was withheld during pregnancy and breastfeeding. Estimated dates of delivery and the last menstrual period (LMP), the date when pregnancy ended, and the pregnancy outcome were collected for each pregnancy. We defined pregnancy start date as the date of the LMP and the pregnancy end date as the self-reported date of delivery or loss, with dates left censored at enrollment or right censored at study exit to capture time with data on sexual activity. Complete data on LMP and pregnancy end were available for 97% of pregnancies. For pregnancies with incomplete dates (3%), we assigned start and/or end dates on the basis of pregnancy outcome and duration, the date of the LMP, and/or estimated the date of delivery.
Statistical Analysis
Pregnancy incidence was calculated as the number of pregnancies per 100 person-years. HIV incidence was calculated as the number of genetically linked HIV infections per 100 person-years. The time of first evidence of HIV was defined as the earliest date of a positive HIV antibody test result or detection of HIV RNA in an archived plasma specimen. Visits were censored once the male partner reported initiation of ART. Women who had HIV RNA detected at enrollment were excluded. Women who acquired HIV that was not genetically linked to their HIV-infected study partner were censored at the visit before first evidence of infection.
Reproductive stage was assessed as a time-varying exposure in all models. Start and end dates for each pregnancy were used to calculate the number of days between HIV tests during (1) early pregnancy (ie, from the start of pregnancy to gestation week 13), (2) late pregnancy (ie, from gestation week 14 to the end of pregnancy), (3) the postpartum period (ie, from delivery to week 24 after delivery, for live births), or (4) the nonpregnant period (ie, when not pregnant or in the postpartum period). The proportion of each HIV testing interval spent across the 4 reproductive stages was calculated as the number of days spent in each stage divided by the total number of days between HIV tests. In cases of contiguous pregnancies, the postpartum period was truncated to assign study time to the early pregnancy state in the subsequent pregnancy. Women who experienced a pregnancy loss were assigned a postpartum duration based on their expected date of ovulation, as follows: pregnancy loss at gestation week <6, no postpartum time [18]; pregnancy loss between gestation weeks 6 and 20, 28 days [19, 20]; and pregnancy loss at gestation week ≥20 or infant death before age 6 months, 42 days [21, 22]. Date of breastfeeding cessation was not collected. The postpartum period was defined to capture hormonal shifts that occur after delivery and a minimum duration of breastfeeding, based on what is customary in East and Southern Africa. To calculate the HIV incidence by reproductive stage, events were assigned to the stage the woman was in at first evidence of HIV.
To determine the total number of sex acts associated with each HIV testing interval, women’s monthly reports of sex acts with their study partners were used from the Partners PrEP Study. Men’s monthly report of sex acts with their study partners, aggregated over the 3 months between women’s HIV tests, were used from the Partners in Prevention HSV/HIV Transmission Study. In this study, correlation between the frequencies of sex reported by the male and female partner was high (P < .001) and concordant (within 1–3 acts of each other at 80% of visits attended by both); use of the monthly report from the male partner allowed for detection of changes in sexual frequency that were not captured by females who attend only quarterly visits.
When >6 weeks elapsed between visits (which occurred for 2.6% of all visits), missing data on sex acts were imputed on the basis of the mean monthly number of sex acts reported at previous visits multiplied by the number of month(s) missed. Rates of sexual activity were calculated as the number of reported sex acts, with and without a condom, per person-month in each reproductive stage. To compare sexual behavior across reproductive stages, we fit Poisson regression models with an independent correlation matrix and robust standard errors. A priori, we adjusted these models for female age and relationship duration. To estimate the per-coital-act probability of HIV acquisition per condomless sex act, we used a complementary log-log model where λ is the per-coital-act infectivity, n is the number of sexual acts, X denotes covariates (including the proportion of time in each reproductive stage and the proportion of sex acts protected by a condom) in the current interval, and p(X;n) describes the overall probability of HIV acquisition by the female partner [23]. HIV acquisition was a rare event; for small values of λ, the β coefficient of each reproductive stage represents the log RR of acquisition for that stage.
The adjusted model included time-varying covariates for male partners’ plasma HIV viral load (carried forward between measurements) and female partners’ use of active PrEP (defined as those who received TDF or FTC/TDF based on randomization arm and dispensation records), given their known influence on HIV transmission. We assessed additional demographic and clinical variables as potential confounders and included variables that changed the risk estimate for any reproductive stage by ≥10% in the adjusted model. HIV viral load was centered at 10000 copies/mL, and female partner age was centered at 25 years. Thus, the reference case for adjusted absolute HIV acquisition probabilities was a condomless sex act between a 25-year-old woman not using PrEP and a male partner with HIV RNA viral load of 10000 copies/mL.
To evaluate the robustness of our findings, we conducted 5 sensitivity analyses. First, given the efficacy of PrEP for HIV prevention, we excluded women randomized to receive active PrEP. Second, to address any potential bias from using male participants’ reports of sex acts, we used female participants’ reports of sex acts for participants in the Prevention HSV/HIV Transmission Study, calculated as the 30-day recall among female participants, multiplied by 3 to account for data not collected between quarterly visits. Third, to address any residual or unmeasured confounding that may result from differences between those who became and those did not become pregnant, we excluded women with no study pregnancies. Fourth, to assess the impact of missing data, we excluded any visit for which data on sex acts were imputed. Fifth, to explore better precision about the timing of infection relative to pregnancy, we used the estimated date of HIV (defined as 17 days before the date on which HIV RNA was first detected, if available, or as the midpoint between the date of the last negative and first positive result of HIV antibody testing) [24] in lieu of the date that infection was first detected by the study and excluded infections for which the estimated date of infection and date of detection differed by >30 days.
All data were analyzed using SAS, version 9.4. The protocols for each study were approved by the Human Subjects Division at the University of Washington and by ethics review committees for each study site. All participants provided written informed consent.
RESULTS
The current analysis included 2751 HIV-uninfected women ages 18–49 years who completed ≥1 follow-up visits before their HIV-infected partner initiated ART. In the month prior to enrollment, the median number of sex acts within study partnerships was 4.0 (IQR, 2.0–8.0 sex acts), and 24.4% reported at least 1 condomless sex act with their study partner (Table 1).
Table 1.
Enrollment Characteristics of 2751 HIV-Uninfected African Women and Their HIV-Infected Male Partners, Individually and as Couples
| Characteristic | HIV-Uninfected Female Partners | HIV-Infected Male Partners |
|---|---|---|
| Age, y | 32.0 (27.0–37.7) | 37.6 (32.7–43.1) |
| Education duration, y | 7.0 (4.0–9.0) | 7.0 (5.0–11.0) |
| Effective contraceptive usea | 1044/2751 (37.9) | … |
| Medical | ||
| Circumcised | … | 909/2750 (33.1) |
| Sexually transmitted infectionb | 852/2017 (30.1) | 220/2751 (8.0) |
| HSV-2 seropositive | 2230/2709 (82.3) | 1167/2751 (42.4)c |
| Sex acts in past 30 d, no. | ||
| Any act with study partner | 4.0 (2.0–8.0) | 4.0 (2.0–8.0) |
| Any act with study partner protected by a condom | 3.0 (1.0–6.0) | 3.0 (2.0–6.0) |
| Any condomless act with study partner | 670/2751 (24.4) | 706/2751 (25.7) |
| Any act with additional partner | 10/2751 (0.4) | 338/2751 (12.3) |
| HIV-associated clinical data | ||
| Plasma HIV RNA viral load, log10 copies/mL | … | 4.2 (3.5–4.8) |
| CD4 T-cell count, cells/mm3 | … | 447.0 (347.0–584.0) |
| Female and Male Partners Combinedd | ||
| Partnership duration, y | 9.7 (4.4–16.6) | |
| Married to study partner | 2536/2751 (92.2) | |
| Living together | 2656/2751 (96.6) | |
| Living children with study partner, no. | 2.0 (1.0–4.0) | |
Data are no. (%) of partners or couples or median values (interquartile ranges).
Abbreviation: HSV-2, herpes simplex virus type 2.
aIncludes implanted devices, injectable agents, oral contraceptive pills, intrauterine devices, and permanent methods.
bLaboratory diagnosis of Neisseria gonorrhoeae, Chlamydia trachomatis, Treponema pallidum, Trichomonas vaginalis, HSV2, or bacterial vaginosis (female partners only).
c1,319 men tested at enrollment. Missing data (men not tested) included in denominator.
dReported by the HIV-uninfected female.
A total of 5069 person-years were accrued, and the median duration of follow-up was 23.5 months (IQR, 13.9–29.8 months). Within the study partnership, there were 4.62, 4.71, 3.24, and 2.32 sex acts per person-month during the nonpregnant period, early pregnancy, late pregnancy, and the postpartum period, respectively (Table 2). After adjustment for age and relationship duration, compared with the nonpregnant period, the average frequency of sex was significantly lower during late pregnancy (adjusted RR [aRR], 0.66; P < .0001) and the postpartum period (aRR, 0.45; P < .0001), and the average frequency of condomless sex with study partners was significantly higher during early pregnancy (aRR, 2.43; P < .0001).
Table 2.
Sexual Activity During Study Follow-up Among 2751 African Heterosexual HIV-Serodiscordant Couples With an Initially HIV-Uninfected Female Partner, by Reproductive Stage
| Variable | Nonpregnant/Nonpostpartum Periodsa | Early Pregnancy | Late Pregnancy | Postpartum Period | |||
|---|---|---|---|---|---|---|---|
| Value | P | Value | P | Value | P | ||
| Total sex acts reported with study partner, no. | 256462 | 7548 | 7205 | 3573 | |||
| Total condomless sex acts reported with study partner, no. | 25946 | 1530 | 947 | 327 | |||
| Total person-months during follow-up, no. | 55504 | 1602 | 2224 | 1541 | |||
| Sex acts with study partner | |||||||
| No./person-month (95% CI) | 4.62 (4.60–4.64) | 4.71 (4.61–4.82) | 3.24 (3.17–3.32) | 2.32 (2.24–2.40) | |||
| RR for the mean frequency | |||||||
| Crude (95% CI) | 1.00 | 1.11 (1.01–1.21) | .02 | 0.70 (0.63–0.78) | <.0001 | 0.48 (0.42–0.55) | <.0001 |
| Adjustedb (95% CI) | 1.00 | 1.04 (0.95–1.14) | .40 | 0.66 (0.59–0.73) | <.0001 | 0.45 (0.39–0.51) | <.0001 |
| Condomless sex acts with study partner | |||||||
| No./person-month (95% CI) | 0.47 (0.46–0.47) | 0.96 (0.91–1.00) | 0.43 (0.40–0.45) | 0.21 (0.19–0.24) | |||
| RR for the mean frequency | |||||||
| Crude (95% CI) | 1.00 | 2.64 (2.10–3.31) | <.0001 | 0.97 (0.70–1.32) | .83 | 0.55 (0.29–1.04) | .07 |
| Adjustedb (95% CI) | 1.00 | 2.43 (1.91–3.10) | <.0001 | 0.91 (0.66–1.25) | .55 | 0.51 (0.27–0.97) | .04 |
Early pregnancy was defined as the interval from the start of pregnancy (typically the time of the last menstrual period) to gestation week 13. Late pregnancy was defined as the interval from gestation week 14 to the end of pregnancy. The postpartum period was defined as the interval from the end of pregnancy to month 6 after delivery (for women with live births), week 6 after pregnancy loss (for women with pregnancy loss at gestation week ≥20 or newborn death), or week 4 after pregnancy loss (for women with pregnancy loss during gestation weeks 6–19).
Abbreviations: CI, confidence interval; RR, relative risk.
aReference group for comparisons is time spent during the nonpregnant and nonpostpartum periods.
bAdjusted for female age and duration of relationship with HIV-infected study partner.
Twenty-two percent of women experienced at least 1 pregnancy during follow-up (Table 3), of whom 89.4% had 1 pregnancy, 9.9% had 2 pregnancies, and 0.3% had 3 or 4 pregnancies each. Ninety-two women were pregnant at enrollment, and 594 incident pregnancies were identified. The pregnancy incidence rate was 12.50 cases/100 person-years (95% confidence interval [CI], 11.52–13.55). Of the 686 pregnancies, 62.1% ended with a live birth, 24.6% resulted in a pregnancy loss, and 13.3% were ongoing at study exit.
Table 3.
Pregnancy Incidence and Outcomes Among 2751 Initially HIV-Uninfected African Women With HIV-Infected Male Partners
| Variable | Value |
| Ever pregnant during follow-up | 615 (22.4) |
| Total person-years of follow-up | 5069 |
| Total person-years pregnant | 318 |
| Total person-years at risk of pregnancy | 4751 |
| Total pregnancies | |
| Overall | 686 |
| Identified at enrollment | 92 (13.4) |
| Identified during follow-up | 594 (86.6) |
| Pregnancy incidence rate per 100 person-years | 12.50 (11.52–13.55) |
| Pregnancy outcome | |
| Pregnant at study exit | 91 (13.3) |
| Live birth | 426 (62.1) |
| Pregnancy loss, by gestation wk | |
| Any | 169 (24.6) |
| <6 wk | 29 (17.2) |
| 6–13 wk | 97 (57.4) |
| 14–19 wk | 24 (14.2) |
| ≥20 wk | 19 (11.2) |
Data are no. (%) of women or median value (interquartile range).
After excluding 42 HIV (16 present at enrollment and 26 that could not be genetically linked between study partners), eighty-two HIV events were observed, for an HIV incidence rate of 1.62 cases/100 person-years (95% CI, 1.29–2.01; Table 4). For 24 HIV events, the first evidence of HIV was detected at a study visit that occurred during the interval from early pregnancy through the postpartum period. The HIV incidence per 100 person-years was 1.25 cases during the nonpregnant period (95% CI, 0.95, 1.62), 3.75 during early pregnancy (95% CI, 1.22, 8.75), 7.02 during late pregnancy (95% CI, 3.74 12.01), and 4.68 during the postpartum period (95% CI, 1.72, 10.18).
Table 4.
Acquisition of HIV Among 2751 African Women With HIV-Infected Male Partners, by Reproductive Stage
| Reproductive Stage | HIV Seroconversion, Cases/Person-Years | HIV Incidence, Cases/100 Person-Years (95% CI) |
|---|---|---|
| Overall | 82/5069 | 1.62 (1.29, 2.01) |
| Nonpregnant/postpartum periods | 58/4622 | 1.25 (0.95, 1.62) |
| Early pregnancy through postpartum period | 24/447 | 5.37 (3.44, 7.99) |
| Early pregnancy | 5/133 | 3.75 (1.22, 8.75) |
| Late pregnancy | 13/185 | 7.02 (3.74, 12.01) |
| Postpartum period | 6/128 | 4.68 (1.72, 10.18) |
Reproductive stage was determined by the stage at the study visit when first evidence of HIV was identified. Early pregnancy was defined as the interval from the start of pregnancy (typically the time of the last menstrual period) to gestation week 13. Late pregnancy was defined as the interval from gestation week 14 to the end of pregnancy. The postpartum period was defined as the interval from the end of pregnancy to month 6 after delivery (for women with live births), week 6 after pregnancy loss (for women with pregnancy loss at gestation week ≥20 or newborn death), or week 4 after pregnancy loss (for women with pregnancy loss during gestation weeks 6–19).
Abbreviation: CI, confidence interval.
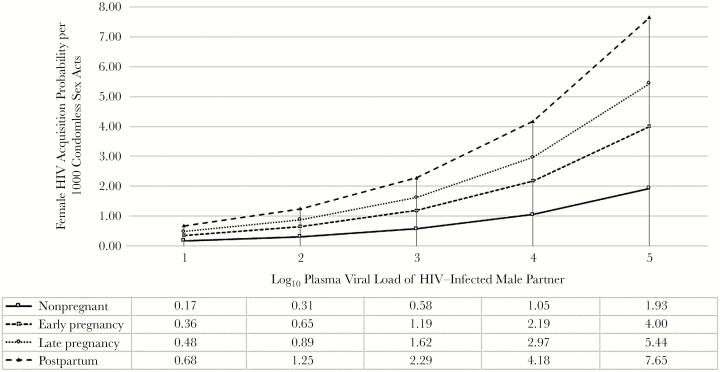
Seventy-eight incident HIV infections were detected during intervals where ≥1 sex act was reported. Table 5 presents estimated HIV acquisition probabilities per condomless sex act. In the base model including the total number of sex acts and the proportion of the acts protected by a condom, HIV acquisition rates per condomless sex act were significantly higher in each pregnancy-related stage relative to the nonpregnant period. After adjustment for age, use of PrEP, and male partner HIV viral load, the probability of HIV acquisition was significantly higher throughout pregnancy and the postpartum period (aRR, 2.76; 95% CI, 1.58–4.81). This increase was driven by late pregnancy (aRR, 2.82; 95% CI, 1.29–6.15; P = .01) and the postpartum period (aRR, 3.97; 95% CI, 1.50–10.51; P = .01). The HIV acquisition probability for the reference case per 1000 sex acts was 1.05, 2.19, 2.97, and 4.18 during the nonpregnant period, early pregnancy, late pregnancy, and the postpartum period, respectively. An additional analysis extending the postpartum period to 25–52 weeks after delivery suggests that the increase in risk that accompanies the postpartum period is concentrated in the initial 6 months (Supplementary Materials). The relationship between male partner HIV viral load and HIV acquisition probability was consistently linear within each reproductive stage (Figure 1).
Table 5.
HIV Acquisition Probability and Relative Risk (RR) of HIV Acquisition Among 2751 African Women With HIV-Infected Male Partners, by Reproductive Stage
| Reproductive Stage | Base Modela | Adjusted Modelb | ||||
|---|---|---|---|---|---|---|
| Probabilityc of HIV Acquisition per Condomless Sex Act (95% CI) | RRd for per-Act Probability of HIV Acquisition (95% CI) | P | Probabilityc of HIV Acquisition per Condomless Sex Act (95% CI) | RRd for per-Act Probability of HIV Acquisition (95% CI) | P | |
| Early pregnancy through postpartum period | 0.0027 (0.0009, 0.0074) | 4.97 (2.95, 8.38) | <.001 | 0.0029 (0.004, 0.0093) | 2.76 (1.58, 4.81) | <.001 |
| Early pregnancy | 0.0018 (0.0003, 0.0070) | 3.20 (1.24, 8.25) | .02 | 0.0022 (0.0004, 0.0093) | 2.07 (0.78, 5.49) | .14 |
| Late pregnancy | 0.0031 (0.0008, 0.0102) | 5.54 (2.62, 11.69) | <.001 | 0.0030 (0.0007, 0.0108) | 2.82 (1.29, 6.15) | .01 |
| Postpartum period | 0.0044 (0.0008, 0.0167) | 7.80 (3.04, 20.02) | <.001 | 0.0042 (0.0007, 0.0177) | 3.97 (1.50, 10.51) | .01 |
| Nonpregnant/nonpostpartum periods | 0.0005 (0.0003, 0.0009) | 1.00 | … | 0.0011 (0.005, 0.0019) | 1.00 | … |
Early pregnancy was defined as the interval from the start of pregnancy (typically the time of the last menstrual period) to gestation week 13. Late pregnancy was defined as the interval from gestation week 14 to the end of pregnancy. The postpartum period was defined as the interval from the end of pregnancy to month 6 after delivery (for women with live births), week 6 after pregnancy loss (for women with pregnancy loss at gestation week ≥20 or newborn death), or week 4 after pregnancy loss (for women with pregnancy loss during gestation weeks 6–19).
Abbreviations: CI, confidence interval; PrEP, preexposure prophylaxis.
aAdjusted for condom use and reproductive stage.
bAdjusted for condom use, reproductive stage, male partner viral load, female partner age, and active PrEP for women randomly assigned to receive and dispensed active PrEP in the Partners PrEP study.
cAdjusted absolute HIV acquisition probabilities among female partners represent infectivity estimates per condomless sex act with an HIV-infected partner with a viral load of 10 000 copies/mL for a 25-year-old female partner not taking PrEP.
dThe reference group for the adjusted model represents a condomless sex act with an HIV-infected partner with a viral load of 10 000 copies/mL for a 25-year-old female not taking PrEP occurring while the woman is not pregnant or is in the postpartum period.
Figure 1.
Adjusted absolute female human immunodeficiency virus type 1 (HIV) acquisition probabilities represent infectivity estimates per 1000 condomless sex acts for a 25-year-old woman not taking preexposure prophylaxis (PrEP) at varying levels of plasma HIV viral load for a male HIV-infected partner.
In sensitivity analyses, the probability of HIV acquisition per act during pregnancy (combined) versus the nonpregnant period remained stable when (1) women randomly assigned to active PrEP were excluded (aRR, 2.92; P = .001), (2) only sex acts reported by female partners were used (aRR, 2.37; P = .003), (3) women who never became pregnant during follow-up were excluded (aRR, 3.37; P = .002), and (4) visits for which data sex acts were imputed were excluded (aRR, 3.11; P = .0002). While the overall trend of increasing risk remained, restriction of the analysis to 43 HIV in which the estimated date of infection occurred within 30 days of detection attenuated the point estimate and the statistical significance of the increased risk during early pregnancy (aRR, 1.08; 95% CI, 0.22–5.50), late pregnancy (aRR, 2.53; 95% CI, 0.82–7.85), and the postpartum period (aRR, 2.85; 95% CI, 0.60–13.48).
DISCUSSION
In this prospective study, the risk of female HIV acquisition per condomless sex act was 3- and 4-fold higher during late pregnancy and the postpartum period, respectively, and remained significant after adjustment for factors known to effect HIV acquisition. Our findings underscore the need for continued counseling on the risk of HIV acquisition and options for enhanced HIV prevention for women during pregnancy and the postpartum period. Additionally, these robust and disaggregated estimates can be used to parameterize mathematical models forecasting the impact and cost-effectiveness of HIV interventions among populations with high pregnancy rates.
Our results suggest that biological factors associated with pregnancy increase female susceptibility to HIV acquisition. The high levels of estrogen and progesterone that accompany pregnancy can induce a cascade of synergistic changes within the female genital tract), including changes in CCR5 and CXCR4 expression, increased inflammation, decreased integrity of the vaginal epithelium, and alterations in vaginal microbiota, all of which have been associated with increased HIV acquisition susceptibility [25]. Pregnancy activates innate immunity, increasing inflammation and HIV target cells in the female genital tract [4, 9], while simultaneously suppressing adaptive immunity and reducing natural killer cells, changes that can persist as long as 9 months after delivery [5, 7]. Results from animal, ex vivo, and human epidemiological studies have demonstrated that such immune activation and the ensuing inflammation are associated with an increased risk of HIV acquisition [10–12, 26]. Our finding that the overall frequency of sex acts declines during pregnancy and the postpartum period is consistent with results from similar populations [27–29]. However, in other studies many African women report resuming sexual activity 6–8 weeks after delivery [27, 30–32]. The postpartum period is accompanied by low levels of estrogen and progesterone. These hormonal shifts can increase the number of HIV receptors, and sex during this period may occur in the presence of macrotrauma and microtrauma caused by vaginal delivery or vaginal dryness from low levels of estrogen during breastfeeding. Although the sexually transmitted infection (STI) status of either partner, including the presence of herpes simplex virus type 2, was not a confounder in the current analysis, STIs are a risk factor for HIV acquisition [33], including during pregnancy [34]. In addition, the prevalence of vaginal infections among pregnant and postpartum women in other populations has been high [35], positing another biological hypothesis for why pregnancy periods could induce greater susceptibility to HIV. Given the increased risk of perinatal transmission through breast milk from acute HIV [36], additional understanding of maternal HIV acquisition during breastfeeding is needed, including detailed study of extended breastfeeding.A study from Rakai, Uganda, estimated the HIV acquisition risk per coital act among female partners in HIV-serodiscordant couples during pregnancy (0.0013) and breastfeeding (0.0009) but found no significant difference between these periods relative to those in nonpregnant periods (0.0007; aRR, 1.42; 95% CI, 0.37, 3.82) [37]. However, annual data collection in this community cohort may have resulted in misclassification of dates for HIV or pregnancy and inaccurate recall of sexual activity. In contrast, data used in the current analysis included >5000 years of follow-up from 2751 HIV-uninfected women for whom sexual activity was reported monthly, and women were evaluated frequently for both HIV and pregnancy. Furthermore, we were able to account for partner characteristics with restriction to genetically linked incident HIV infections and time-varying adjustment for the partner’s HIV viral load. Sexual activity, including frequency and condom use, was subject to self-report, and it is unknown how pregnancy status may have affected the accuracy of this reporting. A previous simulation study suggests that overreporting of condom use would not significantly alter estimates for our reproductive stage covariates in a per-act analysis [23]. Reassuringly, our overall finding of increased risk during pregnancy was consistent across sensitivity analyses that addressed potential confounding or misclassification. Results from our sensitivity analysis using the estimated date of HIV infection lost precision and were attenuated, suggesting that our primary model may be subject to some bias toward HIV detection at later stages of pregnancy; however, the magnitude and dose-response relationship of risk estimates per stage were similar.
Our results have a number of public health implications for integration of HIV prevention strategies into existing reproductive health services. As a highly efficacious [38], cost-effective [39], female-controlled strategy, daily tenofovir-based oral PrEP can play an important role in preventing HIV acquisition, especially for pregnant and postpartum women who are unable to engage their male partner(s) in HIV prevention. Available safety data indicate that maternal tenofovir use is not associated with adverse birth or infant outcomes and that infants are exposed to low levels of tenofovir through breast milk [40, 41]. This evidence has contributed to recent World Health Organization guidelines that promote PrEP use for pregnant and breastfeeding women at high risk of HIV acquisition [42]. Decisions about PrEP use during reproductive periods must carefully evaluate potential risks and benefits, taking into consideration that individual cumulative risk will vary depending on the duration of time pregnant or breastfeeding and the frequency of exposure through condomless sex with an HIV-infected partner. Use of PrEP during pregnancy has been an acceptable risk-reduction strategy for women with known HIV-infected partners [43]. Ongoing and planned studies will assess delivery models for PrEP within routine maternal and child health services [44, 45].
Male partner testing and repeat maternal testing are opportunities to identify women at increased risk of HIV acquisition or undiagnosed HIV. Women with male partners of unknown HIV status may be unaware of the need for HIV prevention [34], and in the absence of repeat testing HIV seroconversions that occur late in pregnancy or during the postpartum period will go undetected. Innovative approaches such as home-based couples testing [46–48] and secondary distribution of self-test kits from pregnant women to their partners [49, 50] have increased male HIV testing. The World Health Organization recommends at least 1 repeat HIV test during the peripartum period for women living in areas of generalized HIV epidemics, but these guidelines lack specificity in terms of when to test and are inconsistently implemented. More-specific normative guidance on repeat maternal HIV testing, including the use of self-testing, is urgently needed, followed by strategies that integrate testing into maternal care, especially during the postpartum period, which our study identified as having the highest per-act risk of HIV acquisition.
In summary, this study provides strong evidence that the risk of HIV acquisition per sex act steadily increases throughout pregnancy and is highest during the postpartum period. While further research is needed to better understand the biological susceptibility that accompanies these periods, scale-up of HIV prevention, counseling, and testing in antenatal and postpartum care in high HIV prevalence settings is warranted to prevent sexual transmission and identify acute maternal HIV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the men and women who participated in the Partners in Prevention HSV/HIV Transmission and Partners PrEP studies, as well as the research teams at each study site.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (OPP47674 and OPP26469 to the Partners in Prevention HSV/HIV Transmission and Partners PrEP studies) and the National Center for Advancing Translational Sciences (TL1TR000422 to K. T.).
Potential conflicts of interest. J. M. B. is on the advisory committee of Gilead Sciences. All other authors report no potential conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Adimora AA, Ramirez C, Auerbach JD et al. ; HIV Prevention Trials Network Women at Risk Committee Preventing HIV infection in women. J Acquir Immune Defic Syndr 2013; 63(Suppl 2):S168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS update. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 3. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groer ME, Jevitt C, Ji M. Immune changes and dysphoric moods across the postpartum. Am J Reprod Immunol 2015; 73:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groer MW, El-Badri N, Djeu J, Williams SN, Kane B, Szekeres K. Suppression of natural killer cell cytotoxicity in postpartum women: time course and potential mechanisms. Biol Res Nurs 2014; 16:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Groer M, El-Badri N, Djeu J, Harrington M, Van Eepoel J. Suppression of natural killer cell cytotoxicity in postpartum women. Am J Reprod Immunol 2010; 63:209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luppi P. How immune mechanisms are affected by pregnancy. Vaccine 2003; 21:3352–7. [DOI] [PubMed] [Google Scholar]
- 8. Balkus J, Agnew K, Lawler R, Mitchell C, Hitti J. Effects of pregnancy and bacterial vaginosis on proinflammatory cytokine and secretory leukocyte protease inhibitor concentrations in vaginal secretions. J Pregnancy 2010; 2010:385981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donders GG, Vereecken A, Bosmans E, Spitz B. Vaginal cytokines in normal pregnancy. Am J Obstet Gynecol 2003; 189:1433–8. [DOI] [PubMed] [Google Scholar]
- 10. Asin SN, Eszterhas SK, Rollenhagen C, Heimberg AM, Howell AL. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis 2009; 200:965–72. [DOI] [PubMed] [Google Scholar]
- 11. Masson L, Passmore JA, Liebenberg LJ et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahle EM, Bolton M, Hughes JP et al. ; Partners in Prevention HSV/HIV Transmission Study Team Plasma cytokine levels and risk of HIV type 1 (HIV-1) transmission and acquisition: a nested case-control study among HIV-1-serodiscordant couples. J Infect Dis 2015; 211:1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Celum C, Wald A, Lingappa JR et al. ; Partners in Prevention HSV/HIV Transmission Study Team Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baeten JM, Donnell D, Ndase P et al. ; Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell MS, Mullins JI, Hughes JP et al. ; Partners in Prevention HSV/HIV Transmission Study Team Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLoS One 2011; 6:e16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eshleman SH, Hudelson SE, Redd AD et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. J Infect Dis 2011; 204:1918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jukic AM, Weinberg CR, Wilcox AJ, Baird DD. Effects of early pregnancy loss on hormone levels in the subsequent menstrual cycle. Gynecol Endocrinol 2010; 26:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lähteenmäki P, Luukkainen T. Return of ovarian function after abortion. Clin Endocrinol (Oxf) 1978; 8:123–32. [DOI] [PubMed] [Google Scholar]
- 20. Donnet ML, Howie PW, Marnie M, Cooper W, Lewis M. Return of ovarian function following spontaneous abortion. Clin Endocrinol (Oxf) 1990; 33:13–20. [DOI] [PubMed] [Google Scholar]
- 21. Jackson E, Glasier A. Return of ovulation and menses in postpartum nonlactating women: a systematic review. Obstet Gynecol 2011; 117:657–62. [DOI] [PubMed] [Google Scholar]
- 22. Gray RH, Campbell OM, Zacur HA, Labbok MH, MacRae SL. Postpartum return of ovarian activity in nonbreastfeeding women monitored by urinary assays. J Clin Endocrinol Metab 1987; 64:645–50. [DOI] [PubMed] [Google Scholar]
- 23. Hughes JP, Baeten JM, Lingappa JR et al. ; Partners in Prevention HSV/HIV Transmission Study Team Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell MS, Kahle EM, Celum C et al. ; Partners in Prevention HSV/HIV Transmission Study Team Plasma viral loads during early HIV-1 infection are similar in subtype C- and non-subtype C-infected African seroconverters. J Infect Dis 2013; 207:1166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hapgood JP, Kaushic C, Hel Z. Hormonal contraception and HIV-1 acquisition: biological mechanisms. Endocr Rev 2018; 39:36–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Estes JD, Schlievert PM et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009; 458:1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinuthia J, Richardson BA, Drake AL et al. Sexual behavior and vaginal practices during pregnancy and postpartum: implications for HIV prevention strategies. J Acquir Immune Defic Syndr 2017; 74:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teasdale CA, Abrams EJ, Chiasson MA, Justman J, Blanchard K, Jones HE. Sexual risk and intravaginal practice behavior changes during pregnancy. Arch Sex Behav 2017; 46:539–48. [DOI] [PubMed] [Google Scholar]
- 29. Keating MA, Hamela G, Miller WC, Moses A, Hoffman IF, Hosseinipour MC. High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: implications for the risk of HIV acquisition. PLoS One 2012; 7:e39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alum AC, Kizza IB, Osingada CP, Katende G, Kaye DK. Factors associated with early resumption of sexual intercourse among postnatal women in Uganda. Reprod Health 2015; 12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osinde MO, Kaye DK, Kakaire O. Influence of HIV infection on women’s resumption of sexual intercourse and use of contraception in the postpartum period in rural Uganda. Int J Gynaecol Obstet 2012; 116:171–2. [DOI] [PubMed] [Google Scholar]
- 32. Ezebialu IU, Eke AC. Resumption of vaginal intercourse in the early postpartum period: determinants and considerations for child spacing in a Nigerian population. J Obstet Gynaecol 2012; 32:353–6. [DOI] [PubMed] [Google Scholar]
- 33. Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med 2004; 12:104–7. [PubMed] [Google Scholar]
- 34. Kinuthia J, Drake AL, Matemo D et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29:2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moodley D, Moodley P, Sebitloane M et al. High prevalence and incidence of asymptomatic sexually transmitted infections during pregnancy and postdelivery in KwaZulu Natal, South Africa. Sex Transm Dis 2015; 42:43–7. [DOI] [PubMed] [Google Scholar]
- 36. Humphrey JH, Marinda E, Mutasa K et al. ; ZVITAMBO Study Group Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ 2010; 341:c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray RH, Li X, Kigozi G et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 2005; 366:1182–8. [DOI] [PubMed] [Google Scholar]
- 38. Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS 2016; 11:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price JT, Wheeler SB, Stranix-Chibanda L et al. Cost-effectiveness of Pre-exposure HIV prophylaxis during pregnancy and breastfeeding in Sub-Saharan Africa. J Acquir Immune Defic Syndr 2016; 72(Suppl 2):S145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mugwanya KK, Hendrix CW, Mugo NR et al. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: a prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med 2016; 13:e1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. AIDS 2017; 31:213–32. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization (WHO). Preventing HIV during pregnancy and breastfeeding in the context of PrEP. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 43. Heffron R, Mugo N, Ngure K et al. PrEP and ART reduce HIV transmission between members of HIV serodiscordant couples during pregnancy and pregnancy attempts. In: 21st International AIDS Conference Durban, South Africa. [Google Scholar]
- 44. US President’s Emergency Plan for AIDS Relief (PEPFAR). DREAMS innovation challenge http://www.dreamspart nership.org/. Accessed 9 September 2017.
- 45. Clinicaltrials.gov. PrEP Implementation for Mothers in Antenatal Care (PrIMA) https://clinicaltrials.gov/ct2/show/results/NCT03070600. Accessed 9 September 2017.
- 46. Osoti AO, John-Stewart G, Kiarie J et al. Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: a randomized clinical trial. AIDS 2014; 28:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mark J, Kinuthia J, Roxby AC et al. Uptake of home-based syphilis and human immunodeficiency virus testing among male partners of pregnant women in Western Kenya. Sex Transm Dis 2017; 44:533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krakowiak D, Kinuthia J, Osoti AO et al. Home-based HIV testing among pregnant couples increases partner testing and identification of serodiscordant partnerships. J Acquir Immune Defic Syndr 2016; 72(Suppl 2):S167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV 2016; 3:e266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med 2016; 13:e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.