Abstract
Objectives
Variation in antibiotic use may reflect inappropriate use. We aimed to systematically describe the variation in measures for antibiotic use among settings or providers. This study was conducted as part of the innovative medicines initiative (IMI)-funded international project DRIVE-AB.
Methods
We searched for studies published in MEDLINE from January 2004 to January 2015 reporting variation in measures for systemic antibiotic use (e.g. DDDs) in inpatient and outpatient settings. The ratio between a study’s reported maximum and minimum values of a given measure [maximum:minimum ratio (MMR)] was calculated as a measure of variation. Similar measures were grouped into categories and when possible the overall median ratio and IQR were calculated.
Results
One hundred and forty-three studies were included, of which 85 (59.4%) were conducted in Europe and 12 (8.4%) in low- to middle-income countries. Most studies described the variation in the quantity of antibiotic use in the inpatient setting (81/143, 56.6%), especially among hospitals (41/81, 50.6%). The most frequent measure was DDDs with different denominators, reported in 23/81 (28.4%) inpatient studies and in 28/62 (45.2%) outpatient studies. For this measure, we found a median MMR of 3.7 (IQR 2.6–5.0) in 4 studies reporting antibiotic use in ICUs in DDDs/1000 patient-days and a median MMR of 2.3 (IQR 1.5–3.2) in 18 studies reporting outpatient antibiotic use in DDDs/1000 inhabitant-days. Substantial variation was also identified in other measures.
Conclusions
Our review confirms the large variation in antibiotic use even across similar settings and providers. Data from low- and middle-income countries are under-represented. Further studies should try to better elucidate reasons for the observed variation to facilitate interventions that reduce unwarranted practice variation. In addition, the heterogeneity of reported measures clearly shows that there is need for standardization.
Introduction
Variation in healthcare delivery among different geographical areas, healthcare facilities and individual providers is a nearly ubiquitous finding that can only be partly explained by differences in patient characteristics or disease epidemiology.1,2 The importance of systematically studying the extent of this variation and its underlying causes in order to improve quality and resource utilization was first recognized in the 1970s by Wennberg and Gittelsohn,3 when they analysed ‘small area variations’ of hospitalization rates and surgical procedures in the US state of Vermont.
With the continuing emergence and spread of MDR organisms across the globe in recent years, there has been increasing interest in medical practice variation regarding antibiotics since inappropriate use is one of the key drivers of antimicrobial resistance.4–8 Indeed, important variation in antibiotic use has been described among countries, hospitals and physicians that share many similarities in their patient populations and economic, geographical and epidemiological characteristics.9,10 While the exact relationship between antibiotic use and emergence and spread of antimicrobial resistance (AMR) is complex, there is, for example, a clear correlation between a country’s level of outpatient antibiotic use and the prevalence of certain antibiotic-resistant bacteria.11,12 Describing and ultimately understanding the variation in antibiotic use could help to target the implementation of interventions to improve antibiotic prescribing where this is most needed. Having a clear picture of the observed variation in measures for antibiotic use is also important to establish benchmarks for antibiotic use measures.13
This systematic review of the literature was conducted as part of the European DRIVE-AB project and its primary objective was to describe the variation in use of antibiotics in outpatient and inpatient settings.1 Further systematic reviews in the context of DRIVE-AB aimed to specifically assess the definition of responsible use of antibiotics14 and to assess quantity metrics and quality indicators for antimicrobial use in the inpatient15,16 and outpatient settings.17,18
Methods
This systematic review is reported following the PRISMA statement.19
Eligibility criteria
We conducted a systematic review of the published literature to identify English language studies that describe the naturally observed variation (i.e. the variation occurring outside the context of a specific interventional study) in measures of systemic antibiotic use for treatment and prophylaxis within and among different settings (e.g. countries, hospitals, hospital units) and providers (e.g. general practitioners, paediatricians, physicians with different specialties) at a given point in time. We included studies describing variation in both paediatric and adult populations. Only studies describing variation among a minimum number of providers or settings were included (Table 1). We predefined different limits for larger entities (such as hospitals and countries) and individual providers (Table 1). The cut-offs were chosen for pragmatic reasons, since we felt that otherwise the number of eligible studies would be too vast without offering much additional information about variation due to the small number of entities.
Table 1.
Minimum number of providers or settings considered for study eligibility
| Setting | Unit/hospital/region/country level | Provider level |
|---|---|---|
| Inpatient | Data from ≥5 hospitals irrespective of their size | ≥20 providers in the same hospital |
| OR | ||
| Data from ≥5 identical units (e.g. ICUs, haematology wards etc.) from ≥5 hospitals irrespective of their size | ||
| OR | ||
| Data from ≥5 units/wards in the same hospital irrespective of their size | ||
| Outpatient | ≥2 countries, regions or districts (same or different country) | ≥50 providers in the same geographical area |
| OR | ||
| ≥5 clinics/primary health care facilities |
The cut-offs were chosen for pragmatic reasons, since we felt that otherwise the number of eligible studies would be too vast without offering much information about variation due to the small number of entities.
We allowed the inclusion of ESAC/ESAC-net (European Surveillance of Antimicrobial Consumption Network) studies reporting data from the same year(s) only when the number of countries participating in the studies was different or the described measures were different.
We tried to group similar measures into categories reflecting:
The quantity of antibiotic use [e.g. DDDs, days of therapy (DOT), length of therapy (LOT) with different denominators or percentage of treated patients].
Prescribing strategies (e.g. percentage of delayed prescriptions, percentage of antibiotics prescribed as empirical treatment).
Compliance with guidelines (percentage of appropriate or compliant prescriptions, percentage of patients treated with antibiotics within a given timeframe).
Process and structural measures for antibiotic stewardship policies (e.g. percentage of prescriptions documented in the medical file, presence of antibiotic stewardship guidelines).
Antibiotic use for medical or surgical prophylaxis (e.g. percentage of patients receiving surgical prophylaxis for >24 h).
We excluded the following studies:
Studies describing exclusively the use of antivirals, antifungals, antimycobacterials, antiparasitic drugs and topical antibiotics.
Studies only describing variation of antibiotic use over time within the same setting.
Studies only describing variation in outcomes associated with antibiotic use (e.g. variation in rates of Clostridium difficile infection).
Studies focusing on the variation in healthcare professionals’ views, beliefs, attitudes and knowledge.
Studies describing self-reported (by patients, caregivers and physicians) behaviours regarding antibiotic use.
Studies whose full text could not be retrieved from any of the libraries of the participating centres (eight different catalogues).
Studies that presented data only graphically (no efforts were made to contact authors).
Interventional studies without extractable pre-intervention data.
Systematic reviews and meta-analyses and studies not reporting original data (narrative reviews, opinion pieces etc.). Their reference lists were, however, screened to identify potentially eligible studies.
Search and information sources
We searched the MEDLINE database using the PubMed interface using a combination of search terms for the concepts (i) ‘antibiotics’, (ii) ‘quality’ and ‘quantity of use’ and (iii) ‘variation’ (for the detailed search strategy see Table S1, available as Supplementary data available at JAC Online). Owing to the large number of potentially eligible studies (>6000) we used the PubMed filters for species (‘humans’) and language (‘English’). Since we were mostly interested in recent findings, we restricted the timeframe to articles whose last year of data collection was 2004 (included) or later with a publication date between 1 January 2004 and 15 January 2015.
Study selection and data collection process
All the steps of this systematic review were carried out using the Distiller SR® software (Evidence Partners, Ottawa, Canada). Duplicates were removed before title and abstract screening using the algorithm provided by Distiller SR®. One reviewer (V. Z.) screened all titles and abstracts. A second reviewer (B. H.) independently screened a random subset of 700 abstracts (13%). For 3/700 (0.4%) references screened by both reviewers there was disagreement regarding inclusion/exclusion. All three references were later excluded at full-text screening level.
All full-text assessments were performed by one reviewer (V. Z.) and, in case of uncertainty, discussed with another investigator (B. H.) until a consensus was reached. Data extraction was performed by one reviewer (V. Z.) using a standardized data extraction form; any uncertainty about extracted data was discussed with another investigator (B. H.).
Data regarding authors, setting (inpatients or outpatients), country/region, last year of data collection, study design, level of variation (providers, units, hospitals etc.), number and characteristics of participants, data source, category of the numerator and denominator of the measure, full description of the measure, mean or median (as available in the text) and measure of variability (maximum–minimum range, IQR or SD as presented in the text) were collected (for definitions see Table S2). We extracted data for overall antibiotic use and if the study also reported variation for specific antibiotic classes we extracted data only for β-lactams, quinolones and macrolides (except if the study described variation of only specific antibiotic classes but no overall antibiotic use) since we felt that extracting data for all individual classes would go beyond the scope of this review. Since we mainly included observational studies and the aim of this review was to describe variation (without causal inferences) we decided not to perform risk of bias assessment for the included studies.
Synthesis of results and summary measures
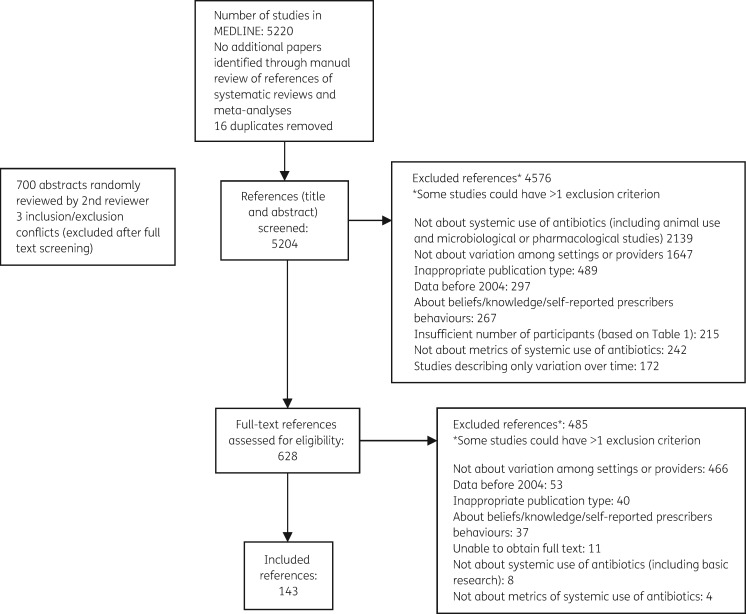
Using the search strategy (Table S1), we identified 5204 studies. After title and abstract screening 628 studies were retained for full-text review; of these 143 met the inclusion criteria (Figure 1). Selected study characteristics are presented in Table 2. Detailed information for all 143 studies is available in Tables S5 to S8. The results of our search are presented by setting (inpatients versus outpatients) and country income level (high versus low to middle income using the World Bank 2015 classification20); for high-income countries we further differentiated between European and North American studies since these were the most represented regions. Data regarding measures for antibiotic use in paediatric patients are presented as a separate category. Measures were extracted from each study and a measure’s category was attributed to each of them. The complete list of measure categories is reported in Table S3.
Figure 1.
Flow chart of study selection.
Table 2.
Characteristics of included studies
| Inpatients |
Outpatients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Level at which the variation is described | units | hospitals/LTCFs | countries | providers | clinics | regions | countries | Total |
| Number of studies | 25 | 50 | 6 | 14 | 13 | 16 | 19 | 143 |
| Number of health facilities/providers/ geographical areas, median (IQR) | 18 (10–40.5) | 37.5 (20–116.5) | 27.5 (19.7–31.5) | 413.5 (107.5–1703) | 15 (11–27.5) | 11 (10–22) | 21 (14.5–32.5) | NA |
| Single country, n (%) | 22/25 (88.0) | 41/50 (82.0) | 0 | 10/14 (71.4) | 10/13 (76.9) | 16/16 (100) | NA | 99/143 (69.2) |
| High-income countries, n (%) | 23/25 (92.0) | 46/50 (92.0) | 6/6 (100) | 12/14 (85.7) | 10/13 (76.9) | 16/16 (100) | 18/19 (94.7) | 131/143 (91.6) |
| Most frequent WHO region, n/N (%) | Europe, 18/25 (72.0) | Europe, 25/50 (50.0) | Europe, 4/6 (66.7) | Europe, 10/14 (71.4) | Europe, 6/13 (46.1) | Europe, 7/16 (43.7) | Europe, 15/19 (78.9) | Europe, 85/143 (59.4) |
| Antibiotic prophylaxis included, n/N (%) | 1/25 (4.0) | 7/50 (14.0) | 1/6 (16.7) | 0 | 0 | 0 | 0 | 9/143 (6.3) |
| Children included, n/N (%) | 8/25 (32.0) | 11/50 (22.0) | 0 | 1/14 (7.1) | 3/13 (23.1) | 3/16 (18.7) | 0 | 26/143 (18.2) |
NA, not applicable.
Given our broad search strategy, we identified a wide range of different antibiotic use measures, often applying to specific medical conditions (e.g. otitis media, urinary tract infections or community-acquired pneumonia) or settings (e.g. ICUs, neonatal ICUs, emergency departments). In addition, most studies presented only aggregated data, making it challenging to summarize variation in a standardized way. In the absence of better alternatives, we decided, whenever possible, to report the extent of variation in a given antibiotic use measure for each study by calculating the ratio between the reported maximum and minimum value of the measure (MMR). For example, in a study describing variation in overall antibiotic use among hospitals in DDDs we divided the maximum value by the minimum value of DDDs. This ratio was then used to calculate the median and IQR ratio whenever measures of the same category could be grouped together.
Results
Overall a total of 44 unique measures grouped in five categories were identified (Table S3). For the purpose of this review the term ‘unique measures’ is used to indicate distinctive measures, meaning measures different from one another with respect to their numerator (e.g. DDDs, DOT, percentage of treated patients). Measures that have the same numerator but different denominators, e.g. DDDs per inpatient day or per admission, were not considered unique measures for the purpose of this review because the numerator is the same. An overview of the frequency of the measures in the different categories both in the in- and outpatient settings is presented in Table S4.
In the context of this review we identified 10 unique measures for quantity, 5 for prescribing strategies, 8 for compliance with guidelines, 18 for process and structural measures of antibiotic stewardship policies and 3 for antibiotic prophylaxis.
Inpatient setting (81 studies)
High-income countries
Overall, 75 studies (75/143, 52.4%) met inclusion criteria for this setting: 47 from Europe, 17 from the USA and Canada, 3 from Australia, 1 from Israel, 1 from Japan and 6 from more than one WHO region (see Table S2 for the definition of WHO regions). Thirty-eight unique measures were extracted for this setting with sometimes different studies reporting identical measures. Overall, 169 ‘non-unique’ measures reporting data about variation (also called ‘variation data’ in this review) were represented for this setting.
The most frequent measures concerned ‘quantity’ of antibiotic use, with 91 variation data extracted from 58 studies. The three most frequent unique measures (out of the 10 belonging to this category) were DDDs (reported 34 times for overall use or for specific antibiotic classes and medical conditions and with different denominators), followed by ‘percentage of treated patients’ (reported 31 times in different settings and for different health conditions) and DOT (reported 13 times both for overall and specific antibiotic classes use and for specific medical indications and with different denominators).
Europe
Forty-seven studies (47/143, 32.9%), including 9 ESAC studies, were included for the inpatient setting, reporting data from 13 different European countries. We identified 18 variation data specifically addressing the paediatric population and 4 variation data concerning antibiotic prophylaxis.
Included studies described variation either among acute care hospitals (18/47, 38.3%), long-term care facilities (LTCFs) (7/47, 14.9%), neonatal ICUs (2/47, 4.3%), adult ICUs (6/47, 12.8%), different hospital units (10/47, 21.3%) or different countries (4/47, 8.5%). No study described variation among providers (e.g. physicians with different specialties). Concerning the nine ESAC studies, almost all (8/9, 88.9%) addressed antibiotic use for treatment in the adult population, describing variation among hospitals (3/9, 33.3%), units (2/9, 22.2%), LTCFs (2/9, 22.2%) or countries (2/9, 22.2%). Both among units and among LTCFs, the most frequently reported measure was overall percentage of patients treated with an antibiotic (only one study specifically addressed nosocomial infections at the unit level).
We identified 58 variation data for this setting for the category ‘quantity’ of antibiotic use from 37 studies (Table S5) for the complete list of measures with calculated levels of variation (MMR). MMRs for the most frequently reported measures referring to the same population, medical condition and setting are presented in Table 3. The highest MMR for this setting was described among LTCFs (six studies) for the measure ‘percentage of patients treated with antibiotics for any condition’, with a median MMR of 13.3 (IQR 8.9–16.5). The same measure presented a median MMR of 2.1 (IQR 1.8–2.4) among hospitals (five studies). We also found a median MMR of 4.2 (IQR 2.3–8.1) among hospitals (six studies) and of 3.7 (IQR 2.6–5.0) among ICUs (four studies) for the measure DDDs/1000 patient-days.
Table 3.
Most frequent measures, Europe and North America: inpatient setting (64 studies)
| Measure | Number of studies (%) | MMR (IQR) (if applicable) | Comments |
|---|---|---|---|
| Europe | |||
| percentage of patients treated with antibiotics (any condition) | 19/47 (40.4) | Hospitals (5 studies29–33): median MMR 2.1 (1.8–2.4) |
|
| LTCFs (6 studies34–39): median MMR 13.6 (8.9–16.5) |
|
||
| total DDDs/1000 patient-days | 16/47 (34.0) | Hospitals (6 studies40–45): median MMR 4.2 (2.3–8.1) | |
| ICUs (4 studies42,46–48): median MMR 3.7 (IQR 2.6–5.0) | |||
| all measures related to antibiotic stewardship | 7/4749–55 (14.9) | NA | Examples of measures: presence of a surveillance system for antibiotic usage or presence of an antibiotic stewardship programme for LTCFs |
| percentage of appropriate prescriptions | 1/47 (2.1) | Emergency departments (1 study56): MMR 1.5 | 56Appropriate antibiotic prescriptions for urinary tract infections in emergency departments of 10 hospitals from different Spanish regions |
| antibiotic days/patient-days | 2/47 (4.2) | Hospitals (1 study55): MMR 2.1 | 55In this study, antibiotic use was measured using number of use-days/100 patient-days during a 7 day period. The study reported the prevalence of patients receiving at least two antimicrobials during the study day in 30 Finnish hospitals |
| number of antibiotics accounting for 75% of total consumption | 1/47 (2.1) | Hospitals (1 study57): MMR 2.1 | 57Drug utilization 75% (DU75%) in 17 European hospitals: results from the ESAC-2 Hospital Care Sub Project |
| percentage of combination therapy | 1/47 (2.1) | Countries (1 study58): MMR 1.2 | 58European Surveillance of Antimicrobial Consumption (ESAC): value of a PPS of antimicrobial use across Europe. The use of combination therapy was related to hospital type, with teaching and tertiary hospitals having a significantly higher use of combination therapy |
| percentage of surgical prophylaxis >24 h | 1/47 (2.1) | Countries (1 study59): MMR 3.1 | 59Prolonged perioperative surgical prophylaxis within European hospitals. For the purpose of the study data were extracted from the ECDC PPS 2011–12 report |
| percentage of patients receiving prophylaxis (medical or surgical) | 4/47 (8.5) | LTCFs (2 studies): MMR 22 (overall60), MMR 55 (for UTI61) | 56Variation was described among 44 Norwegian LTCFs. 10% of residents on the day of the survey were receiving antibiotics for infection prevention and 6% for infection treatment. The indication for prophylaxis was UTI in all but 1 case |
| Hospitals (2 studies): MMR 4.5,62 MMR 1.662,63 | |||
| percentage of patients whose antibiotic prophylaxis was stopped <24 h after surgery | 1/47 (2.1) | Departments (1 study64): MMR 1.2 | 60The measure specifically referred to duration of surgical prophylaxis <24 h in Scottish acute care hospitals |
| North America | |||
| percentage of patients treated with antibiotics | 2/17 (11.8) | Departments (1 study addressing adults65): MMR 26.6 |
|
| LTCFs (1 study66): MMR 4.5 | |||
| DOT/antibiotic days | 6/17 (35.3) | Hospitals (1 study67): MMR 1.8 | 67Variation was measured among 70 US academic centres |
| prophylaxis use before surgery | 3/17 (17.6) | Hospitals (3 studies): adults62 median MMR 4.5 | 62Percentage of patients receiving prophylaxis within 1 h before surgery |
| DDDs/10000 patient-days | 1/17 (5.9) | Hospitals (1 study68): MMR 2.5 | 68Interventional study examining the effect of antibiotic stewardship programme (ASP)-based strategies (all including a component of audit and feedback) on antibiotic consumption of target antibiotics (piperacillin/tazobactam, fluoroquinolones, or cefepime) Data refer to the baseline data in the intervention group (non-target antibiotics) |
| percentage of patients whose antibiotic prophylaxis was stopped <24 h after surgery | Hospitals (1 study62): MMR 4.5 | 62The measure specifically referred to duration of prophylaxis <24 h across 295 US hospital groups (a hospital group comprises all hospitals sharing identical categories for location by state, teaching status, bed size and urban/rural location) | |
PPS, point prevalence study.
The Loeb minimum criteria, developed by a 2001 consensus conference, are minimum standards for initiation of antibiotics in long-term care settings, intended to reduce inappropriate prescribing.
North America
We found 17 North American studies that fulfilled inclusion criteria for the inpatient setting: 14 from the USA and 3 from Canada; 53% (9/17) addressed the paediatric population and 2 described variation in measures for antibiotic use in LTCFs. Three studies included measures for antibiotic prophylaxis. One was an interventional study. The four most frequent unique measures described for this setting are reported in Table 3. Among the paediatric studies the MMR for the measure ‘percentage of febrile neonates treated with antibiotics’ among departments was 1.321 and 1.522 in two studies, respectively.
An MMR of 2.5 was described in one study describing the same measure in children with bronchiolitis presenting at the emergency department. As in Europe, the MMR among LTCFs for the percentage of patients receiving antibiotics was high (4.5 in one study).
Low- to middle-income countries
We found three different measures described for this setting (Table 4), two belonging to the category ‘quantity’ (DDDs and percentage of treated patients) and one belonging to the category ‘compliance with guidelines’ (percentage of appropriate/inappropriate prescription).
Table 4.
Inpatient setting: low- to middle-income countries (six studies)
| Measure | Number of studies (%) | MMR, median (IQR) (if applicable) | Comments |
|---|---|---|---|
| DDDs/100 bed-days | 3/6 (50%) | Hospitals (1 study): adults69→ 5.1 | 69International study taking place in >1 WHO region |
| Percentage of treated patients | 3/6 (50%) | Hospitals (2 studies70,71): 1.9,67 2.368 | 70,71Both studies were from the Western pacific WHO region (Vietnam and China) |
Six studies could be included for this setting (three of which were from the Western Pacific WHO region); one was an international study that included countries from more than one WHO region and the others were from four different countries. No study included data collected after 2011. Three studies addressed the paediatric population. Variation was mainly described among hospitals (four studies, 66.7%) with an MMR of 2.1 (IQR 2–2.2) for the measure ‘percentage of patients treated with antibiotics’ (three studies) and an MMR of 5.1 for the measure DDDs/100 bed-days (one study). The complete list of included studies for this setting is presented in Table S6.
Outpatient setting (62 studies)
High-income countries
Overall, 56 studies (56/143, 39.2%) met the inclusion criteria for this setting: 38 from Europe, 10 from the USA and Canada, 1 each from Bahrain, Israel, Saudi Arabia and South Korea and 4 from more than one WHO region. Overall, 110 variation data were identified (Table S7).
As for the inpatient setting, the category with the highest number of included measures was ‘quantity’ of antibiotic use, with 53 studies reporting 91 variation data. The three most frequent measures were: (i) DDDs (34 variation data in 28 studies); (ii) percentage of antibiotic prescriptions for different medical conditions, populations (e.g. children, adults or elderly people) and sometimes for specific antibiotic classes (18 variation data in 10 studies); and (iii) percentage of treated patients (17 variation data in 12 studies).
Europe
We included 38 studies (38/56, 67.8%) for this setting, including 15 ESAC studies. No study reported data collected after 2011. More than half were international studies (20/38, 52.6%); the rest were single-country studies from nine different countries. Italy was the country with the highest number of studies (6/38, 15.8%) followed by France (4/38, 10.5%).
Most of the studies reported variation among countries (15/38, 39.5%) or geographical areas within the same country (e.g. regions, provinces, districts) (7/38, 18.4%). Ten studies (26.3%) reported variation among providers and six among outpatient clinics. Overall, most studies (36/38, 94.7%) included measures for the category ‘quantity’ of antibiotic use, with the three most represented measures being: (i) DDDs (reported in 21 studies); (ii) percentage of treated patients (reported in 10 studies); and (iii) percentage of antibiotic prescriptions (reported in 9 studies). The second category in terms of frequency of measures was ‘prescribing strategies’ [e.g. percentage of delayed prescriptions for lower respiratory tract infections (LRTIs), days of delay before taking the antibiotic for LRTIs, percentage of patients treated with specific antibiotic classes for respiratory tract infections, and percentage of antibiotics administered through parenteral route] with 10 unique measures included followed by ‘compliance with guidelines’ (e.g. percentage of guideline-compliant prescriptions for LRTIs) with 6 unique measures included. No study reporting measures for the category ‘process and structural measures for antibiotic stewardship policies’ was identified for this setting.
In Table 5 we report the level of variation for the most frequent measures: DDDs/1000 inhabitant-days, which showed a median MMR of 3.2 (IQR 3.0–3.5) among countries (eight studies) and a median MMR of 1.5 (IQR 1.4–1.5) among geographical areas (three studies). For the measure ‘percentage of treated patients’ we found an MMR of 1.4 in two studies reporting variation among geographical areas in the same country.
Table 5.
Outpatient setting: Europe and North America (48 studies)
| Measure | Number of studies (%) | MMR (maximum/minimum ratio) (IQR) (if applicable) | Comments |
|---|---|---|---|
| Europe | |||
| DDDs/1000 inhabitant-days | 19/38 (50.0) | No study included data collected after 2009 8 were ESAC studies | |
| percentage of treated patients | 12/38 (31.6) | Geographical areas (2 studies81,82): MMR 1.4 | |
| percentage of total antibiotic use | 1/38 (2.6) | Providers (1 study83) | 83Specific focus on fluoroquinolone use |
| duration of therapy | 1/38 (2.6) | Providers (1 study84): MMR 6 | 84Antibiotic use for CAP among 94 Italian GPs |
| percentage of compliant prescriptions | 1/38 (2.6) | Clinics (1 study85): MMR 6.6 | 85Antibiotic use for LRTI among GPs in 14 primary care research networks in 13 European countries (GRACE study) |
| percentage of delayed prescriptions | 1/38 (2.6) | Clinics (1 study86): MMR 165.5 | 86Antibiotic use for LRTI among GPs in 14 primary care research networks in 13 European countries (GRACE study) |
| North America | |||
| DDDs/1000 inhabitant-days | 6/10 (60) | Geographical areas (2 studies87,88): MMR 1.9 | |
| prescriptions | 2/10 (20.0) | Geographical areas (1 study89): MMR 1.4 | 89Data on oral antibiotic prescriptions dispensed during 2010 in the USA extracted from a national administrative database |
| percentage of treated patients | 2/10 (20.0) | Providers (1 study90): MMR 2.3 | 90Data on antibiotic use for patients with acute otitis externa among providers in different clinical specialties (otolaryngologists, emergency departments physicians, paediatricians) in the USA |
| percentage of non- compliant prescriptions | 1/10 (10.0) | Clinics (1 study91): MMR 2 | 91Data on inappropriate use of systemic antibiotics for children with otitis media with effusion among 19 clinics in the USA |
| number of antibiotics whose prescription is restricted and requires specific information confirming the diagnosis in order for the patient to be reimbursed by the health system | 1/10 (10.0) | Geographical areas (1 study92): MMR 15 | 92The objective of this study was to assess whether the relative flexibility/stringency of provincial antibiotic formularies had a statistical impact upon provincial prescription volume in Canadaa |
GPs, general practitioners.
In Canada, provinces have the option to list antibiotics as either ‘general benefits’ (available to all by prescription) or as ‘restricted benefit’ (requiring additional information and/or paperwork before prescriptions may be reimbursed). For example, Quebec has only one antibiotic, linezolid, in its formulary that is listed as ‘restricted benefit’ while Saskatchewan has 15 restricted antibiotics in its formulary.
North America
Ten studies (10/62, 16.1% of all studies included for the outpatient setting) describing variation in outpatient antibiotic use in North America were identified: 6 from Canada, 3 from the USA and 1 that included data from both countries. Two studies (20%) included measures addressing the paediatric population. No study described measures for antibiotic prophylaxis.
Nine studies included measures for the category ‘quantity’ of antibiotic use and one study included one measure for the category ‘process and structural measures for antibiotic stewardship policies’. No study described measures for the remaining three categories (‘compliance with guidelines’, ‘prescribing strategies’ and ‘antibiotic prophylaxis’).
The most frequent measure reported for this setting (Table 5) was DDDs/1000 inhabitant-days (six studies), with an MMR of 1.9 among geographical areas (two studies). An MMR of 1.4 was described also for the measure ‘percentage of treated patients’, again among geographical areas in the same country (one study).
Low- to middle-income countries
Six studies fulfilled the inclusion criteria (9.7% of all studies included for the outpatient setting). One was an international study and the rest were single-country studies from three different countries (China, Iran and India). One interventional study was included. No study included data collected after 2010. Variation was described among providers (two studies), countries or smaller geographical areas within the same country (two studies) or outpatient clinics (two studies). We identified three different measures for this setting (Table 6), with the highest MMR (20.5) described among providers (one study) for the measure ‘percentage of treated patients’ in a study from Iran. The complete list of included studies for this setting is presented in Table S8.
Table 6.
Low- to middle-income countries: outpatient setting (6 studies)
| Measure | Number of studies (%) | MMR, median (IQR) (if applicable) | Comments |
|---|---|---|---|
| Percentage of treated patients | 3/6 (50) |
|
|
| DDDs/1000 inhabitant-days | 2/6 (33.3) (one of the studies concerned the paediatric population) | Countries (1 study96): 2.4 | 96Antibiotic use in eight Latin American countries (we considered only data from 2007) |
| Percentage of antibiotics per prescription | 1/6 (16.7) | Providers (1 study97): 2.2 | 97Cluster randomized controlled trial of 159 GPs working in 6 cities, in 2 regions in East Azerbaijan in Iran. The cities were matched and randomly divided into an intervention arm, for an outcome-based education on rational prescribing, and a control arm for a traditional CME programme on the same topic. GPs’ prescribing behaviour was assessed 9 months before and 3 months after the CME programmes |
CME, continuing medical education; GPs, general practitioners.
Measures for antibiotic use for antibiotic prophylaxis and in the paediatric population
Nine studies including measures related to antibiotic prophylaxis were also included (6.3%), all of them from high-income countries, mainly from Europe and North America (six studies, 66.7%); three studies were from more than one WHO region. Six studies focused on perioperative prophylaxis (of which two included measures specifically addressing the paediatric population and one focused on specific surgical procedures: prostate biopsy and cystoscopy) and three studies focused on prophylaxis of urinary tract infections (UTIs) in LTCFs. Three studies (one paediatric) included measures related to compliance with antibiotic prophylaxis guidelines (e.g. percentage of patients given prophylaxis for a duration of <24 h). Variation was described among hospitals (four studies), units (one study), countries (one study) and LTCFs (three studies). The highest MMR was found for the measure ‘percentage of patients receiving prophylaxis’ for UTIs among LTCFs (55 in one study). Twenty-six studies reported measures for antibiotic use in children mainly for the inpatient setting (19/26, 73.1%). Four studies were from low- to middle-income countries. Most of the measures belonged to the category ‘quantity’ of antibiotic use (18/26, 69.2%), two measures (from one study) belonged to the category ‘prescribing strategies’ [e.g. percentage of antibiotics prescribed as empirical treatment for community-acquired pneumonia (CAP)] and four (from two studies) were measures specifically addressing antibiotic prophylaxis. No measure for the category ‘process and structural measures for antibiotic stewardship policies’ was included. The highest MMRs were described for the measure ‘percentage of treated patients’ (median MMR 3.5, IQR 2.5–4.4, among hospitals in four studies and an MMR of 4.7 in one study describing variation among clinics) and for the measure DOT, with an MMR of 5.1 among departments in one study (Table S9).
Discussion
The most important finding of this review was the large variation in measures of antibiotic use even across similar settings and providers. This finding was confirmed also for specific medical conditions and populations both in high- and low- to middle-income countries. A second key finding is the large heterogeneity of reported measures (even without taking into account differences in data sources; e.g. antibiotic dispensing versus administration data), clearly indicating the need for standardization.
Most of the findings of this review concerned evaluation of the quantity of antibiotic consumption, and variation was observed whatever the type of measure used and regardless of the setting. For example, for the European inpatient setting we found high variation in DDDs/1000 patient-days with a median MMR of 4.2 for hospitals (six studies) and 3.7 for ICUs (four studies). In low- to middle-income countries the same measure showed an MMR of 5.1 (one study) among hospitals. Variation was also observed for antibiotic prescribing strategies, guideline compliance and for existence of antimicrobial stewardship policies across healthcare facilities. In most of the cases measures referred to antibiotic use for treatment in the adult population, whereas studies including antibiotic use for prophylaxis or addressing the paediatric population were a minority. It was not surprising to see that most of the information retrieved from the literature came from observational retrospective studies from high-income countries, especially from Europe, and that data from resource-limited settings were under-represented in the literature.23
Despite the fact that our search was performed at the beginning of 2015, most data were from before 2012, most likely reflecting the delay between data collection, data analysis and final publication. The great variety of measures made it difficult to summarize and present the observed variation. The ratio between maximum and minimum values is certainly a suboptimal measure of variation since it is heavily influenced by outliers and the characteristics of the measure (e.g. MMRs will be inherently different for measures with an absolute upper and lower boundary, such as those expressed in percentages, and measures that only have an absolute lower boundary, such as DDDs).
Inferences on the causes of the described variation were beyond the scope of this review. Indeed, few of the examined studies offered clear evidence for the reasons behind the observed variation. It seems clear, however, that much of the observed variation is ‘unwarranted’, in the sense that it is unlikely to be driven by differences in the epidemiology of infectious diseases or patient characteristics but could rather be explained by one or more of the seven determinants of healthcare professionals’ behaviours described by Flottorp et al.24 and that could also be applied to antibiotic prescribing practices. These seven domains are: (i) guideline factors (e.g. guideline characteristics or presence of contradictory guidelines); (ii) individual prescriber preferences (which could be influenced by many factors, such as lack of agreement with specific guidelines, lack of motivation or inertia of previous practices etc.); (iii) patient factors (e.g. patient’s expectations, inability to reconcile patient’s preferences with guideline recommendations); (iv) professional interactions (e.g. leadership, key individuals, team processes); (v) incentives and resources (e.g. economic incentives, technical knowledge, organizational size); (vi) capacity for organizational change (e.g. planning, engaging, executing and evaluating); and (vii) social, political and legal medical norms (e.g. legislation or regulations, priority on societal agenda, corruption, political stability etc.). The impact of combinations of these determinants on antibiotic use has been previously described in both the inpatient and the outpatient setting.25–28
In particular in the inpatient setting, differences in ‘culture’ (country level, e.g. ideas about health, causes of disease, labelling of illness, coping strategies and ‘treatment modalities’ differ across countries), ‘context’ (hospital level, influenced by organizational policies, multi-professional care-delivery system) and ‘behaviour’ (professional level) have all been described.28 In the outpatient setting, socio-cultural differences as well as specific regulatory practices have been associated with varying levels of prescriptions for frequent medical conditions, such as LRTIs.27
Strengths of this review include a broad search strategy and the inclusion of studies from a variety of different settings. There are, however, also several limitations to our work. We only searched the MEDLINE database over a 10 year period and we did not explore other sources of information, such as relevant surveillance websites or single-country data. Another limiting factor is that we did not perform a quality assessment of included studies and we did not explore the relationship between extent of variation in measures and outcomes of antibiotic use, e.g. resistance, costs or rates of C. difficile infections. Although we included a large variety of measures described in the literature, we did not address possible benchmarks for the measures and we did not try to explore reasons for the observed variation as most studies did not provide information regarding this issue. We did not address the relevance of the measures for appropriate use. This is addressed in other work of the DRIVE-AB consortium.14–18
Conclusions
At a time when major stakeholders are trying to find effective solutions to the problem of antibiotic resistance, the large variation in measures of antibiotic use (even across similar settings/providers or for similar clinical conditions) remains poorly understood and can only be partly explained by different patients’ and physicians’ attitudes. Although variation is not something negative by definition and is ‘natural’ up to a certain level, a better understanding of this phenomenon, addressing similarities and differences across specific settings and providers, should be pursued. Furthermore, in order to make informative comparisons among settings, there is need to standardize the measures used to measure the quantity and quality of antibiotic use.
Supplementary Material
Acknowledgements
We acknowledge the input of Elodie von Dach in providing help with the literature search and data collection and all the members of the DRIVE-AB steering committee for critically reviewing the manuscript.
Preliminary results of this study have been presented as a poster presentation (P1294) at the 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), 9–12 April 2016, Amsterdam, The Netherlands.
Funding
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115618 (Driving re-investment in R&D and responsible antibiotic use – DRIVE-AB – www.drive-ab.eu), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution.
Transparency declarations
V. Z., A. A. M., M. E. H., N. A., A. V., C. P., V. V-P., M. L. M., G. T., M. S. B., R. M. and B. H. have none to declare. I. C. G. and S. H. report having received educational grants from Pfizer, outside the submitted work.
This article forms part of a Supplement sponsored by DRIVE-AB.
Supplementary data
Tables S1–S9 are available as Supplementary data at JAC online.
References
- 1. Harbarth S, Theuretzbacher U, Hackett J.. Antibiotic research and development: business as usual? J Antimicrob Chemother 2015; 70: 1604–7. [DOI] [PubMed] [Google Scholar]
- 2. Neuhauser D, Provost L, Bergman B.. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf 2011; 20: i36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wennberg J, Gittelsohn A.. Small area variations in health care delivery. Science 1973; 182: 1102–8. [DOI] [PubMed] [Google Scholar]
- 4. Huttner A, Harbarth S, Carlet J. et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2: 31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuster SP, Rudnick W, Shigayeva A. et al. Previous antibiotic exposure and antimicrobial resistance in invasive pneumococcal disease: results from prospective surveillance. Clin Infect Dis 2014; 59: 944–52. [DOI] [PubMed] [Google Scholar]
- 6. van de Sande-Bruinsma N, Grundmann H, Verloo D. et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 2008; 14: 1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Lastours V, Cambau E, Guillard T. et al. Diversity of individual dynamic patterns of emergence of resistance to quinolones in Escherichia coli from the fecal flora of healthy volunteers exposed to ciprofloxacin. J Infect Dis 2012; 206: 1399–406. [DOI] [PubMed] [Google Scholar]
- 8. Malhotra-Kumar S, Lammens C, Coenen S. et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet 2007; 369: 482–90. [DOI] [PubMed] [Google Scholar]
- 9. Cars O, Molstad S, Melander A.. Variation in antibiotic use in the European Union. Lancet 2001; 357: 1851–3. [DOI] [PubMed] [Google Scholar]
- 10. Adriaenssens N, Coenen S, Versporten A. et al. European Surveillance of Antimicrobial Consumption (ESAC): quality appraisal of antibiotic use in Europe. J Antimicrob Chemother 2011; 66: vi71–7. [DOI] [PubMed] [Google Scholar]
- 11. Blommaert A, Marais C, Hens N. et al. Determinants of between-country differences in ambulatory antibiotic use and antibiotic resistance in Europe: a longitudinal observational study. J Antimicrob Chemother 2014; 69: 535–47. [DOI] [PubMed] [Google Scholar]
- 12. Albrich WC, Monnet DL, Harbarth S.. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis 2004; 10: 514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibrahim OM, Polk RE.. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: methodology, opportunities, and challenges. Infect Dis Clin North Am 2014; 28: 195–214. [DOI] [PubMed] [Google Scholar]
- 14. Monnier AA, Eisenstein BI, Hulscher ME. et al. Towards a global definition of responsible antibiotic use: results of an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi3–vi16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanić Benić M, Milanič R, Monnier AA. et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi50–vi58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monnier AA, Schouten J, Le Maréchal M. et al. Quality indicators for responsible antibiotic use in the inpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi30–vi39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Maréchal M, Tebano G, Monnier AA. et al. Quality indicators assessing antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi40–vi49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Versporten A, Gyssens IC, Pulcini C. et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi59–vi66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 151: 264–9.w64. [DOI] [PubMed] [Google Scholar]
- 20. World Bank. The World Bank. Data: Countries and Economies http://data.worldbank.org/country.
- 21. Jain S, Cheng J, Alpern ER. et al. Management of febrile neonates in US pediatric emergency departments. Pediatrics 2014; 133: 187–95. [DOI] [PubMed] [Google Scholar]
- 22. Goldman RD, Scolnik D, Chauvin-Kimoff L. et al. Practice variations in the treatment of febrile infants among pediatric emergency physicians. Pediatrics 2009; 124: 439–45. [DOI] [PubMed] [Google Scholar]
- 23. Vernet G, Mary C, Altmann DM. et al. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg Infect Dis 2014; 20: 434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flottorp SA, Oxman AD, Krause J. et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 2013; 8: 35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butler CC, Hood K, Verheij T. et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ 2009; 338: b2242.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borg MA. National cultural dimensions as drivers of inappropriate ambulatory care consumption of antibiotics in Europe and their relevance to awareness campaigns. J Antimicrob Chemother 2012; 67: 763–7. [DOI] [PubMed] [Google Scholar]
- 27. Harbarth S, Albrich W, Brun-Buisson C.. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg Infect Dis 2002; 8: 1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulscher ME, Grol RP, van der Meer JW.. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis 2010; 10: 167–75. [DOI] [PubMed] [Google Scholar]
- 29. Kanerva M, Ollgren J, Lyytikainen O.. Antimicrobial use in Finnish acute care hospitals: data from national prevalence survey, 2005. J Antimicrob Chemother 2007; 60: 440–4. [DOI] [PubMed] [Google Scholar]
- 30. Amadeo B, Dumartin C, Venier AG. et al. Factors associated with the prevalence of antibiotic use for the treatment of hospital-acquired infections at 393 French hospitals: a regional variation analysis. Infect Control Hosp Epidemiol 2011; 32: 155–62. [DOI] [PubMed] [Google Scholar]
- 31. van der Kooi TI, Mannien J, Wille JC. et al. Prevalence of nosocomial infections in The Netherlands, 2007-2008: results of the first four national studies. J Hosp Infect 2010; 75: 168–72. [DOI] [PubMed] [Google Scholar]
- 32. Willemsen I, van der Kooij T, van Benthem B. et al. Appropriateness of antimicrobial therapy: a multicentre prevalence survey in the Netherlands, 2008-2009. Euro Surveill 2010; 15: pii=19715. [DOI] [PubMed] [Google Scholar]
- 33. Ansari F, Erntell M, Goossens H. et al. The European Surveillance of Antimicrobial Consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009; 49: 1496–504. [DOI] [PubMed] [Google Scholar]
- 34. Boivin Y, Talon D, Leroy J. et al. Antibiotic prescription in nursing homes for dependent elderly people: a cross-sectional study in Franche-Comte. Med Mal Infect 2013; 43: 163–9. [DOI] [PubMed] [Google Scholar]
- 35. McClean P, Tunney M, Gilpin D. et al. Antimicrobial prescribing in residential homes. J Antimicrob Chemother 2012; 67: 1781–90. [DOI] [PubMed] [Google Scholar]
- 36. McClean P, Hughes C, Tunney M. et al. Antimicrobial prescribing in European nursing homes. J Antimicrob Chemother 2011; 66: 1609–16. [DOI] [PubMed] [Google Scholar]
- 37. McClean P, Tunney M, Gilpin D. et al. Antimicrobial prescribing in nursing homes in Northern Ireland: results of two point-prevalence surveys. Drugs Aging 2011; 28: 819–29. [DOI] [PubMed] [Google Scholar]
- 38. Rummukainen ML, Karki T, Kanerva M. et al. Antimicrobial prescribing in nursing homes in Finland: results of three point prevalence surveys. Infection 2013; 41: 355–60. [DOI] [PubMed] [Google Scholar]
- 39. Latour K, Catry B, Broex E. et al. Indications for antimicrobial prescribing in European nursing homes: results from a point prevalence survey. Pharmacoepidemiol Drug Saf 2012; 21: 937–44. [DOI] [PubMed] [Google Scholar]
- 40. Cooke J, Stephens P, Ashiru-Oredope D. et al. Longitudinal trends and cross-sectional analysis of English national hospital antibacterial use over 5 years (2008-13): working towards hospital prescribing quality measures. J Antimicrob Chemother 2015; 70: 279–85. [DOI] [PubMed] [Google Scholar]
- 41. Gbaguidi-Haore H, Dumartin C, L'Heriteau F. et al. Antibiotics involved in the occurrence of antibiotic-resistant bacteria: a nationwide multilevel study suggests differences within antibiotic classes. J Antimicrob Chemother 2013; 68: 461–70. [DOI] [PubMed] [Google Scholar]
- 42. Pluss-Suard C, Pannatier A, Kronenberg A. et al. Hospital antibiotic consumption in Switzerland: comparison of a multicultural country with Europe. J Hosp Infect 2011; 79: 166–71. [DOI] [PubMed] [Google Scholar]
- 43. Miliani K, L'Heriteau F, Lacave L. et al. Imipenem and ciprofloxacin consumption as factors associated with high incidence rates of resistant Pseudomonas aeruginosa in hospitals in northern France. J Hosp Infect 2011; 77: 343–7. [DOI] [PubMed] [Google Scholar]
- 44. Amadeo B, Dumartin C, Parneix P. et al. Relationship between antibiotic consumption and antibiotic policy: an adjusted analysis in the French healthcare system. J Antimicrob Chemother 2011; 66: 434–42. [DOI] [PubMed] [Google Scholar]
- 45. Dumartin C, L'Heriteau F, Pefau M. et al. Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother 2010; 65: 2028–36. [DOI] [PubMed] [Google Scholar]
- 46. Meyer E, Gastmeier P, Deja M. et al. Antibiotic consumption and resistance: data from Europe and Germany. Int J Med Microbiol 2013; 303: 388–95. [DOI] [PubMed] [Google Scholar]
- 47. Thiebaut AC, Arlet G, Andremont A. et al. Variability of intestinal colonization with third-generation cephalosporin-resistant Enterobacteriaceae and antibiotic use in intensive care units. J Antimicrob Chemother 2012; 67: 1525–36. [DOI] [PubMed] [Google Scholar]
- 48. Benko R, Matuz M, Peto Z. et al. Variations and determinants of antibiotic consumption in Hungarian adult intensive care units. Pharmacoepidemiol Drug Saf 2012; 21: 104–9. [DOI] [PubMed] [Google Scholar]
- 49. Maechler F, Schwab F, Geffers C. et al. Antibiotic stewardship in Germany: a cross-sectional questionnaire survey of 355 intensive care units. Infection 2014; 42: 119–25. [DOI] [PubMed] [Google Scholar]
- 50. Cookson B, Mackenzie D, Kafatos G. et al. Development and assessment of national performance indicators for infection prevention and control and antimicrobial stewardship in European long-term care facilities. J Hosp Infect 2013; 85: 45–53. [DOI] [PubMed] [Google Scholar]
- 51. Nathwani D, Sneddon J, Patton A. et al. Antimicrobial stewardship in Scotland: impact of a national programme. Antimicrob Resist Infect Control 2012; 1: 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ashiru-Oredope D, Sharland M, Charani E. et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: start Smart—Then Focus. J Antimicrob Chemother 2012; 67: i51–63. [DOI] [PubMed] [Google Scholar]
- 53. Struelens MJ, Costers M.. Hospital antibiotic management in Belgium—results of the ABS maturity survey of the ABS International group. Wien Klin Wochenschr 2008; 120: 284–8. [DOI] [PubMed] [Google Scholar]
- 54. Leone M, Ragonnet B, Alonso S. et al. Variable compliance with clinical practice guidelines identified in a 1-day audit at 66 French adult intensive care units. Crit Care Med 2012; 40: 3189–95. [DOI] [PubMed] [Google Scholar]
- 55. Kanerva M, Ollgren J, Lyytikainen O.. Benchmarking antibiotic use in Finnish acute care hospitals using patient case-mix adjustment. J Antimicrob Chemother 2011; 66: 2651–4. [DOI] [PubMed] [Google Scholar]
- 56. Martinez MA, Inglada L, Ochoa C. et al. Assessment of antibiotic prescription in acute urinary tract infections in adults. J Infect 2007; 54: 235–44. [DOI] [PubMed] [Google Scholar]
- 57. Zarb P, Ansari F, Muller A. et al. Drug utilization 75% (DU75%) in 17 European hospitals (2000-2005): results from the ESAC-2 Hospital Care Sub Project. Curr Clin Pharmacol 2011; 6: 62–70. [DOI] [PubMed] [Google Scholar]
- 58. Zarb P, Goossens H.. European Surveillance of Antimicrobial Consumption (ESAC): value of a point-prevalence survey of antimicrobial use across Europe. Drugs 2011; 71: 745–55. [DOI] [PubMed] [Google Scholar]
- 59. Borg MA. Prolonged perioperative surgical prophylaxis within European hospitals: an exercise in uncertainty avoidance? J Antimicrob Chemother 2014; 69: 1142–4. [DOI] [PubMed] [Google Scholar]
- 60. Blix HS, Bergman J, Schjott J.. How are antibacterials used in nursing homes? Results from a point-prevalence prescription study in 44 Norwegian nursing homes. Pharmacoepidemiol Drug Saf 2010; 19: 1025–30. [DOI] [PubMed] [Google Scholar]
- 61. Bergman J, Schjott J, Blix HS.. Prevention of urinary tract infections in nursing homes: lack of evidence-based prescription? BMC Geriatr 2011; 11: 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cataife G, Weinberg DA, Wong HH. et al. The effect of Surgical Care Improvement Project (SCIP) compliance on surgical site infections (SSI). Med Care 2014; 52: S66–73. [DOI] [PubMed] [Google Scholar]
- 63. Cek M, Tandogdu Z, Naber K. et al. Antibiotic prophylaxis in urology departments, 2005-2010. Eur Urol 2013; 63: 386–94. [DOI] [PubMed] [Google Scholar]
- 64. Malcolm W, Nathwani D, Davey P. et al. From intermittent antibiotic point prevalence surveys to quality improvement: experience in Scottish hospitals. Antimicrob Resist Infect Control 2013; 2: 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Magill SS, Edwards JR, Beldavs ZG. et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312: 1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olsho LE, Bertrand RM, Edwards AS. et al. Does adherence to the Loeb minimum criteria reduce antibiotic prescribing rates in nursing homes? J Am Med Dir Assoc 2013; 14: 309.e1–7. [DOI] [PubMed] [Google Scholar]
- 67. Polk RE, Hohmann SF, Medvedev S. et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53: 1100–10. [DOI] [PubMed] [Google Scholar]
- 68. Ostrowsky B, Ruiz R, Brown S. et al. Lessons learned from implementing Clostridium difficile-focused antibiotic stewardship interventions. Infect Control Hosp Epidemiol 2014; 35 Suppl 3: S86–95. [DOI] [PubMed] [Google Scholar]
- 69. Borg MA, Zarb P, Ferech M. et al. Antibiotic consumption in southern and eastern Mediterranean hospitals: results from the ARMed project. J Antimicrob Chemother 2008; 62: 830–6. [DOI] [PubMed] [Google Scholar]
- 70. Thu TA, Rahman M, Coffin S. et al. Antibiotic use in Vietnamese hospitals: a multicenter point-prevalence study. Am J Infect Control 2012; 40: 840–4. [DOI] [PubMed] [Google Scholar]
- 71. Xie DS, Xiong W, Xiang LL. et al. Point prevalence surveys of healthcare-associated infection in 13 hospitals in Hubei Province, China, 2007-2008. J Hosp Infect 2010; 76: 150–5. [DOI] [PubMed] [Google Scholar]
- 72. Versporten A, Bolokhovets G, Ghazaryan L. et al. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis 2014; 14: 381–7. [DOI] [PubMed] [Google Scholar]
- 73. Filippini M, Ortiz LG, Masiero G.. Assessing the impact of national antibiotic campaigns in Europe. Eur J Health Econ 2013; 14: 587–99. [DOI] [PubMed] [Google Scholar]
- 74. Muller A, Coenen S, Monnet DL. et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe, 1998-2005. Euro Surveill 2007; 12: E071011.1. [DOI] [PubMed] [Google Scholar]
- 75. Coenen S, Muller A, Adriaenssens N. et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient parenteral antibiotic treatment in Europe. J Antimicrob Chemother 2009; 64: 200–5. [DOI] [PubMed] [Google Scholar]
- 76. Riedel S, Beekmann SE, Heilmann KP. et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis 2007; 26: 485–90. [DOI] [PubMed] [Google Scholar]
- 77. Goossens H, Ferech M, Coenen S. et al. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis 2007; 44: 1091–5. [DOI] [PubMed] [Google Scholar]
- 78. Gallini A, Taboulet F, Bourrel R.. Regional variations in quinolone use in France and associated factors. Eur J Clin Microbiol Infect Dis 2012; 31: 2911–8. [DOI] [PubMed] [Google Scholar]
- 79. Alvarez M, Pastor E, Eiros JM.. Social and demographic determinants in the prescription of systemic antibiotics. Infez Med 2012; 20: 37–48. [PubMed] [Google Scholar]
- 80. Achermann R, Suter K, Kronenberg A. et al. Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin Microbiol Infect 2011; 17: 855–61. [DOI] [PubMed] [Google Scholar]
- 81. Piovani D, Clavenna A, Cartabia M. et al. The regional profile of antibiotic prescriptions in Italian outpatient children. Eur J Clin Pharmacol 2012; 68: 997–1005. [DOI] [PubMed] [Google Scholar]
- 82. Piovani D, Clavenna A, Sequi M. et al. Reducing the costs of paediatric antibiotic prescribing in the community by implementing guideline recommendations. J Clin Pharm Ther 2013; 38: 373–8. [DOI] [PubMed] [Google Scholar]
- 83. Pulcini C, Lions C, Ventelou B. et al. Approaching the quality of antibiotic prescriptions in primary care using reimbursement data. Eur J Clin Microbiol Infect Dis 2013; 32: 325–32. [DOI] [PubMed] [Google Scholar]
- 84. Potena A, Simoni M, Cellini M. et al. Management of community-acquired pneumonia by trained family general practitioners. Int J Tuberc Lung Dis 2008; 12: 19–25. [PubMed] [Google Scholar]
- 85. Wood J, Butler CC, Hood K. et al. Antibiotic prescribing for adults with acute cough/lower respiratory tract infection: congruence with guidelines. Eur Respir J 2011; 38: 112–8. [DOI] [PubMed] [Google Scholar]
- 86. Francis NA, Gillespie D, Nuttall J. et al. Delayed antibiotic prescribing and associated antibiotic consumption in adults with acute cough. Br J Gen Pract 2012; 62: e639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marra F, Mak S, Chong M. et al. The relationship among antibiotic consumption, socioeconomic factors and climatic conditions. Can J Infect Dis Med Microbiol 2010; 21: e99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Glass-Kaastra SK, Finley R, Hutchinson J. et al. Variation in outpatient oral antimicrobial use patterns among Canadian provinces, 2000 to 2010. Can J Infect Dis Med Microbiol 2014; 25: 95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hicks LA, Taylor TH Jr, Hunkler RJ.. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 368: 1461–2. [DOI] [PubMed] [Google Scholar]
- 90. Collier SA, Hlavsa MC, Piercefield EW. et al. Antimicrobial and analgesic prescribing patterns for acute otitis externa, 2004-2010. Otolaryngol Head Neck Surg 2013; 148: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lannon C, Peterson LE, Goudie A.. Quality measures for the care of children with otitis media with effusion. Pediatrics 2011; 127: e1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Glass-Kaastra SK, Finley R, Hutchinson J. et al. Does variation among provincial drug formulary antimicrobial listings in Canada influence prescribing rates? PLoS One 2014; 9: e107515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sadeghian GH, Safaeian L, Mahdanian AR. et al. Prescribing quality in medical specialists in Isfahan, Iran. Iran J Pharm Res 2013; 12: 235–41. [PMC free article] [PubMed] [Google Scholar]
- 94. Chandy SJ, Thomas K, Mathai E. et al. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: a repeated cross-sectional study in Vellore, South India. J Antimicrob Chemother 2013; 68: 229–36. [DOI] [PubMed] [Google Scholar]
- 95. Dong L, Yan H, Wang D.. Drug prescribing indicators in village health clinics across 10 provinces of Western China. Fam Pract 2011; 28: 63–7. [DOI] [PubMed] [Google Scholar]
- 96. Wirtz VJ, Dreser A, Gonzales R.. Trends in antibiotic utilization in eight Latin American countries, 1997-2007. Rev Panam Salud Publica 2010; 27: 219–25. [DOI] [PubMed] [Google Scholar]
- 97. Esmaily HM, Silver I, Shiva S. et al. Can rational prescribing be improved by an outcome-based educational approach? A randomized trial completed in Iran. J Contin Educ Health Prof 2010; 30: 11–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.