Abstract
Background
Quantifying antibiotic use is an essential element of antibiotic stewardship since it allows comparison between different settings and time windows, and measurement of the impact of interventions. However, quantity metrics (QMs) and methods have not been standardized.
Objectives
To propose a set of QMs for antibiotic use in inpatients (IQMs) that are accepted globally by professionals in a range of disciplines. The study was conducted within the Driving Reinvestment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) project.
Methods
A systematic literature review using MEDLINE identified articles on measuring inpatient antibiotic use, published up to 29 January 2015. A consensually selected list of national and international web sites was screened for additional IQMs. IQMs were classified according to the type of numerator used and presented to a multidisciplinary panel of stakeholders. A RAND-modified Delphi consensus procedure, which consisted of two online questionnaires and a face-to-face meeting, was performed.
Results
The systematic literature review and web site search identified 168 eligible articles from which an initial list of 20 IQMs, composed of 20 different numerators and associated denominators was developed. The consensus procedure resulted in a final set of 12 IQMs. Among this final set, DDDs per 100(0) patient-days and days of therapy per patient-days were most frequently found in the review. The panel recommended that antibiotic use should be expressed in at least two metrics simultaneously.
Conclusions
Our consensus procedure identified a set of IQMs that we propose as an evidence-based global standard.
Introduction
Introduction of antibiotic treatment, together with hygiene measures, was probably one of the main causes for the notable reduction in morbidity and mortality rates caused by bacterial infections in the last century.1 However, infections with bacteria resistant to many commonly used antimicrobials are one of the major treatment challenges nowadays.2 The development of new antibiotics and the responsible use of existing ones represent valuable options to combat the spread and emergence of antimicrobial resistance. Since the development of new antibiotics is a difficult endeavour due to scientific complexity, and financial, time and safety issues, responsible use of existing antibiotics remains crucial to preserve their effectiveness.3
Measuring the quantity of antibiotic use is one of the key strategies in antimicrobial stewardship programmes since measurement of antibiotic use is the first step that leads to control and eventually to an improvement of use.4 Quantity metrics may reflect the volume or costs of antibiotic use and proper comparison of antibiotic consumption is enabled only by standardization of its quantification.4 Zanichelli et al.5 showed a large variation in quantitative metrics for antibiotic use across similar settings and providers and for specific medical conditions and populations, both in high- and low- to middle-income countries. Reducing antimicrobial resistance through responsible antibiotic use and identifying how to incentivize the discovery and development of novel antibiotics through new economic models are the most important goals of the Driving Reinvestment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) project, funded by the Innovative Medicines Initiative.6,7
This study, which is part of the DRIVE-AB project, aimed to develop a set of evidence-based and consensually validated quantity metrics for antibiotic use for the inpatient setting. Additionally, inpatient quality indicators are presented by Monnier et al.8
Materials and methods
Systematic review of the literature
A systematic literature review, followed by a RAND-modified Delphi consensus procedure, was performed according to previously described methods.9–11 This review is reported according to the PRISMA statement.12
The MEDLINE (via the PubMed interface) electronic database from inception until 29 January 2015 was searched. The search strategy was built using the combination of four main search term concepts: (i) antibiotics; (ii) quantity/utilization; (iii) measurement unit; and (iv) inpatient (a detailed list of search terms is provided in Table S1, available as Supplementary data at JAC Online). In addition, the reference lists of included articles identified by the literature search were hand-searched to identify any additional relevant studies.
Inclusion and exclusion criteria
Articles were included if they were written in English, concerned systemic (i.e. not topical, vaginal or inhaled) antibiotic use in humans and reported quantity metrics for antibiotic use/prescribing in the hospital setting. Papers on antiviral, antifungal and antiparasitic drugs were excluded, as well as articles describing antibiotic use in tuberculosis and in pathologies included in the Orphanet list of rare diseases.13
Papers were selected for full-text screening if they fulfilled any of the following criteria:
reviews which described metrics used in literature; OR
papers comparing two or more different quantity metrics for antibiotic use/prescribing; OR
papers which used the quantity metric (one or more) to assess correlation between antibiotic use and selection or propagation of antimicrobial resistance; OR
papers which developed a new (unknown) quantity metric for antibiotic use/prescribing; OR
papers concerning (methodological) issues in quantifying antibiotic use/prescribing.
Papers for which the full text could not be retrieved from any of the libraries of the participating centres (five different catalogues) were also excluded.
Screening process, data collection and analysis
Special systematic review software (DistillerSR®, Evidence Partners, Ottawa, Ontario, Canada) was used for literature screening based on defined inclusion and exclusion criteria. Two reviewers (M. S. B., R. M.) independently screened the titles and abstracts of the records retrieved by the initial search. Discrepancies between the reviewers were resolved through a discussion with a third reviewer (V. V.-P.). Three reviewers (M. S. B., R. M., V. V.-P.) independently extracted relevant data from included articles. A random selection of 10 papers was used as a pilot test to clarify any ambiguities in the process of data extraction. From the eligible studies, the following data were retrieved: study objective; study design (cross-sectional, prospective, retrospective, literature review); the type of quantitative metric (e.g. volume, cost); and the definition of metric by numerator/denominator.
All extracted inpatient quantity metrics (IQMs) from the included studies were grouped into categories based on the numerator. These IQMs were then presented to the stakeholders in the consensus procedure.
Web site search
The web sites of national and international organizations were also searched for data reporting antibiotic IQMs published until 29 January 2015. Two reviewers (M. S. B. and R. M.) screened a selection of relevant web sites. These web sites were selected based on a discussion with all study authors. The list of searched web sites is presented in Table S2. Data were extracted and all extracted IQMs from the included web sites were grouped into categories based on the numerator and presented to the stakeholders in the consensus procedure.
RAND-modified Delphi procedure
The RAND-modified Delphi procedure consisted of a two-round online survey addressed to a multidisciplinary expert panel of stakeholders with a face-to-face meeting held between the two surveys. International stakeholders were invited by e-mail to participate. The selection process of the stakeholders is described elsewhere.8 Fifty-two stakeholders amongst four different groups, aiming at representing all parties involved with antibiotic use, were invited: medical community (n = 15); public health and patients (n = 12); antibiotic research and development (R&D) (n = 14); and payers, policy makers, governments and regulators (n = 11). These are detailed in Table S3. The IQMs extracted from the included studies and web sites were classified according to the category of the numerator and similar IQMs were grouped together (e.g. numerator: Treatment courses categorized together with Treatment period). The list of IQMs was converted into an internet-based questionnaire (Figure S1) using SurveyMonkey® (Palo Alto, CA, USA). Stakeholders assessed the relevance of the extracted IQMs in the first-round internet-based questionnaire using a nine-point Likert scale ranging from 1, ‘Clearly not relevant’, to 9, ‘Clearly relevant’, plus a ‘Cannot assess’ option, and there was a comments box for each IQM. IQMs were selected if the median score was ≥7 with agreement, held for discussion if the median score was ≥7 with disagreement, and rejected if the median score was <7. Agreement was defined as ≥70% of the scores being in the top tertile (score 7–9). Additionally, stakeholders were asked to select relevant denominators (one or more) for each of the proposed IQM numerators. The stakeholders also had an option to propose new IQMs that had not been presented in the first online survey.
After the first online survey, a face-to-face consensus meeting with stakeholders was held to discuss disagreements and to evaluate newly suggested metrics. During the meeting the stakeholders could accept, rephrase or reject presented IQMs.
A detailed overview of the first survey and face-to-face meeting was submitted to the multidisciplinary stakeholder panel in a second internet-based survey (data not shown). Stakeholders could accept or reject the updated set of IQMs and comment on any relevant issues. Finally, IQMs were accepted if >70% of the respondents agreed with their selection.
Results
Systematic review of the literature and web sites
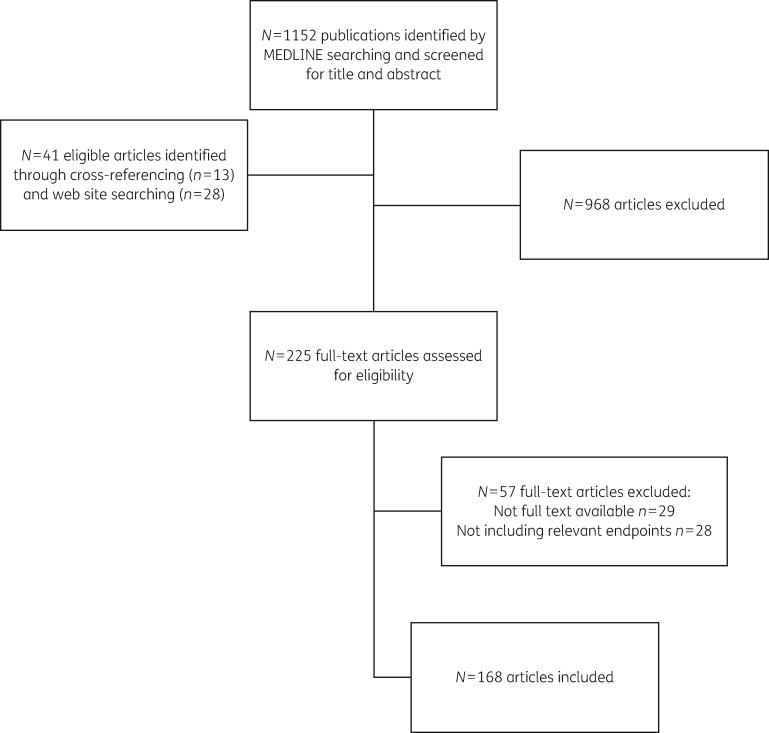
The flow chart of the review process is shown in Figure 1. The initial search of the MEDLINE database identified 1152 articles. An additional 41 articles were identified through cross-referencing (n = 13) and searching of web sites (n = 28). By screening the abstracts, 968 articles and duplicates were excluded. An additional 57 articles were excluded for the following reasons: 29 did not have full text available and 28 did not meet the inclusion criteria. The complete search resulted in 168 articles (140 from MEDLINE and 28 from web sites). Table S4 shows the articles and the following characteristics: author, year of publication, country, socioeconomic setting, study design, specific populations and objectives of the studies included in the review. In addition, summary descriptions of included studies are presented in Table S5. Based on the numerator, 20 different classes of IQMs were generated (74 numerator/denominator combinations). The definitions of all included IQMs are listed in Table 1. The consensus process with final IQMs is described In Table 2.
Figure 1.
The flow chart of the systematic review process of the literature.
Table 1.
Definitions of included numerators of inpatient quantity metrics for antibiotic use following the systematic review
| Defined daily dose (DDD) | An agreed technical statistical unit developed by the WHO Collaborating Centre for Drug Statistics Methodology; it is the assumed average maintenance dose per day for a drug used for its main indication in adults. |
| Hospital-adjusted defined daily dose (haDDD) | Determined from recommendations in regional or national guidelines on antibiotic use in hospital. |
| Prescribed daily dose (PDD) | Derived from actually prescribed/administered dose to a patient. It is the average dose prescribed according to a representative sample of prescriptions. The PDD will give the average daily amount of a drug that is actually prescribed. |
| Recommended daily dose (RDD) | Similar to DDD but the daily dose is defined by local guidelines; usually varies according to the weight of the patient. |
| Doses/units | Expressed in mg/kg/day or g/patient/day or g or mg. |
| Packages | The number or vials, bottles, pills/tablets, infusion bags, packages. |
| Prescriptions | The number or percentage of prescriptions containing antibiotics. |
| Days of therapy (DOT)a | The number of days that a patient receives antibiotics regardless of the dose. When a patient receives more than one antibiotic, more than one DOT may be counted.Synonym: antibiotic days or antimicrobial days. |
| Agent days | The number of days that a patient received a particular agent; defined by subtracting the date of the first dose of the antibiotic course from the date of the last dose. |
| Length of therapy (LOT) or duration of treatment (DOT) or days of treatment (DOT) | The number of days that a patient receives systemic antimicrobial agents, irrespective of the number of different antibiotics. Therefore, LOT will be lower than or equal to days of therapy (DOT) because the DOT is calculated for each antibiotic. |
| Treatment courses | Period during which the same agent (regardless of dose or route) was administered to the same patient on consecutive days. One patient may be given more than one treatment course at a time.Synonym: treatment period. |
| Length of stay (LOS) or antibiotic-related length of stay (ALOS) | Defined as the length of an inpatient episode of care, calculated from the day of admission to the day of discharge, and based on the number of nights spent in hospital. Patients admitted and discharged on the same day have a length of stay of <1 day. |
| Patients | Defined as the number or percentage of patients receiving one or more antibiotics or patients exposed to antibiotics. |
| Drug utilization index (DU) | The number of drugs accounting for 75%/90%/100% of total drug use. |
| Antibiotic costs | Defined as the total amount or percentage of costs of antibiotic per total drug costs. |
| Drug cost index 90% (DC 90%) | The costs of drugs accounting for 90% of total drug costs. |
In the literature, the acronym DOT is also used for duration of treatment and days of treatment, which are used as synonyms of length of therapy.
Table 2.
The consensus process of selecting the inpatient quantity metrics for antibiotic use
| Proposed numerator/denominators | Conclusion after the first survey | Conclusion after the consensus meetinga | Final conclusion after the second survey |
|---|---|---|---|
| I. Defined daily doses (DDDs)b | |||
| No denominator | Labelled for discussion | Selected | Selected |
| 100(0) PDs/BDs/OBDsc | 100(0) PDs/BDs/OBDs (61%) | IQM 1 | |
| Admissions | Admissions (44%) | IQM 2 | |
| 100 BDs per CMId | 100 BDs per CMI (35%) | IQM 3 | |
| 1000 inhabitants per day | |||
| Discharges | |||
| Age-adjusted comorbidity score, 100 PDs/age adjusted comorbidity score | |||
| FCEse | |||
| Number of treatment days | |||
| Patients | |||
| 100 BDs/€ | |||
| 100 admissions per CMI | |||
| II. Hospital-adjusted DDDs (haDDDs) | |||
| No denominator | Rejected | – | – |
| 100 PDs | |||
| 100 discharges | |||
| III. Prescribed daily dose (PDDs) | |||
| No denominator | Labelled for discussion | Selected | Selected |
| 100 PDs | 100 PDs (70%) | IQM 4 | |
| Admissions | Admissions (30%) | ||
| DDDs | DDDs (30%) | ||
| RDDs | |||
| V. Recommended daily doses (RDDs) | |||
| No denominator | Rejected | – | – |
| PDs | |||
| V. Doses/unit (mg/kg/day or g/patient/day or g or mg) | |||
| No denominator | Rejected | – | – |
| Days of therapy | |||
| VI. Packages | |||
| No denominator | Rejected | – | – |
| VII. Prescriptions | |||
| No denominator | Rejected | – | – |
| Admissions | |||
| VIII. Average number of drugs (antibiotics) per prescription | |||
| No denominator | Rejected | – | – |
| IX. Days of therapy (DOT) (synonym: antibiotic days or antimicrobial days) | |||
| No denominator | Selected | Selected | Selected |
| PDs | PDs (52%) | IQM 5 | |
| Patients | Patients (30%) | IQM 6 | |
| Admissions | Admissions (30%) | IQM 7 | |
| Discharges | |||
| Length of therapy (LOT) | |||
| Expected days of therapy (DOT) | |||
| X. Antibiotic days or antimicrobial days | |||
| No denominator | Rejected | – | – |
| Admissions | |||
| PDs | |||
| 1000 FCEs | |||
| XI. Agent days | |||
| No denominator | Rejected | – | – |
| Admissions | |||
| 1000 FCEs | |||
| XII. Length of therapy (LOT) | |||
| No denominator | Labelled for discussion | Selected | Selected |
| PDs | PDs (44%) | ||
| Admissions | Admissions (39%) | IQM 8 | |
| Patients | Patients (30%) | IQM 9 | |
| Discharges | |||
| 1000 FCEs | |||
| XIII. Treatment courses (synonym: treatment period) | |||
| No denominator | Rejected | – | – |
| Admissions | |||
| PDs | |||
| 1000 FCEs | |||
| Total number of treatment courses | |||
| XIV. Percentage of classes of antimicrobials that accounted for more than a certain percentage (10% or 50%) of treatment courses | |||
| – | Rejected | – | – |
| XV. Number or percentage of different antibiotics used/prescribed | |||
| No denominator | Rejected | – | – |
| Patient | |||
| Days of treatment | |||
| 1000 resident care days | |||
| Hospital | |||
| XVI. Length of stay (LOS) or antibiotic-related length of stay (ALOS) | |||
| No denominator | Rejected | – | – |
| PDs | |||
| XVII. Patients | |||
| No denominator | Labelled for discussion | Selected | Selected |
| All patients | All patients (26%) | IQM 10 | |
| Admissions | Admissions (22%) | IQM 11 | |
| XVIII. Drug utilization index (DU) DU 75%, DU 90%, DU 100% | |||
| No denominator | Rejected | – | – |
| XIX. Antibiotic costs | |||
| No denominator | Rejected | – | – |
| PDs | |||
| Overall cost of treatment | |||
| Treatment days (antibiotic days) | |||
| Admissions | |||
| DDDs | |||
| FCEs | |||
| Dose | |||
| XX. Drug cost index 90% (DC 90%) | |||
| No denominator | Rejected | – | – |
| XXI. Antibiotic use should be preferably expressed in at least two metrics simultaneously | |||
| – | Newly suggested | Selected: IQM 12 | |
Top two/three denominators ranked by the stakeholders.
Bold text shows inpatient quantity metrics that were selected as a result of the consensus procedure.
100 or 1000 patient-days (PDs)/bed-days (BDs)/occupied bed-days (OBDs).
CMI, case mix index. This is a relative value assigned to a diagnosis-related group of patients in a medical care environment.
FCE, finished consultant episode. This is an NHS term for a consultant episode that has ended due to discharge, transfer or death.
Results of the first online-based stakeholder survey
Twenty-three of the 52 stakeholders initially invited (44%) completed the survey between August and September 2015.
Out of the 20 IQMs presented for assessment through the first online-based survey questionnaire, ‘Days of therapy (DOT)’, with all denominators, was selected for the second online survey; metrics based on the numerators ‘DDDs’, ‘Prescribed daily dose (PDDs)’, ‘Length of therapy (LOT)’ and ‘Patients exposed to antibiotics’ were labelled for discussion, and the 15 remaining IQMs were excluded. A newly suggested IQM for further assessment was ‘Antibiotic use should be preferably expressed in at least two metrics simultaneously’ (Table 2).
Results of the consensus meeting on the IQMs of antibiotic use
The face-to-face consensus meeting took place on 30 September 2015. Sixteen stakeholders who participated in the first online survey attended the meeting. All four numerators labelled for discussion from the first round survey (‘DDDs’, ‘PDDs’, ‘LOT’, ‘Patients exposed to antibiotics’) with denominators within the top two or three (11 numerator/denominator combinations) and the newly suggested IQM (‘Antibiotic use should be preferably expressed in at least two metrics simultaneously’) were selected for the final-round evaluation (Table 2).
Results of the second online-based stakeholder survey
The second round was completed between December 2015 and February 2016. Overall response rate was 88% (22 out of 25 invited stakeholders completed the survey). The result of the consensus procedure as a final set of 12 IQMs of antibiotic use is shown in Table 3.
Table 3.
The final set of 12 evidence-based and consensually validated quantity metrics for antibiotic use in the inpatient setting
| Inpatient quantity metric (IQM) |
|---|
| IQM 1: Defined daily doses (DDDs) per 100(0) PDs/BDs/OBDsa |
| IQM 2: Defined daily doses (DDDs) per admission |
| IQM 3: Defined daily doses (DDDs) per (100 bed-days per CMIb) |
| IQM 4: Prescribed daily doses (PDDs) per 100 PDs |
| IQM 5: Days of therapy (DOT) per PD |
| IQM 6: Days of therapy (DOT) per patient |
| IQM 7: Days of therapy (DOT) per admission |
| IQM 8: Length of therapy (LOT) per admission |
| IQM 9: Length of therapy (LOT) per patient |
| IQM 10: Patients exposed to antibiotics per all patients |
| IQM 11: Patients exposed to antibiotics per admission |
| IQM 12: Antibiotic use should be preferably expressed in at least two metrics simultaneously |
100(0) patient-days (PD)/bed-days (BDs)/occupied bed-days (OBDs).
CMI, case mix index. This is a relative value assigned to a diagnosis-related group of patients in a medical care environment.
Discussion
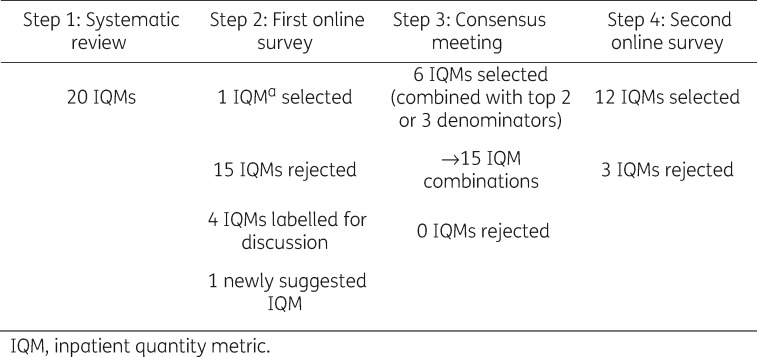
This study identified 12 IQMs as appropriate for quantifying antibiotic use by a Delphi procedure including a multidisciplinary panel of international stakeholders (Figure 2). The 12 IQMs represent only one-sixth of the comprehensive list of 74 IQMs generated through a systematic review of the literature and relevant web sites. In addition, new IQMs were added by the panel. The consensus procedure identified the limitations of individual IQMs, and stakeholders specifically proposed a preference that antibiotic use be quantified in at least two different metrics simultaneously. Other authors have stated that, although each IQM individually measures and describes different aspects of antibiotic prescribing, the actual antibiotic use can be more precisely evaluated in combination with at least one additional IQM.14,15 The three IQMs with DDDs as a numerator selected in the consensus procedure represent widely used metrics that can relatively easily be obtained and calculated and can thus be used to measure the use of different antibiotics, across different centres and countries.16 However, although the Infectious Diseases Society of America released a guideline in which DDDs is recommended for benchmarking purposes, there are still too many IQMs on antibiotic use present in the published literature that preclude a valid comparison of data within and between institutions.5,17 On the other hand, using only DDDs for comparison purposes does not always offer a clear, realistic picture of antibiotic use in practice.18 DDDs as a metric is relevant only for adult populations.19 Some of the factors that may influence variations in antibiotic use measured in DDDs are reduction in administered daily dose (e.g. renal impairment, weight-based dosing in children), different dosing recommendations in different countries and route of administration (oral, parenteral).20,21 Hence, as pointed out by others, in order to enable more informative benchmarking, other IQMs, including PDDs, DOT, LOT and exposed patients, all as numerators, may yield a more valid description of antibiotic exposure than DDDs alone.21–24 Polk et al.21 and Dalton et al.25 analysed similarities and differences between DDDs and DOT. For example, when the administered dosage was less than the DDDs, such as for ceftriaxone in the study by MacDougall and Polk,26 estimates of use based on DDDs/1000 patient-days (PDs) were significantly lower than those based on DOT/1000 PDs. Addition of another unit of measure, such as DOT, might also be helpful to meaningfully interpret data on the impact of antibiotic use, since the effects on the microbiota might depend more on total duration of treatment than on total daily dose.20
| Step 1: Systematic review | Step 2: First online survey | Step 3: Consensus meeting | Step 4: Second online survey |
|---|---|---|---|
| 20 IQMs | 1 IQM selected | 6 IQMs selected (combined with top 2 or 3 denominators) | 12 IQMs selected |
| 15 IQMs rejected | →15 IQM combinations | 3 IQMs rejected | |
| 4 IQMs labelled for discussion | 0 IQMs rejected | ||
| 1 newly suggested IQM |
Figure 2.
Results of the four-step RAND-modified Delphi procedure.
Although the recommendation to provide two IQMs simultaneously is not a metric in itself, we reported it as the 12th IQM according to the analytical process involved in this study. In order to accurately report antibiotic use in the paediatric setting, the 12th metric could be particularly helpful.27 Reporting the quantity of antibiotic use by at least two different IQMs simultaneously gives a reporter an option to use a DDD-based metric as a measure of overall use per unit population accompanied by any of the other proposed IQMs that would best fit the investigator’s requirements and the aim of the reported data, frequently describing an individual, patient-level pattern of antibiotic use.
Stakeholders involved in this survey also chose case-mix index (CMI) as a denominator with DDDs as one of the 12 proposed IQMs. CMI is used as a risk adjustment for antibiotic utilization across different institutions.28–30 Considering the confounding effect of different wards and intra- and inter-institutional patient characteristics, the proposed IQM, DDDs/100 bed-days (BDs) per CMI may help to overcome this barrier and render the measurement of antibiotic use more realistic. Haug et al.31 and Rajmokan et al.32 emphasized that there is no consistent definition and index of CMI. Thus, we recommend that a definition of CMI should be specified in studies involving this indicator.28,29
Morris et al.24 used a somewhat similar methodological approach to assess IQMs that are used to evaluate the impact of antimicrobial stewardship programmes and serve for public reporting. Potential for selection bias in the measures presented for evaluation to the expert panel and the lack of detailed reporting regarding all steps of the systematic review process present important limitations of their study.24 There are also a few other studies that described advantages and disadvantages of commonly used IQMs for antibiotic use that were highlighted by the participants in our consensus procedure.15,33,34 Many factors are recognized as influencing reliable reporting and comparison of antibiotic use worldwide. Different electronic prescribing modules use different filters for data extraction; pharmacy sales data aggregated over short registration intervals may significantly differ from ward stock accounting for the same period; risk adjustment of antibiotic use for patient mix and severity of illness is important.23,31,32,35,36 Metrics describing costs and other low-informative metrics (e.g. DDDs/day; minimum marketed dose; average number of antibiotics per prescription) generated through a systematic review process were both regarded as non-relevant by stakeholder assessment and previous studies.3,24,37
Definitions of involved IQMs for antibiotic use and data sources have an important effect on published results concerning the volume of antibiotic use.25,38 Consistency across definitions and quantity measures used in surveys should be synchronized worldwide and we promote this by providing a complete list of definitions of IQM numerators used internationally and emphasizing specific terms for IQMs. We propose to use the abbreviation ‘DOT’ only for days of therapy, excluding other terms abbreviated to DOT in the literature (Table 1). Another proposal that has arisen from this consensus process is that denominators should be clearly defined. It should be clearly stated whether the calculation of number of admissions and discharges between different departments within one hospital include only 1 day of hospitalization.39 Also, it is important to state whether patient-days are calculated based on calendar days or passages of midnight. Data sources (e.g. dispensing pharmacies data), including also specific providers of data (e.g. manufacturers, wholesalers, pharmacies) should be clearly specified to achieve more realistic insight.25 Kuster et al.39 have made recommendations for reporting methodological information for clear standardized reporting on hospital antibiotic use.
One of the strengths of this study is our methodological approach involving a comprehensive four step RAND-modified Delphi procedure (systematic review, first online survey, consensus meeting and second online survey) in which international multidisciplinary representatives of all concerned stakeholders participated. Both online surveys had a higher response rate (44% and 88%) than what is observed on average.40–42 Both online surveys included a wide range of stakeholders from the medical community, from patients and global public health, from the pharmaceutical industry, and from payers, policy makers, government and regulators. One limitation is that the systematic review process was limited to only one database (MEDLINE), although it was accompanied by a citation-tracking search and the review of 26 relevant web sites. The web site search included the most relevant ones, but not all. One could interpret it as a limitation of this study, but the web site search should rather be seen as complementary to the systematic literature review. It was performed to make sure no metrics were missed in the literature. Initial arbitrary selection of stakeholders could be considered a limitation of this study, but all groups were well represented. The recommendation to report antibiotic use with at least two different quantity metrics does not indicate which ones are preferred; this could be a strength and a limitation simultaneously.
Conclusions
Standardization of surveillance methods, including currently used IQMs, is crucial in order to improve stewardship measures and outcomes, including antibiotic resistance.34,43,44 Based on a comprehensive approach, a set of 12 IQMs on antibiotic use was generated that may serve for more accurate benchmarking practice. The last IQM, the recommendation ‘Antibiotic use should be preferably expressed in at least two metrics simultaneously’, constitutes a valuable new metric. The set of final metrics is a first step towards harmonized, internationally accepted reporting on the quantity of antibiotic use.
Supplementary Material
Acknowledgements
Members of the DRIVE-AB WP1 group
The authors are grateful to all stakeholders that participated in the consensus procedure for sharing their knowledge and insights on the subject: Ad Antonisse, Bojana Beović, Michael Borg, Franky Buyle, Marco Cavaleri, Harpal Dhillon, Catherine Dumartin, Richard Drew, David Findlay, Abdul Ghafur, Lindsay Grayson, Elizabeth Hermsen, Lauri Hicks, Philip Howard, Mike Kenston, Aaron S. Kesselheim, Charles Knirsch, Patrick Lacor, Ramanan Laxminarayan, Mical Paul, Diamantis Plachouras, Garyfallia Poulakou, Christian Rabaud, John H. Rex, Jesus Rodriguez-Baño, Arjun Srinivasan, Cecilia Stålsby Lundborg, Thomas Tängdén, Visanu Thamlikitkul, Alexandra Waluszewski, Sally Wellsteed, Heiman Wertheim and Claudia Wild.
The authors thank the DRIVE-AB steering committee for their critical review of the manuscript.
The authors acknowledge Visanu Thamlikitkul for his valuable comments on the manuscript.
The results of this study were presented in a poster session (poster 2847) at the 26th European Congress of Clinical Microbiology and Infectious Diseases in Amsterdam in Amsterdam, the Netherlands, in April 2016.
Funding
This work was supported by the Innovative Medicines Initiative (IMI) Joint Undertaking (grant agreement no.115618—Driving re-investment in R&D and responsible antibiotic use—DRIVE-AB – www.drive-ab.eu). Resources are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) in-kind contribution.
Transparency declarations
I. C. G. reports having received educational grants from Pfizer outside the submitted work. The remaining authors have none to declare.
This article forms part of a Supplement sponsored by DRIVE-AB.
Supplementary data
Tables S1 to S5 and Figure S1 are available as Supplementary data at JAC Online.
Contributor Information
the DRIVE-AB WP1 group:
Ad Antonisse, Bojana Beović, Michael Borg, Franky Buyle, Marco Cavaleri, Harpal Dhillon, Catherine Dumartin, Richard Drew, David Findlay, Abdul Ghafur, Lindsay Grayson, Elizabeth Hermsen, Lauri Hicks, Philip Howard, Mike Kenston, Aaron S Kesselheim, Charles Knirsch, Patrick Lacor, Ramanan Laxminarayan, Mical Paul, Diamantis Plachouras, Garyfallia Poulakou, Christian Rabaud, John H Rex, Jesus Rodriguez-Baño, Arjun Srinivasan, Cecilia Stålsby Lundborg, Thomas Tängdén, Visanu Thamlikitkul, Alexandra Waluszewski, Sally Wellsteed, Heiman Wertheim, and Claudia Wild
References
- 1. Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 2010; 1: 134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thabit AK, Crandon JL, Nicolau DP.. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother 2015; 16: 159–77. [DOI] [PubMed] [Google Scholar]
- 3. Monnier AA, Eisenstein BI, Hulscher ME. et al. What is responsible antibiotic use? Towards a global definition. In: ESCMID Publications: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 2017. Poster P1063. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
- 4. Centers for Disease Control and Prevention (CDC). Core Elements of Hospital Antibiotic Stewardship Programs 2016. http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. [DOI] [PMC free article] [PubMed]
- 5. Zanichelli V, Monnier AA, Gyssens IC. et al. Variation in antibiotic use among and within different settings: a systematic review. J Antimicrob Chemother 2018; 73Suppl 6: vi17–vi29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Driving Reinvestment in R&D for Antibiotics and Advocating their Responsible Use (DRIVE-AB project). 2016. http://drive-ab.eu/.
- 7. Harbarth S, Theuretzbacher U, Hackett J.. Antibiotic research and development: business as usual? J Antimicrob Chemother 2015; 70: 1604–7. [DOI] [PubMed] [Google Scholar]
- 8. Monnier AA, Schouten J, Le Maréchal M. et al. Quality indicators for responsible antibiotic use in the inpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother2018; 73Suppl 6: vi30–vi39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitch K, Bernstein SJ, Aguilar MD. et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA, USA: RAND, 2001. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf. [Google Scholar]
- 10. Hasson F, Keeney S, McKenna H.. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–15. [PubMed] [Google Scholar]
- 11. Hearnshaw HM, Harker RM, Cheater FM. et al. Expert consensus on the desirable characteristics of review criteria for improvement of health care quality. Qual Health Care 2001; 10: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orphanet. www.orpha.net/.
- 14. Irwin A, Sharland M.. Measuring antibiotic prescribing in hospitalised children in resource-poor countries: a systematic review. J Paediatr Child Health 2013; 49: 185–92. [DOI] [PubMed] [Google Scholar]
- 15. Berrington A. Antimicrobial prescribing in hospitals: be careful what you measure. J Antimicrob Chemother 2010; 65: 163–8. [DOI] [PubMed] [Google Scholar]
- 16. Monnet DL. Measuring antimicrobial use: the way forward. Clin Infect Dis 2007; 44: 671–3. [DOI] [PubMed] [Google Scholar]
- 17. Dellit TH, Owens RC, McGowan JEJ. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 18. Polk RE, Hohmann SF, Medvedev S. et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53: 1100–10. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization (WHO). More about DDDs.http://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd_more/en/.
- 20. Pulcini C, Beovic B, Cavalié P. et al. Amoxicillin dosing recommendations are very different in European countries: a cross-sectional survey. Clin Microbiol Infect 2017; 23: 414–5. [DOI] [PubMed] [Google Scholar]
- 21. Polk RE, Fox C, Mahoney A. et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44: 664–70. [DOI] [PubMed] [Google Scholar]
- 22. de With K, Bestehorn H, Steib-Bauert M. et al. Comparison of defined versus recommended versus prescribed daily doses for measuring hospital antibiotic consumption. Infection 2009; 37: 349–52. [DOI] [PubMed] [Google Scholar]
- 23. Versporten A, Sharland M, Bielicki J. et al. The Antibiotic Resistance and Prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J 2013; 32: e242–53. [DOI] [PubMed] [Google Scholar]
- 24. Morris AM, Brener S, Dresser L. et al. Use of a structured panel process to define quality metrics for antimicrobial stewardship programs. Infect Control Hosp Epidemiol 2012; 33: 500–6. [DOI] [PubMed] [Google Scholar]
- 25. Dalton BR, Sabuda DM, Bresee LC. et al. Assessment of antimicrobial utilization metrics: days of therapy versus defined daily doses and pharmacy dispensing records versus nursing administration data. Infect Control Hosp Epidemiol 2015; 36: 688–94. [DOI] [PubMed] [Google Scholar]
- 26. MacDougall C, Polk RE.. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol 2008; 29: 203–11. [DOI] [PubMed] [Google Scholar]
- 27. Liem TBY, Heerdink ER, Egberts ACG. et al. Quantifying antibiotic use in paediatrics: a proposal for neonatal DDDs. Eur J Clin Microbiol Infect Dis 2010; 29: 1301–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerber JS, Newland JG, Coffin SE. et al. Variability in antibiotic use at children’s hospitals. Pediatrics 2010; 126: 1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuster SP, Ruef C, Bollinger AK. et al. Correlation between case mix index and antibiotic use in hospitals. J Antimicrob Chemother 2008; 62: 837–42. [DOI] [PubMed] [Google Scholar]
- 30. Kanerva M, Ollgren J, Lyytikainen O.. Benchmarking antibiotic use in Finnish acute care hospitals using patient case-mix adjustment. J Antimicrob Chemother 2011; 66: 2651–4. [DOI] [PubMed] [Google Scholar]
- 31. Haug JB, Berild D, Walberg M. et al. Hospital- and patient-related factors associated with differences in hospital antibiotic use: analysis of national surveillance results. Antimicrob Resist Infect Control 2014; 3: 40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajmokan M, Morton A, Marquess J. et al. Development of a risk-adjustment model for antimicrobial utilization data in 21 public hospitals in Queensland, Australia (2006-11). J Antimicrob Chemother 2013; 68: 2400–5. [DOI] [PubMed] [Google Scholar]
- 33. Porta A, Hsia Y, Doerholt K. et al. Comparing neonatal and paediatric antibiotic prescribing between hospitals: a new algorithm to help international benchmarking. J Antimicrob Chemother 2012; 67: 1278–86. [DOI] [PubMed] [Google Scholar]
- 34. Ibrahim OM, Polk RE.. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: methodology, opportunities, and challenges. Infect Dis Clin North Am 2014; 28: 195–214. [DOI] [PubMed] [Google Scholar]
- 35. Berrington A. Hospital antibiotic prescribing data require careful local interpretation. J Antimicrob Chemother 2007; 59: 162–3. [DOI] [PubMed] [Google Scholar]
- 36. Ibrahim OM, Polk RE.. Benchmarking antimicrobial drug use in hospitals. Expert Rev Anti Infect Ther 2012; 10: 445–57. [DOI] [PubMed] [Google Scholar]
- 37. Bumpass JB, McDaneld PM, DePestel DD. et al. Outcomes and metrics for antimicrobial stewardship: survey of physicians and pharmacists. Clin Infect Dis 2014; 59: S108–11. [DOI] [PubMed] [Google Scholar]
- 38. Haug JB, Myhr R, Reikvam A.. Pharmacy sales data versus ward stock accounting for the surveillance of broad-spectrum antibiotic use in hospitals. BMC Med Res Methodol 2011; 11: 166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuster SP, Ruef C, Ledergerber B. et al. Quantitative antibiotic use in hospitals: comparison of measurements, literature review, and recommendations for a standard of reporting. Infection 2008; 36: 549–59. [DOI] [PubMed] [Google Scholar]
- 40. Cho YI, Johnson TP, Vangeest JB.. Enhancing surveys of health care professionals: a meta-analysis of techniques to improve response. Eval Health Prof 2013; 36: 382–407. [DOI] [PubMed] [Google Scholar]
- 41. Scott A, Jeon S-H, Joyce CM. et al. A randomised trial and economic evaluation of the effect of response mode on response rate, response bias, and item non-response in a survey of doctors. BMC Med Res Methodol 2011; 11: 126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cook DA, Wittich CM, Daniels WL. et al. Incentive and reminder strategies to improve response rate for internet-based physician surveys: a randomized experiment. J Med Internet Res 2016; 18: e244.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kritsotakis EI, Gikas A.. Surveillance of antibiotic use in hospitals: methods, trends and targets. Clin Microbiol Infect 2006; 12: 701–4. [DOI] [PubMed] [Google Scholar]
- 44. Pelle B, Gilchrist M, Lawson W. et al. Using defined daily doses to study the use of antibacterials in UK hospitals. Hosp Pharm 2006; 13: 133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.