Using data from PATRICIA and Costa Rica Vaccine trials, the risk of detecting a new HPV-18 infection and associated lesions was compared between women HPV seropositive and seronegative at enrollment. High HPV-18 naturally acquired antibodies were associated with partial protection.
Keywords: human papillomavirus, HPV, immunity, naturally acquired antibodies
Abstract
Background
Studies on the role of antibodies produced after infection with human papillomavirus 18 (HPV-18) and subsequent protection from HPV-18 infection have been conflicting, mainly due to inadequate sample size.
Methods
We pooled data from the control arms of the Costa Rica Vaccine Trial and the PATRICIA trial. Using Poisson regression we compared the risk of newly detected 1-time HPV-18 infection, HPV-18 1-year persistent infection (12MPI), and HPV-18–associated atypical squamous cells of undetermined significance or greater (ASC-US+) lesions between HPV-18 seropositive and seronegative women.
Results
High HPV-18 antibodies at enrollment was associated with reduced subsequent HPV-18 detection (P trend = 0.001; relative rate [RR] = 0.69; 95% confidence interval [CI], 0.47–1.01 for the third quartile; RR = 0.63; 95% CI, 0.43–0.94 for the fourth quartile, compared to seronegative). The risk of 12MPI showed a decreasing trend with increasing antibodies (P trend = 0.06; RR = 0.72; 95% CI, 0.29–1.77; RR = 0.42; 95% CI, 0.13–1.32 for the third and fourth quartiles, respectively). Lastly, we observed a significant decreased risk of HPV-18 ASC-US+ with increasing antibody (P trend = 0.01; RR = 0.46; 95% CI, 0.21–0.97 for the fourth quartile). We also observed a significant decreased risk of HPV-16 infection, 12MPI, and ASC-US+ with increasing HPV-16 antibody level.
Conclusions
High HPV-18 naturally acquired antibodies were associated with partial protection from future HPV-18 infections and associated lesions.
Clinical Trials Registration
NCT00128661 and NCT001226810.
Persistent infection with human papillomavirus (HPV) is a necessary cause of cervical precancers and cancer [1–3]. Approximately 13 HPV types have been classified as oncogenic, (genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) and an additional genotype (66) is classified as “limited evidence in humans” [4]. HPV-16 and HPV-18 cause approximately 70% of invasive cervical cancers worldwide [5, 6].
Most HPV infections clear naturally within approximately 2 years of acquisition [7–11]. Innate and cell-mediated immunity are likely responsible for clearance, defined as lack of detectability using standard polymerase chain reaction (PCR) assays, although the exact mechanism and meaning (ie, whether the virus is truly gone or only controlled) is poorly understood [9, 10].
From a natural history perspective, serologic measures of HPV antibodies can reflect past exposure [12], while DNA-based detection reflect infection at the time of testing. Only a fraction (50%–70%) of women develop antibodies after natural infection [13–15], at much lower levels than induced by vaccination [16].
Earlier reports investigating protection by naturally acquired antibodies have been conflicting, partially attributed to heterogeneity of both antibodies and DNA assays [17–20], while recent studies have consistently shown partial protection from incident HPV detection (defined as new HPV DNA or RNA detection after testing negative) in women who were previously exposed to HPV and mounted an immune response as measured by HPV-16/18–specific antibodies [21–23]. Due to limited sample size, these studies mainly investigated the effect of antibodies on 1-time HPV infection, which in most cases clears within 2 years [9]. It is important to establish whether protection is observed for persistent infections and precancerous lesions that have a higher risk of progressing to cancer [9, 10].
The PApilloma TRIal against Cancer In young Adults (PATRICIA) and Costa Rica HPV-16/18 Vaccine Trial (CVT) are phase III trials of the HPV-16/18 AS04-adjuvanted vaccine cumulatively conducted in over 25000 women. Because of their similar prospective design, procedures, and follow-up, analyses of participants in the control arms of these randomized trials provided a unique opportunity to evaluate the impact of naturally induced antibodies detected at baseline on subsequent HPV detection and related cervical abnormalities in young women.
Separate analysis within CVT and PATRICIA trials independently reported the protective role of naturally acquired HPV-16 antibodies in future HPV-16 detection [21, 22]. A protective role for naturally acquired HPV-18 antibodies in future HPV-18 detection was shown in CVT [21], but it was not clearly established in PATRICIA, due to limited power (lower numbers of incidently detected HPV-18 infections in PATRICIA than CVT). Thus, using pooled data from these trials, the primary objectives of this analysis was to assess the relationship between baseline (study entry) naturally acquired HPV-18 antibodies and (1) newly detected HPV-18 infection, (2) newly detected HPV-18 infections that persisted over 12 months, and (3) newly detected HPV-18 infections with concurrent cytological abnormalities (defined in the Methods section). As secondary objectives, we report findings for HPV-16 incident and persistent detections as well as HPV-16–related lesions. Lastly, we estimated levels of type-specific antibodies required for 50%, 70%, and 90% reduction in incidence of future HPV-18 and HPV-16 detection. In exploratory analyses, we further assessed the relationship between baseline naturally-acquired type-specific antibodies histological outcomes: (1) incidence of new HPV-18 cervical intraepithelial neoplasia (CIN) grade 1 or 2, or 3, squamous cell carcinoma (SCC), adenocarcinoma in situ (AIS), adenocarcinoma (ADC; CIN1+); (2) CIN 2; or (3) SCC, AIS, ADC (CIN2+); and separately HPV-16 CIN1+ and CIN2+. Further analyses were performed to assess whether HPV-18 antibodies are protective against newly detected HPV-45, a type phylogenetically related to HPV-18; and the association of HPV-16 antibodies and newly detected HPV-31 and HPV-33, types phylogenetically related to HPV-16.
METHODS
Study Population
Study participants were women randomized to the control arms of the PATRICIA trial (NCT00122681; 9337 women aged 15–25 years) or CVT trial (NCT00128661; 3736 women aged 18–25 years; 1 participant was 17 years old at enrollment) (Figure 1). The clinical trial methodology, inclusion and exclusion criteria, trial locations, and dates have been described elsewhere [16, 24]. Written informed consents and/or assents were obtained from all participants or their parents, and the protocol and other materials were approved by independent ethics committees or institutional review boards.
Figure 1.
Consort diagram of the study population. Abbreviations: AGC, atypical glandular cells; ASC-H, atypical squamous cells-cannot exclude HSIL; CVT, Costa Rica vaccine trial; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; N, number of subjects; PATRICIA, Papilloma Trial Against Cancer in Young Adults; Sero+, seropositive; Sero−, seronegative; TVC-E, total vaccinated cohort for efficacy.
Briefly, women were randomized to HPV-16/18 AS04-adjuvanted vaccine or control hepatitis-A vaccine and followed for 4 years. Gynecological examinations were performed every 6 (PATRICIA) or 12 months (CVT) on sexually experienced participants. At these visits, cervical liquid-based cytology samples were collected and used for HPV DNA-typing and cytopathological examination using the Bethesda system. Repeated cytology and/or colposcopy referral were carried out according to predefined clinical management algorithms, which were the same for CVT and PATRICIA trials [24, 25]. Participants completed a demographic and behavioral questionnaire, either at enrollment (CVT) or at the second study visit, 1 month after the first vaccination (PATRICIA), and yearly thereafter during the follow-up period for both trials.
Both trials used the broad spectrum PCR SPF10-LiPA25 (version 1) and PCR TS16/TS18 DEIA DNA-based assays to test cervical samples and biopsy material (PATRICIA) for 14 oncogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68/73) and 11 nononcogenic HPV types (6, 11, 34, 40, 42, 43, 44, 53, 54, 70, and 74). These assays were performed at GlaxoSmithKline (GSK) laboratories for PATRICIA, and Delft Diagnostic Laboratory (DDL, under contract from GSK) for CVT; here these are considered to be the same laboratory (“GSK laboratories or GSK-designated laboratory”). Cytology was performed by different laboratories (Quest for PATRICIA and National Cancer Institute [NCI] designated laboratory for CVT).
Serum antibody to HPV-16 and HPV-18 at baseline were determined by enzyme-linked immunosorbent assay (ELISA), performed by GSK laboratories for both PATRICIA and CVT. Seropositivity was defined as an antibody titer greater than or equal to the assay threshold: 7 ELISA units (EU)/mL for HPV-18 and 8 EU/mL for HPV-16 [12, 21, 22].
Statistical Analysis
Analysis Cohort
The analysis was performed on the total vaccinated cohort for efficacy (TVC-E) in the control arm of both trials. The TVC-E included all women who received at least 1 dose of the control vaccine and who had normal or low-grade cytology at baseline. Women who did not have a follow-up visit were excluded from the analysis (Figure 1). Further, women were excluded if they were: (1) over 25 years old at enrollment; (2) self-reported virgins at enrollment and did not begin sexual activity during the follow-up period, or had missing data for this variable (thus included women who had potentially been exposed to HPV infection via sexual intercourse); (3) DNA positive at baseline for the corresponding HPV type considered in the analysis or had missing HPV DNA results for that type (ie, women who were not known to be DNA negative for HPV-18, or HPV-16, were excluded from the respective analyses) (Figure 1). Thus, only newly detected infections over the follow-up were accounted for in the analysis.
Outcome Variables
Endpoints evaluated were newly detected infection with HPV-18 or HPV-16, 12-month persistent HPV-18 or HPV-16 infection (12MPI), and any ASC-US+ associated with HPV-18 or HPV-16 defined as:
– Incident infection: new detection of the HPV type at any time during the follow-up period among women negative by DNA testing for that type until that point.
– 12MPI: detection of the same HPV type in at least 2 samples not interrupted by negative samples over a minimum of 10 months.
– ASC-US+: atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells-cannot exclude HSIL (ASC-H), or atypical glandular cells (AGC) associated with HPV-18 or HPV-16.
In exploratory analyses, we assessed the relationship between baseline type-specific antibodies and histological outcomes of CIN1+, and CIN2+ (Supplementary Tables), and phylogenetically related HPV-45, and HPV-31, and HPV-33 (results not shown).
Exposure Variables
The main exposure variables were HPV-18 and HPV-16 serostatus at study enrollment. Serostatus was expressed as: (1) binary variable (seropositive or seronegative) according to the ELISA assay threshold; (2) quartiles (≥7–10, >10–16, >16–40, and >40–2541 EU/mL for HPV-18, and ≥8–13, >13–24, >24–64, and >64–3202 EU/mL for HPV-16); or (3) a continuous variable (antibody level).
Additional covariates known to be associated with risk of acquiring HPV were also taken into account in the analyses, and defined as shown in Supplementary Table 1. In summary, 8 potential confounders (covariates) were included in the models: study (CVT, PATRICIA), geographical region, marital status, cigarette smoking (packs per year), lifetime number of sexual partners, Chlamydia trachomatis infection at enrollment, age at first sexual intercourse, and previous pregnancy.
All analyses were performed using SAS version 9.2.
Poisson Regression Analysis
Incidence rate was calculated as the number of incidently detected events divided by the total person-time. Person-time was calculated as the sum of the follow-up for each participant expressed in years. The follow-up period started on the day after first vaccination with the control vaccine, and ended on the date of the first occurrence of the endpoint or on the date of the last visit (whichever occurred first).
We used Poisson regression to evaluate the univariate and multivariable effect of the exposure variables on the endpoints and obtain relative incidence rates (relative rates [RR]) and 95% confidence intervals (CI). The final multivariable analysis allowed an estimation of the relative contribution of initial HPV-18 or HPV-16 serostatus while adjusting for the simultaneous effects of the 8 covariates selected as potential confounders. Only subjects with no missing data for the different variables were included in the multivariate analyses.
The relationship between the risk of newly detected infection and the baseline antibody was analyzed using Poisson regression, where we included the antibody titer as a continuous variable. Seronegative women were assigned a value of half the ELISA assay cut-off level. Age at first sexual intercourse, lifetime number of sexual partners, and smoking status were included as covariates. Interaction between antibody level and study (PATRICIA, CVT) was also tested but removed from the final model because it was not statistically significant (P > .10). Predicted antibody titers corresponding to a 50%, 70%, and 90% reduction in incidence were derived from this model.
RESULTS
Participant Characteristics at Study Entry
Mean age at study entry was 20.4 years (standard deviation 3.0). Twenty-four percent (2776/11582) were between 15 and 17 years old and 76% between 17 and 25 years old. The distributions of age and selected exposure variables according to initial serostatus are shown in Supplementary Table 1.
Participant characteristics are shown in Figure 1. A total of 11169 women were included in the HPV-18–related analyses, of whom 1659 (15%) were HPV-18 seropositive at baseline (geometric mean titer 22.4 EU/mL). A total of 10752 women were included in the HPV-16–related analyses, of whom 1960 (18%) were HPV-16 seropositive at baseline (geometric mean titer 31.2 EU/mL) (Figure 1).
Poisson Regression Analyses
HPV-18
Overall, there were 1079 incidently detected HPV-18 infections, 190 12MPI, 426 ASC-US+, 78 CIN1+, and 39 CIN2+ detected during the 4-year follow-up period. Results from the univariate and multivariable Poisson regression models are presented in Table 1. In multivariable models, we observed a 17% decreased risk of newly detected HPV-18 infection for seropositives (P = .06). The risk of infection decreased with increasing quartiles of antibodies (P trend = 0.001; RR = 0.69; 95% CI, 0.47–1.01 for the third quartile and RR = 0.63; 95% CI, 0.43–0.94 for the highest quartile, compared to seronegatives). We did not observe statistically significant associations between enrollment seropositivity and HPV-18 12MPI; however, the risk of 12MPI was lower in the third and fourth quartiles of antibody level (RR = 0.72; 95% CI, 0.29–1.77 and RR = 0.42; 95% CI, 0.13–1.32, respectively) with borderline statistically significant trend (P trend = 0.06). Lastly, we observed a significantly decreased risk of HPV-18 ASC-US+ with increasing quartiles of antibodies (P trend = 0.01; RR = 0.46; 95% CI, 0.21–0.97 for the last quartile compared to seronegatives) but were underpowered to observe associations with HPV-18 CIN1+ or CIN2+ (CIN1+ RR = 0.32; 95% CI, 0.04–2.34 for the last quartile compared to seronegatives; Supplementary Table 2). Results for the full models are presented in Supplementary Table 2.
Table 1.
Univariate and Multivariable Poisson Regression for Incident Infections, 12-Month Persistent Infections, and ASC-US+ Cervical Cytology Associated With HPV-18: TVC-E Control Arm, 15–25 Years Old at Enrollment Among Participants Reporting Ever had Sexual Intercourse
| Univariate Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Variable | Categories | No. of Subjects | No. of Events | Incidence per 100/per Year | Relative Rate (95% CI) | P value | Relative Rate (95% CI) | P value |
| Endpoint: HPV-18 incident infection | ||||||||
| (N = 10846) | (N = 1079) | (N = 10846) | (N = 10046) | |||||
| HPV-18 serostatus | Negative | 9242 | 945 | 2.78 | 1.00 | … | 1.00 | … |
| Positive(≥7 EU/mL) | 1604 | 134 | 2.17 | 0.78 (0.65–0.94) | .008 | 0.83 (0.69–1.01) | .06 | |
| HPV-18 titers | Negative (<7 EU/mL) | 9242 | 945 | 2.78 | 1.00 | … | 1.00 | … |
| Q1a (7–10 EU/mL) | 457 | 47 | 2.72 | 0.98 (0.73–1.31) | .89 | 1.14 (0.85–1.54) | .39 | |
| Q2 (10–16 EU/mL) | 355 | 30 | 2.17 | 0.78 (0.54–1.13) | .19 | 0.88 (0.6–1.27) | .49 | |
| Q3 (16–40 EU/mL) | 394 | 31 | 2.02 | 0.73 (0.51–1.04) | .08 | 0.69 (0.47–1.01) | .06 | |
| Q4 (40–2541 EU/mL) | 398 | 26 | 1.71 | 0.62 (0.42–0.91) | .01 | 0.63 (0.43–0.94) | .02 | |
| P trend .001 | ||||||||
| Endpoint: 12-month persistent HPV-18 infection | ||||||||
| (N = 10461) | (N = 190) | (N = 10461) | (N = 9715) | |||||
| HPV-18 serostatus | Negative | 8915 | 166 | 0.48 | 1.00 | … | 1.00 | … |
| Positive(≥7 EU/mL) | 1546 | 24 | 0.38 | 0.80 (0.52–1.23) | .31 | 0.85 (0.54–1.35) | .50 | |
| HPV-18 titers | Negative (<7 EU/mL) | 8915 | 166 | 0.48 | 1.00 | … | 1.00 | … |
| Q1a (7–10 EU/mL) | 441 | 8 | 0.45 | 0.94 (0.46–1.91) | .86 | 1.13 (0.55–2.33) | .73 | |
| Q2 (10–16 EU/mL) | 342 | 7 | 0.50 | 1.05 (0.49–2.23) | .90 | 1.20 (0.56–2.58) | .64 | |
| Q3 (16–40 EU/mL) | 385 | 6 | 0.38 | 0.80 (0.35–1.81) | .59 | 0.72 (0.29–1.77) | .47 | |
| Q4 (40–2541 EU/mL) | 378 | 3 | 0.19 | 0.41 (0.13–1.28) | .12 | 0.42 (0.13–1.32) | .14 | |
| P trend .06 | ||||||||
| Endpoint: HPV-18 ASC-US+ | ||||||||
| (N = 10679) | (N = 426) | (N = 10679) | (N = 9895) | |||||
| HPV-18 serostatus | Negative | 9100 | 376 | 1.08 | 1.00 | … | 1.00 | … |
| Positive(≥7 EU/mL) | 1579 | 50 | 0.79 | 0.74 (0.55–0.99) | .04 | 0.84 (0.61–1.15) | .27 | |
| HPV-18 titers | Negative (<7 EU/mL) | 9100 | 376 | 1.08 | 1.00 | … | 1.00 | … |
| Q1a (7–10 EU/mL) | 448 | 18 | 1.01 | 0.94 (0.58–1.5) | .79 | 1.12 (0.69–1.84) | .64 | |
| Q2 (10–16 EU/mL) | 348 | 13 | 0.94 | 0.87 (0.5–1.51) | .62 | 1.06 (0.6–1.84) | .85 | |
| Q3 (16–40 EU/mL) | 390 | 12 | 0.77 | 0.71 (0.4–1.26) | .24 | 0.75 (0.41–1.38) | .36 | |
| Q4 (40–2541 EU/mL) | 393 | 7 | 0.45 | 0.42 (0.2–0.88) | .02 | 0.46 (0.21–0.97) | .04 | |
| P trend 0.01 | ||||||||
Relative rates are presented for the multivariable model including serostatus as binary and separately for models that include serostatus as categorical variables.
Full models including titers are available in Supplementary Table 1. Models coadjusted for: study (CVT and PATRICIA), study region, marital status, age at first sexual intercourse, pack-years smoked (0–0.5 vs 0.5+), lifetime sexual partners, previous pregnancy, Chlamydia test results.
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or greater; CI, confidence interval; CVT, Costa Rica vaccine trial; HPV, human papillomavirus; N, number of subjects with available data; PATRICIA, the Papilloma Trial Against Cancer in Young Adults; TVC-E, total vaccinated cohort for efficacy.
aQ1–Q4: Quartiles 1 to 4.
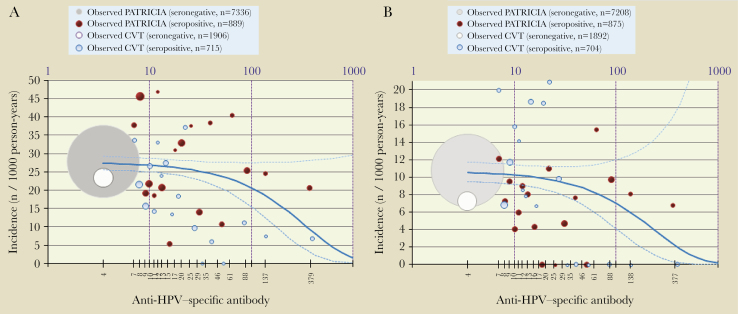
Figure 2A and 2B illustrate the quantitative model that shows a decreased risk of HPV-18 incidently detected infection/ASC-US+ with increasing antibodies levels. The adjusted model-predicted HPV-18 antibody titers associated with a 90% risk reduction of incidently detected infections were 622 EU/mL (95% CI, 334–3838), and antibody titers associated with a 90% risk reduction of HPV-18/ASC-US+ were 480 EU/mL (95% CI, 217–not determined). The corresponding values for 50% and 70% risk reduction were 187 EU/mL (95% CI, 100–1155) and 325 EU/mL (95% CI, 174–2007) for incident detection, and 144 EU/mL (95% CI, 65–not determined) and 251 EU/mL (95% CI, 114–not determined) for ASC-US+.
Figure 2.
Quantitative Poisson regression model showing relationship between initial HPV-18 antibody level and incident HPV-18 infection (A) and HPV-18–associated ASC-US+ (B). TVC-E control arm: 15–25 years old, who reported ever having had sexual intercourse. Infection: the adjusted model-predicted HPV-18 antibody titers associated with a 90% risk reduction of incident infection were 622 EU/mL (95% CI, 334–3838). ASU-CS+: the adjusted model-predicted HPV-18 antibody titers associated with a 90% risk reduction of ASC-US+ were 480 EU/mL (95% CI, 217–not determined). The dot size is proportional to the number of subjects; gray/white dot represents all seronegative subjects, and red/blue dots represent approximately 5-percentile classes of seropositive subjects. The solid blue line corresponds to the Poisson regression model (the dotted lines are 95% confidence limits). Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or greater; CI, confidence interval; CVT, Costa Rica Vaccine Trial; HPV, human papillomavirus; n, number of subjects in a given category; PATRICIA, The PApilloma TRIal against Cancer In young Adults; TVC-E, total vaccinated cohort for efficacy.
HPV-16–Related Endpoints
Overall, there were 1534 incidently detected HPV-16 infections, 517 12MPI, 719 ASC-US+, 222 CIN1+, and 143 CIN2+ detected during the 4-year follow-up period. Newly detected HPV-16 infections were reduced by 37% (RR = 0.63; 95% CI, 0.53–0.73), 12MPI by 30% (RR = 0.70; 95% CI, 0.54–0.92), and ASC-US+ by 43% (RR = 0.57; 95% CI, 0.45–0.73) in HPV-16-seropositive women, and decreased significantly with increasing HPV-16 antibody levels (Table 2); all associations were statistically significant. HPV-16–associated CIN1+ and CIN2+ were also statistically significantly reduced with increasing HPV-16 antibody levels (CIN1+ RR = 0.09; 95% CI, 0.01–0.68 for the last quartile compared to seronegatives and CIN2+ RR = 0.15; 95% CI, 0.02–1.08 for the last quartile compared to seronegatives). Univariate and multivariate models with all covariates assessed, and the different endpoints are presented in Supplementary Table 3.
Table 2.
Univariate and Multivariable Poisson Regression for Incident Infections, 12-Month Persistent Infections, and ASC-US+ Cervical Cytology Associated with HPV-16: TVC-E Control Arm, 15–25 Years Old at Enrollment Among Participants Reporting Ever had Sexual Intercourse
| Univariate Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Variable | Categories | No. of Subjects | No. of Events | Incidence per 100/per Year | Relative Rate (95% CI) | P Value | Relative Rate (95% CI) | P Value |
| Endpoint: HPV-16 incident infection | ||||||||
| (N = 10447) | (N = 1534) | (N = 10447) | (N = 10046) | |||||
| HPV-16 serostatus | Negative | 8564 | 1338 | 4.37 | 1.00 | … | 1.00 | … |
| Positive(≥8 EU/mL) | 1883 | 196 | 2.81 | 0.64 (0.55–0.75) | <.0001 | 0.63 (0.53–0.73) | <.0001 | |
| HPV-16 titers | Negative (<8 EU/mL) | 8564 | 1338 | 4.37 | 1.00 | … | 1.00 | … |
| Q1a (8–13 EU/mL) | 530 | 63 | 3.28 | 0.75 (0.58–0.97) | .03 | 0.76 (0.59–0.99) | .04 | |
| Q2 (13–24 EU/mL) | 428 | 61 | 3.88 | 0.89 (0.69–1.15) | .37 | 0.86 (0.66–1.12) | .27 | |
| Q3 (24–64 EU/mL) | 467 | 44 | 2.56 | 0.59 (0.43–0.79) | .0005 | 0.58 (0.43–0.8) | .0007 | |
| Q4 (64–3202 EU/mL) | 458 | 28 | 1.59 | 0.36 (0.25–0.53) | <.0001 | 0.32 (0.22–0.48) | <.0001 | |
| P trend <.0001 | ||||||||
| Endpoint: 12-month persistent infection | ||||||||
| (N = 10082) | (N = 517) | (N = 10082) | (N = 9344) | |||||
| HPV-16 serostatus | Negative | 8268 | 448 | 1.41 | 1.00 | … | 1.00 | … |
| Positive(≥8 EU/mL) | 1814 | 69 | 0.97 | 0.68 (0.53–0.88) | .003 | 0.70 (0.54–0.92) | .01 | |
| HPV-16 titers | Negative (<8 EU/mL) | 8268 | 448 | 1.41 | 1.00 | … | 1.00 | … |
| Q1a (8–13 EU/mL) | 510 | 26 | 1.31 | 0.93 (0.63–1.38) | .72 | 0.97 (0.64–1.47) | .89 | |
| Q2 (13–24 EU/mL) | 414 | 16 | 0.98 | 0.70 (0.42–1.15) | .16 | 0.74 (0.45–1.22) | .24 | |
| Q3 (24–64 EU/mL) | 443 | 19 | 1.08 | 0.77 (0.48–1.21) | .25 | 0.81 (0.5–1.3) | .38 | |
| Q4 (64–3202 EU/mL) | 447 | 8 | 0.45 | 0.32 (0.16–0.64) | .001 | 0.29 (0.14–0.61) | .001 | |
| P trend 0.0002 | ||||||||
| Endpoint: HPV-16 ASC-US+ | ||||||||
| (N = 10288) | (N = 719) | (N = 10288) | (N = 9516) | |||||
| HPV-16 serostatus | Negative | 8426 | 632 | 1.98 | 1.00 | … | 1.00 | … |
| Positive (≥8 EU/mL) | 1862 | 87 | 1.21 | 0.61 (0.49–0.77) | <.0001 | 0.57 (0.45–0.73) | <.0001 | |
| HPV-16 titers | Negative (<8 EU/mL) | 8426 | 632 | 1.98 | 1.00 | … | 1.00 | … |
| Q1a (8–13 EU/mL) | 526 | 30 | 1.50 | 0.76 (0.53–1.09) | .14 | 0.78 (0.54–1.13) | .19 | |
| Q2 (13–24 EU/mL) | 422 | 24 | 1.49 | 0.75 (0.5–1.13) | .17 | 0.73 (0.48–1.1) | .13 | |
| Q3 (24–64 EU/mL) | 458 | 23 | 1.30 | 0.66 (0.43–0.99) | .05 | 0.60 (0.38–0.93) | .02 | |
| Q4 (64–3202 EU/mL) | 456 | 10 | 0.56 | 0.28 (0.15–0.52) | <.0001 | 0.21 (0.1–0.42) | <.0001 | |
| P trend <.0001 | ||||||||
Relative rates are presented for the multivariable model including serostatus as binary and separately for models that include serostatus as categorical variables.
Full models including titers are available in Supplemental Table 2. Models coadjusted for: study (CVT and PATRICIA), study region, marital status, age at first sexual intercourse, pack-years smoked (0–0.5 vs 0.5+), lifetime sexual partners, previous pregnancy, Chlamydia test results.
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or greater; CI, confidence interval; CVT, Costa Rica vaccine trial; HPV, human papillomavirus; N, number of subjects with available data; PATRICIA, Papilloma Trial Against Cancer in Young Adults; TVC-E, total vaccinated cohort for efficacy.
aQ1–Q4: quartiles 1 to 4.
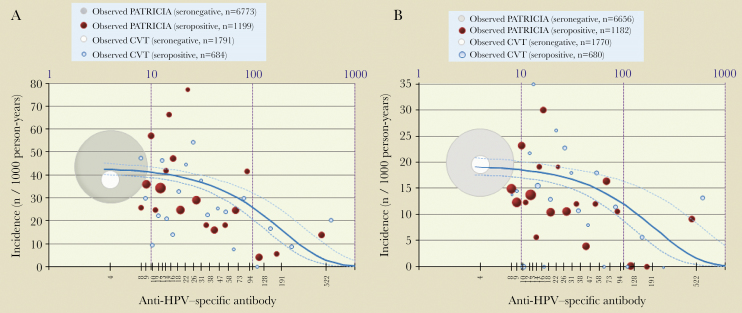
The quantitative model showed a decreased risk of incident HPV-16 infection/ASC-US+ with increasing antibody levels (Figure 3). The adjusted model-predicted HPV-16 antibody titers associated with a 90% risk reduction of incident infections were 371 EU/mL (95% CI, 268–606). The corresponding values for 12MPI and for ASC-US+ were 299 EU/mL (95% CI, 184–822) and 384 EU/mL (95% CI, 245–886). The corresponding values for 50% and 70% risk reduction were 112 EU/mL (95% CI, 81–182) and 194 EU/mL (95% CI, 140–317) for incident infection, and 116 EU/mL (95% CI, 74–267) and 201 EU/mL (95% CI, 128–463) for ASC-US+.
Figure 3.
Quantitative Poisson regression model showing relationship between initial HPV-16 antibody level and incident HPV-16 infection (A) and ASC-US+ (B). TVC-E control arm: 15–25 years old, who reported ever having had sexual intercourse. Infection: the adjusted model-predicted HPV-16 antibody titers associated with a 90% risk reduction of incident infection were 371 EU/mL (95% CI, 268–606). ASC-US+: the adjusted model-predicted HPV-16 antibody titers associated with a 90% risk reduction of ASC-US+ were 384 EU/mL (95% CI, 245–886). The dot size is proportional to the number of subjects; the gray/white dot represents all seronegative subjects, and red/blue dots represent approximately 5-percentile classes of seropositive subjects. The solid blue line corresponds to the Poisson regression model (the dotted lines are 95% confidence limits). Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or greater; CI, confidence interval; CVT, Costa Rica Vaccine Trial; HPV, human papillomavirus; n, number of subjects in a given category; PATRICIA, The PApilloma TRIal against Cancer In young Adults; TVC-E, total vaccinated cohort for efficacy.
DISCUSSION
In this large study of over 10000 women, we demonstrated that women with naturally acquired antibodies to HPV-18 had a lower risk of newly detected HPV-18 infection or associated cervical lesions over 4 years. Risk decreased as antibody levels increased and higher antibody titers were associated with lower risk of most endpoints evaluated. There was also a clear pattern of reduced risk of an incidently detected or persistent HPV-18 infection, associated ASC-US+, or CIN1+ with increasing quartiles of HPV-18 antibody titers. Results were independent of the behavioral and other risk factors accounted for in the analysis. Too few cases of CIN2+ were observed to perform an inferential analysis; as expected in women of this age group (15–25 years old at study entry), there were no cases of cervical cancer in these randomized trials where women were closely followed up. Thus, the results of this study firmly demonstrate for the first time that naturally acquired HPV-18 antibodies also play a protective role in the prevention of subsequent new detection and associated abnormalities over the 4 years’ follow-up.
Furthermore, our analyses confirmed the relationship between HPV-16 antibody titer and detection of new HPV-16 infection and associated abnormalities, similar to previous observations in CVT [21] and PATRICIA [22]. In addition to confirmation of past findings, this analysis also served as a check of the validity of our HPV-18 findings.
We did not observe any association between HPV-18 antibodies and newly detected phylogenetically closely related genotype HPV-45, nor between HPV-16 antibodies and the new detection of phylogenetically closely related genotypes HPV-31 and HPV-33 (data not shown), indicating that the lower antibody levels following natural infection may be too low to provide immune crossprotection for phylogenetically related HPV types.
This study is among the first to demonstrate a statistically significant quantitative relationship between naturally acquired antibodies and protection from future new HPV-18 detection, persistent HPV-18 infections, and HPV-18–associated ASC-US+. These models allowed us to estimate antibody titers associated with a certain level of risk reduction. We estimated that a naturally acquired antibody titer of approximately 622 EU/mL was associated with a 90% reduction of HPV-18 infection detection, whilst a titer of 480 EU/mL was associated with a 90% reduction in risk of HPV-18–related ASC-US+. The results of analyses of the quantitative relationship between antibodies and various outcomes are interesting as they consistently showed that higher antibody levels are needed for protection, and not that ASC-US+ lesions may need a higher or lower absolute level. Moreover, the apparently higher titer needed for protection from newly detected infection could be due to misclassifications of titers, especially at the lower end of the assay cut-off. The corresponding values for HPV-16 were 371 EU/mL and 384 EU/mL. However, we note that absolute values are assay and laboratory specific, and not transferable between assays and laboratories. Furthermore, these values should not be interpreted as correlates of protection with regard to vaccination, as there are key differences between naturally acquired versus vaccine-induced antibody production that might affect the values conferring protection [26]. However, while both natural and vaccine-induced antibody levels are lower for HPV-18 than HPV-16, the long-term vaccine-induced antibody level is clearly higher than our 90% reduction model-derived thresholds for both HPV-18 and HPV-16, particularly with the HPV-16/18 AS04-adjuvanted vaccine [27]. Considering the mentioned limitations of difficulty in comparing values across assays, we note that a higher antibody level seems to be necessary to prevent HPV-18 detection and associated ASC-US+ lesions than HPV-16. This may be related to misclassification of both the DNA and the serology assays, or some of these infections may be depositions and not real infections, or may be random occurrence.
HPV is a mucosal infection, the virus remains in the cervical epithelium, and it is not exposed to the systemic immune system; thus, lower levels of antibodies are detected following natural infection than vaccination. In a vaccination setting, an individual is exposed to a large quantity of the virus-like particles systemically, and therefore the mechanism of exposure of the immune system to HPV antigens via vaccination leads to the induction of a higher antibody level. Properties of the antibodies (eg, affinity) may also be different.
Whether those with the highest naturally acquired antibodies level should be vaccinated is an interesting question, but it should be noted that, currently, the assays that measure HPV antibodies are variable, measuring qualitatively and quantitatively different subsets of the immune system [28–30]. As such, there is currently no utility in measuring antibodies in the context of natural infection for risk stratification purposes or to indicate vaccination, primarily due to the variability in the available assays.
The large sample size and well-characterized population from both vaccine trials, along with harmonized laboratory (both DNA and serological assessments) and study procedures are substantial strengths. There are some important differences, including populations (PATRICIA is a multinational study, whereas the population in CVT is more homogeneous); participants’ age (15–25 years old in PATRICIA, 18–25 years old in CVT), and different cytology/histology laboratories (for ASC-US+ and CIN1+ analyses); however, the studies had similar information on important behavioral risk factors known to be associated with incident HPV infections. The analysis was based on antibody levels measured at study baseline, and we did not determine how antibody titers changed over the course of the study. If naturally acquired antibody titers decline considerably over time, then it may be expected that the protection offered will wane. However, we could not determine this within the study.
Many women with HPV infection never develop detectable antibodies [12, 31], therefore some women found to be seronegative may have been previously exposed to infection. Two possible explanations are (1) that these women may have mounted an immune response against HPV other than antibodies, and/or (2) antibodies produced are lower than the assay’s detection limit. Both possibilities may have resulted in an underestimate of the protective effect. However, this limitation would not be relevant to the observation of increasing protection with increasing titers.
Measurement of the antibody response is highly dependent upon the assay used. Neutralizing antibodies are likely to form the main basis for protection against HPV infection, but the assay used in our study measures total IgG response. Nevertheless, good correlation between this assay and an assay measuring only neutralizing anti-HPV antibodies has been shown previously [32].
It is not possible to distinguish between reinfection (a new HPV infection) and reactivation or redetection of an existing infection that persisted at a low, undetectable level. It has been shown that women can experience reactivation or redetection of a type-specific infection after a period of nondetection [33]. The choice of the DNA assay is important in distinguishing between new versus reactivated/redetected infection; more sensitive whole-genome sequencing can be helpful in these efforts. Another study limitation is the 6-month (PATRICIA) or 1-year (CVT) interval between obtaining cervical samples. Transient infections arising and clearing between measurements might not have been detected.
In summary, our pooled analysis showed that naturally acquired HPV-18 antibodies provide immune protection from future newly detected HPV-18 infections and associated lesions over the 4-year follow-up, as previously observed for HPV-16 [21–23]. Although a correlate of vaccine protection cannot be inferred from natural history studies, it may help set a bench mark for protection, and may be useful in assessing the levels in 1-dose vaccinations. It is also reassuring that the antibody titer that appears to offer 90% protection against infection and ASC-US+ is several-fold lower than the titer observed after long-term follow-up of women who received the HPV-16/18 AS04-adjuvanted vaccine. This suggests that levels required for vaccine protection may be lower than those obtained with the current efficacious 3-dose regimen vaccines. Our data offer guidance in the continuing search for correlates of natural- and vaccine-induced immunity against genital HPV infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Contributions. GlaxoSmithKline Biologicals SA designed the PATRICIA trial, in collaboration with investigators, and coordinated collection, analysis, and interpretation of data, and preparation of the manuscript. Investigators from the PATRICIA study group collected data for the trial and cared for the subjects. The CVT is a long-standing collaboration between investigators in Costa Rica and the NCI. The CVT was sponsored by the NCI. GlaxoSmithKline Biologicals SA provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company. The NCI and Costa Rica investigators were responsible for the design and conduct of the study and collection, management, analysis, and interpretation of samples and data. All authors of this report had full access to all the trial data for the trial they participated in and access to summary-level trial data for both trials. All authors gave final approval of the manuscript.
The Costa Rica HPV Vaccine Trial and the PATRICIA study groups: A Chatterjee (Department of Pediatrics, University of South Dakota, Sanford School of Medicine and Sanford Children’s Specialty Clinic, South Dakota, USA), S-N Chow (Department of Obstetrics and Gynecology, College of Medicine and the Hospital, National Taiwan University, Taipei, Taiwan), N De Carvalho (Department of Gynecology and Obstetrics, Federal University of Paraná, Infectious Diseases in Gynecology and Obstetrics Sector, Curitiba, Parana, Brazil), MR Del Rosario Raymundo (Department of Obstetrics and Gynecology, San Pablo Colleges Medical Center, San Pablo City, Laguna, Philippines), F Diaz Mitoma (Advanced Medical Research Institute of Canada, Sudbury, Ontario, Canada), G Dubin (GSK, Wavre, Belgium at the time of the study), S Garland (Microbiology and Infectious Diseases Department, Royal Women’s Hospital and Department of Obstetrics and Gynaecology, University of Melbourne, Murdoch Childrens Research Institute Melbourne, VIC, Australia), MJ Germar (University of the Philippines College of Medicine, Philippine General Hospital, Manila, Philippines), P Gonzalez (Proyecto Epidemiologico Guanacaste / FUNIN, Liberia – Guanacaste, Costa Rica), DM Harper (Geisel School of Medicine at Dartmouth, Hanover, NH, USA), U Jaisamrarn (Department of Obstetrics and Gynaecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand), AR Kreimer (National Cancer Institute, Division of Cancer Epidemiology and Genetics, USA), M Lehtinen (University of Tampere, Tampere, Finland), P Naud (University Federal of Rio Grande do Sul, Hospital de Clínica de Porto Alegre, Porto Alegre, Brazil), J Paavonen (Department of Obstetrics and Gynaecology, University of Helsinki, Helsinki, Finland), K Peters (Facharzt für Frauenheilkunde und Geburtshilfe, Hamburg Germany), W Poppe (Department of Gynaecology, University Hospital KU Leuven Gasthuisberg, Leuven, Belgium), C Porras (Fundación INCIENSA, Proyecto Epidemiológico Guanacaste, San José, Costa Rica), J Salmeròn (Unidad de Investigación Epidemiológica y en Servicios de Salud, Instituto Mexicano del Seguro Social, Morelos, Mexico), M Sherman (National Cancer Institute, Division of Cancer Prevention, Bethesda, USA), S.R Skinner (Vaccine Trials Group, Telethon Kids Institute, Perth, W.A and Sydney Medical School, Discipline of Paediatrics and Child Health, Children’s Hospital at Westmead, Sydney, NSW, Australia), F Struyf (GSK, Wavre, Belgium), J Teixeira (Departamento de Tocoginecologia da Unicamp, University of Campinas, Campinas, Sao Paulo, Brazil), W Tjalma (Multidisciplinary Breast Clinic – Gynecological Oncology Unit, Department of Obstetrics and Gynecology, Antwerp University Hospital – University of Antwerp, Antwerp, Belgium), CM Wheeler (Departments of Pathology and Obstetrics and Gynecology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA)
Acknowledgments. The authors thank all study participants, investigators, contributors, and coordinators from the CVT, extending a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the CVT group involved in this project (http://www.acibcr.com/ACIB/en/2017/10/18/costa-rica-hpv-vaccine-trial-cvt/). The authors would also like to thank all investigators, participants, and their families from the PATRICIA trial and acknowledge the work of the central and local study coordinators and staff members of the sites that participated in this study.
The authors also thank Business and Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK. J. Ghesquiere coordinated manuscript development and editorial support. The authors thank the joined Costa Rica HPV Vaccine Trial and the PATRICIA study group members for their critical review of the publication.
Financial support. The CVT was funded by the NCI (contract N01-CP-11005) and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals SA provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the 4-year, randomized blinded phase of our study. The PATRICIA trial was funded by GlaxoSmithKline Biologicals SA.
This work was supported by GlaxoSmithKline Biologicals SA and the NCI Intramural Research Program.
Potential conflicts of interest. M. S. is now employed by Roche. X. C. reports having received funding through his institution from the GSK group of companies for the purpose of conducting this study. X. C. also received grants from Merck & Co., Inc. outside the submitted work and occasional speaking honoraria from VIANEX and Merck Sharp & Dohme. M. H. S. reports having received HPV typing of specimens from Roche and BD at no cost for studies conducted by NCI. D. R. is an employee of the GSK group of companies and holds stocks and shares in the GSK group of companies. L. B. was employee of the GSK group of companies at the time of the study and holds shares in the GSK group of companies. M.-C. B. reports having received consulting fees from the GSK group of companies through her institution. All other authors from the core writing team report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 29th International Papillomavirus Conference and Clinical Workshop, Seattle, Washington, 20–25 August 2014. Abstract PH.PPO2.86(Epidemiology):312.
Contributor Information
Costa Rica HPV Vaccine Trial and the PATRICIA study groups:
A Chatterjee, S-N Chow, N De Carvalho, MR Del Rosario Raymundo, F Diaz Mitoma, G Dubin, S Garland, M J Germar, P Gonzalez, D M Harper, U Jaisamrarn, A R Kreimer, M Lehtinen, P Naud, J Paavonen, K Peters, W Poppe, C Porras, J Salmeròn, M Sherman, S R Skinner, F Struyf, J Teixeira, W Tjalma, and C M Wheeler
References
- 1. Walboomers JM, Jacobs MV, Manos MM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–9. [DOI] [PubMed] [Google Scholar]
- 2. Wallin KL, Wiklund F, Angström T et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med 1999; 341:1633–8. [DOI] [PubMed] [Google Scholar]
- 3. Koutsky LA, Holmes KK, Critchlow CW et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–8. [DOI] [PubMed] [Google Scholar]
- 4. Bouvard V, Baan R, Straif K et al. ; WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens–Part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 5. de Sanjose S, Quint WG, Alemany L et al. ; Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. [DOI] [PubMed] [Google Scholar]
- 6. Tjalma WA, Fiander A, Reich O et al. ; HERACLES/SCALE Study Group Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer 2013; 132:854–67. [DOI] [PubMed] [Google Scholar]
- 7. Franco EL, Villa LL, Sobrinho JP et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180:1415–23. [DOI] [PubMed] [Google Scholar]
- 8. Sellors JW, Karwalajtys TL, Kaczorowski J et al. ; Survey of HPV in Ontario Women Group Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ 2003; 168:421–5. [PMC free article] [PubMed] [Google Scholar]
- 9. Rodríguez AC, Schiffman M, Herrero R et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez AC, Schiffman M, Herrero R et al. ; Proyecto Epidemiológico Guanacaste Group Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008; 100:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaisamrarn U, Castellsagué X, Garland SM et al. ; HPV PATRICIA Study Group Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS One 2013; 8:e79260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coseo S, Porras C, Hildesheim A et al. ; Costa Rica HPV Vaccine Trial (CVT) Group Seroprevalence and correlates of human papillomavirus 16/18 seropositivity among young women in Costa Rica. Sex Transm Dis 2010; 37:706–14. [DOI] [PubMed] [Google Scholar]
- 13. Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst 1994; 86:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viscidi RP, Kotloff KL, Clayman B, Russ K, Shapiro S, Shah KV. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin Diagn Lab Immunol 1997; 4:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carter JJ, Koutsky LA, Hughes JP et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000; 181:1911–9. [DOI] [PubMed] [Google Scholar]
- 16. Paavonen J, Jenkins D, Bosch FX et al. ; HPV PATRICIA study group Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161–70. [DOI] [PubMed] [Google Scholar]
- 17. Ho GY, Studentsov Y, Hall CB et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis 2002; 186:737–42. [DOI] [PubMed] [Google Scholar]
- 18. Viscidi RP, Schiffman M, Hildesheim A et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev 2004; 13:324–7. [DOI] [PubMed] [Google Scholar]
- 19. Viscidi RP, Snyder B, Cu-Uvin S et al. Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV-positive and HIV-negative women. Cancer Epidemiol Biomarkers Prev 2005; 14:283–8. [PubMed] [Google Scholar]
- 20. Olsson SE, Kjaer SK, Sigurdsson K et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin 2009; 5:696–704. [DOI] [PubMed] [Google Scholar]
- 21. Safaeian M, Porras C, Schiffman M et al. ; Costa Rican Vaccine Trial Group Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castellsagué X, Naud P, Chow SN et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis 2014; 210:517–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wentzensen N, Rodriguez AC, Viscidi R et al. A competitive serological assay shows naturally acquired immunity to human papillomavirus infections in the Guanacaste Natural History Study. J Infect Dis 2011; 204:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paavonen J, Naud P, Salmerón J et al. ; HPV PATRICIA Study Group Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–14. [DOI] [PubMed] [Google Scholar]
- 25. Herrero R, Wacholder S, Rodríguez AC et al. ; Costa Rica Vaccine Trial Group Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schiller JT, Lowy DR. Raising expectations for subunit vaccine. J Infect Dis 2015; 211:1373–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Einstein MH, Takacs P, Chatterjee A et al. ; HPV-010 Study Group Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother 2014; 10:3435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis 2009; 200:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Safaeian M, Ghosh A, Porras C et al. Direct comparison of HPV16 serological assays used to define HPV-naïve women in HPV vaccine trials. Cancer Epidemiol Biomarkers Prev 2012; 21:1547–54. [DOI] [PubMed] [Google Scholar]
- 30. Robbins HA, Kemp TJ, Porras C et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front Oncol 2014; 3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carter JJ, Koutsky LA, Wipf GC et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis 1996; 174:927–36. [DOI] [PubMed] [Google Scholar]
- 32. Dessy FJ, Giannini SL, Bougelet CA et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425–34. [DOI] [PubMed] [Google Scholar]
- 33. Insinga RP, Perez G, Wheeler CM et al. ; FUTURE I Investigators Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev 2010; 19:1585–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.