Abstract
Background
Conducted as part of the Driving Reinvestment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) project, this study aimed to identify key elements for a global definition of responsible antibiotic use based on diverse stakeholder input.
Methods
A three-step RAND-modified Delphi method was applied. First, a systematic review of antibiotic stewardship literature and relevant organization web sites identified definitions and synonyms of responsible use. Identified elements of definitions were presented by questionnaire to a multidisciplinary international stakeholder panel for appraisal of their relevance. Finally, questionnaire results were discussed in a consensus meeting.
Results
The systematic review and the web site search identified 17 synonyms (e.g. appropriate, correct) and 22 potential elements to include in a definition of responsible use. Elements were grouped into patient-level (e.g. Indication, Documentation) or societal-level elements (e.g. Education, Future Effectiveness). Forty-eight stakeholders with diverse backgrounds [medical community, public health, patients, antibiotic research and development (R&D), regulators, governments] from 18 countries across all continents participated in the questionnaire. Based on relevance scores, 21 elements were retained, 9 were rephrased and 1 was added. Together, the 22 elements and associated best-practice descriptions comprise an exhaustive list of elements to be considered when defining responsible use.
Conclusions
Combination of concepts from the literature and stakeholder opinion led to an international multidisciplinary consensus on a global definition of responsible antibiotic use. The widely diverging perspectives of stakeholders providing input should ensure the comprehensiveness and relevance of the definition for both individual patients and society. An aspirational goal would be to address all elements.
Introduction
The human impact of antimicrobial resistance is increasing worldwide with more and more antimicrobials losing their power to cure infections. At the same time, the pipelines for new antibiotics are running dry.1,2 A steep growth of initiatives aiming at improving antimicrobial use and tackling antimicrobial resistance indicates that the ‘tipping point’3 on this major global health threat may have been reached. Examples of such international initiatives include the WHO’s Global Action Plan on Antimicrobial Resistance and the Transatlantic Taskforce on Antimicrobial Resistance.4,5 Altogether, these initiatives contributed to a worldwide call to address antimicrobial resistance, reaching the agenda of the United Nations General Assembly as a major global health priority in September 2016.6
While all use of antimicrobial drugs contributes to the development of resistance, major forces driving the increasing resistance include inappropriate infection prevention and inappropriate use.1,7 Inappropriate use is also known to drive increased costs of care, morbidity and mortality.8–10 In order to define inappropriate use, a clear understanding of what appropriate, correct or responsible use entails is crucial. The definition of rational use of drugs, as per the WHO, states that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community.11 More recently, the WHO introduced the concept of responsible use of medicines, implying that the activities, capabilities and existing resources of health system stakeholders should be aligned to ensure patients receive the right medicines at the right time, use them appropriately, and benefit from them.12
However, antimicrobials are one-of-a-kind drugs as they are the only drugs that do not directly and exclusively affect the patient. Indeed, antimicrobials target the biology of microorganisms, both pathogens and commensals, carried by the patient, which can also be shared with a larger human or animal community. Antimicrobial therapy should therefore consider factors related to these microorganisms and the societal ramifications of antibiotic use in addition to patient and drug-related characteristics. The ‘pyramid of infectious diseases’ illustrates the many interplays between the bug, the drug and the patient.13 These interactions are the basis of the complexity of antimicrobial prescription and use but are not explicitly addressed in the WHO definition of rational drug use.
Activities aiming at reducing the undesired consequences of inappropriate use have been undertaken since the early 1970s.14 In the mid-1990s, the term ‘antimicrobial stewardship’ was introduced, describing a collection of strategies, policies, guidelines or tools that could improve antimicrobial prescribing with the aim of decreasing antimicrobial resistance and use.15,16 Stewardship addresses how improved antimicrobial use should be achieved. A definition of responsible antibiotic use would provide clear goals of what should be improved and should thereby steer stewardship activities.
While the medical community, including the antibiotic prescribers, is crucial in assessing responsible antibiotic use, they are not the only stakeholders concerned by the global antibiotic resistance health threat. Great expectations are arising for public health organizations, developers and producers of antibiotics to help solve the issue. Increased political attention is leading to the involvement of policy makers and governments. It is therefore important that all these different cross-disciplinary perspectives should be accounted for in any attempt to define responsible antibiotic use.
This study was conducted within the Driving Reinvestment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) project focusing on human antibiotic use.17,18 The aim of this study was to develop a consensus-driven definition of responsible antibiotic use considering different perspectives, including those of the medical community, public health, patients, antibiotic developers, regulators and governments. The definition should account for diverse socioeconomic settings, thereby ensuring a global scope.
Materials and methods
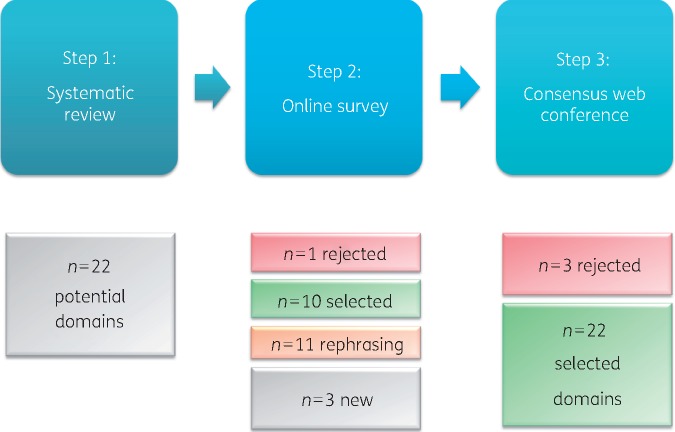
A three-step RAND-modified Delphi method19 was applied to reach consensus on a global definition of responsible antibiotic use (Figure 1). The consensus procedure combined the individual opinions of four groups of stakeholders.
Figure 1.
The number of elements of the definition of responsible use resulting from each step of the RAND-modified Delphi method.
Step 1 – Literature and web site search
A systematic review was performed in the MEDLINE database (since 1966) to identify elements of and definitions of responsible antibiotic use and its synonyms in the scientific literature. Articles were screened in title and abstract with the following search strategy: ‘antibiotic stewardship’ OR ‘antimicrobial stewardship’ OR ‘antibiotic policies’ OR ‘antibiotic policy’. In the context of antibiotic use, policy is a synonym and a predecessor of the term stewardship, a term coined in the mid-1990s.15,16 The search was performed on 25 March 2015. Two researchers (A. A. M. and M. E. H.) independently screened papers discussing general principles of antibiotic stewardship within a random sample of 25% of the found literature. After reaching consensus on this 25% sample, one researcher (M. E. H.) continued the selection process of the remaining literature. Exclusion criteria were papers: not written in English, not discussing antibiotics, not describing general principles of antibiotic stewardship and not containing statements on responsible antibiotic use or its synonyms. Papers were also excluded for which the full-text version was not accessible from one of the following libraries: Radboud University Medical Center, University of Rijeka, University of Antwerp, University of Genève, University of Leuven, University of Lorraine and Google Scholar®. A complementary search was performed on web sites of relevant (inter)national organizations and institutions active in the field of antibiotic stewardship and/or public health. Organizations, institutions and their web sites were identified using lists provided in four publications on antibiotic stewardship and antibiotic research and development (R&D) activities.18,20–22 Ultimately, an exhaustive search of references of included web pages was done. The searches were restricted to web sites in English. Relevant sections of the web sites were searched by one researcher (A. A. M.) using the search terms ‘antibiotic’ and ‘use’.
The data extraction of synonyms and definitions of responsible antibiotic use was performed by one researcher (A. A. M.). For the papers included in the systematic review, the extraction process was repeated by the same researcher 1 month later for 10% of the references. No discrepancies were found, thus ensuring comprehensiveness of the data and intra-rater reproducibility. The data extraction from web sites was performed by the same researcher twice in order to ensure the comprehensiveness of the data. The extracted data were compiled and definition components were clustered into different non-overlapping logical elements (e.g. Microbiologic Diagnostics, Indication), each of which appeared distinctly relevant to defining responsible use. The categorization into elements was done by one researcher (A. A. M.) and then validated by a second researcher (I. C. G.). Discrepancies were discussed until consensus was reached. For each potential element, an explanatory phrase describing the goal for responsible use was proposed by combining different phrasings extracted from the literature. The phrasing was done in consensus between three authors (A. A. M., I. C. G. and M. E. H.). The explanatory phrases for each element were formulated to complete the sentence ‘Responsible antibiotic use includes …’ (e.g. Responsible use includes using microbiology diagnostic tools to provide diagnostic testing.). Finally, the wording of the element names and corresponding explanatory phrases was reviewed by two native English speakers and experts in the field of antibiotic stewardship, one from the UK and one from the USA. Preliminary results of the systematic review were discussed at a ‘train-the-trainer event’ in collaboration with the BSAC during the 26th European Congress of Clinical Microbiology and Infectious Diseases. Senior members of the European medical antibiotic stewardship community were asked for feedback.23
Step 2 – Online questionnaire
The consensus procedure took place from July until September 2016. Seventy-four international stakeholders were invited by e-mail to participate. Reminders were sent 4 and 2 weeks before the closing of the questionnaire. Stakeholders were invited based either on demonstrated experience and expertise on the topic of antibiotic use and/or stewardship, e.g. relevant publications, or involvement in national stewardship activities, or on different perspectives on antibiotic use, e.g. having a prominent role within a relevant organization, institute, society or company. Stakeholders from the extended international network of academic and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners of the DRIVE-AB project were solicited.
Individuals amongst four different stakeholder groups, aiming at representing all parties involved with antibiotic use, were identified: medical community (n = 18); public health and patients (n = 18); antibiotic R&D (n = 21); payers, policy makers, governments and regulators (n = 17). The invited stakeholders originated from 20 countries across all continents.
A web-based questionnaire on responsible antibiotic use was designed in SurveyMonkey®. Together with the invitation e-mail, stakeholders were sent a document providing the scientific references from the systematic review for each of the identified elements. The stakeholders were asked to appraise the relevance of each element to be included in the definition of responsible antibiotic use. The relevance was graded using a nine-point Likert scale (1 = clearly not relevant, 9 = clearly relevant). Relevance scores were calculated for each element following the RAND agreement criteria.19 Median scores were analysed across the four stakeholder groups. If the element had a median of 8 or 9 and >70% of the stakeholders scored in the upper tertile (i.e. 7, 8 or 9), the element was selected. If the element had a median <8 or <70% of the stakeholders scored in the upper tertile, the element was rejected.
Stakeholders could comment on the elements as well as make suggestions for new elements. If comments referred to the clarity of the element and/or wording of its explanatory phrase, these elements were labelled for discussion. Newly proposed elements that did not present any overlap with other elements were selected.
Step 3 – Consensus meeting
The first 20 stakeholders that filled in the questionnaire were asked for their availability to take part in a consensus meeting after the summer of 2016. The aim was to have balanced numbers of participants across the four stakeholders groups. The meeting was held on 28 September 2016 using a web conferencing interface. During the meeting, the stakeholders discussed elements labelled for discussion and newly proposed elements. For the elements labelled for discussion, the researchers prepared a new wording proposal based on the comments made by the stakeholders in the questionnaire. These comments were categorized for each stakeholder group and shown during the consensus meeting to expedite the discussions. Typically, modifications to the new wording proposal were made until agreement was reached. An audio recording of the meeting was made and used to make sure no relevant suggestions were missed.
Results
Step 1 – Literature and web site search
The systematic literature search identified 1700 articles of which 343 were considered eligible for full-text screening. After exclusion and inclusion criteria were applied, 161 articles (10%) were included and data extraction was performed. The flowchart of the systematic review is shown in Figure S1 (available as Supplementary data at JAC Online). The web sites of 50 institutions and organizations were identified and searched for definitions. Fifteen web sites (30%) were ultimately included for data extraction (Table S1).
The systematic review and the complementary web site search led to the identification of 17 synonyms of responsible antibiotic use: adequate, appropriate, better, correct, effective, focused, improved, judicious, optimal, optimized, proper, proportionate, prudent, rational, right, safe, thoughtful. Furthermore, this first step led to the identification of 22 potential elements of responsible antibiotic use for appraisal by the stakeholders (Figure 1; Table 1).
Table 1.
Results of the consensus procedure on the elements of responsible antibiotic use.
| Final no. | Element name | References | Result after the online survey | Result after the consensus meeting | Element description |
|---|---|---|---|---|---|
| 1 | Microbiological Diagnostics | 41–61 | Labelled for rephrasing | Rephrased and selected | Using high quality available microbiology laboratory facilities to provide routine diagnostic testing. |
| Rephrased: Using microbiology diagnostic tools to provide diagnostic testing. | |||||
| 2 | Indication | 29,46–50,52,53,55,57,59–96 | Labelled for rephrasing | Rephrased and selected | Restricting antibiotic use to prevent or cure suspected or microbiologically proven bacterial infections. |
| Rephrased: Using antibiotics only to prevent or cure infections for which antibiotic treatment provides a proven benefit. | |||||
| 3 | Antibacterial Activity | 29,41–43,45,46,48,50,52–55,59,64,66,67,69–71,75,76,79,80,85,87,89,93,94,97–152 | Labelled for rephrasing | Rephrased and selected | Rationally selecting antibiotics based on their antibacterial activity. |
| Rephrased: Selecting antibiotics based on their antibacterial activity. | |||||
| 4 | Antibacterial Spectrum | 29,41–43,45,47–51,56,58,67,69,74,76,78,79,86,94,105,123,127,139,143,153–156 | Selected | – | Selecting antibiotics based on their antibacterial spectrum (as narrow as possible). |
| 5 | Dosing, PK/PD, Interval | Labelled for rephrasing | Rephrased and selected | Dosing and dosing frequency of the antibiotic regimen based on clear PK/PD principles (ensuring sufficient free concentrations of antibiotic at the site of infection). | |
| Rephrased: Dose and dosing frequency of the antibiotic regimen based on available knowledge on PK/PD (ensuring sufficient free concentrations of antibiotic at the site of infection). | |||||
| 6 | Duration | 16,29,41–43,45–55,59,60,64–67,69–71,73–76,78–80,85–89,91,94–99,101,102,104–107,109–111,113,115–121,124–138,140,142–151,155,156,158,159,161 | Labelled for rephrasing | Rephrased and selected | Using the shortest possible duration of the antibiotic regimen. |
| Rephrased: Using the shortest possible evidence-based duration of the antibiotic regimen. | |||||
| 7 | Route | 29,41,43,46,48,51–54,64,66,68,70,74–76,79,80,86,93,97,98,101,102,104,105,109,111,113,115–117,120,123–125,127,128,132,139,140,143,144,146–150,152,162 | Labelled for rephrasing | Rephrased and Selected | Selecting the proper route (parenteral or oral) based on antibiotic and patient characteristics. |
| Rephrased: Selecting the proper route (e.g. parenteral or oral) based on antibiotic, severity or type of infection and patient characteristics. | |||||
| 8 | Timing | 29,49,52,66,68,71,74,78,83,89,94,121,123,124,126,127,142,153,156 | Labelled for rephrasing | Selected | Administering antibiotics in a timely manner. |
| 9 | Interactions | 45,76,159 | Selected | – | Selecting antibiotics taking into account possible interactions with other medication(s). |
| 10 | Toxicity | 20,29,41,45,48–50,52–55,59,60,62,73,75,76,83,85,86,93,97,99,101,106,109,115,116,118,119,122,123,125,126,128,131,133,134,136–138,141,148,149,151–154,159,160,162–180 | Selected | – | Selecting the antibiotic with the least toxicity possible. |
| 11 | Unintended Consequences | 20,29,41,48,49,53,55,66,68,69,71,73,75,76,85,89,91,94,99,103,110,114,117,119,123,125–127,132–134,136,138,140,141,143,144,146,147,149,151–153,160,163,164,168,172,173,177,179–189 | Selected | – | Selecting the antibiotic with the lowest risk of secondary infections such as C. difficile diarrhoea. |
| 12 | Documentation | 41,47,49,88 | Selected | – | Fully documenting the antibiotic regimen including indication in the medical record. |
| 13 | Patient Compliance | 47,50,60,154 | Selected | – | Ensuring patient compliance with the antibiotic prescription. |
| 14 | Patient Outcome | 20,29,41,42,45,47,48,50,52–54,57,59,62,66,71–74,77,79,83,85,89,91,93,97,98,101,106,107,109,110,114–116,118,119,122,123,125,126,128,129,131–134,136–138,140,142–144,146–151,153,154,156,158,162,164–170,172–185,187–199 | Labelled for rephrasing | Rephrased and selected | Optimizing outcome (reduced morbidity, mortality and length of hospital stay) following the treatment or prevention of bacterial infections. |
| Rephrased: Optimizing patient outcome (reduced morbidity, mortality and length of hospital stay) by treating or preventing bacterial infections. | |||||
| 15 | Access-Availability | 57,60,61,200 | Selected | – | Ensuring access and routine availability of quality antibiotics. |
| – | Costs | 20,41,45,47,50,52–54,57,60,66,69,71,85,89,97,110,112,115,117,118,126,133,134,141–143,148–150,153–155,158,162–170,175,179–181,188,189,191–193,195,201 | Rejected | – | Using the most cost-effective antibiotic regimen. |
| 16 | Resistance | 16,29,41,42,44,45,48–50,53–55,57,59,60,62,66,69,71,73,75,76,81,83,85,89,91,97,99,101,103,106,109,110,112,114,115,117,120,123,125–127,131–134,136–138,140–142,144–151,153,160,163–174,176,178–195,197–200,202,203 | Labelled for rephrasing | Selected | Limiting the emergence of antibiotic resistance. |
| 17 | Future Effectiveness | 41,49,53,57,62,93,103,105,120,128,134,193,200,204–207 | Selected | – | Conserving the effectiveness of antibiotics for the future. |
| 18 | Resistance Surveillance | 48,50,57,60,61,86,94,103,105,123,130,134,139,189,208,209 | Labelled for rephrasing | Rephrased and selected | Using resistance surveillance data for empirical prescribing. |
| Rephrased: Using local antibiotic resistance surveillance data for guidelines on empirical antibiotic prescribing. | |||||
| 19 | Evidence-based Guidelines | Selected | - | Ensuring the availability and use of local (or national) evidence-based treatment guidelines. | |
| Rephrased: Ensuring educational programmes on antibiotic use from an early stage for the public and all relevant professionals, including trainees in healthcare curricula. | |||||
| 20 | Expertise and Resources | 49,60,70,123,139 | Selected | – | Using available infectious disease expertise and resources. |
| 21 | Education | 29,60–62,77,91,93,95,98,103,123,160,161,208 | Labelled for rephrasing | Rephrased and selected | Ensuring educational programmes on antibiotic use for all relevant professionals and the public. |
| Rephrased: Ensuring educational programmes on antibiotic use from an early stage for the public and all relevant professionals, including trainees in healthcare curricula. | |||||
| 22 | Waste Disposal | Proposed in the online survey | - | Rephrased and selected | Disposing waste antibiotics to prevent selection in the environment. |
| Rephrased: Safely disposing of unused antibiotics and waste products containing antibiotics to prevent selection in the environment. | |||||
| – | Alternatives | Proposed in the online survey | – | Rejected* | Considering alternatives for antibiotics to prevent infections (e.g. vaccines, hygiene, infection control). |
| – | Multidisciplinarity | Proposed in the online survey | – | Rejected | Stimulating collaboration between different types of healthcare professionals (e.g. nurses, doctors, pharmacists). |
Element 1-14: patient level elements; elements 15-22: societal elements.
Indicates rejected as an element of responsible antibiotic use but added to the figure as an additional aspect.
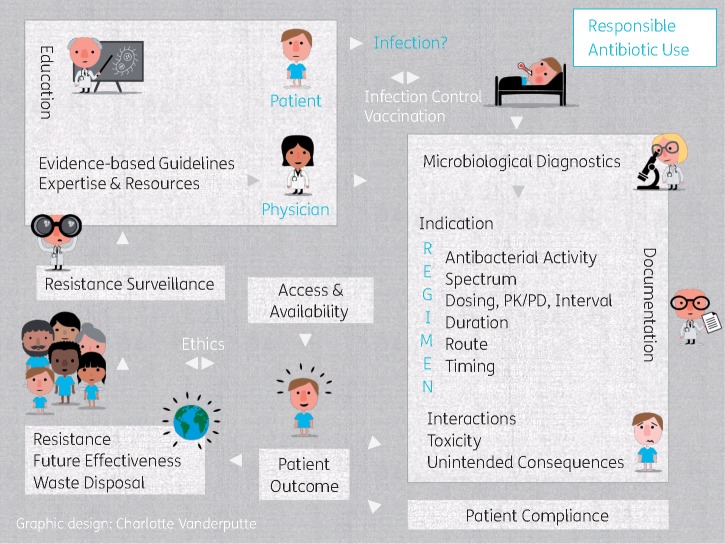
Delegates from 19 EU countries attended the train-the-trainer event. During discussions at this event it was acknowledged that the balance between the elements Patient Outcome, Resistance and Future Effectiveness of the antibiotic drug implied important ethical considerations, which should be made visible. As a result, Ethics: Making the balance between Patient Outcome, Future Effectiveness and Resistance based on ethical considerations was added as an aspect of importance to the definition of responsible antibiotic use as shown in the infographic (Figure 2) and Table 2.
Figure 2.
The final 22 elements included in the definition of responsible antibiotic use. The elements of responsible antibiotic use are shown in black text; two additions, suggested during stakeholder consultations, were inserted into the infographic on responsible antibiotic use: Ethics and alternatives (Infection Control, Vaccination) were considered of important value without directly defining responsible use. On the right: patient-level elements; on the left: societal elements.
Table 2.
The final 22 elements of the definition of responsible antibiotic use and their explanatory phrase
| Element | Explanatory phrase |
|---|---|
| Microbiological Diagnostics | Using microbiology diagnostic tools to provide diagnostic testing. |
| Indication | Using antibiotics only to prevent or cure infections for which antibiotic treatment provides a proven benefit. |
| Antibacterial Activity | Selecting antibiotics based on their antibacterial activity. |
| Antibacterial Spectrum | Selecting antibiotics based on their antibacterial spectrum (as narrow as possible). |
| Dosing, PK/PD, Interval | Dose and dosing frequency of the antibiotic regimen based on available knowledge on PK/PD (ensuring sufficient free concentrations of antibiotic at the site of infection). |
| Duration | Using the shortest possible evidence-based duration of the antibiotic regimen. |
| Route | Selecting the proper route (e.g. parenteral or oral) based on antibiotic, severity or type of infection and patient characteristics. |
| Timing | Administering antibiotics in a timely manner. |
| Interactions | Selecting antibiotics taking into account possible interactions with other medication(s). |
| Toxicity | Selecting the antibiotic with the least toxicity possible. |
| Unintended Consequences | Selecting the antibiotic with the lowest risk of secondary infections such as C. difficile diarrhoea. |
| Documentation | Fully documenting the antibiotic regimen including indication in the medical record. |
| Patient Compliance | Ensuring patient compliance with the antibiotic prescription. |
| Patient Outcome | Optimizing patient outcome (reduced morbidity, mortality and length of hospital stay) by treating or preventing bacterial infections. |
| Access-Availability | Ensuring access and routine availability of quality antibiotics. |
| Resistance | Limiting the emergence of antibiotic resistance. |
| Future Effectiveness | Conserving the effectiveness of antibiotics for the future. |
| Resistance Surveillance | Using local antibiotic resistance surveillance data for guidelines on empirical antibiotic prescribing. |
| Evidence-based Guidelines | Ensuring the availability and use of local (or national) evidence-based treatment guidelines. |
| Expertise and Resources | Using available infectious disease expertise and resources. |
| Education | Ensuring educational programmes on antibiotic use from an early stage for the public and all relevant professionals, including trainees in health care curricula. |
| Waste Disposal | Safely disposing of unused antibiotics and waste products containing antibiotics to prevent selection in the environment. |
Step 2 – Online questionnaire
In the online questionnaire, a multidisciplinary panel of 50 stakeholders (response rate 68%) from 18 countries across all continents appraised the relevance of the 22 potential elements of responsible antibiotic use. The online questionnaire is shown in Figure S2. These 50 stakeholders were distributed as follows: 13 belonged to the medical community group including professional societies, hospital pharmacists, infectious disease physicians, clinical microbiologists and a nurse; 12 belonged to the public health and patients group including the WHO, Médecins Sans Frontières, national public health institutes and ethicists; 13 belonged to antibiotic R&D organizations including small and medium enterprises, pharmaceutical companies and economists; and 12 were payers, policy makers, governments and regulators including the European Centre for Disease Prevention and Control, the Centers for Disease Control and Prevention, the US Food and Drug Administration, European Medicines Agency, governments and a national health insurance advisor. The answers of two stakeholders were incomplete; as a result 48 answers were used for data analysis. A detailed list of all the stakeholders and their affiliations is shown in Table S2.
The results of the questionnaire are shown in Table 1. Based on relevance scores, 21 elements to be included in the definition of responsible use were selected and one element, Costs: Using the most cost-effective antibiotic regimen, was rejected. Comments provided by stakeholders to explain their low relevance scores for this element included e.g. ‘Ceftriaxone has been a major driver of inappropriate use due to cost’ and ‘Cost-efficiency is not a great criterion for being responsible’.
Ten elements were selected without suggestions for rephrasing: Access-Availability, Antibacterial Spectrum, Documentation, Evidence-based Guidelines, Expertise and Resources, Future Effectiveness, Interactions, Patient Compliance, Toxicity and Unintended Consequences.
Among the 21 selected elements, 11 were labelled for rephrasing of the explanatory text based on comments made by stakeholders: Antibacterial Activity, Dosing-PK/PD-Interval, Duration, Education, Indication, Microbiological Diagnostics, Patient Outcome, Route, Resistance, Resistance Surveillance and Timing. Three new potential elements were suggested: Waste Disposal, Alternatives and Multidisciplinarity.
Step 3 – Consensus meeting
Ten stakeholders discussed the 11 elements labelled for rephrasing as well as the 3 newly suggested elements. The stakeholders represented all groups: medical community (n = 3); public health and patients (n = 3); antibiotic R&D (n = 1); payers, policy makers, governments and regulators (n = 3). The details of the consensus procedure including the final selection and rejection as well as the rephrasing of the elements are shown in Table 1. Nine elements were rephrased and two remained unchanged (Resistance, Timing). The newly suggested element Waste Disposal was rephrased to Safely disposing of unused antibiotics and waste products containing antibiotics to prevent selection in the environment and selected. The other two suggested elements, Multidisciplinarity and Alternatives, were rejected as these were not found to be defining elements of responsible antibiotic use. Multidisciplinarity: Stimulating collaboration between different types of healthcare professionals (e.g. nurses, doctors, pharmacists) was rejected as it was argued that the opposite, antibiotic stewardship performed without any multidisciplinary aspect, could not be considered bad clinical practice. Alternatives: considering alternatives for antibiotics to prevent infections (e.g. vaccines, hygiene, infection control) was recognized as extremely important for reducing the number of infections and thereby reducing antibiotic resistance; however, it also did not directly contribute to defining responsible use. Therefore, Infection Control and Vaccination were added to the infographic as additional aspects of importance related to the definition of responsible antibiotic use (Figure 2). The final 22 elements of responsible use resulting from the Delphi procedure are shown in Table 2 and illustrated in Figure 2. Fourteen elements were patient-level elements that related to aspects of responsible use of antibiotics: Antibacterial Activity, Antibacterial Spectrum, Documentation, Dosing-PK/PD-Interval, Duration Indication, Interactions, Microbiological Diagnostics, Patient Compliance, Patient Outcome, Route, Timing, Toxicity and Unintended Consequences. Eight elements were considered societal-level as relating to responsible antibiotic use in a broader societal context: Access-Availability, Education, Evidence-based Guidelines, Expertise and Resources, Future Effectiveness, Resistance, Resistance Surveillance and Waste Disposal.
Discussion
In this study a list of 22 key elements and their associated best-practice descriptions were developed that, taken together, need to be included in the definition of responsible antibiotic use. This exhaustive list was the result of a systematic review followed by an international and multidisciplinary consensus. Fourteen elements corresponded to patient-level and 8 to societal-level elements. Patient-level elements reflect individual care parameters whereas societal-level elements typically affect large populations. At present, all the identified elements should be considered relevant and an aspirational goal would be to address them when using antibiotics.
Two additions, suggested during stakeholder consultations, were inserted in the infographic of responsible antibiotic use: Ethics and Alternatives were considered of important value without directly defining responsible use. The ethical dimensions of the balance between present and future patients have been addressed previously by others.24,25 In a recent perspective on responsible use, Dyar et al.26 also highlight two relevant dimensions of ‘responsible’: the responsible individual practices and the societal implications of being responsible. Regarding alternatives to antibiotics, the importance of infection control in parallel to stewardship activities has been highlighted by, among others, the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America.20,27 In addition, vaccines are effective against bacterial diseases and therefore reduce the need for antibiotic usage.28
The global scope of the definition was emphasized by considering both its comprehensiveness and its worldwide relevance. Socio-economic, cultural or care-setting specific factors as well as feasibility or practical implications were not considered in this study, so that the resulting elements would be relevant to any setting worldwide. The elements of responsible antibiotic use should be considered as a consensus-derived set of principles of what responsible antibiotic use should entail. From these elements, generic quality and quantity measures can be developed, for both current and newly developed antibiotic drugs in the future. Finally, the definition constitutes a valuable educational tool for use in different healthcare curricula, including undergraduate education.29
Until now, such a consensus-driven definition of responsible antibiotic use was lacking within the infectious disease and antibiotic stewardship community. The limitations of the WHO definition of rational use were previously addressed in the introduction of this manuscript. A strength of this work is the use of a systematic and stepwise method combining both concepts from the literature and stakeholder opinion. An additional innovative aspect is that the perspectives of a wide range of stakeholders involved with antibiotics were accounted for. This approach contrasts, however, with previous research efforts, which have mainly involved medical and public health communities. Previously, scientists have called for a multi-stakeholder approach including the producers and regulators of antibiotics, without any concrete success to date.30,31
The adjective ‘responsible’ was the terminology used in the Innovative Medicines Initiative (IMI) call, and therefore it was a logical continuation for use in the DRIVE-AB project. In our study, 17 synonyms of responsible antibiotic use were identified. This diversity in vocabulary is also illustrated by the fact that currently the WHO opts for ‘rational’ and ‘appropriate’7, while the ECDC uses the term ‘prudent’32 and the CDC ‘appropriate’.33 According to the authors, the identified synonyms should be considered as interchangeable as long as all the 22 elements are being considered.
A limitation of this work is the focus on human medicine only. As human health, animal health and the environment are closely interrelated, a One Health approach is of paramount importance. Antibiotic resistance has been recognized as the quintessential One Health issue, illustrating its principles better than any other public health threat.34 In the global definition of responsible use, the element Waste Disposal of human antibiotics addresses the environment. Over the last 20 years, the importance of Safely disposing of unused antibiotics and waste products containing antibiotics to prevent selection in the environment has been demonstrated by several studies reporting pollution with antibiotics in effluents of drug manufacturers, which is driving antibiotic selection pressure in the environment.35 Another limitation is that the veterinary sector was not addressed in any of the elements of the definition. However, the principles illustrated by the human elements of responsible antibiotic use are equally pertinent for animal health. Aspects relating to applicability or implementation in clinical practice were not included in this definition. While this contributes to the simplicity required for the global scope, including the coverage of low-income settings, and could be considered a strength, this should also be addressed as a flaw. A methodological limitation of this study is the use of a single literature database (MEDLINE) for the systematic review. However, both the complementary web site search and the opportunity given to the stakeholders to propose new elements should have ensured that no relevant element was missed. Another limitation is that the screening of the literature and websites and the data extraction process were performed by a single researcher. However, measures to address intra-rater bias included a second data extraction process for a proportion of the articles and for all the web sites, and inter-rater bias was reduced by performing the screening of a proportion of the articles in duplicate. Finally, language subtleties might have been missed or might have contributed to a lack of understanding of the elements, as the researchers as well as some of the stakeholders were non-native English speakers.
In conclusion, a global list of elements key to the definition of responsible antibiotic use was developed considering the perspectives of a wide range of stakeholders involved with antibiotics. DRIVE-AB identified measures for assessing the quality and quantity of antibiotic use.36–39 Together, these tools will be proposed as a global standard of responsible use for old and new antibiotics. Indeed, the ultimate goal of the DRIVE-AB initiative is to reconcile the long-term conservation of antibiotics through responsible use and incentives for novel antibiotic development.40
Supplementary Material
Acknowledgements
The authors acknowledge all stakeholders that participated in the consensus procedure: Diane Ashiru-Oredope, Luis Bavestrello, Radu Botgros, Christian Brun-Buisson, Franky Buyle, Sujith John Chandy, Claudie Charbonneau, Barry Cookson, Pieter-Jan Cortoos, John Farley, David Findlay, Jérôme Gabard, Abdul Ghafur, Elizabeth Hermsen, David Heymann, Lauri Hicks, Karianne Johansen, Rupa Kanapathipillai, Aaron S. Kesselheim, Marie Paule Kieny, Charles Knirsch, David Kronlid, Patrick Lacor, Gabriel Levy Hara, Marc Lemonnier, Marc Mendelson, Cliodna McNulty, Blandina Theophil Mmbaga, Sumathi Nambiar, Iruka N. Okeke, Jean-Pierre Paccaud, Charles Penn, David Payne, Diamantis Plachouras, John H. Rex, France Roblot, Jesús Rodríguez-Baño, Monique Rothan-Tondeur, Babette Rump, Arjun Srinivasan, Thomas Tängdén, Visanu Thamlikitkul, Karin Thursky, Gert Jan van der Wilt, Theo Verheij, Alexandra Waluszewski, Sally Wellsteed and Suwit Wibulpolprasert.
The authors acknowledge John H. Rex for valuable contributions to the manuscript; Jeroen Schouten for helping build the search strategy and chairing the consensus meeting; Irah Noy for helping retrieve full-text publications for the systematic review. With special thanks to Charlotte Vanderputte for designing the infographic of responsible antibiotic use as well as to Mike Sharland for piloting the questionnaire and providing linguistic feedback.
The preliminary results of this study were presented at the DRIVE-AB ‘train-the-trainer event’ held during the 26th European Congress of Clinical Microbiology and Infectious Diseases in Amsterdam, the Netherlands, in April 2016. The final results were presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases in Vienna, Austria, in April 2017 (presentation number: P1063).
Members of the DRIVE-AB WP1A group
The authors thank the fellow DRIVE-AB Work Package 1A researchers for their contributions: Niels Adriaenssens, Benedikt Huttner, Marion Le Maréchal, Romina Milanič, Céline Pulcini, Mirjana Stanić Benić, Gianpiero Tebano, Ann Versporten, Vera Vlahović-Palčevski and Veronica Zanichelli.
The authors thank the DRIVE-AB steering committee for their critical review of the manuscript.
Funding
This work was supported by the Innovative Medicines Initiative (IMI) Joint Undertaking (grant agreement no. 115618 - Driving re-investment in R&D and responsible antibiotic use—DRIVE-AB—www.drive-ab.eu). Resources are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) in-kind contribution.
Transparency declarations
A. A. M. and M. E. H. have no disclosures to report. I. C. G. reports an educational grant from Pfizer, outside the submitted work. B. I. E. is presently Chair of the Scientific Advisory Board, CARB-X. While working on this project, he was employed at Cubist Pharmaceuticals and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
This article forms part of a Supplement sponsored by DRIVE-AB.
Author contributions
A. A. M. performed the literature and web site searches, the data extraction, sent the questionnaire, collected and analysed the data and drafted the wording of the elements and the manuscript. M. E. H., I. C. G. and B. I. E. co-designed the study. M. E. H. performed the screening for the systematic review and provided methodological support. I. C. G. validated the classifications of the definitions into the elements. B. I. E. piloted the questionnaire. All authors have critically reviewed and approved the final manuscript.
Supplementary data
Figures S1 and S2 and Tables S1 and S2 are available as Supplementary data at JAC Online.
Contributor Information
DRIVE-AB WP1 group:
Niels Adriaenssens, Benedikt Huttner, Marion Le Maréchal, Romina Milanič, Céline Pulcini, Mirjana Stanić Benić, Gianpiero Tebano, Ann Versporten, Vera Vlahović-Palčevski, Veronica Zanichelli, Diane Ashiru-Oredope, Luis Bavestrello, Radu Botgros, Christian Brun-Buisson, Franky Buyle, Sujith John Chandy, Claudie Charbonneau, Barry Cookson, Pieter-Jan Cortoos, John Farley, David Findlay, Jérôme Gabard, Abdul Ghafur, Elizabeth Hermsen, David Heymann, Lauri Hicks, Karianne Johansen, Rupa Kanapathipillai, Aaron S Kesselheim, Marie Paule Kieny, Charles Knirsch, David Kronlid, Patrick Lacor, Gabriel Levy Hara, Marc Lemonnier, Marc Mendelson, Cliodna McNulty, Blandina Theophil Mmbaga, Sumathi Nambiar, Iruka N Okeke, Jean-Pierre Paccaud, Charles Penn, David Payne, Diamantis Plachouras, John H. Rex, France Roblot, Jesús Rodríguez-Baño, Monique Rothan-Tondeur, Babette Rump, Arjun Srinivasan, Thomas Tängdén, Visanu Thamlikitkul, Karin Thursky, Gert Jan van der Wilt, Theo Verheij, Alexandra Waluszewski, Sally Wellsteed, and Suwit Wibulpolprasert
References
- 1. Laxminarayan R, Duse A, Wattal C. et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013; 13: 1057–98. [DOI] [PubMed] [Google Scholar]
- 2. Huttner A, Harbarth S, Carlet J. et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2: 31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, NY, USA: Little, Brown and Company, 2000. [Google Scholar]
- 4. Transatlantic Taskforce on Antimicrobial Resistance (TATFAR) Progress report on recommendations for further collaboration between the US and EU. http://www.cdc.gov/drugresistance/pdf/tatfar-progress_report_2014.pdf. [Google Scholar]
- 5. World Health Organization. Global Action Plan on Antimicrobial Resistance 2015. http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua=1. [DOI] [PubMed]
- 6. United Nations. High-Level Meeting on Antimicrobial Resistance http://www.un.org/pga/70/events/high-level-meeting-on-antimicrobial-resistance/.
- 7. World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf.
- 8. McDonald LC, Yu HT, Yin HC. et al. Use and abuse of surgical antibiotic prophylaxis in hospitals in Taiwan. J Formos Med Assoc 2001; 100: 5–13. [PubMed] [Google Scholar]
- 9. Kunin CM, Lipton HL, Tupasi T. et al. Social, behavioral, and practical factors affecting antibiotic use worldwide: report of Task Force 4. Rev Infect Dis 1987; 9 Suppl 3: S270–85. [DOI] [PubMed] [Google Scholar]
- 10. Ledger WJ. Prophylactic antibiotics in obstetrics-gynecology: a current asset, a future liability? Expert Rev Anti Infect Ther 2006; 4: 957–64. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. The Rational Use of Drugs. Report of the Conference of Experts http://apps.who.int/medicinedocs/documents/s17054e/s17054e.pdf.
- 12. World Health Organization. The Pursuit of Responsible Use of Medicines: Sharing and Learning from Country Experiences http://apps.who.int/iris/bitstream/10665/75828/1/WHO_EMP_MAR_2012.3_eng.pdf.
- 13. Gyssens IC. Quality measures of antimicrobial drug use. Int J Antimicrob Agents 2001; 17: 9–19. [DOI] [PubMed] [Google Scholar]
- 14. McGowan JE, Finland M.. Usage of antibiotics in a general hospital: effect of requiring justification. J Infect Dis 1974; 130: 165–8. [DOI] [PubMed] [Google Scholar]
- 15. McGowan JE, Gerding DN.. Does antibiotic restriction prevent resistance? New Horiz 1996; 4: 370–6. [PubMed] [Google Scholar]
- 16. Shlaes DM, Gerding DN, John JF Jr. et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis 1997; 25: 584–99. [DOI] [PubMed] [Google Scholar]
- 17. Harbarth S, Theuretzbacher U, Hackett J.. Antibiotic research and development: business as usual? J Antimicrob Chemother 2015; 70: 1604–7. [DOI] [PubMed] [Google Scholar]
- 18. Harbarth S, Hackett J. Introduction: DRIVE-AB’s definition, metrics and indicators to monitor responsible antibiotic use. This supplement. [DOI] [PMC free article] [PubMed]
- 19. Fitch K, Bernstein SJ, Aguilar MD. et al. The RAND/UCLA Appropriateness Method User’s Manual Santa Monica, CA, USA: RAND Corporation, 2001.
- 20. Dellit TH, Owens RC, McGowan JE Jr. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 21. Pagani L, Gyssens IC, Huttner B. et al. Navigating the Web in search of resources on antimicrobial stewardship in health care institutions. Clin Infect Dis 2009; 48: 626–32. [DOI] [PubMed] [Google Scholar]
- 22. Miller A. Antibacterial development: a changing landscape. Microbe 2016; 11: 111–18. [Google Scholar]
- 23. DRIVE-AB. Train-the-Trainer Event: Defining and Implementing Responsible Antibiotic Use http://drive-ab.eu/events/train-the-trainer-event-defining-and-implementing-responsible-antibiotic-use/. [DOI] [PMC free article] [PubMed]
- 24. Leibovici L, Paul M.. Ethical dilemmas in antibiotic treatment: focus on the elderly. Clin Microbiol Infect 2015; 21: 27–9. [DOI] [PubMed] [Google Scholar]
- 25. Littmann J, Buyx A, Cars O.. Antibiotic resistance: an ethical challenge. Int J Antimicrob Agents 2015; 46: 359–61. [DOI] [PubMed] [Google Scholar]
- 26. Dyar OJ, Obua C, Chandy S. et al. Using antibiotics responsibly: are we there yet? Future Microbiol 2016; 11: 1057–71. [DOI] [PubMed] [Google Scholar]
- 27. Barlam TF, Cosgrove SE, Abbo LM. et al. Implementing an Antibiotic Stewardship Program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipsitch M, Siber GR.. How can vaccines contribute to solving the antimicrobial resistance problem? MBio 2016; 7: pii: e00428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulcini C, Gyssens IC.. How to educate prescribers in antimicrobial stewardship practices. Virulence 2013; 4: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norrby SR, Nord CE, Finch R.. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis 2005; 5: 115–9. [DOI] [PubMed] [Google Scholar]
- 31. Gyssens IC. All EU hands to the EU pumps: the Science Academies of Europe (EASAC) recommend strong support of research to tackle antibacterial resistance. Clin Microbiol Infect 2008; 14: 889–91. [DOI] [PubMed] [Google Scholar]
- 32. European Centre for Disease Prevention and Control (ECDC). Proposals for EU Guidelines on the Prudent Use of Antimicrobials in Humans http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1643.
- 33.Centers for Disease Control and Prevention (CDC). Adult Appropriate Use Summary: Physician Information Sheet. https://www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-approp-summary.html.
- 34. Robinson TP, Bu DP, Carrique-Mas J. et al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg 2016; 110: 377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larsson DJ. Pollution from drug manufacturing: review and perspectives. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130571.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Maréchal M, Tebano G, Monnier AA. et al. Quality indicators assessing antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi40–vi49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monnier AA, Schouten S, Le Maréchal M. et al. Quality indicators for responsible antibiotic use in the inpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi30–vi39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Versporten A, Gyssens IC, Pulcini C. et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi59–vi66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stanić Benić M, Milanič R, Monnier AA. et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi50–vi58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Driving Reinvestment in R&D for Antibiotics and Advocating Their Responsible Use (DRIVE-AB Project) www.drive-ab.eu.
- 41. Aryee A, Price N.. Antimicrobial stewardship—can we afford to do without it? Br J Clin Pharmacol 2015; 79: 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bassetti M, De Waele JJ, Eggimann P. et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med 2015; 41: 776–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brett A, Bielicki J, Newland JG. et al. Neonatal and pediatric antimicrobial stewardship programs in Europe-defining the research agenda. Pediatr Infect Dis J 2013; 32: e456–65. [DOI] [PubMed] [Google Scholar]
- 44. Charani E, Castro-Sanchez E, Holmes A.. The role of behavior change in antimicrobial stewardship. Infect Dis Clin North Am 2014; 28: 169–75. [DOI] [PubMed] [Google Scholar]
- 45. Fishman N. Antimicrobial stewardship. Am J Infect Control 2006; 34: S55–63; discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 46. Goff DA. Antimicrobial stewardship: bridging the gap between quality care and cost. Curr Opin Infect Dis 2011; 24 Suppl 1: S11–20. [DOI] [PubMed] [Google Scholar]
- 47. Gould LM. Minimum antibiotic stewardship measures. Clin Microbiol Infect 2001; 7 Suppl 6: 22–6. [PubMed] [Google Scholar]
- 48. Gyssens IC. Antibiotic policy. Int J Antimicrob Agents 2011; 38 Suppl: 11–20. [DOI] [PubMed] [Google Scholar]
- 49. Hand K. Antibiotic stewardship. Clin Med (Lond) 2013; 13: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levy-Hara G, Amabile-Cuevas CF, Gould I. et al. “Ten commandments” for the appropriate use of antibiotics by the practicing physician in an outpatient setting. Front Microbiol 2011; 2: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luyt CE, Brechot N, Trouillet JL. et al. Antibiotic stewardship in the intensive care unit. Crit Care 2014; 18: 480.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Retamar P, Martin ML, Molina J. et al. Evaluating the quality of antimicrobial prescribing: is standardisation possible? Enferm Infecc Microbiol Clin 2013; 31 Suppl 4: 25–30. [DOI] [PubMed] [Google Scholar]
- 53. Sipahi OR. Economics of antibiotic resistance. Expert Rev Anti Infect Ther 2008; 6: 523–39. [DOI] [PubMed] [Google Scholar]
- 54. Teng CB, Lee W, Yeo CL. et al. Guidelines for antimicrobial stewardship training and practice. Ann Acad Med Singapore 2012; 41: 29–34. [PubMed] [Google Scholar]
- 55. Tzialla C, Borghesi A, Perotti GF. et al. Use and misuse of antibiotics in the neonatal intensive care unit. J Matern Fetal Neonatal Med 2012; 25 Suppl 4: 35–7. [DOI] [PubMed] [Google Scholar]
- 56. World Health Organization. Control of antibiotic-resistant bacteria: memorandum from a WHO meeting. Bull World Health Organ 1983; 61: 423–33. [PMC free article] [PubMed] [Google Scholar]
- 57.Action on Antibiotic Resistance (ReAct). https://www.reactgroup.org/.
- 58.Review of Antimicrobial Resistance. https://amr-review.org/.
- 59.The Public Health Agency of Canada. https://www.canada.ca/en/public-health.html.
- 60.World Health Organization (WHO). http://who.int/.
- 61.World Alliance Against Antibiotic Resistance (WAAAR). http://www.ac2bmr.fr/index.php/en/.
- 62. Ashiru-Oredope D, Cookson B, Fry C. et al. Developing the first national antimicrobial prescribing and stewardship competences. J Antimicrob Chemother 2014; 69: 2886–8. [DOI] [PubMed] [Google Scholar]
- 63. Ayliffe AG. Antibiotic policies. J Antimicrob Chemother 1975; 1: 255–7. [DOI] [PubMed] [Google Scholar]
- 64. Bielicki J, Lundin R, Patel S. et al. Antimicrobial stewardship for neonates and children: a global approach. Pediatr Infect Dis J 2015; 34: 311–3. [DOI] [PubMed] [Google Scholar]
- 65. Bebell LM, Muiru AN.. Antibiotic use and emerging resistance: how can resource-limited countries turn the tide? Glob Heart 2014; 9: 347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cakmakci M. Antibiotic stewardship programmes and the surgeon’s role. J Hosp Infect 2015; 89: 264–6. [DOI] [PubMed] [Google Scholar]
- 67. Campbell KA, Stein S, Looze C. et al. Antibiotic stewardship in orthopaedic surgery: principles and practice. J Am Acad Orthop Surg 2014; 22: 772–81. [DOI] [PubMed] [Google Scholar]
- 68. Davey P, Sneddon J, Nathwani D.. Overview of strategies for overcoming the challenge of antimicrobial resistance. Expert Rev Clin Pharmacol 2010; 3: 667–86. [DOI] [PubMed] [Google Scholar]
- 69. Doron S, Davidson LE.. Antimicrobial stewardship. Mayo Clin Proc 2011; 86: 1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. DePestel DD, Eiland EH 3rd, Lusardi K. et al. Assessing appropriateness of antimicrobial therapy: in the eye of the interpreter. Clin Infect Dis 2014; 59 Suppl 3: S154–61. [DOI] [PubMed] [Google Scholar]
- 71. Drekonja DM, Filice GA, Greer N. et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36: 142–52. [DOI] [PubMed] [Google Scholar]
- 72. Dryden MS, Cooke J, Davey P.. Antibiotic stewardship—more education and regulation not more availability? J Antimicrob Chemother 2009; 64: 885–8. [DOI] [PubMed] [Google Scholar]
- 73. Dumartin C, Rogues AM, Amadeo B. et al. Antibiotic usage in south-western French hospitals: trends and association with antibiotic stewardship measures. J Antimicrob Chemother 2011; 66: 1631.. [DOI] [PubMed] [Google Scholar]
- 74. Edwards B, Gould IM.. Antimicrobial stewardship: lessons from human healthcare. Rev Sci Tech 2012; 31: 135–44. [DOI] [PubMed] [Google Scholar]
- 75. Edwards R, Drumright L, Kiernan M. et al. Covering more territory to fight resistance: considering nurses’ role in antimicrobial stewardship. J Infect Prev 2011; 12: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gilchrist M, Seaton RA.. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother 2015; 70: 965–70. [DOI] [PubMed] [Google Scholar]
- 77. Gould IM. Antibiotic policies and control of resistance. Curr Opin Infect Dis 2002; 15: 395–400. [DOI] [PubMed] [Google Scholar]
- 78. Han KS, Ramsamy Y.. Surveillance alone plays a key role in curbing the overuse of antimicrobials: the major role of antibiotic stewardship. S Afr Med J 2013; 103: 368.. [DOI] [PubMed] [Google Scholar]
- 79. Ho PL, Cheng JC, Ching PT. et al. Optimising antimicrobial prescription in hospitals by introducing an antimicrobial stewardship programme in Hong Kong: consensus statement. Hong Kong Med J 2006; 12: 141–8. [PubMed] [Google Scholar]
- 80. Hojgard S. Antibiotic resistance—why is the problem so difficult to solve? Infect Ecol Epidemiol 2012; 2: 18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kolmos HJ. Interaction between the microbiology laboratory and clinician: what the microbiologist can provide. J Hosp Infect 1999; 43 Suppl: S285–91. [DOI] [PubMed] [Google Scholar]
- 82. Llor C, Bjerrum L.. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5: 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nair GB, Niederman MS.. Year in review 2013: critical care—respiratory infections. Crit Care 2014; 18: 572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nathwani D, Christie P.. The Scottish approach to enhancing antimicrobial stewardship. J Antimicrob Chemother 2007; 60 Suppl 1: i69–71. [DOI] [PubMed] [Google Scholar]
- 85. Owens RC., Jr. Antimicrobial stewardship: concepts and strategies in the 21st century. Diagn Microbiol Infect Dis 2008; 61: 110–28. [DOI] [PubMed] [Google Scholar]
- 86. Patel SJ, Saiman L.. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012; 36: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Remesh A, Gayathri AM, Singh R. et al. The knowledge, attitude and the perception of prescribers on the rational use of antibiotics and the need for an antibiotic policy—a cross sectional survey in a tertiary care hospital. J Clin Diagn Res 2013; 7: 675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schlemmer B. Impact of registration procedures on antibiotic policies. Clin Microbiol Infect 2001; 7 Suppl 6: 5–8. [PubMed] [Google Scholar]
- 89. Srinivasan A. Engaging hospitalists in antimicrobial stewardship: the CDC perspective. J Hosp Med 2011; 6 Suppl 1: S31–3. [DOI] [PubMed] [Google Scholar]
- 90. Tamma PD, Holmes A, Ashley ED.. Antimicrobial stewardship: another focus for patient safety? Curr Opin Infect Dis 2014; 27: 348–55. [DOI] [PubMed] [Google Scholar]
- 91. Wickens HJ, Farrell S, Ashiru-Oredope DA. et al. The increasing role of pharmacists in antimicrobial stewardship in English hospitals. J Antimicrob Chemother 2013; 68: 2675–81. [DOI] [PubMed] [Google Scholar]
- 92.Alliance for the Prudent Use of Antibiotics. https://apua.org/.
- 93.Centers for Disease Control and Prevention (CDC). https://www.cdc.gov.
- 94.Department of Health United-Kingdom. https://www.gov.uk/government/organisations/department-of-health.
- 95.European Centre for Disease Prevention and Control (ECDC). https://ecdc.europa.eu/.
- 96.Pew Charitable Trusts. http://www.pewtrusts.org/en.
- 97. Aitken SL, Palmer HR, Topal JE. et al. Call for antimicrobial stewardship in solid organ transplantation. Am J Transplant 2013; 13: 2499.. [DOI] [PubMed] [Google Scholar]
- 98. Ashiru-Oredope D, Sharland M, Charani E. et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. J Antimicrob Chemother 2012; 67 Suppl 1: i51–63. [DOI] [PubMed] [Google Scholar]
- 99. Dodds Ashley ES, Kaye KS, DePestel DD. et al. Antimicrobial stewardship: philosophy versus practice. Clin Infect Dis 2014; 59 Suppl 3: S112–21. [DOI] [PubMed] [Google Scholar]
- 100. Borg MA, Cookson BD, Gur D. et al. Infection control and antibiotic stewardship practices reported by south-eastern Mediterranean hospitals collaborating in the ARMed project. J Hosp Infect 2008; 70: 228–34. [DOI] [PubMed] [Google Scholar]
- 101. Bradley JS. Antibiotic stewardship in pediatrics: a necessity. Pediatr Infect Dis J 2007; 26: 538–9. [DOI] [PubMed] [Google Scholar]
- 102. Cantey JB, Patel SJ.. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am 2014; 28: 247–61. [DOI] [PubMed] [Google Scholar]
- 103. Charani E, Cooke J, Holmes A.. Antibiotic stewardship programmes—what’s missing? J Antimicrob Chemother 2010; 65: 2275–7. [DOI] [PubMed] [Google Scholar]
- 104. Cosgrove SE, Hermsen ED, Rybak MJ. et al. Guidance for the knowledge and skills required for antimicrobial stewardship leaders. Infect Control Hosp Epidemiol 2014; 35: 1444–51. [DOI] [PubMed] [Google Scholar]
- 105. Cotta MO, Roberts JA, Tabah A. et al. Antimicrobial stewardship of β-lactams in intensive care units. Expert Rev Anti Infect Ther 2014; 12: 581–95. [DOI] [PubMed] [Google Scholar]
- 106. D'Agata EM. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial 2013; 26: 457–64. [DOI] [PubMed] [Google Scholar]
- 107. Daneman N, Rochon P.. Antimicrobial stewardship: opportunities in long-term care homes. Drugs Aging 2011; 28: 765–7. [DOI] [PubMed] [Google Scholar]
- 108. Deguchi T, Matsumoto T.. Antimicrobial stewardship in urology. Int J Urol 2014; 21: 628–9. [DOI] [PubMed] [Google Scholar]
- 109. Doron S, Nadkarni L, Lyn Price L. et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther 2013; 35: 758–65.e20. [DOI] [PubMed] [Google Scholar]
- 110. Drekonja D, Filice G, Greer N. et al. VA Evidence-based Synthesis Program reports Antimicrobial Stewardship Programs in Outpatient Settings: A Systematic Review. Washington, DC, USA: Department of Veterans Affairs, 2014. [PubMed] [Google Scholar]
- 111. Drew RH, White R, MacDougall C. et al. Insights from the Society of Infectious Diseases Pharmacists on antimicrobial stewardship guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Pharmacotherapy 2009; 29: 593–607. [DOI] [PubMed] [Google Scholar]
- 112. Dumartin C, Rogues AM, Amadeo B. et al. Antibiotic stewardship programmes: legal framework and structure and process indicator in southwestern French hospitals, 2005-2008. J Hosp Infect 2011; 77: 123–8. [DOI] [PubMed] [Google Scholar]
- 113. Dyar OJ, Pagani L, Pulcini C.. Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect 2015; 21: 10–9. [DOI] [PubMed] [Google Scholar]
- 114. File TM Jr, Solomkin JS, Cosgrove SE.. Strategies for improving antimicrobial use and the role of antimicrobial stewardship programs. Clin Infect Dis 2011; 53 Suppl 1: S15–22. [DOI] [PubMed] [Google Scholar]
- 115. Fishman N. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33: 322–7. [DOI] [PubMed] [Google Scholar]
- 116. Fleming A, Tonna A, O'Connor S. et al. A cross-sectional survey of the profile and activities of Antimicrobial Management Teams in Irish Hospitals. Int J Clin Pharm 2014; 36: 377–83. [DOI] [PubMed] [Google Scholar]
- 117. Fu P, Brown G, Legal M. et al. Antibiotic Stewardship without an Antibiotic Stewardship Program? Can J Hosp Pharm 2014; 67: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gerding DN. Good antimicrobial stewardship in the hospital: fitting, but flagrantly flagging. Infect Control Hosp Epidemiol 2000; 21: 253–5. [DOI] [PubMed] [Google Scholar]
- 119. Gerding DN. The search for good antimicrobial stewardship. Jt Comm J Qual Improv 2001; 27: 403–4. [DOI] [PubMed] [Google Scholar]
- 120. Griffith M, Postelnick M, Scheetz M.. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther 2012; 10: 63–73. [DOI] [PubMed] [Google Scholar]
- 121. Hermsen ED, McDaneld PM, Eiland EH 3rd. et al. Breaking down the barriers: challenges with development and implementation of an industry-sponsored antimicrobial stewardship data collection and analysis tool. Clin Infect Dis 2014; 59 Suppl 3: S179–84. [DOI] [PubMed] [Google Scholar]
- 122. Hoffmann C, Khadem T, Schweighardt A. et al. New thoughts on the ‘forgotten’ aspect of antimicrobial stewardship: adverse event reporting. Pharmacotherapy 2015; 35: 59–63. [DOI] [PubMed] [Google Scholar]
- 123. Hyun DY, Hersh AL, Namtu K. et al. Antimicrobial stewardship in pediatrics: how every pediatrician can be a steward. JAMA Pediatr 2013; 167: 859–66. [DOI] [PubMed] [Google Scholar]
- 124. Isturiz RE. Optimizing antimicrobial prescribing. Int J Antimicrob Agents 2010; 36 Suppl 3: S19–22. [DOI] [PubMed] [Google Scholar]
- 125. Kaki R, Elligsen M, Walker S. et al. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 2011; 66: 1223–30. [DOI] [PubMed] [Google Scholar]
- 126. Kollef MH, Micek ST.. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012; 16: 179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Llewelyn MJ, Hand K, Hopkins S. et al. Antibiotic policies in acute English NHS trusts: implementation of ‘Start Smart—Then Focus’ and relationship with Clostridium difficile infection rates. J Antimicrob Chemother 2015; 70: 1230–5. [DOI] [PubMed] [Google Scholar]
- 128. Manning ML. The urgent need for nurse practitioners to lead antimicrobial stewardship in ambulatory health care. J Am Assoc Nurse Pract 2014; 26: 411–3. [DOI] [PubMed] [Google Scholar]
- 129. Moehring RW, Anderson DJ.. Antimicrobial stewardship as part of the infection prevention effort. Curr Infect Dis Rep 2012; 14: 592–600. [DOI] [PubMed] [Google Scholar]
- 130. Moro ML, Petrosillo N, Gandin C.. Antibiotic policies in Italian hospitals: still a lot to achieve. Microb Drug Resist 2003; 9: 219–22. [DOI] [PubMed] [Google Scholar]
- 131. Newland JG, Hersh AL.. Purpose and design of antimicrobial stewardship programs in pediatrics. Pediatr Infect Dis J 2010; 29: 862–3. [DOI] [PubMed] [Google Scholar]
- 132. O'Brien DJ, Gould IM.. Maximizing the impact of antimicrobial stewardship: the role of diagnostics, national and international efforts. Curr Opin Infect Dis 2013; 26: 352–8. [DOI] [PubMed] [Google Scholar]
- 133. Ohl CA, Dodds Ashley ES.. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011; 53 Suppl 1: S23–8; quiz S29–30. [DOI] [PubMed] [Google Scholar]
- 134. Ohl CA, Luther VP.. Antimicrobial stewardship for inpatient facilities. J Hosp Med 2011; 6 Suppl 1: S4–15. [DOI] [PubMed] [Google Scholar]
- 135. Owens RC., Jr. Antimicrobial stewardship: application in the intensive care unit. Infect Dis Clin North Am 2009; 23: 683–702. [DOI] [PubMed] [Google Scholar]
- 136. Owens RC Jr, Ambrose PG.. Antimicrobial stewardship and the role of pharmacokinetics-pharmacodynamics in the modern antibiotic era. Diagn Microbiol Infect Dis 2007; 57: 77S–83S. [DOI] [PubMed] [Google Scholar]
- 137. Owens RC Jr, Fraser GL, Stogsdill P.. Antimicrobial stewardship programs as a means to optimize antimicrobial use. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2004; 24: 896–908. [DOI] [PubMed] [Google Scholar]
- 138. Paskovaty A, Pflomm JM, Myke N. et al. A multidisciplinary approach to antimicrobial stewardship: evolution into the 21st century. Int J Antimicrob Agents 2005; 25: 1–10. [DOI] [PubMed] [Google Scholar]
- 139. Patel SJ, Saiman L.. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin Perinatol 2010; 37: 547–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pile JC. Antimicrobial stewardship: optimizing antibiotic use in an era of increasing resistance and rising costs. J Hosp Med 2011; 6 Suppl 1: S1–3. [DOI] [PubMed] [Google Scholar]
- 141. Richey EA, Dudley L, Liu SK.. Quality improvement in hospital management of community-acquired pneumonia: focus on new strategies and current challenges. Curr Infect Dis Rep 2014; 16: 395.. [DOI] [PubMed] [Google Scholar]
- 142. Rohde JM, Jacobsen D, Rosenberg DJ.. Role of the hospitalist in antimicrobial stewardship: a review of work completed and description of a multisite collaborative. Clin Ther 2013; 35: 751–7. [DOI] [PubMed] [Google Scholar]
- 143. Ruhnke M. Antifungal stewardship in invasive Candida infections. Clin Microbiol Infect 2014; 20 Suppl 6: 11–8. [DOI] [PubMed] [Google Scholar]
- 144. Septimus EJ, Owens RC Jr.. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis 2011; 53 Suppl 1: S8–14. [DOI] [PubMed] [Google Scholar]
- 145. Shlaes DM, Gerding DN, John JF Jr. et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol 1997; 18: 275–91. [DOI] [PubMed] [Google Scholar]
- 146. Trivedi KK, Rosenberg J.. The state of antimicrobial stewardship programs in California. Infect Control Hosp Epidemiol 2013; 34: 379–84. [DOI] [PubMed] [Google Scholar]
- 147. Trivedi KK, Dumartin C, Gilchrist M. et al. Identifying best practices across three countries: hospital antimicrobial stewardship in the United Kingdom, France, and the United States. Clin Infect Dis 2014; 59 Suppl 3: S170–8. [DOI] [PubMed] [Google Scholar]
- 148. Van Gastel E, Balligand E, Costers M. et al. Antibiotic management teams in Belgian hospitals: continued improvement in the period from 2007 to 2011. Eur J Clin Microbiol Infect Dis 2015; 34: 673–7. [DOI] [PubMed] [Google Scholar]
- 149. Wagner B, Filice GA, Drekonja D. et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35: 1209–28. [DOI] [PubMed] [Google Scholar]
- 150. Van Gastel E, Costers M, Peetermans WE. et al. Nationwide implementation of antibiotic management teams in Belgian hospitals: a self-reporting survey. J Antimicrob Chemother 2010; 65: 576–80. [DOI] [PubMed] [Google Scholar]
- 151. Wong SY, Allen DM.. Antimicrobial stewardship: the next big thing? Ann Acad Med Singapore 2012; 41: 1–3. [PubMed] [Google Scholar]
- 152. You J. Antimicrobial stewardship programs—cost-minimizing or cost-effective? Expert Opin Pharmacother 2015; 16: 155–7. [DOI] [PubMed] [Google Scholar]
- 153. Deege MP, Paterson DL.. Reducing the development of antibiotic resistance in critical care units. Curr Pharm Biotechnol 2011; 12: 2062–9. [DOI] [PubMed] [Google Scholar]
- 154. Emmerson AM. More about antibiotic policies. J Antimicrob Chemother 1980; 6: 6–7. [DOI] [PubMed] [Google Scholar]
- 155. Gandhi TN, DePestel DD, Collins CD. et al. Managing antimicrobial resistance in intensive care units. Crit Care Med 2010; 38: S315–23. [DOI] [PubMed] [Google Scholar]
- 156. Lawrence KL, Kollef MH.. Antimicrobial stewardship in the intensive care unit: advances. Am J Respir Crit Care Med 2009; 179: 434–8. [DOI] [PubMed] [Google Scholar]
- 157. Lautenbach E, Polk RE.. Resistant gram-negative bacilli: a neglected healthcare crisis? Am J Health Syst Pharm 2007; 64: S3–21; quiz S22-4. [DOI] [PubMed] [Google Scholar]
- 158. Shah RC, Shah P.. Antimicrobial stewardship in institutions and office practices. Indian J Pediatr 2008; 75: 815–20. [DOI] [PubMed] [Google Scholar]
- 159. Thursky K. Use of computerized decision support systems to improve antibiotic prescribing. Expert Rev Anti Infect Ther 2006; 4: 491–507. [DOI] [PubMed] [Google Scholar]
- 160. Hamilton KW, Gerber JS, Moehring R. et al. Point-of-prescription interventions to improve antimicrobial stewardship. Clin Infect Dis 2015; 60: 1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Community for Open Antimicrobial Drug Discovery. http://www.co-add.org/.
- 162. Smith RG, Joseph WS.. Antibiotic stewardship: the lower-extremity physician’s prescription for effectively treating infection. J Am Podiatr Med Assoc 2014; 104: 77–84. [DOI] [PubMed] [Google Scholar]
- 163. Abbo LM, Ariza-Heredia EJ.. Antimicrobial stewardship in immunocompromised hosts. Infect Dis Clin North Am 2014; 28: 263–79. [DOI] [PubMed] [Google Scholar]
- 164. Allerberger F, Gareis R, Jindrak V. et al. Antibiotic stewardship implementation in the EU: the way forward. Expert Rev Anti Infect Ther 2009; 7: 1175–83. [DOI] [PubMed] [Google Scholar]
- 165. Avent ML, Hall L, Davis L. et al. Antimicrobial stewardship activities: a survey of Queensland hospitals. Aust Health Review 2014; 38: 557–63. [DOI] [PubMed] [Google Scholar]
- 166. Bannan A, Buono E, McLaws ML. et al. A survey of medical staff attitudes to an antibiotic approval and stewardship programme. Intern Med J 2009; 39: 662–8. [DOI] [PubMed] [Google Scholar]
- 167. Bumpass JB, McDaneld PM, DePestel DD. et al. Outcomes and metrics for antimicrobial stewardship: survey of physicians and pharmacists. Clin Infect Dis 2014; 59 Suppl 3: S108–11. [DOI] [PubMed] [Google Scholar]
- 168. Dixon J, Duncan CJ.. Importance of antimicrobial stewardship to the English National Health Service. Infect Drug Resist 2014; 7: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Flannery DD, Swami S, Chan S. et al. Prescriber perceptions of a pediatric antimicrobial stewardship program. Clin Pediatr (Phila) 2014; 53: 747–50. [DOI] [PubMed] [Google Scholar]
- 170. Gyssens IC, Kern WV, Livermore DM. et al. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematologica 2013; 98: 1821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. James RS, McIntosh KA, Luu SB. et al. Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps. Med J Aust 2013; 199: 692–5. [DOI] [PubMed] [Google Scholar]
- 172. Johannsson B, Beekmann SE, Srinivasan A. et al. Improving antimicrobial stewardship: the evolution of programmatic strategies and barriers. Infect Control Hosp Epidemiol 2011; 32: 367–74. [DOI] [PubMed] [Google Scholar]
- 173. Ladenheim D, Rosembert D, Hallam C. et al. Antimicrobial stewardship: the role of the nurse. Nurs Stand 2013; 28: 46–9. [DOI] [PubMed] [Google Scholar]
- 174. Manning ML, Giannuzzi D.. Keeping patients safe: antibiotic resistance and the role of nurse executives in antibiotic stewardship. J Nurs Adm 2015; 45: 67–9. [DOI] [PubMed] [Google Scholar]
- 175. Njoku JC, Hermsen ED.. Antimicrobial stewardship in the intensive care unit: a focus on potential pitfalls. J Pharm Pract 2010; 23: 50–60. [DOI] [PubMed] [Google Scholar]
- 176. Pawluk S, Black E, El-Awaisi A.. Strategies for improving antibiotic use in Qatar: a survey of pharmacists’ perceptions and experiences. Int J Pharm Pract 2015; 23: 77–9. [DOI] [PubMed] [Google Scholar]
- 177. Pollack LA, Srinivasan A.. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59 Suppl 3: S97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ponto JA. ASHP statement on the pharmacist’s role in antimicrobial stewardship and infection prevention and control. Am J Health Syst Pharm 2010; 67: 575.. [DOI] [PubMed] [Google Scholar]
- 179. van Limburg M, Sinha B, Lo-Ten-Foe JR. et al. Evaluation of early implementations of antibiotic stewardship program initiatives in nine Dutch hospitals. Antimicrob Resist Infect Control 2014; 3: 33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Infectious Diseases Society of America (IDSA) and the Society of Healthcare Epidemiology of America (SHEA). http://www.idsociety.org/Antimicrobial_Agents/.
- 181. Allerberger F, Mittermayer H.. Antimicrobial stewardship. Clin Microbiol Infect 2008; 14: 197–9. [DOI] [PubMed] [Google Scholar]
- 182. Charani E, Holmes AH.. Antimicrobial stewardship programmes: the need for wider engagement. BMJ Qual Saf 2013; 22: 885–7. [DOI] [PubMed] [Google Scholar]
- 183. Cho JC, Stovall SH.. Need for pediatric antimicrobial stewardship. Am J Health Syst Pharm 2015; 72: 347.. [DOI] [PubMed] [Google Scholar]
- 184. Hurst JM, Bosso JA.. Antimicrobial stewardship in the management of community-acquired pneumonia. Curr Opin Infect Dis 2013; 26: 184–8. [DOI] [PubMed] [Google Scholar]
- 185. Le Saux N. Antimicrobial stewardship in daily practice: managing an important resource. Can J Infect Dis Med Microbiol 2014; 25: 241–5. [PMC free article] [PubMed] [Google Scholar]
- 186. Piacenti FJ, Leuthner KD.. Antimicrobial stewardship and Clostridium difficile-associated diarrhea. J Pharm Pract 2013; 26: 506–13. [DOI] [PubMed] [Google Scholar]
- 187. Trivedi KK, Kuper K.. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am 2014; 28: 281–9. [DOI] [PubMed] [Google Scholar]
- 188. Vitrat V, Hautefeuille S, Janssen C. et al. Optimizing antimicrobial therapy in critically ill patients. Infect Drug Resist 2014; 7: 261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Transatlantic Taskforce on Antimicrobial Resistance (TATFAR). https://www.cdc.gov/drugresistance/tatfar/index.html. [DOI] [PMC free article] [PubMed]
- 190. Davey P, Brown E, Charani E. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; issue 4: CD003543. [DOI] [PubMed] [Google Scholar]
- 191. Goncalves-Pereira J, Paiva JA.. Dose modulation: a new concept of antibiotic therapy in the critically ill patient? J Crit Care 2013; 28: 341–6. [DOI] [PubMed] [Google Scholar]
- 192. Hersh AL, Beekmann SE, Polgreen PM. et al. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol 2009; 30: 1211–7. [DOI] [PubMed] [Google Scholar]
- 193. Hull CM, Purdy NJ, Moody SC.. Mitigation of human-pathogenic fungi that exhibit resistance to medical agents: can clinical antifungal stewardship help? Future Microbiol 2014; 9: 307–25. [DOI] [PubMed] [Google Scholar]
- 194. James R, Upjohn L, Cotta M. et al. Measuring antimicrobial prescribing quality in Australian hospitals: development and evaluation of a national antimicrobial prescribing survey tool. J Antimicrob Chemother 2015; 70: 1912–8. [DOI] [PubMed] [Google Scholar]
- 195. Lesprit P, Brun-Buisson C.. Hospital antibiotic stewardship. Curr Opin Infect Dis 2008; 21: 344–9. [DOI] [PubMed] [Google Scholar]
- 196. Leuthner KD, Doern GV.. Antimicrobial stewardship programs. J Clin Microbiol 2013; 51: 3916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Saunderson RB, Gouliouris T, Cartwright EJ. et al. Impact of infectious diseases consultation on the management of Staphylococcus aureus bacteraemia in children. BMJ Open 2014; 4: e004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Paterson DL. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals. Clin Infect Dis 2006; 42 Suppl 2: S90–5. [DOI] [PubMed] [Google Scholar]
- 199. Tonna AP, Gould IM, Stewart D.. A cross-sectional survey of antimicrobial stewardship strategies in UK hospitals. J Clin Pharm Ther 2014; 39: 516–20. [DOI] [PubMed] [Google Scholar]
- 200. Global Antibiotic Resistance Partnership (GARP). http://www.cddep.org/garp/home.
- 201. Nebraska Medical Center. https://www.nebraskamed.com/.
- 202. Bryant PA, Australasian Stewardship of Antimicrobials in Paediatrics group. Antimicrobial stewardship resources and activities for children in tertiary hospitals in Australasia: a comprehensive survey. Med J Aust 2015; 202: 134–8. [DOI] [PubMed] [Google Scholar]
- 203. Charani E, Edwards R, Sevdalis N. et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis 2011; 53: 651–62. [DOI] [PubMed] [Google Scholar]
- 204. Bartlett JG, Gilbert DN, Spellberg B.. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis 2013; 56: 1445–50. [DOI] [PubMed] [Google Scholar]
- 205. Burgess DS, Rapp RP.. Bugs versus drugs: addressing the pharmacist’s challenge. Am J Health Syst Pharm 2008; 65: S4–15. [DOI] [PubMed] [Google Scholar]
- 206. Khadem TM, Dodds Ashley E, Wrobel MJ. et al. Antimicrobial stewardship: a matter of process or outcome? Pharmacotherapy 2012; 32: 688–706. [DOI] [PubMed] [Google Scholar]
- 207. Ohl CA, Luther VP.. Health care provider education as a tool to enhance antibiotic stewardship practices. Infect Dis Clin North Am 2014; 28: 177–93. [DOI] [PubMed] [Google Scholar]
- 208. May L, Cosgrove S, L'Archeveque M. et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med 2013; 62: 69–77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Nathwani D. Antimicrobial prescribing policy and practice in Scotland: recommendations for good antimicrobial practice in acute hospitals. J Antimicrob Chemother 2006; 57: 1189–96. [DOI] [PubMed] [Google Scholar]
- 210. McGowan JE. Antimicrobial stewardship—the state of the art in 2011: focus on outcome and methods. Infect Control Hosp Epidemiol 2012; 33: 331–7. [DOI] [PubMed] [Google Scholar]
- 211. Tamma PD, Cosgrove SE.. Antimicrobial stewardship. Infect Dis Clin North Am 2011; 25: 245–60. [DOI] [PubMed] [Google Scholar]
- 212. Bruce J, MacKenzie FM, Cookson B. et al. Antibiotic stewardship and consumption: findings from a pan-European hospital study. J Antimicrob Chemother 2009; 64: 853–60. [DOI] [PubMed] [Google Scholar]
- 213. Canton R, Bryan J.. Global antimicrobial resistance: from surveillance to stewardship. Part 1: surveillance and risk factors for resistance. Expert Rev Anti Infect Ther 2012; 10: 1269–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.