Abstract
Background
Circulating concentrations of B vitamins and factors related to one-carbon metabolism have been found to be strongly inversely associated with lung cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. The extent to which these associations are present in other study populations is unknown.
Methods
Within 20 prospective cohorts from the National Cancer Institute Cohort Consortium, a nested case-control study was designed including 5364 incident lung cancer case patients and 5364 control subjects who were individually matched to case patients by age, sex, cohort, and smoking status. Centralized biochemical analyses were performed to measure circulating concentrations of vitamin B6, folate, and methionine, as well as cotinine as an indicator of recent tobacco exposure. The association between these biomarkers and lung cancer risk was evaluated using conditional logistic regression models.
Results
Participants with higher circulating concentrations of vitamin B6 and folate had a modestly decreased risk of lung cancer risk overall, the odds ratios when comparing the top and bottom fourths (OR4vs1) being 0.88 (95% confidence interval [CI] = 0.78 to 1.00) and 0.86 (95% CI = 0.74 to 0.99), respectively. We found stronger associations among men (vitamin B6: OR4vs1 = 0.74, 95% CI = 0.62 to 0.89; folate: OR4vs1 = 0.75, 95% CI = 0.61 to 0.93) and ever smokers (vitamin B6: OR4vs1 = 0.78, 95% CI = 0.67 to 0.91; folate: OR4vs1 = 0.87, 95% CI = 0.73 to 1.03). We further noted that the association of folate was restricted to Europe/Australia and Asia, whereas no clear association was observed for the United States. Circulating concentrations of methionine were not associated with lung cancer risk overall or in important subgroups.
Conclusions
Although confounding by tobacco exposure or reverse causation cannot be ruled out, these study results are compatible with a small decrease in lung cancer risk in ever smokers who avoid low concentrations of circulating folate and vitamin B6.
The most important and effective means for lung cancer control is by reducing the number of people who smoke tobacco products. However, even among subjects who have quit smoking, the lifetime risk of lung cancer remains high (1–3), and in some regions where antitobacco campaigns have been successful, such as the United States, more than 50% of lung cancer case patients now occur among former and never smokers. Given these considerations, exploring additional means of primary prevention of lung cancer is important for subgroups at high risk—in particular former smokers—who seek additional means to further reduce their risk.
One-carbon metabolism (OCM) encompasses a series of biochemical reactions involving B vitamins that are essential to ensure balanced DNA synthesis and methylation (4,5). Changes in specific OCM factors have been implicated in cancer development, as well as other noncommunicable diseases, including neural tube defect, cardiovascular diseases, inflammation, and dementia (4,6–9). If imbalances in B vitamins such as folate (vitamin B9) and vitamin B6 are proved to be causally implicated in lung cancer etiology, they would provide an appealing target for chemoprevention as they are modifiable by changes in diet or supplementation (10,11).
Only two prospective studies have been published on lung cancer and circulating biomarkers of OCM, the EPIC study (12) and the Alpha-Tocopherol, Beta-Carotene, Prevention Cancer (ATBC) study of smoking men (13), both of which reported that subjects in the upper categories of circulating vitamin B6 concentrations had approximately half the risk of lung cancer compared with those in the lower concentration categories. In particular, the EPIC nested case-control study of 899 case patients and 1770 control subjects reported that the inverse association of vitamin B6 with lung cancer risk was strong and consistent regardless of tobacco smoking history, and also reported strong inverse associations of methionine and folate with risk (12).
In order to provide conclusive evidence on the importance of circulating B vitamins and lung cancer risk, we initiated the Lung Cancer Cohort Consortium (LC3) with a combined cohort population of greater than 2 000 000 participants from North America, Europe, Asia, and Australia to retrieve blood samples and conduct biochemical analysis on more than 5000 case–controls pairs.
Methods
Study Population
We invited all prospective cohort studies with cryopreserved baseline plasma and serum samples that in 2009 were members in the US National Cancer Institute (NCI) Cohort Consortium to participate in the study. Twenty cohorts fulfilled those criteria and agreed to participate, resulting in a combined cohort population of more than 2 000 000 participants from North America, Europe, Asia, and Australia. Written informed consent was provided by all study participants, and the research was approved by the relevant institutional review boards. Brief details on design of the cohorts and their follow-up procedures are provided in the Supplementary Methods (available online).
Selection of Case Patients and Control Subjects
Lung cancer case patients were defined on the basis of the International Classification of Diseases for Oncology, 3rd ed. (ICD-O-2), and included all invasive cancers coded as C34.0-C34-9 (14). We selected a total of 5545 lung cancer case patients, and to optimize the statistical power in smoking stratified analyses, never and former smoking case patients were oversampled. For each case patients, one control subject was matched by cohort, sex, race (US cohorts only), date of blood collection (±1 month, relaxed to ± 3 months for sets without available control subjects), and date of birth (±1 year, relaxed to ± 3 years), as well as smoking status in five categories; never smokers, short- and long-term quitters among former smokers (<10 years, 10 years since quitting), and light and heavy smokers among current smokers (<15 years, 15 cigarettes per day). After various exclusions (see the Supplementary Methods, available online), 5364 lung cancer case-control pairs remained eligible for the risk analysis.
Biochemical Analyses
Concentrations of vitamin B6, methionine, and cotinine were determined by liquid chromatography/tandem mass spectrometry (LC-MS/MS) and gas chromatography/tandem mass spectrometry (GC-MS/MS) (15,16), and microbiological methods were used to determine concentrations of folate (Lactobacillus casei) (17). Further details are provided in the Supplementary Methods (available online).
Statistical Analyses
Hierarchical linear models were used to describe the variation in average biomarker concentrations between the cohorts and the extent to which these could be explained by differences in baseline characteristics. Relative risks of lung cancer were estimated by calculating odds ratios (ORs) and 95% confidence intervals (CIs) using conditional logistic regression (conditioning on matched case-control sets) with the first quartile as the referent. Additional covariates were included to account for confounding by risk factors, including indicators of educational attainment (in six categories) and tobacco exposure (in addition to matching to smoking status by design: cotinine concentrations [quartiles defined in current smokers]). Including additional covariates of body mass index (BMI) and alcohol intake did not appreciably alter the results, and these were not included in the final models. As a sensitivity analysis, we fitted models that were additionally adjusted for smoking duration or pack-years of smoking among ever smokers. All risk analyses were conducted overall and stratified by smoking status and region (US, EU/AU, Asia).
As an indication of the overall statistical strength of association between each biomarker and risk, we calculated a Ptrend value by including the base-2 logarithm (log2) of the biomarker concentration as a continuous variable in a separate conditional logistic regression model. The same approach was used in stratified risk analyses according to other predefined demographic characteristics and risk factors. Odds ratio estimates per log2 unit (log2 OR) may be interpreted as the relative risk associated with a doubling in the concentration of a circulating biomarker.
All statistical analyses were conducted using SAS 9.2 (Cary, NC) (18), R version 3.1.3 (19), or Stan version 2.9.0 (20). A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided. Additional details on the statistical methods are provided in the Supplementary Methods (available online).
Results
Baseline Characteristics
Baseline characteristics of the study subjects separated by geographic areas are shown in Table 1. The final study population included 5364 lung cancer case patients and 5364 individually matched control subjects. Median age at cohort enrolment was 60 years, and the median time between blood draw and lung cancer diagnosis was 6.3 years. Because we oversampled never and former smokers, approximately half of the study population were current smokers at recruitment (2519 case-control pairs, 47.0%), whereas the other half were either former (1518 case-control pairs, 28.3%) or never smokers (1327 case-control pairs, 24.7%). Overall, 45.8% of the participants were women, though the sex distribution varied by region; the US cohorts contributed more women, and the cohorts from Europe and Asia contributed more men.
Table 1.
Baseline and clinical characteristics of study participants overall and per continent
| Variable | US cohorts* |
EU/AU cohorts |
Asian cohorts |
Overall |
||||
|---|---|---|---|---|---|---|---|---|
| Case patients (n = 2400) | Matched control subjects (n = 2400) | Case patients (n = 1189) | Matched control subjects (n = 1189) | Case patients (n = 1775) | Matched control subjects (n = 1775) | Case patients (n = 5364) | Matched control subjects (n = 5364) | |
| Discrete variables | ||||||||
| Sex, No. (%) | ||||||||
| Men | 991 (41.3) | 991 (41.3) | 688 (57.9) | 688 (57.9) | 1229 (69.2) | 1229 (69.2) | 2908 (54.2) | 2908 (54.2) |
| Women | 1409 (58.7) | 1409 (58.7) | 501 (42.1) | 501 (42.1) | 546 (30.8) | 546 (30.8) | 2456 (45.8) | 2456 (45.8) |
| Smoking status, No. (%) | ||||||||
| Never | 569 (23.7) | 569 (23.7) | 156 (13.1) | 156 (13.1) | 602 (33.9) | 602 (33.9) | 1327 (24.7) | 1327 (24.7) |
| Former | 1007 (42) | 1007 (42) | 335 (28.2) | 335 (28.2) | 176 (9.9) | 176 (9.9) | 1518 (28.3) | 1518 (28.3) |
| Current | 824 (34.3) | 824 (34.3) | 698 (58.7) | 698 (58.7) | 997 (56.2) | 997 (56.2) | 2519 (47.0) | 2519 (47.0) |
| Education, No. (%) | ||||||||
| Less than high school | 237 (9.9) | 215 (9.0) | 661 (55.6) | 596 (50.2) | 898 (50.6) | 883 (49.7) | 1797 (33.5) | 1696 (31.6) |
| Completed high school | 357 (14.9) | 374 (15.6) | 159 (13.4) | 180 (15.2) | 243 (13.7) | 230 (13) | 759 (14.1) | 784 (14.6) |
| Vocational school | 422 (17.6) | 435 (18.1) | 180 (15.2) | 200 (16.8) | 289 (16.3) | 279 (15.7) | 891 (16.6) | 914 (17) |
| Some college | 402 (16.8) | 393 (16.4) | 107 (9.0) | 129 (10.9) | 171 (9.6) | 196 (11) | 680 (12.7) | 718 (13.4) |
| College graduate | 357 (14.9) | 319 (13.3) | 63 (5.3) | 64 (5.4) | 104 (5.9) | 113 (6.4) | 524 (9.8) | 496 (9.2) |
| Graduate studies | 574 (23.9) | 637 (26.5) | 10 (0.8) | 8 (0.7) | 62 (3.5) | 65 (3.7) | 646 (12.0) | 710 (13.2) |
| Unknown | 51 (2.1) | 27 (1.1) | 8 (0.7) | 10 (0.8) | 8 (0.5) | 9 (0.5) | 67 (1.2) | 46 (0.9) |
| Alcohol use (any type), No. (%) | ||||||||
| Never | 250 (10.4) | 252 (10.5) | 292 (24.6) | 286 (24.1) | 946 (53.3) | 974 (54.9) | 1488 (27.7) | 1512 (28.2) |
| Ever | 2013 (83.9) | 2041 (85.0) | 643 (54.1) | 643 (54.1) | 829 (46.7) | 801 (45.1) | 3485 (65.0) | 3485 (65.0) |
| Unknown | 137 (5.7) | 107 (4.5) | 254 (21.4) | 260 (21.9) | 0 (0.0) | 0 (0.0) | 391 (7.3) | 367 (6.8) |
| Body mass index, No. (%), kg/m2 | ||||||||
| <18.5 | 30 (1.3) | 31 (1.3) | 14 (1.2) | 9 (0.8) | 157 (8.8) | 113 (6.4) | 201 (3.7) | 153 (2.9) |
| 18.5–25 | 1088 (45.3) | 1020 (42.5) | 521 (43.8) | 435 (36.6) | 1203 (67.8) | 1192 (67.2) | 2812 (52.4) | 2647 (49.3) |
| 25–30 | 841 (35.0) | 858 (35.8) | 468 (39.4) | 536 (45.1) | 369 (20.8) | 424 (23.9) | 1678 (31.3) | 1818 (33.9) |
| ≥30 | 378 (15.8) | 430 (17.9) | 185 (15.6) | 207 (17.4) | 46 (2.6) | 46 (2.6) | 609 (11.4) | 683 (12.7) |
| Unknown | 63 (2.6) | 61 (2.5) | 1 (0.1) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 64 (1.2) | 63 (1.2) |
| Continuous variables | ||||||||
| Median age at recruitment, y (5th–95th percentile) | 60 (42–74) | 60 (42–74) | 60 (45–70) | 60 (45–70) | 60 (46–72) | 60 (46–72) | 60 (44–72) | 60 (44–72) |
| Median blood concentrations for biomarkers (5th–95th percentile) | ||||||||
| Vitamin B6 (Pyridoxal 5'–phosphate), nmol/L | 47.6 (15.2–266) | 49.9 (16.4–271) | 28.9 (12.6–127) | 30.9 (13.3–102) | 29.0 (11.1–114) | 31.2 (12.4–119) | 35.2 (12.6–205) | 37.1 (13.9-197) |
| Vitamin B9 (folate), nmol/L | 32.9 (8.3–114.2) | 33.7 (8.1–114.9) | 10.2 (4.4–30.1) | 10.8 (4.9–32.9) | 13.9 (6.2–40.5) | 15.1 (6.7–40.7) | 17.0 (6.0–91.8) | 17.9 (6.3-90.3) |
| Methionine, µmol/L | 25.8 (18.4–39.6) | 26.2 (18.8–39.7) | 26.1 (19.1–37.8) | 26.3 (18.7–37.8) | 27.3 (18.0–42.4) | 27.4 (18.2–41.9) | 26.3 (18.4–40.4) | 26.6 (18.6-40.3) |
| Clinical characteristics, case participants only | ||||||||
| Median age at diagnosis (range), y | 70 (55–83) | 69 (54–79) | 69 (52–80) | 69.8 (53.6–82.0) | ||||
| Time from blood draw to diagnosis, y | 5.2 (1–15.5) | 10.0 (1.5–16.0) | 5.8 (0.7–16.5) | 6.3 (1.0–16.0) | ||||
| Hystology, No. (%) | ||||||||
| Large cell carcinoma | 112 (4.6) | 46 (4.0) | 16 (1.0) | 174 (3.3) | ||||
| Small cell carcinoma | 245 (10.4) | 150 (12.5) | 99 (5.5) | 492 (9.2) | ||||
| Squamous cell carcinoma | 291 (11.9) | 231 (19.5) | 319 (17.9) | 836 (15.5) | ||||
| Adenocarcinoma | 1034 (42.7) | 419 (34.5) | 615 (34.6) | 2056 (38.4) | ||||
| Other/unknown | 735 (31.4) | 357 (29.5) | 726 (41.0) | 1806 (33.6) | ||||
US cohorts included 83% white, 11% African American, and 6% participants indicated as “Other.”
Variation in Circulating Biomarkers
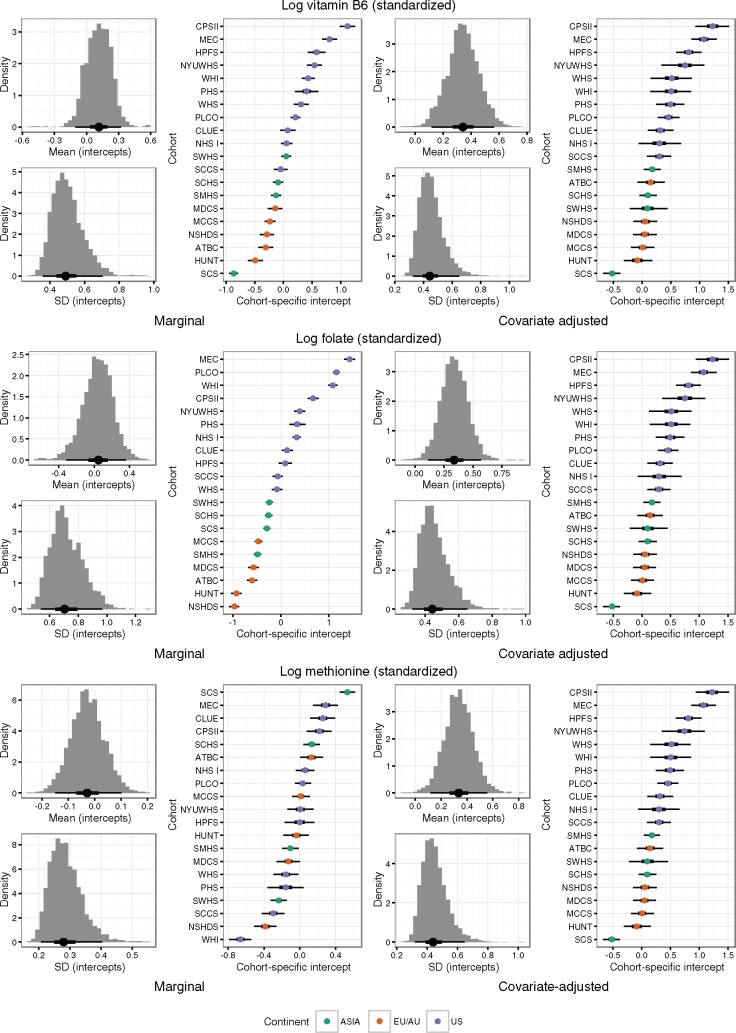
There was substantial between-cohort variation in concentrations of all biomarkers (Table 1), in particular for folate and vitamin B6 concentrations that were substantially higher in US cohorts than for EU/AU or Asian cohorts. This could only partly be explained by differences in age, sex, smoking status, or BMI (Figure 1). For circulating vitamin B6 and methionine, the between-cohort variability was similar before and after covariate adjustment (Figure 1). Conversely, for circulating folate, adjustment for age, sex, smoking status, and BMI reduced the between-cohort variability, the SD being 0.70 (90.0% CI = 0.54 to 0.97) without adjustment and 0.44 (90.0% CI = 0.32 to 0.65) with adjustment. Limited data on supplementation use suggested that a part of the differences in vitamin B6 and folate concentrations between US and non-US cohorts can be explained by multivitamin use (vitamin B6 median concentrations among US multivitamin users: 76.2 nmol/L, nonusers: 39.2 nmol/L; folate median concentrations users: 46.5 nmol/L, nonusers: 25.5 nmol/L) (Supplementary Table 1, available online).
Figure 1.
Estimated between-cohort variation in mean concentration of vitamin B6, folate, and methionine. Before (left) and after (right) adjustment for age, sex, smoking status, and body mass index. Histograms show the posterior distribution of the overall mean and standard deviation of the cohort-specific intercepts. The “caterpillar plots” show the estimated cohort-specific mean concentrations, along with 50% and 90% credible intervals (thick and thin bars, respectively). WHI = the Women's Health Initiative; SCCS = the Southern Community Cohort Study; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; NYUWHS = New York University Women's Health Study; CPS-II = the American Cancer Society Cancer Prevention Study-II Nutrition Cohort; CLUE = the Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II); MEC = the Multiethnic Cohort; WHS = the Women's Health Study; PHS = Physicians' Health Study; NHS = the Nurses' Health Study; HPFS = Health Professionnals Follow-up Study; MCCS = the Melbourne Collaborative Cohort Study; MDCS = the Malmö Diet and Cancer Study; NSHDS = the Northern Sweden Health and Disease Study Cohort; ATBC = the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; HUNT = the Nord-Trøndelag Health Study; SMHS = the Shanghai Men's Health Study; SWHS = the Shanghai Women's Health Study; SCHS = the Singapore Chinese Health Study; SCS = the Shanghai Cohort Study.
Risk Analysis Overall and Stratified by Region
Participants with higher circulating vitamin B6 and folate had approximately a 20.0% lower risk of lung cancer overall (Tables 2 and 3), the odds ratios when comparing the top and bottom quartiles (OR4vs1) being 0.81 for vitamin B6 (95% CI = 0.72 to 0.92) and 0.80 for folate (95% CI = 0.70 to 0.92). These odds ratio estimates were slightly attenuated after adjustment for circulating cotinine and education (vitamin B6: OR4vs1 = 0.88, 95% CI = 0.78 to 1.00; folate: OR4vs1 = 0.86, 95% CI = 0.74 to 0.99). We did not observe any clear association between circulating methionine and risk of lung cancer (OR4vs1 = 0.95, 95% CI = 0.85 to 1.07), apart from US men (OR4vs1 = 0.68, 95% CI = 0.52 to 0.89) (Table 4). Among participants with available information on smoking duration and pack-years of smoking (90% of ever smokers), further adjustment by continuous number of years of smoking or pack-years of smoking did not notably affect the estimates (Supplementary Table 2 and 3, available online).
Table 2.
Odds ratios of lung cancer for circulating concentrations of vitamin B6
| LC3 participants and quartile (range) | Case/control participants | OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| Case patients compared with matched control subjects unadjusted* | Case patients compared with matched control subjects adjusted for cotinine and education† | Never smokers‡ | Former smokers‡ | Current smokers† | Men† | Women† | ||
| (n = 5364/5364)* | (n = 5364/5364)† | (n = 1327/1327) | (n = 1518/1518) | (n = 2519/2519) | (n = 2908/2908) | (n = 2456/2456) | ||
| United States (n = 11 cohorts) | ||||||||
| Vitamin B6 (Pyridoxal 5'-phosphate), nmol/L§ | ||||||||
| 1 (5.08–29.89) | 629/600 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (29.90–49.90) | 618/600 | 0.98 (0.83 to 1.15) | 1.00 (0.84 to 1.18) | 1.45 (0.99 to 2.13) | 0.88 (0.68 to 1.14) | 0.95 (0.73 to 1.24) | 0.82 (0.63 to 1.06) | 1.14 (0.91 to 1.41) |
| 3 (49.91–90.05) | 574/600 | 0.90 (0.77 to 1.07) | 0.93 (0.78 to 1.10) | 1.19 (0.82 to 1.72) | 0.87 (0.67 to 1.13) | 0.89 (0.67 to 1.17) | 0.77 (0.59 to 1.00) | 1.04 (0.84 to 1.30) |
| 4 (>90.05) | 579/600 | 0.91 (0.76 to 1.07) | 0.95 (0.8 to 1.13) | 1.19 (0.82 to 1.72) | 0.90 (0.69 to 1.17) | 0.85 (0.63 to 1.15) | 0.75 (0.56 to 0.99) | 1.11 (0.89 to 1.38) |
| Ptrend‖ | .14 | .40 | .77 | .15 | .52 | .02 | .47 | |
| Europe/Australia (n = 5 cohorts) | ||||||||
| Vitamin B6 (Pyridoxal 5'-phosphate), nmol/L¶ | ||||||||
| 1 (4.37–22.41) | 378/297 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (22.42–30.90) | 269/297 | 0.70 (0.56 to 0.88) | 0.73 (0.58 to 0.92) | 1.32 (0.59 to 2.98) | 0.97 (0.59 to 1.60) | 0.62 (0.47 to 0.82) | 0.73 (0.54 to 0.99) | 0.71 (0.49 to 1.03) |
| 3 (30.91–44.95) | 258/298 | 0.65 (0.51 to 0.83) | 0.69 (0.54 to 0.88) | 1.19 (0.56 to 2.54) | 0.61 (0.37 to 0.99) | 0.71 (0.52 to 0.97) | 0.74 (0.53 to 1.02) | 0.61 (0.42 to 0.89) |
| 4 (>44.96) | 284/297 | 0.73 (0.57 to 0.92) | 0.78 (0.62 to 1.00) | 1.57 (0.77 to 3.19) | 0.73 (0.46 to 1.17) | 0.76 (0.55 to 1.04) | 0.68 (0.50 to 0.94) | 0.91 (0.64 to 1.31) |
| Ptrend‖ | .12 | .36 | .56 | .42 | .41 | .17 | .97 | |
| Asia (n = 4 cohorts) | ||||||||
| Vitamin B6 (Pyridoxal 5'-phosphate), nmol/L# | ||||||||
| 1 (4.81–20.49) | 528/443 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (20.50–30.82) | 440/444 | 0.78 (0.64 to 0.96) | 0.90 (0.73 to 1.10) | 1.33 (0.80 to 2.21) | 0.67 (0.32 to 1.41) | 0.92 (0.72 to 1.17) | 0.89 (0.71 to 1.11) | 1.29 (0.74 to 2.25) |
| 3 (31.23–46.99) | 398/444 | 0.67 (0.55 to 0.84) | 0.79 (0.63 to 0.98) | 1.59 (0.95 to 2.68) | 0.60 (0.31 to 1.17) | 0.66 (0.50 to 0.86) | 0.68 (0.52 to 0.87) | 1.45 (0.84 to 2.51) |
| 4 (>47.00) | 409/444 | 0.68 (0.55 to 0.87) | 0.82 (0.65 to 1.02) | 1.55 (0.99 to 2.72) | 0.54 (0.28 to 1.02) | 0.66 (0.48 to 0.93) | 0.75 (0.58 to 0.98) | 1.34 (0.79to 2.29) |
| Ptrend‖ | .002 | .08 | .06 | .29 | .003 | .008 | .50 | |
| All cohorts (n = 20 cohorts) | ||||||||
| Vitamin B6 (Pyridoxal 5'-phosphate), nmol/L** | ||||||||
| 1 (4.37–23.93) | 1501/1341 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (23.94–37.05) | 1319/1341 | 0.85 (0.76 to 0.95) | 0.90 (0.81 to 1.01) | 1.53 (1.15 to 2.05) | 0.73 (0.58 to 0.93) | 0.86 (0.74 to 1.00) | 0.77 (0.66 to 0.89) | 1.14 (0.96 to 1.37) |
| 3 (37.06–62.50) | 1254/1341 | 0.80 (0.72 to 0.90) | 0.87 (0.77 to 0.97) | 1.52 (1.15 to 2.00) | 0.66 (0.53 to 0.84) | 0.83 (0.70 to 0.98) | 0.77 (0.66 to 0.91) | 1.03 (0.86 to 1.23) |
| 4 (>62.51) | 1290/1341 | 0.81 (0.72 to 0.92) | 0.88 (0.78 to 1.00) | 1.51 (1.14 to 2.01) | 0.72 (0.57 to 0.91) | 0.79 (0.65 to 0.95) | 0.74 (0.62 to 0.89) | 1.10 (0.92 to 1.31) |
| Ptrend‖ | 9 × 10−4 | .06 | .12 | .05 | .005 | 2 × 10−4 | .37 | |
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set. CI = confidence interval; OR = odds ratio.
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for circulating cotinine (in quartiles) and education (in seven categories).
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for education (in seven categories).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 2400 individually matched control subjects (US cohorts; n = 11).
Ptrend assessed by the base 2 logarithm of the circulating concentrations. All statistical tests were two-sided.
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1189 individually matched control subjects (European/Australian cohorts; n = 5).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1775 individually matched control subjects (Asian cohorts; n = 4).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 5364 individually matched control subjects (pooled cohorts; n = 20).
Table 3.
Odds ratios of lung cancer for circulating concentrations of folate
| LC3 participants and quartile (range) | Case/control participants | OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| Case patients compared with matched control subjects unadjusted* | Case patients compared with matched control subjects adjusted for cotinine and education† | Never smokers‡ | Former smokers‡ | Current smokers† | Men† | Women† | ||
| (n = 5364/5364) | (n = 5364/5364) | (n = 1327/1327) | (n = 1518/1518) | (n = 2519/2519) | (n = 2908/2908) | (n = 2456/2456) | ||
| United States (n = 11 cohorts) | ||||||||
| Folate (vitamin B9), nmol/L§ | ||||||||
| 1 (1.70–17.45) | 633/600 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (17.46–33.35) | 578/600 | 0.90 (0.77 to 1.07) | 0.93 (0.78 to 1.10) | 0.83 (0.54 to 1.29) | 0.95 (0.72 to 1.24) | 0.92 (0.72 to 1.18) | 0.86 (0.66 to 1.12) | 0.98 (0.79 to 1.22) |
| 3 (33.36–59.10) | 598/600 | 0.93 (0.78 to 1.10) | 0.95 (0.80 to 1.14) | 0.87 (0.56 to 1.36) | 0.86 (0.66 to 1.13) | 1.05 (0.78 to 1.42) | 0.92 (0.69 to 1.22) | 0.98 (0.78 to 1.24) |
| 4 (>59.10) | 591/600 | 0.91 (0.75 to 1.10) | 0.95 (0.78 to 1.15) | 0.92 (0.57 to 1.47) | 0.87 (0.65 to 1.16) | 1.02 (0.72 to 1.45) | 0.78 (0.56 to 1.07) | 1.07 (0.84 to 1.37) |
| Ptrend‖ | .27 | .52 | .84 | .27 | .88 | .44 | .85 | |
| Europe /Australia (n = 5 cohorts) | ||||||||
| Folate (vitamin B9), nmol/L¶ | ||||||||
| 1 (0.24–7.78) | 348/297 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (7.79–10.77) | 291/297 | 0.82 (0.65 to 1.03) | 0.84 (0.66 to 1.06) | 1.30 (0.65 to 2.60) | 0.71 (0.44 to 1.14) | 0.81 (0.60 to 1.10) | 0.82 (0.59 to 1.12) | 0.85 (0.59 to 1.22) |
| 3 (10.78–15.98) | 282/298 | 0.78 (0.61 to 0.98) | 0.80 (0.63 to 1.02) | 0.65 (0.33 to 1.25) | 0.69 (0.44 to 1.10) | 0.90 (0.65 to 1.23) | 0.76 (0.55 to 1.04) | 0.85 (0.58 to 1.23) |
| 4 (>15.98) | 268/297 | 0.73 (0.57 to 0.93) | 0.77 (0.60 to 0.99) | 0.98 (0.49 to 1.97) | 0.58 (0.36 to 0.93) | 0.83 (0.60 to 1.16) | 0.69 (0.49 to 0.98) | 0.87 (0.60 to 1.27) |
| Ptrend‖ | .007 | .02 | .89 | .007 | .37 | .02 | .47 | |
| Asia (n = 4 cohorts) | ||||||||
| Folate (vitamin B9), nmol/L# | ||||||||
| 1 (0.17–10.59) | 525/443 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (10.60–15.00) | 461/444 | 0.84 (0.70 to 1.02) | 0.88 (0.72 to 1.06) | 0.96 (0.65 to 1.40) | 0.40 (0.21 to 0.74) | 0.96 (0.75 to 1.23) | 0.85 (0.68 to 1.06) | 1.01 (0.68 to 1.5) |
| 3 (15.01–21.00) | 366/444 | 0.67 (0.55 to 0.81) | 0.73 (0.59 to 0.89) | 0.76 (0.52 to 1.10) | 0.45 (0.23 to 0.85) | 0.80 (0.65 to 1.12) | 0.75 (0.59 to 0.95) | 0.78 (0.53 to 1.14) |
| 4 (>21.01) | 423/444 | 0.77 (0.63 to 0.94) | 0.84 (0.68 to 1.03) | 0.89 (0.61 to 1.29) | 0.65 (0.35 to 1.18) | 0.84 (0.64 to 1.11) | 0.74 (0.58 to 0.94) | 1.12 (0.76 to 1.65) |
| Ptrend‖ | .001 | .02 | .26 | .26 | .09 | .008 | .97 | |
| All cohorts (n = 20 cohorts) | ||||||||
| Folate (vitamin B9), nmol/L** | ||||||||
| 1 (0.17–10.92) | 1489/1341 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (10.93–17.89) | 1331/1341 | 0.87 (0.78 to 0.97) | 0.90 (0.81 to 1.01) | 0.87 (0.67 to 1.13) | 0.74 (0.58 to 0.95) | 0.96 (0.83 to 1.12) | 0.90 (0.77 to 1.04) | 0.92 (0.77 to 1.10) |
| 3 (17.90–34.92) | 1243/1341 | 0.79 (0.70 to 0.89) | 0.84 (0.74 to 0.95) | 0.81 (0.62 to 1.06) | 0.73 (0.57 to 0.93) | 0.88 (0.74 to 1.04) | 0.77 (0.65 to 0.91) | 0.94 (0.78 to 1.13) |
| 4 (>34.97) | 1301/1341 | 0.80 (0.70 to 0.92) | 0.86 (0.74 to 0.99) | 0.86 (0.63 to 1.17) | 0.66 (0.51 to 0.85) | 0.97 (0.77 to 1.21) | 0.75 (0.61 to 0.93) | 0.97 (0.79 to 1.19) |
| Ptrend‖ | 2 × 10−4 | .01 | .37 | .01 | .15 | .001 | .66 | |
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set. CI = confidence interval; OR = odds ratio.
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for circulating cotinine (in quartiles) and education (in seven categories).
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for education (in seven categories).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 2400 individually matched control subjects (US cohorts; n = 11).
Ptrend assessed by the base 2 logarithm of the circulating concentrations. All statistical tests were two-sided.
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1189 individually matched control subjects (European/Australian cohorts; n = 5).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1775 individually matched control subjects (Asian cohorts; n = 4).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 5364 individually matched control subjects (pooled cohorts; n = 20).
Table 4.
Odds ratios of lung cancer for circulating concentrations of methionine
| LC3 participants quartile (range) | Case/control participants | OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| Case patients compared with matched control subjects unadjusted* | Case patients compared with matched control subjects adjusted for cotinine and education† | Never smokers‡ | Former smokers‡ | Current smokers† | Men† | Women† | ||
| (n = 5364/5364) | (n = 5364/5364) | (n = 1327/1327) | (n = 1518/1518) | (n = 2519/2519) | (n = 2908/2908) | (n = 2456/2456) | ||
| United States (n = 11 cohorts) | ||||||||
| Methionine, µmol/L§ | ||||||||
| 1 (11.83–22.77) | 632/600 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (22.78–26.16) | 617/600 | 0.97 (0.82 to 1.14) | 0.97 (0.82 to 1.14) | 1.10 (0.80 to 1.51) | 0.82 (0.62 to 1.08) | 1.02 (0.78 to 1.35) | 0.90 (0.68 to 1.19) | 1.00 (0.82 to 1.23) |
| 3 (26.17–30.47) | 591/600 | 0.92 (0.78 to 1.09) | 0.93 (0.78 to 1.10) | 1.04 (0.73 to 1.48) | 0.78 (0.60 to 1.01) | 1.01 (0.75 to 1.36) | 0.88 (0.67 to 1.16) | 0.95 (0.77 to 1.19) |
| 4 (>30.47) | 560/600 | 0.87 (0.73 to 1.03) | 0.87 (0.73 to 1.04) | 1.05 (0.68 to 1.62) | 0.79 (0.60 to 1.02) | 0.84 (0.63 to 1.13) | 0.68 (0.52 to 0.89) | 1.10 (0.86 to 1.39) |
| Ptrend‖ | .11 | .12 | .93 | .08 | .36 | .008 | .65 | |
| Europe/Australia (n = 5 cohorts) | ||||||||
| Methionine, µmol/L¶ | ||||||||
| 1 (10.63–22.81) | 295/297 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (22.82–26.29) | 316/297 | 1.08 (0.86 to 1.35) | 1.07 (0.85 to 1.35) | 1.01 (0.54 to 1.90) | 0.88 (0.57 to 1.35) | 1.21 (0.88 to 1.65) | 1.08 (0.76 to 1.52) | 1.07 (0.78 to 1.48) |
| 3 (26.30–29.91) | 282/298 | 0.94 (0.74 to 1.20) | 0.91 (0.71 to 1.16) | 0.82 (0.43 to 1.57) | 1.06 (0.67 to 1.65) | 0.87 (0.62 to 1.20) | 0.94 (0.67 to 1.33) | 0.87 (0.60 to 1.24) |
| 4 (>29.91) | 296/297 | 0.99 (0.78 to 1.26) | 1.00 (0.78 to 1.27) | 0.96 (0.49 to 1.89) | 1.11 (0.72 to 1.70) | 0.95 (0.68 to 1.32) | 1.02 (0.74 to 1.42) | 0.96 (0.65 to 1.41) |
| Ptrend‖ | .92 | .92 | .86 | .74 | .83 | .92 | .99 | |
| Asia (n = 4 cohorts) | ||||||||
| Methionine, µmol/L# | ||||||||
| 1 (12.32–23.17) | 451/443 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (23.18–27.39) | 440/444 | 0.99 (0.81 to 1.20) | 1.02 (0.84 to 1.24) | 0.85 (0.63 to 1.16) | 0.70 (0.38 to 1.29) | 1.32 (0.98 to 1.77) | 1.15 (0.89 to 1.49) | 0.86 (0.62 to 1.17) |
| 3 (27.39–32.22) | 420/444 | 0.92 (0.75 to 1.12) | 0.93 (0.76 to 1.15) | 0.99 (0.71 to 1.38) | 0.73 (0.40 to 1.32) | 1.00 (0.74 to 1.34) | 0.94 (0.73 to 1.22) | 1.02 (0.71 to 1.47) |
| 4 (>32.22) | 464/444 | 1.02 (0.82 to 1.22) | 1.04 (0.85 to 1.26) | 1.11 (0.79 to 1.57) | 0.59 (0.32 to 1.10) | 1.16 (0.88 to 1.53) | 1.13 (0.88 to 1.45) | 0.86 (0.6 to 1.22) |
| Ptrend‖ | .82 | .83 | .50 | .17 | .73 | .61 | .70 | |
| All cohorts (n = 20 cohorts) | ||||||||
| Methionine, µmol/L** | ||||||||
| 1 (10.63–22.89) | 1377/1341 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 (22.90–27.40) | 1382/1341 | 1.00 (0.90 to 1.12) | 1.01 (0.91 to 1.13) | 0.99 (0.80 to 1.21) | 0.88 (0.72 to 1.09) | 1.12 (0.95 to 1.32) | 1.02 (0.87 to 1.21) | 1.00 (0.86 to 1.16) |
| 3 (27.40–31.00) | 1303/1341 | 0.93 (0.83 to 1.05) | 0.94 (0.84 to 1.05) | 0.98 (0.78 to 1.22) | 0.80 (0.65 to 0.99) | 1.01 (0.84 to 1.20) | 0.89 (0.76 to 1.05) | 1.00 (0.85 to 1.17) |
| 4 (>31.01) | 1302/1341 | 0.93 (0.83 to 1.04) | 0.95 (0.85 to 1.07) | 1.04 (0.81 to 1.33) | 0.83 (0.67 to 1.03) | 0.99 (0.83 to 1.17) | 0.92 (0.79 to 1.08) | 0.99 (0.83 to 1.18) |
| Ptrend‖ | .22 | .42 | .52 | .07 | .69 | .22 | .88 | |
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set.
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for circulating cotinine (in quartiles) and education (in seven categories).
Assessed by analyzing lung cancer case patients and their individually matched control subjects by conditional logistic regression, conditioning on individual case set and adjusting for education (in seven categories).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 2400 individually matched control subjects (US cohorts; n = 11).
Ptrend assessed by the base 2 logarithm of the circulating concentrations. All statistical tests were two-sided.
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1189 individually matched control subjects (European/Australian cohorts; n = 5).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 1775 individually matched control subjects (Asian cohorts; n = 4).
Quartile cutoff points were determined based on the blood concentration distribution of each biomarker for 5364 individually matched control subjects (pooled cohorts; n = 20).
In risk analyses stratified by region (Tables 2–4), the overall inverse associations of vitamin B6 and folate were mainly driven by Asian and EU/AU participants. The adjusted OR4vs1 for vitamin B6 was 0.82 for the Asian cohorts (95% CI = 0.65 to 1.02), 0.78 for the EU/AU cohorts (95% CI = 0.62 to 1.00), and 0.95 for the US cohorts (95% CI = 0.80 to 1.13). Similarly, for folate, the adjusted OR4vs1 was 0.84 for the Asian cohorts (95% CI = 0.68 to 1.03), 0.77 for the EU/AU cohorts (95% CI = 0.60 to 0.99), and 0.95 for the US cohorts (95% CI = 0.78 to 1.15).
Risk Analysis Stratified by Smoking Status and Sex
In analyses stratified by smoking status, we observed an inverse association between vitamin B6 and overall risk for former (OR4vs1 = 0.72, 95% CI = 0.57 to 0.91) and current smokers (OR4vs1 = 0.79, 95% CI = 0.65 to 0.95) (Table 2). Folate was also inversely associated with risk for former (OR4vs1 = 0.66, 95% CI = 0.51 to 0.85) but not for current smokers (OR4vs1 = 0.97, 95% CI = 0.77 to 1.21) (Table 3). When analyzing ever smokers overall (ie, former and current smokers combined), we observed an inverse association for circulating vitamin B6 (OR4vs1 = 0.78, 95% CI = 0.67 to 0.91) and folate (OR4vs1 = 0.87, 95% CI = 0.73 to 1.03). Of note, while no clear association was seen between folate and risk for never smokers (OR4vs1 = 0.86, 95% CI = 0.63 to 1.17), we observed a positive association between vitamin B6 and risk for never smokers, the OR4vs1 being 1.51 (95% CI = 1.14 to 2.01) (Table 2).
Further analyses stratified by sex showed that any inverse association between folate or vitamin B6 and lung cancer risk was restricted to ever smokers and men. First, the association of vitamin B6 in men overall (OR4vs1 = 0.74, 95% CI = 0.62 to 0.89) was consistently observed in cohorts from each region (Table 2), with similar observations for folate (men overall OR4vs1 = 0.75, 95% CI = 0.61 to 0.93) (Table 3). Conversely, no association was seen among women overall, nor for women separated by region (Tables 2–3). No clear differences in odds ratio estimates were seen in risk analyses conducted on cohorts providing plasma compared with cohorts providing serum (Pheterogeneity > .33, data not shown).
Stratified Risk Analysis by Demographic and Diagnostic Parameters
To assess potential of effect modification, we fitted models that included interaction terms between circulating folate or vitamin B6 and various covariates (Supplementary Figures 1, A–D, and 2, A–D, available online). Sex modified the associations between circulating concentrations of vitamin B6 and folate and lung cancer risk (Pheterogeneity ≤ .05) (Supplementary Figures 1A and 2A, available online). When assessing blood samples taken up to 10 years prior to diagnosis, we observed that the overall inverse association between vitamin B6 and lung cancer risk was driven by case patients diagnosed closer to blood collection (Pheterogeneity = .001) (Supplementary Figure 1A, available online), as well as by former (ORlog2 = 0.81, 95% CI = 0.70 to 0.94) (Supplementary Figure 1C, available online) and current smokers (ORlog2 = 0.79, 95% CI = 0.69 to 0.91) (Supplementary Figure 1D, available online). Stratified analyses by histology showed a stronger inverse association between vitamin B6 and folate and risk of lung squamous cell cancer (vitamin B6: ORlog2 = 0.86, 95% CI = 0.77 to 0.95; folate: ORlog2 = 0.88, 95% CI = 0.78 to 0.98) than for other histological types (Supplementary Figures 1A and 2A, available online). Stratified analysis by period of blood collection before and after 1996, when folate fortification of food items took effect in the United States, did not reveal any discernable difference in lung cancer risk for circulating folate (Supplementary Figure 3, available online), nor did analysis stratified on multivitamin use (data not shown). The associations between biomarker concentrations and lung cancer risk for individual cohorts are presented in Supplementary Figure 4, A–C (available online). We also estimated the potential risk increase associated with being clinically deficient in vitamin B6 and folate and observed a 23% increase in risk of lung cancer for those deficient in vitamin B6 (OR = 1.23, 95% CI = 1.09 to 1.38) that was more apparent for being diagnosed with squamous cell cancer (OR = 1.54, 95% CI = 1.18 to 2.02), and also those for developing lung cancer within the first three years following blood collection (OR = 1.83, 95% CI = 1.42 to 2.36) (Supplementary Figure 5A, available online). Similarly, for folate we observed an increased risk for those classified as deficient (OR = 1.27, 95% CI = 1.07 to 1.49) (Supplementary Figure 5B, available online). Further stratified analyses are presented in Supplementary Figure 6, A and B (available online).
Discussion
Our aim was to evaluate the associations between lung cancer risk and circulating concentrations of folate, vitamin B6, and methionine within a consortium of 20 prospective cohorts from the United States, Europe, Asia, and Australia. We found that individuals with low concentrations of vitamin B6 and folate have a small risk increase of lung cancer, particularly among men, current and former smokers, and participants living outside the United States.
To date, this is the largest cohort consortium initiative for which circulating biomarkers were assessed in relation to a single cancer outcome with coordinated biochemical analysis conducted in one centralized laboratory. The background to this study was the initial findings from the EPIC cohort involving 899 lung cancer case patients and 1770 controls subjects (12). The EPIC study reported a strong inverse association between vitamin B6 and lung cancer regardless of smoking history, and additionally found similar inverse associations with risk for folate and methionine. The results of the EPIC study were noteworthy because they suggested a potentially important role of the one-carbon metabolism pathway in lung cancer etiology, in addition to tobacco exposure. Along with additional evidence from the ATBC cohort (13), this motivated the organization of the Lung Cancer Cohort Consortium (LC3) in order to conclusively evaluate the extent to which these findings translate into other populations from Asia, the United States, Europe, and Australia.
The current study discerned relatively weak inverse associations between folate, vitamin B6, and lung cancer risk overall, associations that differed substantially between groups of different tobacco exposure history. For former and current smokers, we observed slightly lower risk among those study participants with higher concentrations of folate and vitamin B6 compared with those having lower concentrations. This inverse association for lung cancer risk with vitamin B6 or folate can also be interpreted as an increased risk among participants in the lowest category of concentrations compared with those in the remaining three higher categories (Tables 2 and 3). In contrast, methionine was inversely associated with risk in US men, whereas no associations were noted overall or in other important subgroups.
Previous studies have shown that being a current smoker is clearly associated with lower circulating concentrations of folate and vitamin B6 (12), and our analysis confirms this relation (data not shown). This highlights the importance of carefully accounting for tobacco exposure in risk analyses of B vitamins and lung cancer. We used circulating cotinine, an objective and accurate measure of current tobacco exposure (21), and the weak inverse associations of vitamin B6 and folate with lung cancer risk remained after adjusting for cotinine. Further accounting for smoking duration and intensity did not substantively alter the odds ratio estimates. In contrast to current smokers, former smokers tend to have similar circulating B vitamin concentrations as never smokers (12). This suggests that the potential confounding effect of tobacco exposure on the associations between circulating vitamin B6/folate and lung cancer risk is of less concern for former smokers than for current smokers. Despite these considerations, and given the important impact of both past and current tobacco exposure on lung cancer risk, our results do not allow ruling out confounding by tobacco smoking as a possible explanation for the observed associations.
Another potential explanation for the observed association of vitamin B6 with risk is reverse causation. In analyses stratified on smoking status, we observed a gradually stronger inverse association between vitamin B6 and risk of lung cancer in both former and current smokers when measured in blood drawn closer to diagnosis (Supplementary Figure 1, A, C, and D, available online), results that would be consistent with preclinical metabolic changes due to the underlying disease progression. In contrast, no such clear relation by time from blood draw to diagnosis was observed for folate.
In interpreting these results, we note that they are compatible with a small benefit in terms of lung cancer risk for smokers who avoid low circulating concentrations of vitamin B6 and folate. However, we cannot rule out the possibility that residual confounding by tobacco exposure and/or reverse causation due to disease progression underlie their associations with risk.
The most notable difference between our results and those from the previous EPIC study was for never smokers, where vitamin B6 was positively associated with risk among women in the current study, the opposite of what was observed in the previous EPIC study. The reason for this stark discrepancy is unclear and not explained by differences in study characteristics.
Strengths of this study included the large study sample of 5364 case-control pairs, the prospective study design and the use of prediagnostic plasma or serum, the centralized biochemical analysis with a robust quality control protocol, and the wide participation of cohorts from different geographical regions. We benefitted from oversampling never and former smokers, as well as matching by history of tobacco exposure, thus allowing for well-powered stratified analysis. However, this oversampling also meant that the individual cohorts provided different proportions of subjects by smoking status. For instance, the Women’s Health Initiative only contributed never-smoking women, and the ATBC study only contributed current-smoking men. Another particular feature of this study was that circulating vitamin B6 and folate varied substantially across cohorts and continents. Indeed, US participants had about 65% higher B6 and almost threefold the median folate concentrations of European and Asian study participants (see Table 1). A likely explanation of this is that up to 50% of US citizens age 50 years or older regularly consume dietary supplements such as multivitamin pills (22) and because of the folate fortification in the United States (23). Differences in baseline characteristics only partially accounted for these differences, and this means that we could not efficiently compare participants at extremes of the distribution of folate concentrations because the control subjects were matched to case patients within the same cohort, and we were reluctant to break that matching.
This study highlights the importance of re-evaluating promising associations of putative risk factors indicated in initial studies across multiple study populations in a coordinated fashion. The LC3 clearly demonstrates that it is feasible to gather cohorts from around the world and provide robust information on disease risk association before resorting to intervention studies that are costly and risk harming the study participants. While our results were compatible with a modest decrease in lung cancer risk among former and current smokers who avoid low circulating concentrations of vitamin B6 and folate, we could not rule out the possibility that confounding due to tobacco or reverse causation may have given rise to spurious associations. Given that any potential beneficial effect of vitamin B6 or folate—if real—is likely to be small, our findings do not support the conduct of additional prevention studies with the view of using B vitamins for primary prevention of lung cancer.
Funding
The Lung Cancer Cohort Consortium (LC3) was supported by National Institutes of Health/National Cancer Institute grant No. 1U01CA155340-01.
Notes
Authors: Anouar Fanidi*, David C. Muller*, Jian-Min Yuan*, Victoria L. Stevens*, Stephanie J. Weinstein*, Demetrius Albanes*, Ross Prentice*, Cynthia A. Thomsen, Mary Pettinger, Qiuyin Cai, William J. Blot, Jie Wu, Alan A. Arslan, Anne Zeleniuch-Jacquotte, Marjorie L. McCullough, Loic Le Marchand, Lynne R. Wilkens, Christopher A. Haiman, Xuehong Zhang, Jiali Han, Meir J. Stampfer, Stephanie A. Smith-Warner, Edward Giovannucci, Graham G. Giles, Allison M. Hodge, Gianluca Severi, Mikael Johansson, Kjell Grankvist, Arnulf Langhammer, Steinar Krokstad, Marit Næss, Renwei Wang, Yu-Tang Gao, Lesley M. Butler, Woon-Puay Koh, Xiao-Ou Shu, Yong-Bing Xiang, Honglan Li, Wei Zheng, Qing Lan, Kala Visvanathan, Judith Hoffman Bolton, Per Magne Ueland, Øivind Midttun, Arve Ulvik, Neil E. Caporaso, Mark Purdue, Regina G. Ziegler, Neal D. Freedman, Julie E. Buring, I-Min Lee, Howard D. Sesso, J. Michael Gaziano, Jonas Manjer, Ulrika Ericson, Caroline Relton, Paul Brennan†, Mattias Johansson†
*Authors contributed equally to this work.
†Authors jointly supervised this work.
Affiliations of authors: Genetic Epidemiology Group, International Agency for Research on Cancer, Lyon, France (AF, DCM, PB, MaJ); Department of Epidemiology and Biostatistics, Imperial College London, London, UK (DCM); Division of Cancer Control and Population Sciences, University of Pittsburgh Cancer Institute, Pittsburgh, PA (JMY, RW, LMB); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA (JMY, LMB); Epidemiology Research Program, American Cancer Society, Inc., Atlanta, GA (VLS, MLM); Division of Cancer Epidemiology and Genetics (SJW, DA, NEC, MP, RGZ, NDF) and Division of Cancer Epidemiology and Genetics (YBX, HL, QL), National Cancer Institute, Bethesda, MD; Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (RP, MP); Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ (CAT); Division of Epidemiology, Department of Medicine (XOS, WZ), Vanderbilt Epidemiology Center (QC, WJB, JW), Vanderbilt-Ingram Cancer Center (QC, WJB, JW), Vanderbilt University School of Medicine, Nashville, TN; International Epidemiology Institute, Rockville, MD (WJB); Departments of Obstetrics and Gynecology, Population Health and Environmental Medicine (AAA) and Population Health (AZJ), New York University School of Medicine, New York, NY; Epidemiology Program, Cancer Research Center of Hawaii, University of Hawaii, Honolulu, HI (LLM, LRW, CAH); Channing Division of Network Medicine (XZ, MJS, EG), Division of Preventive Medicine (JEB, IML, HDS, JMG), and Division of Aging (JEB, HDS, JMG), Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA; Department of Epidemiology (JH, MJS, SASW, EG, JEB, IML, HDS) and Department of Nutrition (MJS, SASW, EG), Harvard T. H. Chan School of Public Health, Boston, MA; Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Australia (GGG, AMH, GS); Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia (GGG); Molecular end Epidemiology Unit, HuGeF, Human Genetics Foundation, Torino, Italy (GS); Inserm (Institut National de la Sante et de la Recherche Medicale), Centre for Research in Epidemiology and Population Health, Villejuif, France (GS); Umeå, University, Umeå, Sweden (MiJ, KG); HUNT Research Centre, Department of Public Health and General Practice, NTNU, Norwegian University of Science and Technology, Levanger, Norway (AL, SK, MN); Department of Epidemiology, Shanghai Cancer Institute, Shanghai Jiaotong University, Shanghai, China (YTG); Duke-NUS Graduate Medical School Singapore, Singapore (WPK); Department of Epidemiology, George W Comstock Center for Public Health Research and Prevention Health Monitoring Unit, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (KV, JHB); Laboratory of Clinical Biochemistry, Department of Clinical Science, University of Bergen, Bergen, Norway (PMU); Haukeland University Hospital, Bergen, Norway (PMU); Bevital AS, Bergen, Norway (ØM, AU); Boston VA Medical Center, Boston, MA (JMG); Department of Surgery, Skåne University Hospital Malmö, Lund University, Malmö, Sweden (JM); Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden (UE); Institute of Genetic Medicine, Newcastle University, Newcastle, UK (CR); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (CR).
The funding organization had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript.
Drs Ueland and Midttun report that they are members of the steering board of the nonprofit Foundation to Promote Research Into Functional Vitamin B12 Deficiency. No other disclosures were reported.
PB and MaJ initiated, acquired the main funding for, and designed this investigation. PMU and ØM led the laboratory analysis. AF and DCM conducted the statistical analysis under the supervision of MaJ and PB. AF, MaJ, JMY, VLS, SJW, DA, DCM, RP, and PB drafted the first version of the manuscript. All authors were involved with the collection of data, data interpretation, critical revisions of the paper, and approval of the final version. PB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. PB and MaJ are the overall coordinators of the LC3 consortium, which was implemented in collaboration with the main investigators in the collaborating cohorts.
We thank the study participants and LC3 consortium members for their contributions in making this study possible.
Supplementary Material
Contributor Information
The Lung Cancer Cohort Consortium:
Anouar Fanidi, David C Muller, Jian-Min Yuan, Victoria L Stevens, Stephanie J Weinstein, Demetrius Albanes, Ross Prentice, Cynthia A Thomsen, Mary Pettinger, Qiuyin Cai, William J Blot, Jie Wu, Alan A Arslan, Anne Zeleniuch-Jacquotte, Marjorie L McCullough, Loic Le Marchand, Lynne R Wilkens, Christopher A Haiman, Xuehong Zhang, Jiali Han, Meir J Stampfer, Stephanie A Smith-Warner, Edward Giovannucci, Graham G Giles, Allison M Hodge, Gianluca Severi, Mikael Johansson, Kjell Grankvist, Arnulf Langhammer, Steinar Krokstad, Marit Næss, Renwei Wang, Yu-Tang Gao, Lesley M Butler, Woon-Puay Koh, Xiao-Ou Shu, Yong-Bing Xiang, Honglan Li, Wei Zheng, Qing Lan, Kala Visvanathan, Judith Hoffman Bolton, Per Magne Ueland, Øivind Midttun, Arve Ulvik, Neil E Caporaso, Mark Purdue, Regina G Ziegler, Neal D Freedman, Julie E Buring, I-Min Lee, Howard D Sesso, J Michael Gaziano, Jonas Manjer, Ulrika Ericson, Caroline Relton, Paul Brennan, and Mattias Johansson
References
- 1. Brennan P, Crispo A, Zaridze D, et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am J Epidemiol. 2006;164(12):1233–1241. [DOI] [PubMed] [Google Scholar]
- 2. Crispo A, Brennan P, Jockel KH, et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br J Cancer. 2004;91(7):1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebbert JO, Yang P, Vachon CM, et al. Lung cancer risk reduction after smoking cessation: Observations from a prospective cohort of women. J Clin Oncol. 2003;21(5):921–926. [DOI] [PubMed] [Google Scholar]
- 4. Kim YI. Folate and colorectal cancer: An evidence-based critical review. Mol Nutrit Food Res. 2007;51(3):267–292. [DOI] [PubMed] [Google Scholar]
- 5. Locasale JW. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer. 2013;13(8):572-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutrition. 2005;135(11):2703–2709. [DOI] [PubMed] [Google Scholar]
- 7. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: Meta-analyses of data on 50,000 individuals. Lancet. 2013;381(9871):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueland PM, McCann A, Midttun O, et al. Inflammation, vitamin B6 and related pathways. Mol Aspects Med. 2016; in press. [DOI] [PubMed] [Google Scholar]
- 9. Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development—folate review. J Nutr. 2015;145(7):1636S–1680S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galluzzi L, Vacchelli E, Michels J, et al. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene. 2013;32(42):4995–5004. [DOI] [PubMed] [Google Scholar]
- 11. Kalmbach RD, Choumenkovitch SF, Troen AM, et al. Circulating folic acid in plasma: Relation to folic acid fortification. Am J Clin Nutrit. 2008;88(3):763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303(23):2377–2785. [DOI] [PubMed] [Google Scholar]
- 13. Hartman TJ, Woodson K, Stolzenberg-Solomon R, et al. Association of the B-vitamins pyridoxal 5'-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am J Epidemiol. 2001;153(7):688–694. [DOI] [PubMed] [Google Scholar]
- 14. International Classification of Diseases for Oncology. 3rd ed., 1st revision. Geneva: WHO Press; 2013. [Google Scholar]
- 15. Midttun O, Hustad S, Ueland PM.. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(9):1371–1379. [DOI] [PubMed] [Google Scholar]
- 16. Ueland PM, Midttun O, Windelberg A, et al. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med. 2007;45(12):1737–1745. [DOI] [PubMed] [Google Scholar]
- 17. Molloy AM, Scott JM.. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 18. SAS Institute Inc. SAS 9.4 Help and Documentation. 9.4 ed.Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- 19. Stan Development Team. RStan: The R Interface to Stan Version 2.5. US: Stan Development Team; 2014. [Google Scholar]
- 20. Stan Development Team. Stan Modeling Language Users Guide and Reference Manual Version 2.5.0. US: Stan Development Team; 2014. [Google Scholar]
- 21. Boffetta P, Clark S, Shen M, et al. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1184–1188. [DOI] [PubMed] [Google Scholar]
- 22. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutrition. 2011;141(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340(19):1449–1454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.