Temporal trends in human papillomavirus vaccine coverage were assessed among 2011–2016 National Health and Nutrition Examination Surveys participants aged 9–26 years. Coverage substantially increased among males, but minimal gains were observed among females. Coverage remains below national targets.
Keywords: NHANES, human papillomavirus (HPV), sexually transmitted infections (STIs), vaccination, cancer prevention, trends
Abstract
In the United States, human papillomavirus (HPV) vaccination has been recommended for females since 2006 and males since 2011. We assessed temporal trends in HPV vaccine coverage (defined as receipt of ≥1 dose) among 9–26-year-old participants in the 2011–2016 National Health and Nutrition Examination Surveys. While coverage increased overall, from 37.7% to 45.7%, among females (adjusted prevalence difference [aPD], 7.1%; 95% confidence interval [CI], .1%–13.7%), there was no change among female adolescents aged 9–17 years. For males, coverage increased overall, from 7.8% to 27.4% (aPD, 18.8%; 95% CI, 14.1%–23.5%), and among every stratum of age, race/ethnicity, health insurance status, poverty level, and immigration status (P ≤ .05). The increase in HPV vaccine coverage observed among males is encouraging, but coverage remains below national targets for both males and females.
Human papillomavirus (HPV) vaccination is a powerful tool for cancer prevention. Three prophylactic HPV vaccines are licensed in the United States (a bivalent formulation [Cervarix], a quadrivalent formulation [Gardasil], and a nonavalent formulation [Gardasil-9]). Each vaccine confers protection against HPV types 16 (HPV-16) and HPV-18, which are responsible for 63% of HPV-associated cancers in the United States [1]. The nonavalent vaccine confers protection against additional oncogenic HPV types (ie, HPV-31, 33, 45, 52, and 58) that cause approximately 10% of HPV-associated cancers [1]. The quadrivalent and nonavalent vaccines also confer protection against HPV-6 and HPV-11, which cause genital warts. HPV vaccination is most effective when initiated before individuals reach sexual debut, because there is a lower likelihood of prior HPV exposure among recipients [2]. Young adolescents aged 9–14 years have also been shown to mount a stronger immune response to HPV vaccination, compared to older individuals aged 15–26 years [3]. As evidence of HPV vaccine immunogenicity, safety, and effectiveness has rapidly accumulated [4, 5], recommendations for early vaccination have evolved accordingly.
The Advisory Committee on Immunization Practices (ACIP) has, since 2006, recommended routine HPV vaccination for females and, since 2011, for males [6]. Routine HPV vaccination is recommended for individuals aged 11–12 years, but the vaccination schedule can be initiated as early as 9–10 years of age. Catch-up HPV vaccination is recommended for all females aged 13–26 years and all males aged 13–21 years. Additionally, men aged 22–26 years are permissively eligible for catch-up HPV vaccination, particularly if they have an immunocompromising condition or report having sex with men. Adequate HPV vaccination for all ages previously required a 3-dose schedule. However, since October 2016, immunocompetent adolescents who receive the first dose before their 15th birthday only require a 2-dose schedule to be considered adequately vaccinated.
HPV vaccine uptake in the United States has been suboptimal, especially in comparison to uptake of other recommended vaccines [6, 7]. Disparities in HPV vaccination have been noted by sex, race/ethnicity, sexual orientation, and geographic region [6–8]. Nationally, there has only been a modest indirect population-level effect of HPV vaccination (ie, via herd immunity), as assessed among young adults participating in the National Health and Nutrition Examination Survey (NHANES) [9]. Data from the National Immunization Survey (NIS)–Teen suggest that HPV vaccine coverage has recently increased among adolescent males aged 13–17 years [6]. However, it is unclear whether there have been changes in HPV vaccine uptake among other age-eligible members of the general US population, particularly in the era of gender-neutral vaccination.
In this study, we describe sex-specific trends in HPV vaccination during 2011–2016 in a national sample of females and males aged 9–26 years in the United States.
METHODS
Study Design and Population
NHANES is a complex household survey of the noninstitutionalized US civilian population. Details on study design and procedures for each 2-year NHANES cycle have been described elsewhere [2, 5, 10]. This study used data collected during the household interview in the 3 most recent NHANES cycles (2011–2016), which had overall interview response rates of 61.3%–72.6%. Participants aged 9–26 years at the time of the interview were eligible for inclusion in this analysis. Oral and written informed consent was obtained from participants aged ≥18 years or from the parent/guardian of participants aged <18 years. The NHANES study protocols were approved by the Research Ethics Board of the National Center for Health Statistics, Centers for Disease Control and Prevention. The present analysis was exempted from review by the Johns Hopkins University School of Medicine Institutional Review Board.
HPV Vaccination History
Data on HPV vaccination have been collected using a computer-assisted personal interview system since 2007 for females and since 2011 for males; participants aged 9–59 years were eligible to complete the questionnaire. Participants aged ≥16 years and emancipated minors were interviewed directly. A parent/guardian provided responses for participants aged <16 years and for participants who could not independently respond. Participants were asked if they ever received a dose of an HPV vaccine (Table S1). Participants who responded “yes” were further queried about the age at which they received their first dose and the number of doses they received. For this analysis, participants who refused to answer, did not know their HPV vaccination status, or had no recorded information regarding their HPV vaccination history were considered to have inadequate HPV vaccination data (Table S2).
Statistical Analysis
Data analysis was conducted in Stata/SE, version 14.2 (StataCorp, College Station, TX). Estimates were weighted to adjust for the unequal probability of selection, nonresponse to the household interview, and noncoverage of the non-institutionalized U.S. civilian population. Weights provided by NCHS were further adjusted to account for the use of three NHANES cycles. Taylor series linearization was used for variance estimation. The primary analysis excluded participants with inadequate HPV vaccination data.
All analyses were stratified by sex. The survey period was the exposure of interest. The primary outcome was the percentage of participants with a history of receiving ≥1 dose of any HPV vaccine. Secondary outcomes were the percentage of participants with a history of receiving ≥2 doses and 3 doses of any HPV vaccine. Temporal trends in HPV vaccine coverage across the 3 survey periods were assessed by a test for linear trend (Ptrend). The probability of HPV vaccination was also examined by multivariable logistic regression. The difference in the predicted margins of HPV vaccination in 2015–2016 as compared to 2011–2012 (the reference period) is reported as an adjusted prevalence difference (aPD). Multivariable models included adjustment for potential sociodemographic confounders determined a priori (ie, age group, race/ethnicity, health insurance, poverty status, and immigration status). Subgroup analyses were conducted after stratification by sociodemographic characteristics.
Several sensitivity analyses were performed. First, to assess the potential impact of excluding participants with inadequate HPV vaccination data, a sensitivity analysis was conducted using multiple imputation with chained equations. Second, we compared sex-specific estimates of HPV vaccination among 13–17-year-old participants in this study versus estimates from the NIS-Teen, which annually collects provider-verified data. Finally, by restricting the analysis to participants aged 9–26 years, we may have excluded older individuals who were vaccinated before the recommended upper age limit. Thus, we performed a sensitivity analysis that eliminated the age-based inclusion criterion and examined sex-specific trends in the proportion of participants aged 9–59 years who reported initiating the HPV vaccine series before their 27th birthday.
RESULTS
Study Population
There were 4033 females and 4007 males aged 9–26 years who participated in the household interview. Adequate HPV vaccination data were ascertained for 3780 females and 3588 males. Prevalence of inadequate HPV vaccination data did not vary significantly by survey period for males or females overall (Table S3). However, upon stratification, there was an increase over time in the percentage of 18–26-year-old males, non-Hispanic Asian males, and Hispanic males with inadequate HPV vaccination data (Table S4).
Temporal Trends in HPV Vaccination Among All Age-Eligible Participants
The percentage of females who received ≥1 dose increased overall, from 37.7% in 2011–2012 to 45.7% in 2015–2016 (aPD, 7.1%; P = .034; Table 1). In stratified analyses, temporal increases in the percentage of females who received ≥1 dose were observed among non-Hispanic black females, Mexican American females, and US-born females (Ptrend ≤ .05; Table 1).
Table 1.
Temporal Trends in Human Papillomavirus (HPV) Vaccine Coverage (≥1 Dose) Among Females and Males Aged 9–26 Years, 2011–2016 National Health and Nutrition Examination Surveys (NHANES)
| Characteristic, by Sex | 2011–2012 NHANES | 2013–2014 NHANES | 2015–2016 NHANES | P trend a | 2011–2012 NHANES vs 2015–2016 NHANES, aPD (95% CI)b | |||
|---|---|---|---|---|---|---|---|---|
| Participants, No. | Coverage, % (95% CI) | Participants, No. | Coverage, % (95% CI) | Participants, No. | Coverage, % (95% CI) | |||
| Female | ||||||||
| Overall | 1233 | 37.7 (33.8–41.7) | 1331 | 38.7 (33.1–44.6) | 1216 | 45.7 (41.3–50.1) | .010 | 7.1 (0.1, 13.7) |
| Race/ethnicityc | ||||||||
| Non-Hispanic white | 307 | 41.5 (34.1–49.4) | 376 | 39.8 (31.3–48.9) | 359 | 48.4 (42.1–54.7) | .180 | 5.2 (−6.2, 16.5) |
| Non-Hispanic black | 377 | 34.1 (29.5–39.0) | 325 | 39.4 (31.4–47.9) | 249 | 49.2 (44.7–53.7) | <.001 | 16.4 (9.8, 22.9) |
| Non-Hispanic Asian | 153 | 34.8 (25.6–45.3) | 136 | 33.1 (20.4–49.0) | 109 | 39.6 (28.0–52.4) | .548 | 7.2 (−7.7, 22.2) |
| Mexican American | 188 | 30.8 (22.8–40.2) | 287 | 39.8 (33.2–46.7) | 263 | 42.4 (37.1–47.8) | .034 | 9.2 (0.7, 17.6) |
| Other Hispanic | 141 | 34.5 (24.8–45.8) | 132 | 36.9 (28.6–46.1) | 164 | 42.6 (34.5–51.1) | .238 | 11.2 (−2.0, 24.4) |
| Health insurance | ||||||||
| Yes, private | 529 | 37.4 (32.0–43.2) | 582 | 39.4 (30.7–48.8) | 491 | 46.0 (40.0–52.1) | .045 | 7.8 (−0.6, 16.2) |
| Yes, Medicaid | 324 | 41.2 (30.4–52.9) | 382 | 44.1 (36.7–51.7) | 382 | 46.1 (41.0–51.3) | .426 | 3.7 (−7.8, 15.2) |
| Yes, other | 177 | 44.9 (33.6–56.8) | 152 | 33.6 (24.6–43.8) | 194 | 47.3 (38.1–56.6) | .734 | 4.4 (−8.0, 16.8) |
| No health insurance | 200 | 29.8 (20.2–41.6) | 214 | 33.0 (25.3–41.7) | 148 | 41.8 (31.1–53.3) | .149 | 12.2 (−3.6, 28.0) |
| Poverty status | ||||||||
| At or above poverty level | 718 | 38.5 (34.1–43.1) | 791 | 39.3 (32.8–46.2) | 804 | 46.6 (41.6–51.8) | .019 | 6.9 (−0.3, 14.1) |
| Below poverty level | 431 | 38.3 (33.0–44.0) | 446 | 38.5 (31.7–45.8) | 312 | 41.8 (34.5–49.5) | .481 | 6.6 (−3.4, 16.6) |
| Immigration status | ||||||||
| US born | 1046 | 38.3 (34.0–42.8) | 1176 | 39.9 (33.9–46.2) | 1059 | 46.8 (42.4–51.4) | .009 | 7.2 (0.1, 14.3) |
| Foreign born | 187 | 32.7 (22.5–44.8) | 155 | 25.7 (17.2–36.6) | 156 | 33.6 (23.9–45.0) | .959 | 8.7 (−5.2, 22.5) |
| Male | ||||||||
| Overall | 1214 | 7.8 (6.0–10.2) | 1248 | 19.7 (17.5–22.3) | 1126 | 27.4 (23.3–31.9) | <.001 | 18.8 (14.1, 23.5) |
| Race/ethnicityc | ||||||||
| Non-Hispanic white | 315 | 6.4 (4.3–9.2) | 379 | 19.7 (15.9–24.2) | 338 | 27.8 (21.7–34.9) | <.001 | 21.5 (14.4, 28.5) |
| Non-Hispanic black | 345 | 11.9 (9.1–15.2) | 313 | 20.8 (16.5–25.9) | 257 | 28.1 (23.0–33.9) | <.001 | 16.9 (9.9, 23.9) |
| Non-Hispanic Asian | 166 | 7.7 (3.4–16.7)d | 113 | 16.5 (12.0–22.2) | 124 | 26.4 (19.7–34.6) | <.001 | 19.5 (9.0, 29.9) |
| Mexican American | 205 | 7.3 (3.2–15.8)d | 252 | 20.5 (15.6–26.5) | 218 | 28.0 (23.8–32.7) | <.001 | 19.6 (11.8, 27.4) |
| Other Hispanic | 121 | 8.5 (5.0–14.0) | 119 | 24.9 (16.2–36.1) | 125 | 27.8 (19.9–37.3) | <.001 | 12.8 (3.7, 21.9) |
| Health insurance | ||||||||
| Yes, private | 516 | 7.5 (4.6–11.8) | 555 | 20.7 (17.4–24.3) | 468 | 29.8 (22.9–37.8) | <.001 | 23.9 (14.8, 33.0) |
| Yes, Medicaid | 290 | 13.1 (8.2–20.3) | 351 | 25.6 (20.0–32.1) | 321 | 30.7 (22.8–39.8) | .002 | 20.8 (10.4, 31.2) |
| Yes, other | 163 | 9.9 (4.9–19.0)d | 123 | 19.5 (11.5–30.9) | 188 | 26.7 (18.9–36.4) | .004 | 17.4 (4.8, 30.1) |
| No health insurance | 241 | 3.5 (1.6–7.8)d | 218 | 10.4 (7.2–14.6) | 146 | 13.7 (9.6–19.2) | <.001 | 11.5 (4.6, 18.4) |
| Poverty status | ||||||||
| At or above poverty level | 713 | 6.7 (4.6–9.7) | 759 | 19.7 (16.3–23.6) | 756 | 27.3 (22.7–32.4) | <.001 | 23.1 (16.3, 29.8) |
| Below poverty level | 398 | 9.2 (5.4–15.5) | 397 | 20.4 (14.8–27.6) | 268 | 23.9 (16.4–33.4) | .002 | 13.5 (5.6, 21.3) |
| Immigration status | ||||||||
| US born | 1018 | 7.9 (5.9–10.5) | 1122 | 20.7 (18.3–23.3) | 988 | 28.4 (23.9–33.3) | <.001 | 22.0 (16.0, 27.9) |
| Foreign born | 196 | 7.1 (4.3–11.5) | 126 | 8.4 (5.1–13.6) | 138 | 17.7 (11.4–26.6) | .012 | 10.1 (2.9, 17.3) |
Abbreviation: CI, confidence interval.
aBy the test for linear trend across the 3 survey periods. Values of ≤ .05 denote statistically significant differences.
bAdjusted prevalence differences (aPDs) represent the change in prevalence of HPV vaccination (≥1 dose) between the 2011–2012 period (reference) and the 2015–2016 period (ie, an absolute measure of the effect of time) for a given subgroup. Each multivariable model included the survey period, age group, race/ethnicity, health insurance, poverty status, and immigration status.
cData for the "other/multiracial" group, as categorized by NCHS, are not shown because of insufficient sample sizes. However, these participants are included in all other estimates.
dRelative standard error, 30%–40%.
The percentage of males who received ≥1 dose increased overall, from 7.8% in 2011–2012 to 27.4% in 2015–2016 (aPD, 18.8%; P < .001; Table 1). There was an increase in the percentage of males who received ≥1 dose for each stratum of race/ethnicity, health insurance, poverty status, and immigration status (Ptrend ≤ .05; Table 1).
Temporal Trends in HPV Vaccination by Age Group
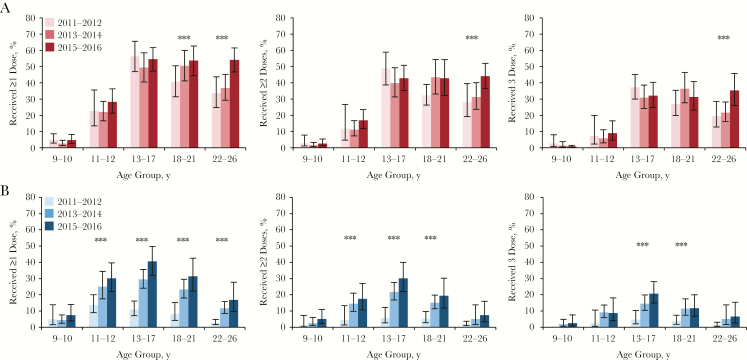
Age-specific temporal trends in HPV vaccination are shown for females in Figure 1A and for males in Figure 1B. The percentage of females who received ≥1 dose remained stable between 2011–2016 among the groups aged 9–10, 11–12, and 13–17 years (Figure 1A). Although there was a significant increasing trend in the percentage of females that received ≥1 dose among the cohorts aged 18–21 and 22–26 years (Ptrend ≤ .05; Figure 1A), the absolute increase between 2011–2012 and 2015–2016 was only statistically significant for the group aged 22–26 years in multivariable analysis (aPD, 19.4%; P = .002; Table S5). In contrast, there was a statistically significant increase between 2011–2012 and 2015–2016 in the percentage of males who received ≥1 dose among all age groups (9–10 years: aPD, 5.5% [P = .050]; 11–12 years: aPD, 16.9% [P = .005]; 13–17 years: aPD, 30.3% [P < .001]; 18–21 years: aPD, 22.1% [P < .001]; and 22–26 years: aPD, 10.7% [P = .005]; Table S5). Similar age-specific temporal trends were observed in the percentage of females and males who received ≥2 or 3 HPV vaccine doses between 2011 and 2016 (Figure 1).
Figure 1.
Age-specific temporal trends in human papillomavirus virus (HPV) vaccine coverage among females and males aged 9–26 years, National Health and Nutrition Examination Surveys, 2011–2016. A and B, Data for female (A) and male (B) participants. The percentages of females and males who reported previously receiving ≥1, ≥2, and 3 HPV vaccine doses are shown by age group and survey period. ***P ≤ .05 for linear trend across the 3 survey periods.
Sensitivity Analyses
Sex-specific estimates of HPV vaccine coverage (≥1 dose) in the primary analysis were similar to estimates calculated using imputed vaccination data (Table S6). Sex-specific estimates of HPV vaccine coverage (≥1 dose) among 13–17-year-old participants were comparable but slightly lower than estimates among 13–17-year-old participants in the NIS-Teen (Figure S1). Among participants aged 9–59 years, the proportion who reported initiating the HPV vaccine series prior to their 27th birthday increased over time for both females and males (Table S7).
Discussion
In this nationally representative study, there were substantial increases in HPV vaccine coverage between 2011–2012 and 2015–2016 among age-eligible males. This finding overall is promising. However, it should be noted that increases in HPV vaccine coverage were lowest among males without health insurance, males below the poverty level, and males born outside the United States. These populations may have less exposure to primary healthcare settings, making them more likely to miss the opportunity to be vaccinated. Although this could not be assessed directly, the observed increase in HPV vaccination among males is likely attributable to the introduction of gender-neutral recommendations for HPV vaccination in 2011. In addition to increases in the frequency of visits during which providers recommend HPV vaccination to their patients, changing attitudes about the vaccine during the study period likely also played a role [11]. While the sex disparity in HPV vaccination is closing, HPV vaccine coverage remains lower among males as compared to females. This delay in scaling HPV vaccination among males should be considered when assessing sex-based differences in age-specific patterns of vaccine-associated HPV infections.
During the study period, a modest increase in HPV vaccine coverage (≥1 dose) was observed overall among females aged 9–26 years. The prevalence of HPV vaccination was stable over time among adolescent females, even in the age group (ie, 11–12 years) for which there is a routine recommendation from the ACIP. It is concerning that a previous NHANES study also observed no change in HPV vaccination prevalence between 2007–2010 and 2011–2014 among females aged 11–12 years [2]. Possible reasons for the stagnating trends in HPV vaccination among adolescent females may include parental vaccine hesitancy, lack of strong provider recommendations, and limited exposure to healthcare providers. It was recently shown that delayed initiation of the HPV vaccination series among female participants in NIS-Teen 2013 was linked to a lack of well-child/check-up visits between ages 11 and 12 years [12].
This study has strengths and limitations. Here, we present the most up-to-date national data on HPV vaccination. To the best of our knowledge, NHANES is the only nationally representative database that has consistently collected data on HPV vaccination for all age-eligible populations. This particular study examined trends in HPV vaccination among populations for whom data are limited (ie, 9–12-year-old individuals and non-Hispanic Asians). A limitation of all NHANES studies, however, is the inability to monitor trends by geographic region. The primary limitation of the present study is that HPV vaccination data were ascertained by self-report or parent/guardian report and may be subject to reporting biases. In a previous analysis of 13–17-year-old female participants in NIS-Teen conducted during 2008–2013, parent report of HPV vaccine coverage (≥1 dose) had 78% sensitivity and 90% specificity as compared to provider-verified data [13]. Estimates for HPV vaccination among 13–17-year-old participants in this study were comparable but consistently lower than estimates from the NIS-Teen. We speculate that this could be due to potential underreporting of HPV vaccination in this study, but it may also be reflective of limitations of the NIS-Teen, such as poor household response rates (ie, 33%–35% in 2015–2016) and incomplete data linkage with providers [6]. Finally, since the interval between doses was unknown, the prevalence of “adequate” HPV vaccination, as it is currently defined by ACIP, could not be estimated.
The increases in HPV vaccine coverage documented among male adolescents and young adults in this study are indeed encouraging. However, HPV vaccine coverage among males and females in the general US population clearly remains well below national targets, including the Healthy People 2020 goal to achieve up-to-date coverage among >80% of adolescents aged 13–15 years [14]. To maximize individual-level and population-level benefits, there is a critical need to develop and implement evidence-based strategies to overcome residual barriers to early HPV vaccination. Coupled with interventions at the systems level, clinicians should desexualize the HPV vaccine and strongly advocate early HPV vaccination for cancer prevention when educating patients and their parents [15]. Continuing to monitor trends in HPV vaccination is needed to guide effective policy on vaccine implementation and to estimate the projected burden of vaccine-associated HPV infections and incidence of HPV-related cancers.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH; grants 1R01AI120938 and 1R01AI128779 to A. A. R. T. and grant K01AI125086 to M. K. G.) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrosky EY, Liu G, Hariri S, Markowitz LE. Human papillomavirus vaccination and age at first sexual activity, National Health and Nutrition Examination Survey. Clin Pediatr (Phila) 2017; 56:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. [DOI] [PubMed] [Google Scholar]
- 4. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet 2017; 390:2143–59. [DOI] [PubMed] [Google Scholar]
- 5. Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vielot NA, Butler AM, Brookhart MA, Becker-Dreps S, Smith JS. Patterns of use of human papillomavirus and other adolescent vaccines in the United States. J Adolesc Health 2017; 61:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniel-Ulloa J, Gilbert PA, Parker EA. Human papillomavirus vaccination in the United States: uneven uptake by gender, race/ethnicity, and sexual orientation. Am J Public Health 2016; 106:746–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol 2017; JCO2017750141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis 2017; 215:1070–9. [DOI] [PubMed] [Google Scholar]
- 11. Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among U.S. adolescents?J Adolesc Health 2017; 61:288–93. [DOI] [PubMed] [Google Scholar]
- 12. Beachler DC, Gonzales FA, Kobrin SC, Kreimer AR. HPV vaccination initiation after the routine-recommended ages of 11–12 in the United States. Papillomavirus Res 2016; 2:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirth J, Kuo YF, Laz TH, et al. Concordance of adolescent human papillomavirus vaccination parental report with provider report in the National Immunization Survey-Teen (2008–2013). Vaccine 2016; 34:4415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:850–8. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein HH, Bocchini JA Jr; Committee On Infectious D Practical approaches to optimize adolescent immunization. Pediatrics 2017; 139:doi: 10.1542/peds.2016-4187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.