Abstract
Background
In smokers, the lung parenchyma is characterized by inflammation and emphysema, processes that can result in local gain and loss of lung tissue. CT measures of lung density might reflect lung tissue changes; however, longitudinal data regarding the effects of CT lung tissue on FEV1 in smokers with and without COPD are scarce.
Methods
The 15th percentile of CT lung density was obtained from the scans of 3,390 smokers who completed baseline and 5-year follow-up of the Genetic Epidemiology of COPD (COPDGene) study visits. The longitudinal relationship between total lung capacity-adjusted lung density (TLC-PD15) and FEV1 was assessed by using multivariable mixed models. Separate models were performed in smokers at risk, smokers with preserved ratio and impaired spirometry (PRISm), and smokers with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system.
Results
The direction of the relationship between lung density and lung function was GOLD stage dependent. In smokers with PRISm, a 1-g/L decrease in TLC-PD15 was associated with an increase of 2.8 mL FEV1 (P = .02). In contrast, among smokers with GOLD III to IV COPD, a 1-g/L decrease in TLC-PD15 was associated with a decrease of 4.1 mL FEV1 (P = .002).
Conclusions
A decline in TLC-PD15 was associated with an increase or decrease in FEV1 depending on disease severity. The associations are GOLD stage specific, and their presence might influence the interpretation of future studies that use CT lung density as an intermediate study end point for a decline in lung function.
Trial Registry
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov.
Key Words: CT scan, lung density, smoking
Abbreviations: AATD, alpha1-antitrypsin deficiency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ILA, interstitial lung abnormalities; PD15, 15th percentile of lung density; PRISm, smokers with preserved ratio and impaired spirometry; TLCCT, total lung capacity by CT scan; TLC-PD15, 15th percentile of lung density adjusted for total lung capacity
Emphysema is defined as abnormal permanent dilation of the distal airspaces,1 and numerous investigations have shown that CT imaging is able to provide in vivo assessments of this pathologic process.2, 3 Densitometric measures of the lung parenchyma have been used in clinical, epidemiologic, and genetic investigations, and this research has repeatedly demonstrated the ability of CT densitometry to be a biomarker for disease stratification.4, 5, 6 Subjects with greater amounts of pathologically low attenuating lung tissue on CT imaging have more severe COPD.7 These cross-sectional data strongly suggest that CT scanning could also be used as an intermediate study end point in which change in lung density is related to loss of lung function. There are few published data, however, supporting that contention.
Although the relationship between changes in CT lung density and lung function has been observed in smokers with alpha1-antitrypsin deficiency (AATD),8, 9, 10 this association has not been convincingly shown in longitudinal observational studies of smokers without AATD.11, 12, 13, 14, 15 This finding may be, in part, due to the relative severity of AATD vs non-AATD parenchymal remodeling but also the complex nature of parenchymal remodeling in smokers. Inflammation might precede centrilobular airspace dilation. When observed macroscopically using CT imaging, the remodeling will effectively result in a local gain and then loss of lung density as it evolves from normal tissue to emphysema.
One way to determine the presence of such a phenomenon is to stratify a cohort according to disease severity with the assumption that those with less severe disease are at a different stage of parenchymal remodeling than those with more severe COPD. We therefore sought to determine if longitudinal CT densitometry was related to loss of lung function using data from the Genetic Epidemiology of COPD (COPDGene) study, one of the largest observational investigations in which subjects underwent volumetric CT scanning and detailed clinical assessments at baseline and a 5-year interval follow-up. A priori, we decided to undertake these analyses stratified according to COPD severity to fully leverage the breadth of smoking-related lung disease represented in the COPDGene study cohort.
Methods
Study Population
The COPDGene study has been described in detail previously16 and is expanded on in e-Appendix 1. At baseline, subjects underwent detailed characterization, including the following: volumetric inspiratory CT scans of the chest; questionnaires; and spirometric measures of lung function. COPDGene subjects were asked to return for a 5-year interval visit to repeat the characterization performed at baseline. We used the first 5,000 dataset of subjects who completed the second visit for this analysis.
Classification of Smokers
Based on their spirometric measures (see e-Appendix 1), subjects were then classified as follows: (1) smokers at risk (ie, normal spirometric data): FEV1/FVC ≥ 0.7 and FEV1 % predicted ≥ 80; (2) smokers with preserved ratio and impaired spirometry (PRISm)17: FEV1/FVC ≥ 0.7 and FEV1 % predicted < 80; and (3) smokers with COPD: FEV1/FVC < 0.7. COPD severity was further classified into Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages I through IV.18, 19 GOLD III and IV were combined because of smaller sample sizes.
Clinical Assessment
Subjects’ demographic and clinical data (including smoking history, history of congestive heart failure, and acute respiratory disease events20) were obtained with standardized questionnaires (available at www.COPDGene.org). More details on this topic are available in e-Appendix 1.
CT Assessment
Volumetric inspiratory CT scans of the chest were acquired at maximal inflation according to standardized coaching and practiced breath-holding.16 Baseline and follow-up inspiratory CT scans were used in this analysis. Details on CT protocols, visual assessment for interstitial lung abnormalities (ILA), and lung density measurements are provided in e-Appendix 1. Briefly, on baseline CT scans, ILA and interstitial lung disease were identified as previously described.21, 22 We used the lung density at 15th percentile (PD15) of the Hounsfield unit distribution adjusted for predicted total lung capacity (TLC) (measured by using CT imaging) on baseline and follow-up scans (hereafter referred to as TLC-PD15) as the main CT measure.12
Statistical Analysis
More details on statistical analysis are described in e-Appendix 1. Multivariable linear mixed models were used to assess the longitudinal relationship between the FEV1 and TLC-PD15. The primary predictor of interest, TLC-PD15, was separated into within- and between-subject components, and the former was used to derive the main estimates for the relationship of interest. Model building was performed based on previous research,23 and the covariates are discussed in e-Appendix 1. Secondary analyses were conducted by using similar modeling but with unadjusted PD15 as the densitometric measure and TLCCT as a covariate for lung volume. Models were generally stratified according to group, although some models that included all severity groups in one model were also fit by including lung density-by-severity group interaction terms to determine whether rates of change differed significantly between groups. P values < .05 were considered significant. The analysis was performed by using SAS 7 package (SAS Institute, Cary, NC).
Results
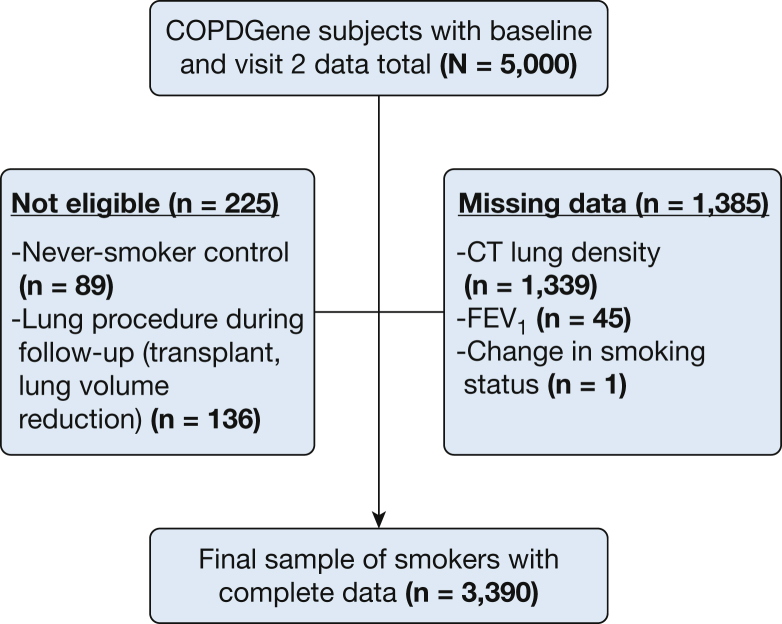
Complete longitudinal CT, clinical, and spirometric data were available on 3,390 of the 5,000 subjects (Fig 1). Participants with missing data were more likely to be African American (26% vs 33%) and have lower FEV1 (2.35 vs. 2.25 L; P = .0002), with no differences in age, pack-years, or sex and smoking status proportions (P > .05). The details of the remaining cohort are provided in Table 1.
Figure 1.
Diagram of subject selection. COPDGene, Genetic Epidemiology of COPD.
Table 1.
Subject Characteristics According to Smoking Group
| Characteristic | All Subjects (N = 3,390) | Smoking Group |
||||
|---|---|---|---|---|---|---|
| At Risk (n = 1,628) | PRISm (n = 372) | GOLD I COPD (n = 306) | GOLD II COPD (n = 656) | GOLD III-IV COPD (n = 428) | ||
| Age, y | 60 ± 9 | 58 ± 8 | 58 ± 8 | 63 ± 8 | 63 ± 8 | 63 ± 8 |
| Male | 50 | 48 | 44 | 56 | 53 | 57 |
| Non-Hispanic white race | 74 | 72 | 66 | 82 | 79 | 79 |
| BMI, kg/m2 | 29 ± 6 | 29 ± 6 | 32 ± 7 | 27 ± 5 | 29 ± 6 | 28 ± 6 |
| Pack-years of smoking | 43 ± 23 | 37 ± 20 | 41 ± 22 | 45 ± 23 | 49 ± 23 | 54 ± 26 |
| Current smoking status | 45 | 47 | 53 | 47 | 44 | 35 |
| Smoking status change | ||||||
| No change | 87 | 87 | 87 | 86 | 88 | 82 |
| Quit smoking | 11 | 10 | 10 | 10 | 10 | 17 |
| Resume smoking | 2 | 3 | 3 | 3 | 2 | 1 |
| History of CHF | 2 | 1 | 5 | 0 | 2 | 4 |
| ILA | 6 | 5 | 8 | 8 | 6 | 3 |
| >1 Acute respiratory disease episode in the prior year | 17 | 9 | 19 | 11 | 24 | 42 |
| FEV1, L | 2.4 ± 0.9 | 2.9 ± 0.7 | 2.1 ± 0.5 | 2.6 ± 0.7 | 1.9 ± 0.5 | 1.1 ± 0.3 |
| FEV1, % predicted | 80 ± 23 | 98 ± 11 | 71 ± 8 | 91 ± 9 | 66 ± 8 | 39 ± 9 |
| FVC, L | 3.4 ± 1.0 | 3.7 ± 0.9 | 2.7 ± 0.7 | 4.1 ± 1.0 | 3.3 ± 0.9 | 2.6 ± 0.8 |
| FVC, % predicted | 90 ± 17 | 97 ± 12 | 72 ± 9 | 107 ± 12 | 86 ± 13 | 70 ± 13 |
| FEV1/FVC ratio | 0.69 ± 0.1 | 0.78 ± 0.1 | 0.76 ± 0.1 | 0.65 ± 0.04 | 0.59 ± 0.1 | 0.43 ± 0.1 |
| TLCCT, L | 5.6 ± 1.4 | 5.4 ± 1.3 | 4.7 ± 1.1 | 6.2 ± 1.5 | 5.8 ± 1.4 | 6.2 ± 1.4 |
| TLCCT change, mL | –31 ± 500 | –8 ± 478 | 39 ± 519 | –84 ± 557 | –27 ± 485 | 10 ± 425 |
| CT lung density, g/L | 77.0 ± 21.8 | 84.7 ± 20.5 | 84.1 ± 17.6 | 77.4 ± 18.9 | 69.9 ± 19.9 | 53.6 ± 22.2 |
Data are mean ± SD or %. The data are for the baseline visit unless otherwise specified. CHF = congestive heart failure; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ILA = interstitial lung abnormalities; PRISm = smokers with preserved ratio and impaired spirometry; TLCCT = total lung capacity by CT scan.
Missing data, ILA, 159.
Changes in TLC-PD15
In the overall cohort, there was a small, variable change in TLC-PD15 (mean ± SD) over 5.4 years of follow-up (–0.81 ± 11.4 g/L). The inspection of the distribution of TLC-PD15 showed no major violations of normality (e-Fig 1). When examined in univariate analysis according to smoking group, smokers with GOLD III to IV COPD had the greatest decrease (–4.43 ± 9.6 g/L), whereas those at risk had a modest gain in TLC-PD15 (1.07 ± 11.42 g/L) over the same period of observation. For the other smoking groups, the changes in TLC-PD15 were as follows: PRISm, –1.37 ± 12.1 g/L; GOLD I COPD, –1.22 ± 11.66 g/L; and GOLD II COPD, –2.11 ± 11.06 g/L.
The decrease in TLC-PD15 was greater in African-American subjects than in non-Hispanic white subjects (–1.60 ± 12.1 g/L vs –0.41 ± 11.1 g/L; P = .008) and current than former smokers (–2.37 ± 11.85 g/L vs 0.66 ± 10.81 g/L; P < .0001). Those who quit smoking between the baseline and follow-up visits (n = 376) had a greater decline in TLC-PD15 than those who continued to smoke (–5.60 ± 12.1 g/L vs –1.33 ± 11.6 g/L; P < .0001). The time elapsed since quitting was not related to TLC-PD15 at visit 2 (P = .37). Former smokers who resumed smoking (n = 82) between study visits gained TLC-PD15 (5.97 ± 16.1 g/L). We observed no differences in TLC-PD15 change between sexes (P = .46). Finally, unadjusted lung density change was inversely correlated with change in TLCCT (r = –0.53; P < .0001). These correlations were in the same direction and remained significant in each smoking group (r range, –0.43 [COPD GOLD III-IV] to –0.57 [smokers at risk]).
Changes in FEV1
The change in FEV1 over the study period (5.4 years, on average) was variable, with a mean of –200 ± 274 mL (–37.1 ± 51.2 mL per year). The greatest decrease was observed in ever smokers with GOLD I COPD (–261 ± 268 mL per 5 years) and those with normal lung function (–220 ± 255 mL per 5 years). The smallest decrease in lung function was observed in those ever smokers with either PRISm (–104 ± 271 mL per 5 years) or GOLD III to IV COPD (–139 ± 248 mL per 5 years). Additional analyses are shown in e-Appendix 1.
Associations Between FEV1 and TLC-PD15 According to Smoking Group
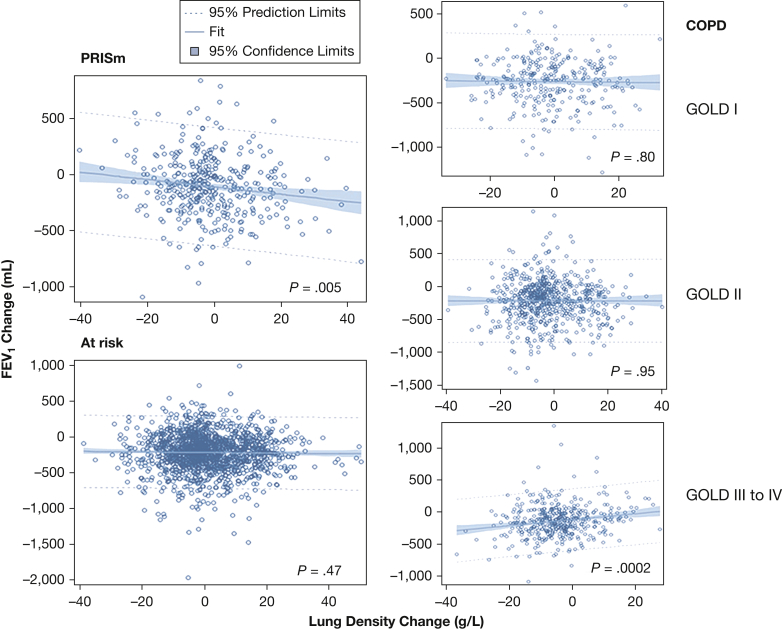
In the entire cohort with complete data, the interaction between TLC-PD15 and smoking group was significant (P < .0001) in the adjusted models (similar results were found for the interaction between unadjusted PD15 and severity group [P < .0001]). Subsequent analyses were then conducted stratified according to smoking groups. Figure 2 demonstrates the relationship of change in FEV1 and change in TLC-PD15 according to smoking group. We explored these associations further using mixed models in which there were substantial differences in both the direction and strength of the longitudinal associations between FEV1 and TLC-PD15. In smokers with PRISm, a 1-g/L decrease in TLC-PD15 was significantly associated with an increase in FEV1 of 2.8 mL. In contrast, among smokers with GOLD III to IV COPD, a 1-g/L decrease in TLC-PD15 was associated with a decrease in FEV1 of 4.1 mL. The associations between FEV1 and TLC-PD15 in smokers at risk and those with GOLD I and II COPD were not significant in fully adjusted models (e-Table 1, Table 2). When excluding subjects with ILA, congestive heart failure, and change in smoking status during follow-up, the effect of TLC-PD15 on FEV1 remained significant (Table 3). Additional results among those who had a change in lung volume < 20%, as well as when using TLCCT as a separate covariate for lung volume and the non-volume-adjusted metric PD15 (e-Table 2), are shown in e-Appendix 1.
Figure 2.
Plots of 5-year changes in FEV1 as a function of the 15th percentile of lung density adjusted for total lung capacity (TLC-PD15) changes according to smoking group. The plots were performed by using unadjusted linear regression analysis. The longitudinal association between FEV1 and within-subject TLC-PD15 remained significant in adjusted mixed models (see text and Table 2) for smokers with PRISm and smokers with GOLD III to IV COPD. GOLD, Global Initiative for Chronic Obstructive Lung Disease; PRISm, smokers with preserved ratio and impaired spirometry.
Table 2.
Effects of Within-Subject TLC-PD15 on FEV1 Over 5 Years of Follow-up According to Smoking Group in All Participants
| Smoking Group/COPD Severity Group | TLC-PD15 Effect (per 1 g/L) |
||
|---|---|---|---|
| Estimate (mL of FEV1) | SE | P Value | |
| PRISm | –2.8 | 1.2 | .02 |
| At risk | –0.3 | 0.7 | .61 |
| COPD stage | |||
| GOLD I | –0.4 | 1.4 | .78 |
| GOLD II | 1.9 | 1.2 | .11 |
| GOLD III-IV | 4.1 | 1.3 | .002 |
Estimates are from multivariable mixed models, which included the following covariates: between-subject CT 15th percentile of lung density adjusted for total lung capacity (TLC-PD15), age, age2, sex, race (non-Hispanic white, African American), height, height2, BMI, pack-years smoked, current smoking status at baseline, change in smoking status during follow-up, ILA, history of CHF, acute respiratory disease in the year prior to enrollment, scanner make, time (categorical: visits 1 and 2), time*sex, time*race, and time*acute respiratory disease in the year prior to enrollment. In addition, a random intercept term was included for scanner model (see Statistical Analysis section for more details). The full model is shown for smokers with PRISm and those with GOLD III to IV COPD in e-Appendix 1. See Table 1 legend for expansion of other abbreviations.
Table 3.
Effects of Within-Subject TLC-PD15 on FEV1 Over 5 Years of Follow-up According to Smoking Group, Excluding Those With ILA, CHF, and Change in Smoking Status
| Smoking Group/COPD Severity Group | TLC-PD15 Effect (per 1 g/L) |
||
|---|---|---|---|
| Estimate (mL of FEV1) | SE | P Value | |
| PRISm | –2.8 | 1.3 | .03 |
| At risk | –0.9 | 0.7 | .23 |
| COPD stage | |||
| GOLD I | 0.5 | 1.7 | .77 |
| GOLD II | 2.4 | 1.3 | .06 |
| GOLD III-IV | 3.8 | 1.4 | .01 |
Estimates are from multivariable mixed models excluding subjects with change in smoking status during follow-up, ILA, and history of CHF, which included the following covariates: between-subject CT TLC-PD15, age, age2, sex, race (non-Hispanic white, African American), height, height2, BMI, pack-years smoked, current smoking status at baseline, acute respiratory disease in the year prior to enrollment, scanner make, time (categorical: visits 1 and 2), time*sex, time*race, and time*acute respiratory disease in the year prior to enrollment. In addition, a random intercept term was included for scanner model (see Statistical Analysis section for more details). See Table 1 and 2 legends for expansion of abbreviations.
Discussion
We analyzed the clinical and imaging data from > 3,300 subjects in the COPDGene study, one of the largest cohorts of smokers with a full range of disease. The data suggest two important features that must be considered when using CT imaging as an intermediate study end point for smokers. The first is that the progression of parenchymal disease on CT scan is not consistent across GOLD stages and in fact may be characterized by an increase or decrease in low attenuating tissue over a limited period of observation. Those smokers at risk for the development of COPD tended to have an increase in the radiographic estimates of lung tissue per unit volume (grams per liter) over a 5-year period of observation, whereas those smokers with GOLD III to IV COPD exhibited the more “prototypic” loss in lung tissue over the same time period. We are unaware of this bidirectional phenomenon being reported previously. The second feature is that the association between TLC-PD15 and lung function is dependent on GOLD stage. A decrease in FEV1 was associated with an increase in TLC-PD15 in the PRISm group, whereas a decrease in FEV1 was related to a loss of TLC-PD15 in GOLD III to IV ever smokers. These data corroborate and expand on previous investigations of a similar nature.9, 10, 12, 13
We begin by focusing on the paradoxical observation that lung density may increase or decrease on serial CT scanning. Although the biologic basis for this observation is not revealed in our current analyses, previous histopathologic investigations may suggest a potential etiology. The precursor to centrilobular emphysema is a gain of tissue due to inflammation and remodeling of the respiratory bronchiole and surrounding alveolar structures.24 In addition, smokers develop pathologic and radiologic evidence of fibrosis that increases lung density.21, 25 Normal lung tissue becomes inflamed, and the irregular deposition of the matrix precedes airway dropout and airspace dilation.26 The aggregate of these data suggest that airspace remodeling in AATD emphysema is not characterized by a simple monotonic loss of tissue. Subjects may appear to have gained lung tissue (when inflammation and remodeling in the respiratory acinus leads to excess tissue or to a certain degree of microscopic fibrosis25), have no net change in the amount of tissue (when gains due to inflammation and remodeling are offset by a loss of healthy parenchyma), or exhibit a loss of lung tissue (when airspace and airway destruction exceeds the inflammation/remodeling process) depending on the sampling interval. In addition to these changes in lung tissue, the lungs become hyperinflated, which further decreases lung density.27 It is therefore possible that in smokers at risk, the 5-year interval between the COPDGene visits spans a period in which parenchymal inflammation and remodeling predominate and there is an increase in tissue grams per liter on CT imaging. In those with more advanced COPD, this same 5-year window may span an interval characterized by tissue loss in excess of inflammation/destruction.
This gain or loss of lung tissue in grams per liter on CT imaging affected subsequent analyses focused on the relationship between change of CT features and change in FEV1. These results were contrary to expectations that a decrease in lung tissue density on CT scan should be uniformly associated with a loss of lung function. Exclusive of our findings in PRISm (in which gain in lung density was associated with a decrease in FEV1), our longitudinal data are most consistent with those reported by Shaker et al,15 who found a greater decline in lung density in a small group of GOLD III subjects than smokers without airflow obstruction. Other investigations such as those reported by Coxson et al12 and Mohamed Hoesein et al13 reported no association between GOLD III and change in CT emphysema. Explanations for the discrepant results between observational studies likely include differences in sample size and the duration of follow-up.
The aggregate COPDGene data are consistent with other studies, which report that the densitometric evolution of parenchymal injury in smokers is a subtle process.12, 15 In our investigation, subjects only exhibited a 1- to 4.4-g/L change in TLC-PD15 over a 5-year period of observation even when stratified according to GOLD stage. In contrast, studies of subjects with AATD report more dramatic radiologic changes over a much smaller time interval.9, 10 For example, the Randomized, placebo-controlled trial of augmentation therapy in Alpha-1 Proteinase Inhibitor Deficiency (RAPID-RCT) showed a clear decline in lung attenuation in several hundred subjects over a 1-year period of observation (placebo group, –2.12 g/L).9 The observed differences between AATD and non-AATD-related COPD are likely due to the greater severity of the former condition and that the panlobular form of emphysema more commonly found in patients with AATD evolves in a faster and more linear manner than centrilobular emphysema typically found in non-AATD smokers.
There are limitations to our data, including the lack of a histopathologic corroboration of the lung density findings on CT imaging. Although this corroboration would be ideal for such an investigation, it is impractical to do so in large cohorts, and it is not feasible to serially sample the same region of lung for histologic examination. Although the COPDGene study includes smokers at risk, PRISm, and the full-range of COPD stages, care should be taken when extrapolating these findings to non-heavy smokers because those subjects were not included in the present study. Although 29% of the subjects had missing data, and a potential selection bias for this analysis cannot be completely excluded, the large sample size included the full range of COPD severity and those with no disease, making this bias less likely. Although the subjects were followed up for 5 years, the imaging and lung function assessments were only performed at two time points, which prevents us from constructing true radiologic trajectories to understand disease progression. Airspace dilation may be a monotonic process in smokers without AATD, but CT scanning lacks the ability to disambiguate the alveolar walls from the cellular infiltrate. This method is unlike histopathologic measures such as the mean linear intercept, which “sees through” inflammation by ignoring alveolar cellular infiltrate when performing a grid counting assessment of the alveolar structure. As such, a therapy may seem promising in the early stages of development but then fail in human studies because it does not meet an expected CT end point.
Our findings do not preclude the utility of longitudinal CT scanning as a biomarker for intermediate study end point but merely suggests that efforts to identify disease progression may be further enhanced by using a multidimensional metric of disease severity and its change over time. The observed relationship between FEV1 and TLC-PD15 will have implications for therapeutic studies using longitudinal CT scanning. The natural trajectory of CT scan-based assessments of parenchymal disease in smokers may involve a gain or loss of lung density. Part of this process may be predicted by baseline measures of disease severity. This factor will be important to consider in future studies because a loss of lung density may signify therapeutic benefit in some and be deleterious in others. Finally, there is significant interest in the development of therapies for early-stage disease, and although such interventions may have the greatest impact in less advanced COPD, there are clear challenges to serial densitometry in this cohort.
Acknowledgments
Author contributions: A. A. D., M. S., and G. R. W. served as guarantors and take full responsibility for the content of the manuscript, including the data analysis. A. A. D., M. S., H. O. C., J. C. R., R. S. J. E., D. L., E. M. V. R., I. O. R., G. M. H., R. K. P., H. H., A. Y., G. L. K., J. E. H., E. K. S., J. C., and G. R. W. were responsible for the conception and design of this study and creation, revision, and final approval of the manuscript. A. A. D., M. S., E. K. S., and G. R. W. performed the analysis and interpretation. A. A. D., D. L., E. M. V. R., I. O. R., G. M. H., R. K. P., H. H., E. K. S., and J. C. were responsible for data acquisition. A. A. D., H. O. C., D. L., E. K. S., and G. R. W. drafted the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. A. D. has received speaker fees from Novartis Inc unrelated to this work. E. M. V. R. is cofounder, shareholder, and managing director of Thirona, a company providing analysis of medical images. G. M. H. has served as a consultant for the Gerson-Lehrman Group and has served on scientific advisory boards for Genentech and Boehringer Ingelheim. H. H. receives research grants from Toshiba Medical System, Inc, Konica-Minolta Inc, and Canon USA, Inc. A. Y. provided a one-time-fee-for-service consultation to Dynavax. E. K. S. received honoraria from Novartis and grant and travel support from GlaxoSmithKline. G. R. W. receives grant support from the National Heart, Lung, and Blood Institute, Boehringer Ingelheim, BTG Interventional Medicine, and Janssen Pharmaceuticals; and has served on consultancies/advisory boards with Boehringer Ingelheim, Genentech, PulmonX, Regeneron, ModoSpira, Janssen Pharmaceuticals, Toshiba, and GlaxoSmithKline. He is also a founder and co-owner of Quantitative Imaging Solutions, a company that provides image-based consulting and develops software to enable data sharing. None declared (M. S., H. O. C., J. C. R., R. S. J. E., D. L., I. O. R., R. K. P., G. L. K., J. E. H., J. C.).
Role of sponsors: The sponsors had no role in the design, conduct, and data analysis of this study, and in creation of this manuscript.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Diaz and Strand contributed equally to this work.
FUNDING/SUPPORT: This work was supported by National Institutes of Health grants: The Genetic Epidemiology of COPD (COPDGene) study [Grants R01HL089897 and R01HL089856]; Dr Diaz [Grant K01HL118714] and the Brigham and Women’s Hospital Minority Faculty Career Development Award; Dr San José Estépar [Grant R01 HL116473]; and Dr Washko [Grants R01 HL116473 and R01 HL107246].
Supplementary Data
References
- 1.Hogg J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 2.Gevenois P.A., De Vuyst P., de Maertelaer V. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 3.Coxson H.O., Leipsic J., Parraga G., Sin D.D. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med. 2014;190(2):135–144. doi: 10.1164/rccm.201402-0256PP. [DOI] [PubMed] [Google Scholar]
- 4.Castaldi P.J., Cho M.H., San Jose Estepar R. Genome-wide association identifies regulatory loci associated with distinct local histogram emphysema patterns. Am J Respir Crit Care Med. 2015;190(4):399–409. doi: 10.1164/rccm.201403-0569OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaldi P.J., San Jose Estepar R., Mendoza C.S. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188(9):1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley R.A., Barker B.L., Newby C. Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: a single-center study. J Allergy Clin Immunol. 2016;137(5):1413–1422.e12. doi: 10.1016/j.jaci.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano Y., Muro S., Sakai H. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 8.Dirksen A., Piitulainen E., Parr D.G. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]
- 9.Chapman K.R., Burdon J.G., Piitulainen E. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 10.Stolk J., Stockley R.A., Piitulainen E., Stoel B.C. Relationship between change in lung density and long-term progression of lung function. Am J Respir Crit Care Med. 2015;192(1):114–116. doi: 10.1164/rccm.201502-0370LE. [DOI] [PubMed] [Google Scholar]
- 11.Stolk J., Putter H., Bakker E.M. Progression parameters for emphysema: a clinical investigation. Respir Med. 2007;101(9):1924–1930. doi: 10.1016/j.rmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Coxson H.O., Dirksen A., Edwards L.D. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed Hoesein F.A., Zanen P., de Jong P.A. Rate of progression of CT-quantified emphysema in male current and ex-smokers: a follow-up study. Respir Res. 2013;14:55. doi: 10.1186/1465-9921-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura M., Makita H., Nagai K. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 15.Shaker S.B., Dirksen A., Lo P., Skovgaard L.T., de Bruijne M., Pedersen J.H. Factors influencing the decline in lung density in a Danish lung cancer screening cohort. Eur Respir J. 2012;40(5):1142–1148. doi: 10.1183/09031936.00207911. [DOI] [PubMed] [Google Scholar]
- 16.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan E.S., Castaldi P.J., Cho M.H. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabe K.F., Hurd S., Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.Wan E.S., Hokanson J.E., Murphy J.R. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184(1):57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowler R.P., Kim V., Regan E. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146(4):941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washko G.R., Hunninghake G.M., Fernandez I.E. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washko G.R., Lynch D.A., Matsuoka S. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17(1):48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestbo J., Edwards L.D., Scanlon P.D. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 24.Leopold J.G., Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12(3):219–235. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auerbach O., Garfinkel L., Hammond E.C. Relation of smoking and age to findings in lung parenchyma: a microscopic study. Chest. 1974;65(1):29–35. doi: 10.1378/chest.65.1.29. [DOI] [PubMed] [Google Scholar]
- 26.McDonough J.E., Yuan R., Suzuki M. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madani A., Van Muylem A., Gevenois P.A. Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology. 2010;257(1):260–268. doi: 10.1148/radiol.10091446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.