Abstract
The worldwide prevalence of obesity has increased rapidly in the last 3 decades, and this increase has led to important changes in the pathogenesis and clinical presentation of many common diseases. This review article examines the relationship between obesity and lung disease, highlighting some of the major findings that have advanced our understanding of the mechanisms contributing to this relationship. Changes in pulmonary function related to fat mass are important, but obesity is much more than simply a state of mass loading, and BMI is only a very indirect measure of metabolic health. The obese state is associated with changes in the gut microbiome, cellular metabolism, lipid handling, immune function, insulin resistance, and circulating factors produced by adipose tissue. Together, these factors can fundamentally alter the pathogenesis and pathophysiology of lung health and disease.
Key Words: inflammation, lung function, metabolism, obesity
Abbreviations: SCFA, short-chain fatty acid; TNF-α, tumor necrosis factor-alpha; Treg, regulatory T cell
The world is facing an unprecedented obesity epidemic, with the highest prevalence occurring in developed countries. More than one-third of the US adult population is obese and another one-third is overweight,1 but most troubling is that 32% of US children and adolescents are either overweight or obese.2 Obesity is a major risk factor for the development of a number of respiratory diseases, including asthma, pulmonary hypertension, sleep apnea, obesity hypoventilation syndrome, pneumonia, and ARDS,3 and it complicates the pathogenesis of other diseases such as COPD. These outcomes occur because obesity profoundly alters normal lung homeostasis through a combination of mass loading and hormonal, metabolic, inflammatory, neurologic, and dietary factors.
The present review highlights some of the major findings that have advanced our understanding of mechanisms linking obesity, as well as its associated metabolic disorders, to common lung diseases.
The Mechanical Effects of Obesity on Lung Function
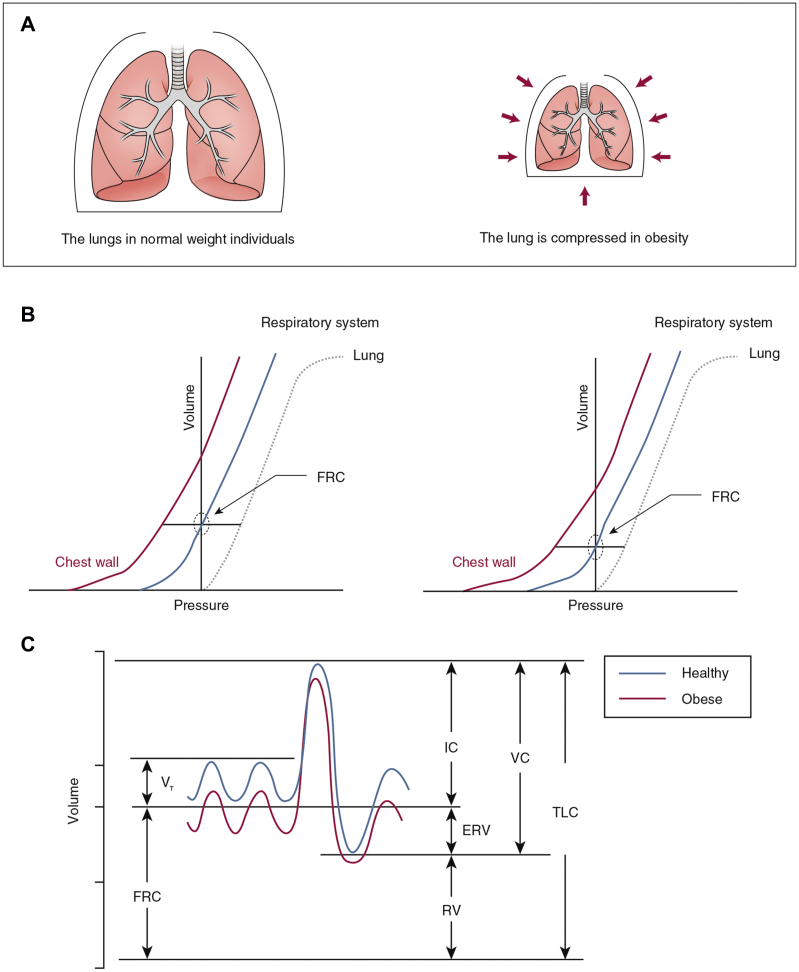
Obesity directly alters the mechanical properties of the lungs and chest wall through the accumulation of fat in the mediastinum and in the abdominal and thoracic cavities.4 This action elevates the diaphragm and also limits its downward excursion, causing pleural pressure to increase5 and functional residual capacity to decrease (Fig 1).6 These effects are substantial: functional residual capacity is reduced by 10%, 22%, and 33% in overweight, mildly obese, and severely obese subjects without asthma, respectively. The mechanical effects of obesity are also reflected in significant decrements in the compliance of the lungs, chest wall, and entire respiratory system,7 which likely contribute to the respiratory symptoms experienced by many obese people.
Figure 1.
A-C, Mechanical effects of lung compression in obese individuals compared with normal-weight individuals. The volume of the chest compartment is invariably decreased in obesity (A), which lowers the operating volume of the lungs (B). Consequently, the FRC and the ERV are reduced, but all other subdivisions of lung volume are remarkably well preserved (C). ERV = expiratory reserve volume; FRC = functional residual capacity; IC = inspiratory capacity; TLC = total lung capacity; VC = vital capacity; VT = tidal volume.
Long-term obesity may also affect lung growth. Forno et al8 showed that obese children had evidence of dysanapsis (a dissociation of lung airway growth with lung size) that may be contributing to lung disease in obese children. Nevertheless, not all obese people develop lung disease. The reasons for this are not fully understood but presumably reflect the fact that obesity, rather than simply being a state of excess mass, is a complex syndrome associated with changes in diet, gut microbiome, systemic and cellular metabolism, and immune function.
Role of Adipose Tissue Mediators
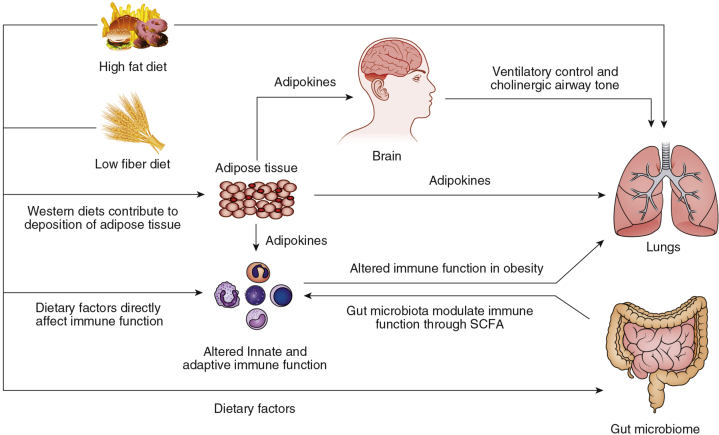
Obesity manifests as increased quantity and change in function of adipose tissue. Adipose tissue in obese individuals produces proinflammatory cytokines and hormones that can have direct effects on the lung (Fig 2). These mediators are released from both adipocytes and from the leukocytes that infiltrate adipose tissue. The inflammatory factors that are increased in obese adipose tissue and then enter the circulation include leptin, tumor necrosis factor-alpha (TNF-α),9 IL-6 and IL-8,10 C-reactive protein, and monocyte chemoattractant protein-1.11 The numbers of inflammatory cells in adipose tissue are also increased in obesity: CD8 and CD4 T-lymphocyte numbers are significantly increased in the adipose tissue of obese humans and mice,12, 13 and CD68 macrophages account for up to 50% of cells in the subcutaneous adipose tissue of obese individuals.12 This proinflammatory milieu affects lung disease. For example, increased infiltration of subcutaneous and visceral adipose tissue by macrophages has been reported in obese individuals with asthma14 and COPD,15 suggesting that adipose tissue inflammation may play an important role in the lung function impairment seen in these patients, possibly through the production of circulating adipokines and cytokines.
Figure 2.
Etiology of lung disease in obesity. High-fat and low-fiber diets are important contributors to increased adiposity. Increased adipose tissue mass can lead to lung function impairment through the release of adipokines that modulate immune response, ventilation, and airway smooth muscle tone. In addition, obesogenic diets have been associated with fundamental changes in the gut microbiome, which could also alter pulmonary host immune response. SCFA = short-chain fatty acid.
Leptin, originally identified as a satiety hormone, is increased in obesity and thus has become a focus of interest relative to obesity-related lung disease. Understanding the pathogenic role of leptin is complicated by the fact that obesity represents a state of leptin resistance; this scenario raises the question as to whether it is leptin per se, or alterations in its downstream targets, that is the primary abnormality. Nevertheless, leptin is involved in normal physiologic function of the lung, promoting neonatal lung development and surfactant protein A production.16 Leptin also affects ventilatory drive17 and cholinergic airway tone.18 We found that leptin expression in visceral fat is closely correlated with airway reactivity in obese subjects with asthma,14 and Shore et al19 have found that administration of leptin increases airway reactivity in a lean allergic mouse model of asthma. Elevated leptin levels and/or leptin resistance might thus affect lung disease through a number of pathways.
Levels of the antiinflammatory adipokine adiponectin are decreased in obesity and have also been studied in the context of respiratory disease. Adiponectin downregulates eosinophil recruitment in the airways to inhibit allergic airway inflammation20, 21 and is markedly reduced in obese patients with asthma14 and COPD.22 Conversely, mice deficient in adiponectin develop airway eosinophilia and airway inflammation coupled with increased vascular remodeling and pulmonary hypertension,20 as well as an emphysema phenotype with increased alveolar macrophage TNF-α expression.23 A role for obesity-associated adiponectin deficiency has also been suggested in the pathogenesis of ARDS, a condition for which the obese are at increased risk,24 likely from pulmonary vascular priming due to impaired endothelial function.25 These data suggest a role for adiponectin in normal lung function; they also suggest that adiponectin deficiency may contribute to diseases of the pulmonary airways, parenchyma, and vasculature.
Circulating levels of certain key inflammatory cytokines produced by adipose tissues are increased in obesity. One example is TNF-α, which may have important implications for lung disease in obesity as we have shown that constitutive overexpression of TNF-α, in normal-weight mice, leads to the development of a combination of both emphysema and pulmonary fibrosis.26 Furthermore, Williams et al27 found that TNF-α is involved in airway responses to ozone in obese mice by augmenting airway neutrophilic inflammation while blunting airway reactivity. Another example of an inflammatory cytokine produced by adipose tissue is IL-6, which epidemiologic studies suggest is associated with reduced lung function and worse outcomes in COPD.28 Furthermore, Peters et al29 recently showed that systemic, but not sputum, IL-6 levels are associated with asthma severity. This outcome is also true, however, of some individuals with normal BMI, illustrating the important point that BMI is only an indirect measure of metabolic dysfunction in patients with respiratory disease. Whether IL-6 has direct effects on the lung, or is simply a marker of another mediator of metabolic dysfunction, remains to be determined.
Although it is clear that factors produced by obese adipose tissue can have pleiotropic effects contributing to lung disease in obesity, it is also possible that obesity and lung disease have common pathogenic pathways. For example, chitinase-3-like protein 1 is involved in both visceral fat accumulation and the TH2-type inflammation associated with allergic asthma.30 Expression of chitinase 3-like-1 is regulated by the histone deacetylase Sirtuin 1 (Sirt1), a signaling pathway that mediates the activity of transcription factors involved in inflammation, metabolism, and endocrine signaling. This suggests there may be common genetic factors contributing to the development of both excess adipose tissue and to asthma. There are thus numerous levels at which biological integration between metabolic dysfunction and respiratory function can occur.
Role of Insulin Resistance and Dyslipidemia
Lung disease and insulin resistance may be linked through common pathways. Diabetes has long been associated with a preemptive decrease in lung function,31 and poorly controlled diabetes is associated with loss of lung function in cystic fibrosis.32 Recent data in mice and people suggest that insulin resistance also contributes to the development of pulmonary hypertension.33, 34 Thus, diabetes seems to affect both airway and pulmonary vascular disease.
Dyslipidemia may have profound effects on immune function and respiratory disease. Children with asthma have a high prevalence of hypertriglyceridemia and risk of insulin resistance,35 which mediates TH1-polarization, thus likely changing the typical TH2 responses associated with asthma in children.36 Abnormal lipid metabolism may also contribute to pulmonary vascular disease: Brittain et al37 discovered abnormal circulating lipid levels and altered fatty acid metabolism in the right ventricle of patients with pulmonary hypertension. This human study supports research in mouse models linking familial primary pulmonary hypertension with abnormal lipid metabolism.38
Insulin resistance and dyslipidemia can induce airway reactivity even in the absence of obesity. Singh et al39 showed that a high-fructose diet induced airway reactivity associated with reduced bioavailability of arginase in the airway, an indicator of increased nitrosative stress.
These findings relating insulin resistance and abnormal lipid metabolism with lung disease likely have implications for treatment. Han et al40 recently reported that a high-fat diet induced dyslipidemia and augmented allergen-induced airway disease in a mouse model of asthma, and these findings could be mitigated with simvastatin treatment, a cholesterol-lowering inhibitor of 3-hydroxy-3-methyl-3-glutaryl coenzyme A reductase.
Investigation is required to determine whether therapies intervening with insulin resistance or dyslipidemia might be effective for treating obesity-related lung disease in patients.
Role of Cellular Immune Function in Obesity
Metabolism is tightly linked to immune function. This connection has important implications for host defense in general, and for respiratory diseases in particular, in obesity.
Innate Immune Response
In addition to the well-documented changes in macrophage behavior in obesity, particularly in adipose tissue and atherosclerotic vascular lesions,12 there seems to be broad effects of obesity on innate immunity in general. Two lung diseases in which these effects are most evident are ARDS and pneumonia. In the case of ARDS, the effects of obesity are complex and indeed even counterintuitive; although obese subjects are at increased risk of developing ARDS, once afflicted, they have reduced mortality from this condition.24 Underlying these opposing effects on risk and outcome seem to be baseline “priming” of both neutrophils41 and the pulmonary vasculature,25 as well as defects in both neutrophil chemotaxis and survival.42, 43 How these effects are interrelated remains poorly understood, but they may also play a role in the pathogenesis of lung infection in obese individuals. Following the influenza A pH1N1 pandemic, it became clear that obesity is associated with increased susceptibility to, and worse outcomes from, this disease.44 Subsequent research has shown that obese subjects are more susceptible to bacterial pneumonia as well.45 Animal models of obesity have recapitulated these findings, implicating both an exuberant initial innate immune response (H1N1)46 and defects in neutrophil function (bacterial pneumonia)43, 45 that alter the pathogenesis and course of pneumonia in obesity.
Adaptive Immune Response
Changes in adaptive immune function are also pertinent to respiratory diseases in obesity because cellular metabolism and circulating adipokines both regulate immune cell function. Tight regulation of immune cell glycolytic and mitochondrial pathways are required to mount an effective immune response.47 For example, aerobic glycolysis is critical to fuel the biosynthetic and energy requirements of effector T-cell cytokine production.
Altered immune cell metabolism in obesity is likely to have significant implications for lung disease. The adipokine leptin has been studied in detail in this context because it regulates immune cellular metabolism by activating intracellular signaling cascades such as the mammalian target of rapamycin pathway.48 The differentiation and activity of regulatory T cells (Tregs) is regulated largely by the mammalian target of rapamycin and by adipokines, particularly leptin.49 Tregs express increased amounts of the leptin receptor,50 allowing the leptin/leptin receptor axis to inhibit Treg generation and proliferation following stimulation of T-cell receptors. The Treg population is significantly increased in leptin-deficient mice, which protects them against autoimmune diseases, but how this finding relates to pulmonary disease remains unclear.
There is some evidence for skewing away from TH2 responses. We have found that decreased serum adiponectin levels in obesity correlated positively with markers of TH1 and negatively with markers of TH2 immune response, suggesting that perturbations of adipokine levels in obesity could skew the immune response toward a TH1 phenotype.51 Compared with healthy control subjects and normal-weight patients with asthma, obese children also demonstrated evidence of TH1-polarized inflammation that correlated with obesity-mediated inflammatory markers such as leptin and IL-6.52 However, overall, we have found evidence of dampened adaptive immune responses in obese humans and in mouse models of obese asthma. Cytokine production from CD4 T cells increases significantly after bariatric surgery,53 and cytokine production from house dust mite-stimulated splenocytes was decreased in an obese mouse model of allergic asthma.54 How this finding contributes to altered allergic airway disease in obesity requires further study, but it suggests that TH2 pathways are dampened in the setting of obesity. Indeed, mice fed a high-fat diet exhibit visceral fat adipose tissue T cells that seem to take on a senescent phenotype,55 and obese children reportedly exhibit increased senescent T cells in the circulation.56 Both findings are indicative of immune ageing; thus, changes in effector T-cell function are likely to be important not only for asthma but also for respiratory disease in general.
Adaptive immune dysfunction in obesity likely contributes to altered responses to viral pathogens. Obese subjects also have impaired responses to influenza vaccine. CD4 and CD8 T cells from overweight and obese individuals express lower levels of CD69, CD28, CD40 ligand, and IL-12 receptor, and produce lower levels of interferon-γ and granzyme B, compared with healthy-weight individuals in response to influenza vaccination.57 This has major implications for public health.
Role of Diet and Gut Microbiome in Obesity
Obesogenic diets are typically high in dietary fats and sugars while being low in soluble fiber and antioxidants. Studies in humans have shown that consumption of a high-fat meal can have rapid effects on airway inflammation (increasing airway neutrophilia)58 and airway smooth muscle function (decreasing bronchodilator responsiveness).59 The mechanisms behind these rapid effects are not entirely clear, but in humans, they are associated with increased Toll-like receptor-4 expression in the sputum, and an increased expression of a number of other immune genes,60, 61 suggesting that a high-fat diet directly induces inflammatory pathways in the lung (Fig 2). In mice, a high-fat diet leads to activation of the NLRP3 inflammasome, and an increase in innate lymphoid cells producing IL17-A, which seem to mediate airway reactivity in this model.62
Dietary factors may also affect the lung indirectly through effects on the gut microbiome (Fig 2). There are significant differences in the gut microbiota of lean and obese adults, the latter group having an increased propensity to harvest energy from the diet, which then exacerbates changes in glucose metabolism and weight gain.63 Diet also affects the metabolites produced by gut microbes that ferment food products to produce short-chain fatty acids (SCFAs). The amount and type of SCFAs produced depends on the specific microbial composition of the gut, which in turn depends on dietary composition. SCFAs modulate immune function and affect neutrophil function, leukocyte recruitment, and chemotaxis. Bacteroidetes bacteria, a major producer of SCFA, are reduced in the gut in obesity63 and in the lungs in asthma.64
Analysis of human intestinal microbiota have also revealed significant decreases in bacterial diversity and the Bacteroidetes/Firmicutes ratio in obese vs lean individuals, and a significant increase in potential proinflammatory Proteobacteria.65 The relevance of this finding to respiratory disease is illustrated by the finding that a diet high in soluble fiber in mice decreases allergic airway disease through increased levels of the SCFA propionate that impairs the capacity of dendritic cells to promote TH2 effector cell responses.66 Gaining a better understanding of the effects of a Western diet on the gut microbiome, and subsequent immune function, is crucial if we are to advance our understanding of the link between obesity and lung disease.
Conclusions
The world is facing an unprecedented obesity epidemic that is changing the clinical presentation and nature of many common lung diseases. Given the complex changes associated with the obese state, which include changes in the microbiome, cellular metabolism and immune function, lipid handling, and circulating factors, as well as mechanical derangements, it is perhaps not surprising that obesity will profoundly change the nature of respiratory diseases. There is an urgent need to better understand the effects of the systemic and cellular metabolic disarray of obesity on the respiratory system; in doing so, we will gain fascinating new insights into the central role of metabolic factors in regulating normal pulmonary function and physiology.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. H. T. B. is a member of the advisory board and shareholder in OscillaVent, LLC. He has also received honorarium from Boehringer Ingelheim. None declared (U. P., B. T. S., A. E. D.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grants HL130847 and HL133920].
References
- 1.Ogden C.L., Carroll M.D., Fryar C.D., Flegal K.M. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 2.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon A.E., Clerisme-Beaty E.M. Humana Press; Totowa, NJ: 2013. Obesity and Lung Disease: a Guide to Management. [Google Scholar]
- 4.Watson R.A., Pride N.B., Thomas E.L. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol (1985) 2010;108(6):1605–1612. doi: 10.1152/japplphysiol.01267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behazin N., Jones S.B., Cohen R.I., Loring S.H. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010;108(1):212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones R.L., Nzekwu M.M. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 7.Sharp J.T., Henry J.P., Sweany S.K., Meadows W.R., Pietras R.J. The total work of breathing in normal and obese men. J Clin Invest. 1964;43:728–739. doi: 10.1172/JCI104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forno E., Weiner D.J., Mullen J. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med. 2017;195(3):314–323. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulló M., Garcia-Lorda P., Salas-Salvadó J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol. 2002;146(3):325–331. doi: 10.1530/eje.0.1460325. [DOI] [PubMed] [Google Scholar]
- 10.Bastard J.P., Jardel C., Bruckert E. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85(9):3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 11.Roth C.L., Kratz M., Ralston M.M., Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60(4):445–452. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers R.L., Motta A.C., Betts J.A., Bouloumié A., Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes (Lond) 2015;39(5):762–769. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sideleva O., Suratt B.T., Black K.E. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186(7):598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulain M., Doucet M., Drapeau V. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2008;5(1):35–41. doi: 10.1177/1479972307087205. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Zhang J.P., Huang H., Wang Z.H., Cheng R., Cai W.B. Leptin promotes fetal lung maturity and upregulates SP-A expression in pulmonary alveoli type-II epithelial cells involving TTF-1 activation. PloS One. 2013;8(7):e69297. doi: 10.1371/journal.pone.0069297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Q., Pho H., Kirkness J. Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep. 2016;39(5):1097–1106. doi: 10.5665/sleep.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteaga-Solis E., Zee T., Emala C.W., Vinson C., Wess J., Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metabolism. 2013;17(1):35–48. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shore S.A., Schwartzman I.N., Mellema M.S., Flynt L., Imrich A., Johnston R.A. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115(1):103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Medoff B.D., Okamoto Y., Leyton P. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41(4):397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M., Hug C., Kasahara D.I. Impact of adiponectin deficiency on pulmonary responses to acute ozone exposure in mice. Am J Respir Cell Mol Biol. 2010;43(4):487–497. doi: 10.1165/rcmb.2009-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krommidas G., Kostikas K., Papatheodorou G. Plasma leptin and adiponectin in COPD exacerbations: associations with inflammatory biomarkers. Respir Med. 2010;104(1):40–46. doi: 10.1016/j.rmed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Summer R., Little F.F., Ouchi N. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1035–L1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhi G., Xin W., Ying W., Guohong X., Shuying L. “Obesity paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PloS One. 2016;11(9):e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah D., Romero F., Duong M. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci Rep. 2015;5:11362. doi: 10.1038/srep11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lundblad L.K., Thompson-Figueroa J., Leclair T. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Car Med. 2005;171(12):1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams A.S., Mathews J.A., Kasahara D.I. Innate and ozone-induced airway hyperresponsiveness in obese mice: role of TNF-α. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1168–L1177. doi: 10.1152/ajplung.00393.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari R., Tanni S.E., Caram L.M., Correa C., Correa C.R., Godoy I. Three-year follow-up of interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res. 2013;14:24. doi: 10.1186/1465-9921-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters M.C., McGrath K.W., Hawkins G.A. Plasma IL6 levels, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahangari F., Sood A., Ma B. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191(7):746–757. doi: 10.1164/rccm.201405-0796OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaigham S., Nilsson P.M., Wollmer P., Engstrom G. The temporal relationship between poor lung function and the risk of diabetes. BMC Pulmonary Med. 2016;16(1):75. doi: 10.1186/s12890-016-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavie M., Fisher D., Vilozni D. Glucose intolerance in cystic fibrosis as a determinant of pulmonary function and clinical status. Diabetes Res Clin Pract. 2015;110(3):276–284. doi: 10.1016/j.diabres.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Meng Q., Lai Y.C., Kelly N.J. Development of a mouse model of metabolic syndrome, pulmonary hypertension, and heart failure with preserved ejection fraction (PH-HFpEF) Am J Respir Cell Mol Biol. 2017;56(4):497–505. doi: 10.1165/rcmb.2016-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brittain E.L., Nwabuo C., Xu M. Echocardiographic Pulmonary Artery Systolic Pressure in the Coronary Artery Risk Development in Young Adults (CARDIA) study: associations with race and metabolic dysregulation. J Am Heart Assoc. 2017;6(4) doi: 10.1161/JAHA.116.005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cottrell L., Neal W.A., Ice C., Perez M.K., Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183(4):441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi D., Fraser S., Oh J. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191(2):149–160. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brittain E.L., Talati M., Fessel J.P. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation. 2016;133(20):1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talati M.H., Brittain E.L., Fessel J.P. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med. 2016;194(6):719–728. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh V.P., Aggarwal R., Singh S. Metabolic syndrome is associated with increased oxo-nitrative stress and asthma-like changes in lungs. PloS One. 2015;10(6):e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han W., Li J., Tang H., Sun L. Treatment of obese asthma in a mouse model by simvastatin is associated with improving dyslipidemia and decreasing leptin level. Biochem Biophys Res Commun. 2017;484(2):396–402. doi: 10.1016/j.bbrc.2017.01.135. [DOI] [PubMed] [Google Scholar]
- 41.Palvinskaya T., Antkowiak M., Burg E. Effects of acute and chronic low density lipoprotein exposure on neutrophil function. Pulm Pharmacol Ther. 2013;26(4):405–411. doi: 10.1016/j.pupt.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kordonowy L.L., Burg E., Lenox C.C. Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am J Respir Cell Mol Biol. 2012;47(1):120–127. doi: 10.1165/rcmb.2011-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ubags N.D., Burg E., Antkowiak M. A comparative study of lung host defense in murine obesity models. insights into neutrophil function. Am J Respir Cell Mol Biol. 2016;55(2):188–200. doi: 10.1165/rcmb.2016-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fezeu L., Julia C., Henegar A. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12(8):653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 45.Ubags N.D., Stapleton R.D., Vernooy J.H. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang A.J., To K.K., Li C. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207(8):1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 47.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maciolek J.A., Pasternak J.A., Wilson H.L. Metabolism of activated T lymphocytes. Curr Opin Immunol. 2014;27:60–74. doi: 10.1016/j.coi.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Galgani M., De Rosa V., La Cava A., Matarese G. Role of metabolism in the immunobiology of regulatory T cells. J Immunol. 2016;197(7):2567–2575. doi: 10.4049/jimmunol.1600242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Rosa V., Procaccini C., Calì G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26(2):241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Dixon A.E., Johnson S.E., Griffes L.V. Relationship of adipokines with immune response and lung function in obese asthmatic and non-asthmatic women. J Asthma. 2011;48(8):811–817. doi: 10.3109/02770903.2011.613507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastogi D., Canfield S.M., Andrade A. Obesity-associated asthma in children: a distinct entity. Chest. 2012;141(4):895–905. doi: 10.1378/chest.11-0930. [DOI] [PubMed] [Google Scholar]
- 53.Dixon A.E., Pratley R.E., Forgione P.M. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128(3):508–515.e1-2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ather J.L., Chung M., Hoyt L.R. Weight loss decreases inherent and allergic methacholine hyperresponsiveness in mouse models of diet-induced obese asthma. Am J Respir Cell Mol Biol. 2016;55(2):176–187. doi: 10.1165/rcmb.2016-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shirakawa K., Yan X., Shinmura K. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126(12):4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spielmann G., Johnston C.A., O'Connor D.P., Foreyt J.P., Simpson R.J. Excess body mass is associated with T cell differentiation indicative of immune ageing in children. Clin Exp Immunol. 2014;176(2):246–254. doi: 10.1111/cei.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paich H.A., Sheridan P.A., Handy J. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity. 2013;21(11):2377–2386. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott H.A., Gibson P.G., Garg M.L., Wood L.G. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38(3):594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 59.Wood L.G., Garg M.L., Gibson P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127(5):1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 60.Halnes I., Baines K.J., Berthon B.S., MacDonald-Wicks L.K., Gibson P.G., Wood L.G. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients. 2017;9(1) doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q., Baines K.J., Gibson P.G., Wood L.G. Changes in expression of genes regulating airway inflammation following a high-fat mixed meal in asthmatics. Nutrients. 2016;8(1) doi: 10.3390/nu8010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H.Y., Lee H.J., Chang Y.J. IL-17 producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nature Med. 2014;20(1):54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 64.Hilty M., Burke C., Pedro H. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verdam F.J., Fuentes S., de Jonge C. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 2013;21(12):E607–E615. doi: 10.1002/oby.20466. [DOI] [PubMed] [Google Scholar]
- 66.Trompette A., Gollwitzer E.S., Yadava K. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]