Summary
Both glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2) are bona fide self-renewal factors for spermatogonial stem cells, whereas retinoic acid (RA) induces spermatogonial differentiation. In this study, we investigated the functional differences between FGF2 and GDNF in the germline niche by providing these factors using a drug delivery system in vivo. Although both factors expanded the GFRA1+ subset of undifferentiated spermatogonia, the FGF2-expanded subset expressed RARG, which is indispensable for proper differentiation, 1.9-fold more frequently than the GDNF-expanded subset, demonstrating that FGF2 expands a differentiation-prone subset in the testis. Moreover, FGF2 acted on the germline niche to suppress RA metabolism and GDNF production, suggesting that FGF2 modifies germline niche functions to be more appropriate for spermatogonial differentiation. These results suggest that FGF2 contributes to induction of differentiation rather than maintenance of undifferentiated spermatogonia, indicating reconsideration of the role of FGF2 in the germline niche.
Keywords: fibroblast growth factor 2, glial cell line-derived neurotrophic factor, retinoic acid, spermatogonial differentiation, spermatogonial self-renewal, germline niche
Graphical Abstract
Highlights
-
•
FGF2 and GDNF induce morphologically distinct spermatogonial clusters in vivo
-
•
FGF2-expanded undifferentiated spermatogonia are prone to be RARG+ in vivo
-
•
FGF2 is more permissive than GDNF for Rarg expression in cultured spermatogonia
-
•
FGF2 suppresses GDNF production and retinoic acid metabolism in the germline niche
Takashima and colleagues demonstrate the novel functions of FGF2 in the germline niche. Although FGF2 induces self-renewal of spermatogonial stem cells in vitro, this molecule expands “differentiation-prone” GFRA1+RARG+ spermatogonia and facilitates retinoic acid actions required for differentiation in vivo. The present study suggests that FGF2 contributes to spermatogonial differentiation, indicating reconsideration of the role of FGF2 in the germline niche.
Introduction
Spermatogonial stem cells (SSCs) are a subpopulation of undifferentiated spermatogonia and the origin of spermatogenesis. They reside in a specialized microenvironment called the germline niche located at the periphery of seminiferous tubules in mammalian testes. The germline niche permits SSCs to produce both stem cells and committed progenitors called differentiating spermatogonia by repeated self-renewal and differentiation. Differentiating spermatogonia further amplify their population by several mitotic divisions and enter meiotic division to produce spermatozoa (de Rooij and Russell, 2000).
It is well established that glial cell line-derived neurotrophic factor (GDNF) ensures the survival and self-renewal of SSCs. Meng et al. (2000) demonstrated that GDNF is indispensable for SSC self-renewal by analyzing Gdnf transgenic/deficient mice. Kanatsu-Shinohara et al. (2003) took advantage of GDNF functions to establish cultured SSCs termed germline stem (GS) cells from mouse pup testes. This technique enables identification of cytokines and chemicals that affect the behavior of SSCs.
Our recent study revealed that fibroblast growth factor 2 (FGF2) is another bona fide self-renewal factor for SSCs (Takashima et al., 2015). FGF2-cultured spermatogonia (F-SPG) cultured for more than 4 months under GDNF-free conditions colonized infertile mouse testes and restored fertility by spermatogonial transplantation. Compared with GDNF-cultured spermatogonia (G-SPG), F-SPG exhibit more differentiated characteristics such as lower SSC activity and higher KIT expression in addition to a difference in MAP2K1/2 dependency. These data suggest that FGF2 plays a distinct role in regulating undifferentiated spermatogonia in vivo.
It is also known that retinoic acid (RA) is required for spermatogonial differentiation (Hogarth et al., 2011). Of three RA receptor isotypes, retinoic acid receptor γ (RARG) has been reported to specifically contribute to this process (Gely-Pernot et al., 2012). Recently, Ikami et al. (2015) demonstrated that RARG expression is sufficient for induction of RA susceptibility in undifferentiated spermatogonia expressing GFRA1 that triggers GDNF signaling in combination with transmembrane receptor tyrosine kinase RET (Sariola and Saarma, 2003). For further understanding, it should be elucidated how RARG+ spermatogonia are derived from GFRA1+ undifferentiated spermatogonia and how RA signals are regulated within the germline niche.
Although FGF2 is considered to be a possible candidate that induces the RARG+ subset of undifferentiated spermatogonia, the absence of an animal model has hampered the analysis. Fgf2-transgenic mice are fertile and not reported to have abnormal testes (Coffin et al., 1995). This is because transgenic mice might not show extremely strong transgene expression depending on the gene. Furthermore, FGF2-knockout mice show no obvious defects in testicular functions (Zhou et al., 1998), which may be because of functional complementation by other FGF molecules.
To overcome such circumstances, we used the biodegradable gelatin microsphere (BGM) system to provide strong FGF2 stimuli only in the testis. This system was established and reported primarily by Tabata et al. (1999). BGMs were prepared by glutaraldehyde-mediated crosslinking of acidic gelatin (microsphere diameter: 30–100 μm). FGF2 can be adsorbed onto BGMs and released depending on the biodegradation of BGMs (Yamamoto et al., 2001). BGMs were first applied to deliver FGF2 into the skin to induce neovascularization (Tabata et al., 1999) and later applied to deliver bone morphogenetic protein 2 and transforming growth factor β1 for heart and bone regeneration (Ueda et al., 2002, Yamamoto et al., 2006, Marui et al., 2007). Uchida et al. (2016) also employed a similar system using different material to induce hyperproliferation of undifferentiated spermatogonia by GDNF treatment, as observed in the previous report of Meng et al. (2000). These reports encouraged employment of the BGM system.
In this study, we analyzed the molecular functions of FGF2 in the mouse germline niche using BGMs for direct FGF2 stimulation in vivo. Although we found that FGF2 expanded GFRA1+ spermatogonia, these cells exhibited a phenotype distinct from that of GDNF-expanded GFRA1+ spermatogonia. Moreover, FGF2 modified germline niche functions to be more appropriate for spermatogonial differentiation.
Results
Fgf2 Expression in the Testis
To identify the origin of FGF2 in the testis, we conducted qRT-PCR analyses. For Sertoli cell analysis, we used R26-CAG-LoxP-hCD271; Amh-Cre mice to purify Sertoli cells by magnetic-activated cell sorting (MACS) (Figure S1A) (Kuroki et al., 2015). Although purified Sertoli cells expressed Gdnf, Fgf2 expression was very low compared with whole testis cells, suggesting that other cell types express Fgf2 (Figures S1B and S1C). In contrast, germ cell depletion decreased Fgf2 expression to some extent, suggesting that germ cells and other components of the germline niche express Fgf2 (Figures S1D and S1E). We also detected FGF2 in the testes of rodents, domestic animals, and humans, suggesting conservation of FGF2 production in mammalian testes (Figure S1F).
FGF2 Expands GFRA1+ Spermatogonia In Vivo
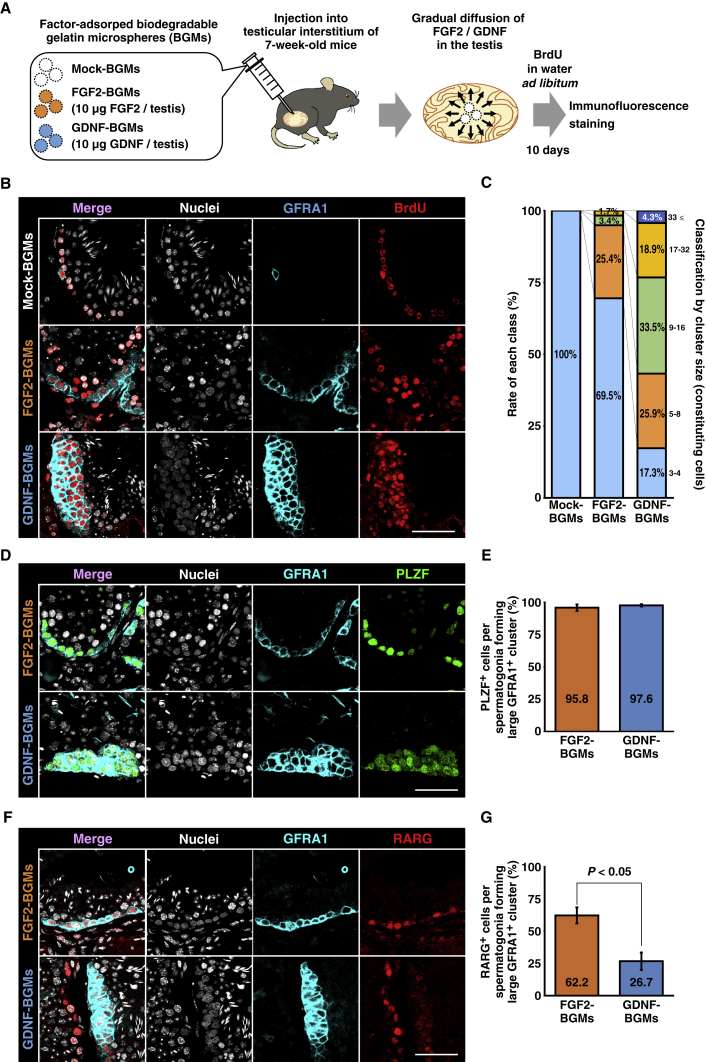
We examined the functions of FGF2 in the testis by applying BGMs for continual growth factor stimulation in vivo (Figure 1A) (Tabata et al., 1999). Growth factor-adsorbed BGMs were transplanted directly into the testicular interstitium of C57BL6/N mice (7 weeks old) under isoflurane anesthesia. Until sacrifice, bromodeoxyuridine (BrdU) was administered via water ad libitum to label proliferating cells. At 10 days after the procedure, BGM-treated testes were harvested and examined by immunofluorescence staining (Figure 1B). Although six testes were included in each group (mock-, FGF2-, and GDNF-BGMs), five testes (mock-BGM), six testes (FGF2-BGM), and four testes (GDNF-BGM) were successfully transplanted with BGMs. Testes were harvested independently from three mice for each group.
Figure 1.
FGF2 Expands GFRA1+ Spermatogonia Phenotypically Distinct from Those Induced by GDNF
(A) Flow chart of biodegradable gelatin microsphere (BGM) experiments. Mock-, FGF2-, or GDNF-adsorbed BGMs were bilaterally injected into the interstitium of 7-week-old mouse testes. BrdU was administered via water ad libitum until mice were sacrificed. At 10 days after injection, testes were analyzed by immunofluorescence staining. Results were obtained independently from three mice.
(B and C) Cluster formation and proliferation of GFRA1+ spermatogonia induced by BGM transplantation. (B) Immunofluorescence staining. GFRA1 and incorporated BrdU are visualized by cyan and red, respectively. Nuclei were counterstained with Hoechst 33,342 (gray). (C) Classification of large GFRA1+ clusters by the number of constituting spermatogonia. Mock-BGMs, n = 5 testes; FGF2-BGMs, n = 6 testes; GDNF-BGMs, n = 4 testes. A total of 1,434 tubules with 261 large GFRA1+ clusters carrying 2749 GFRA1+ spermatogonia were analyzed.
(D–G) Characterization of BGM-induced large GFRA1+ clusters. (D and E) PLZF expression in large GFRA1+ clusters. (D) Immunofluorescence staining. GFRA1 is visualized by cyan, while PLZF is visualized by green. Nuclei were counterstained with Hoechst 33,342 (gray). (E) Quantitative analysis of PLZF expression in large GFRA1+ clusters. FGF2-BGMs, n = 5 testes; GDNF-BGMs, n = 4 testes. A total of 957 tubules with 190 large GFRA1+ clusters carrying 2,111 GFRA1+ spermatogonia were analyzed. In this experiment, one FGF2-BGM-treated testis, which did not carry large GFRA1+ clusters, was excluded from the statistical analysis. (F and G) RARG expression in large GFRA1+ clusters. (F) Immunofluorescence staining. GFRA1 is visualized by cyan, while RARG is visualized by red. Nuclei were counterstained with Hoechst 33,342 (gray). (G) Quantitative analysis of RARG expression in large GFRA1+ clusters. FGF2-BGMs, n = 6 testes; GDNF-BGMs, n = 4 testes. A total of 1,347 tubules with 214 large GFRA1+ clusters carrying 2,147 GFRA1+ spermatogonia were analyzed.
Results are presented as means ± SEM. Scale bars, 50 μm (B, D, and F). See also Figures S1 and S2; Table S1.
In adult mice, most GFRA1+ spermatogonia exist as single or paired cells, and spermatogonial clusters consisting of three or more cells are rare (Suzuki et al., 2009, Nakagawa et al., 2010, Grasso et al., 2012). In agreement with these previous studies, transplantation of mock-BGMs had no effect on GFRA1+ spermatogonia. Most GFRA1+ spermatogonia were found as single or paired cells (Figure 1B). Next, we defined GFRA1+ spermatogonial clusters consisting of three or more cells as “large GFRA1+ clusters.” In this regard, we also classified large GFRA1+ clusters into five classes based on the number of constituting GFRA1+ spermatogonia (Figures 1C and S2A). We found only 17 large GFRA1+ clusters consisting of three or four spermatogonia in 507 tubule sections from five testes (Figure 1C).
GDNF-BGMs often induced large GFRA1+ clusters exhibiting a multi-layered, dome-like morphology (Figures 1B, S2A, and S2B). This result is consistent with a previous report showing that intratesticular transplantation of GDNF-soaked beads induces hyperproliferation of GFRA1+ spermatogonia (Uchida et al., 2016). A total of 185 large GFRA1+ clusters were found in 457 tubule sections from four testes, and 82.7% (153/185) of clusters consisted of more than four spermatogonia (Figure 1C). Although FGF2-BGMs also induced large GFRA1+ clusters, these cells were prone to form two-dimensional flat colonies along the basement membrane of seminiferous tubules (Figures 1B and S2A–S2D). Fifty-nine large GFRA1+ clusters were found in 470 tubule sections from six testes, and 30.5% (18/59) of them consisted of more than four spermatogonia (Figure 1C). In the most extreme case, GFRA1+ spermatogonia covered the entire circumference of the seminiferous tubule (Figure S2D). In GDNF- and FGF2-BGM-treated testes, large GFRA1+ clusters were prone to reside adjacent to BGMs (Figures S2B–S2D). We also observed that GFRA1+ spermatogonia that formed large clusters frequently incorporated BrdU (Figure 1B). These data strongly suggest that FGF2 also acts as growth factor for GFRA1+ spermatogonia.
Phenotypic Differences between FGF2- and GDNF-Expanded GFRA1+ Spermatogonia
We also found morphological differences between FGF2- and GDNF-expanded large GFRA1+ clusters, suggesting functional differences of these factors. Therefore, we compared the phenotypes of FGF2- and GDNF-expanded GFRA1+ spermatogonia that formed large clusters. We found some large GFRA1+ clusters in mock-BGM-treated testes. However, consistent with previous reports (Suzuki et al., 2009, Nakagawa et al., 2010), the number of large GFRA1+ clusters was inadequate for statistical analysis. Therefore, mock-BGM samples were omitted, and comparisons of FGF2-BGM and GDNF-BGM samples were conducted. In this experiment, we examined expression of two spermatogonial markers, promyelocytic leukemia zinc finger (PLZF) protein (marker for undifferentiated spermatogonia) and RARG (marker for a subset of undifferentiated spermatogonia and differentiating spermatogonia). The former is expressed in a broad range of undifferentiated spermatogonia and is essential for their maintenance (Buaas et al., 2004, Costoya et al., 2004). The latter can monitor the differentiation of GFRA1+ undifferentiated spermatogonia into differentiating spermatogonia and is necessary and sufficient for acquisition of RA signals for differentiation into differentiating spermatogonia (Gely-Pernot et al., 2012, Ikami et al., 2015). Ikami et al. (2015) demonstrated that genetically modified GFRA1+ spermatogonia expressing RARG are persistently competent for RA-mediated spermatogonial differentiation. Therefore, RARG/Rarg is considered to be an appropriate marker to monitor the differentiation of GFRA1+ spermatogonia. Immunofluorescence staining revealed that both populations exhibited the PLZF+ phenotype typical for undifferentiated spermatogonia (Figures 1D and 1E). However, a greater percentage of FGF2-expanded GFRA1+ spermatogonia expressed RARG than cells expanded with GDNF (Figures 1F and 1G). In this regard, Ikami et al. (2015) also identified the “GFRA1+RARG+ subset” of undifferentiated spermatogonia as an intermediate between the GFRA1+ primitive subset and more differentiated Neurog3-EGFP+ (RARG/Rarg) subset. Considering that RARG is indispensable for proper spermatogonial differentiation (Gely-Pernot et al., 2012), FGF2 might expand a differentiating subset of GFRA1+ spermatogonia.
FGF2 Is Functionally More Permissive for Rarg Expression
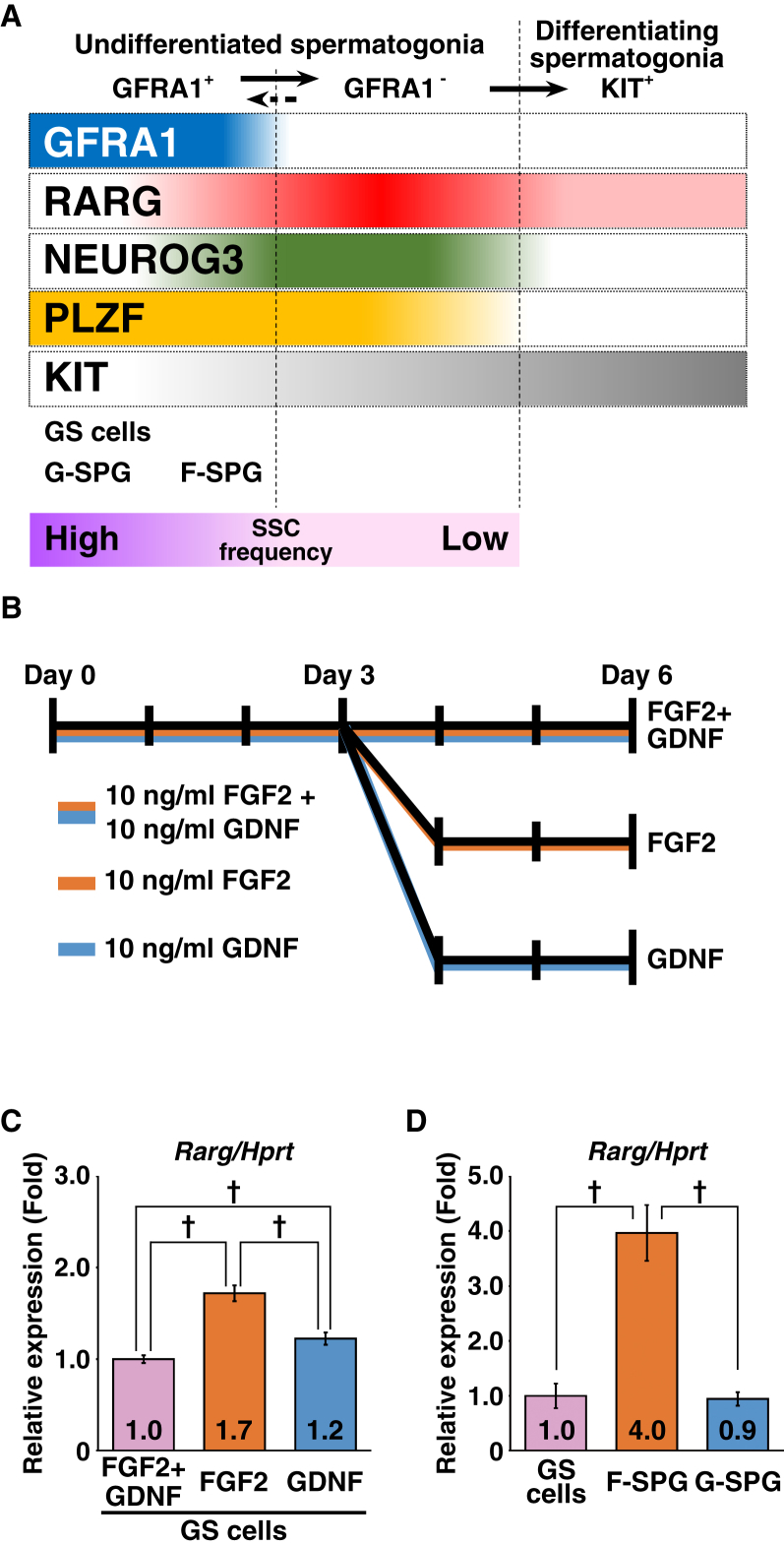
Next, we examined how the GFRA1+RARG+ subset was expanded. Undifferentiated spermatogonia can be divided into GFRA1+ and GFRA1− subsets (Garbuzov et al., 2018). The GFRA1+ subset possesses a higher SSC frequency and a more immature phenotype than the GFRA1− subset, and the GFRA1− subset can produce the GFRA1+ subset (Figure 2A). Hara et al. (2014) also observed a similar phenomenon whereby the GFRA1+ subset produces a Neurog3-EGFP+ subset and the latter can produce the former. Based on these contexts, we hypothesized three possible mechanisms that expand the GFRA1+RARG+ subset: (1) FGF2 induces RARG expression in the GFRA1+RARG− subset; (2) FGF2 expands the GFRA1+RARG+ subset within the GFRA1+ undifferentiated spermatogonial population; and (3) FGF2 induces reversion of GFRA1-undifferentiated spermatogonia into the GFRA1+RARG+ subset. To test the former two hypotheses, we employed SSC cell lines, GS cells, F-SPG, and G-SPG. These cells are powerful tools for biochemical and molecular analyses of undifferentiated spermatogonia (Kanatsu-Shinohara et al., 2003, Takashima et al., 2015). GS cells were established and maintained consistently with both GDNF and FGF2, while F-SPG and G-SPG were established and maintained consistently with FGF2 or GDNF, respectively. Considering the persistent expression of GFRA1 in culture, these cells recapitulated the characteristics of the GFRA+ subset of undifferentiated spermatogonia (Figure 2A).
Figure 2.
FGF2 Is Permissive for Rarg Expression in Cultured Spermatogonial Cell Lines
(A) Schematic representation of the phenotype of spermatogonial subsets and SSC cell lines based on previous reports (Gely-Pernot et al., 2012, Hara et al., 2014, Ikami et al., 2015, Takashima et al., 2015, Garbuzov et al., 2018).
(B and C) Effects of FGF2 and GDNF on Rarg expression in GS cells. (B) GS cell culture conditions. Cells were cultured on laminin-coated dishes. (C) qRT-PCR analysis. After normalization to Hprt expression, values of FGF2 + GDNF were set to 1.0 (n = 8 independent cultures for each group).
(D) qRT-PCR analysis of Rarg expression in cultured spermatogonial cell lines. After normalization to Hprt expression, the value of GS cells was set to 1.0 (GS cells, n = 13 independent cultures; F-SPG, n = 14 independent cultures; G-SPG, n = 18 independent cultures). Results were obtained from three (GS cells and F-SPG) and four (G-SPG) independently established sublines for each group.
Results are presented as means ± SEM. Daggers indicate statistical significance (†p < 0.05).
First, we cultured GS cells on laminin-coated dishes with or without the two growth factors and found that growth factor stimulation suppressed Rarg in GS cells (Figures S3A and S3B). Next, we determined which factor suppressed Rarg in GS cells. After deprivation of growth factors for 2 days, GS cells were cultured for another 2 days with FGF2 and/or GDNF at various concentrations. This experiment revealed that both factors had a Rarg-suppressive activity, and the concentration or combination of the two factors did not show any change in suppression of Rarg (Figures S3C and S3D). However, GS cells cultured under GDNF-free conditions for 3 days (orange column) expressed higher levels of Rarg than those cultured under FGF2-free (blue column) or FGF2 + GDNF (purple column) conditions (Figures 2B and 2C). Considering that F-SPG expressed a higher level of Rarg than GS cells and G-SPG (Figure 2D), it is likely that FGF2 is more permissive than GDNF for expression of Rarg/RARG in GFRA1+ spermatogonia.
FGF2 Acts on the Germline Niche to Suppress GDNF and Permits RA Actions
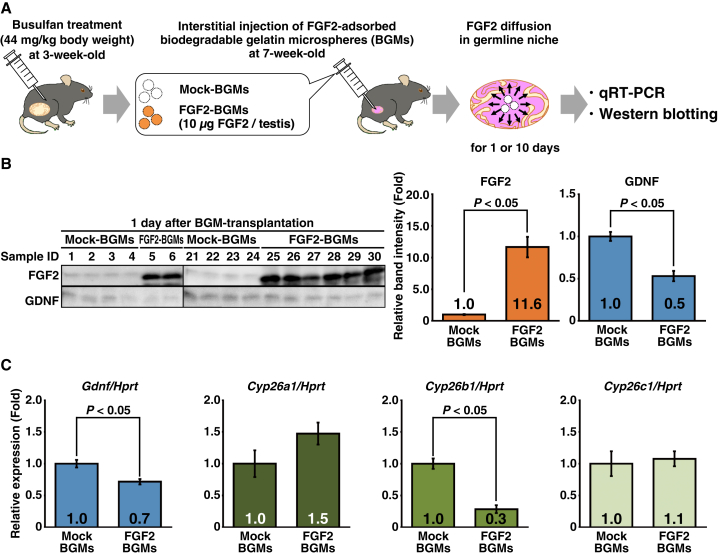
We also determined FGF2 functions in the germline niche. In this experiment, we used the testes of busulfan-treated mice, because Fgf2 expression is attenuated and the germline niche remains functional. However, we did not examine GDNF functions because GFRA1 is expressed exclusively in undifferentiated spermatogonia of mouse testes (Figures S2A–S2D). Mock- or FGF2-BGMs were transplanted into testes of busulfan-treated mice, and the testes were harvested at 1 or 10 days after transplantation. Gene expression related to germline niche functions was assessed by qRT-PCR and western blotting (Figure 3A).
Figure 3.
FGF2 Acts on the Germline Niche to Suppress Gdnf/GDNF and Cyp26b1
(A) Flow chart of the BGM experiment to assess FGF2 functions in the germline niche. Busulfan-treated testes, in which germ cells are depleted and germline niches remain functional, were used in this analysis. At 1 or 10 days after BGM transplantation, testes were harvested and analyzed by qRT-PCR and western blotting.
(B) Western blot analyses of testes at 1 day after BGM treatment. Band intensities of the Mock-BGM group were set to 1.0 (n = 8 testes for each condition).
(C) qRT-PCR analysis of testes at 1 day after BGM treatment. After normalization to Hprt expression, values of the Mock-BGM group were set to 1.0 (n = 8 testes for each condition).
Results are presented as means ± SEM. See also Figure S4; Tables S1 and S2.
Although we analyzed BGM-transplanted testes harvested at 10 days after the procedure, almost all injected FGF2 had disappeared (Figure S4A). This result was different from a study indicating that BGMs continue to release FGF2 in vivo for up to 14 days after the procedure (Yamamoto et al., 2001). In this situation, gene expression related to germline niche functions was unchanged (Figure S4B). However, this BGM system was supposed to be adequate to assess the molecular functions of FGF2 in the germline niche, because this procedure succeeded in inducing hyperproliferation of GFRA1+ spermatogonia by GDNF/FGF2 (Figures 1B, 1C, and S2A–S2D). In contrast, testes harvested at 1 day after the procedure still retained a substantial and non-physiological amount of FGF2, and Gdnf/GDNF was downregulated (Figures 3B and 3C). These results are consistent with our previous report showing that Fgf2 knockdown in mouse testes induces GDNF production (Takashima et al., 2015). Moreover, we found suppression of Cyp26b1 that encodes an RA-metabolizing enzyme (Figure 3C). Although the role of Cyp26c1 in mice remains to be elucidated, considering that deficiency of Cyp26b1, but not Cyp26a1, in Sertoli cells compromises spermatogenesis (Hogarth et al., 2015a), it has been suggested that FGF2 plays an important role in regulating RA metabolism in the germline niche by regulating Cyp26b1. To further understand the regulation of Gdnf and Cyp26 genes, we assessed RA functions in the germline niche. In this experiment, busulfan-treated mice were intraperitoneally injected with 750 μg of RA and their testes were harvested at 11 hr after treatment. qRT-PCR analysis revealed that Cyp26a1 and Cyp26c1 were induced by RA while Cyp26b1 was unaffected, suggesting that Cyp26b1 is specifically regulated by FGF2 in the germline niche (Figure S4C). RA injection also suppressed Gdnf, which is consistent with a previous study (Hasegawa et al., 2013). Taken together, these results suggest that FGF2 modifies germline niche functions to be more appropriate for spermatogonial differentiation by suppressing GDNF and RA metabolism.
Discussion
We investigated the functions of FGF2 in the germline niche. Although FGF2 expanded GFRA1+ spermatogonia in vivo, these cells exhibited a more differentiated phenotype (RARG expression) than those expanded by GDNF. Moreover, FGF2 suppressed RA metabolism and GDNF production. These results suggest that FGF2 acts on both GFRA1+ spermatogonia and their niche to facilitate spermatogonial differentiation, despite the fact that FGF2 is a bona fide self-renewal factor for SSCs.
We applied BGMs for sustained stimulation by growth factors in vivo. Although our previous report employed lentivirus-mediated Fgf2 overexpression in testes (Takashima et al., 2015), it was difficult to judge whether it was sufficient for functional analysis. In contrast, Uchida et al. (2016) demonstrated that transplantation of GDNF-soaked beads induces expansion of GFRA1+ spermatogonia. This observation led us to apply this procedure using BGMs.
BGM performance in vivo has already been demonstrated in several studies. In mouse dermal tissue, BGMs achieved prolonged FGF2 release for 14 days and induced angiogenesis, while 80% of FGF2 disappeared from the site of injection within 24 hr, and angiogenesis was not induced without using BGMs (Yamamoto et al., 2001). Based on these observations, we applied this system to achieve prolonged FGF2 stimuli in the testis. Although our data demonstrated that most of the injected FGF2 was consumed by 10 days after the procedure, a substantial amount of FGF2 remained at 1 day after the procedure. Even though this system might not perform as intended (expected stimuli for about 2 weeks), FGF2 stimulation induced hyperproliferation of GFRA1+ undifferentiated spermatogonia and modulated gene expression in the germline niche. Considering that release kinetics are different depending on the growth factor (Yamamoto et al., 2001), it is anticipated that the release kinetics of GDNF are also different from those of FGF2. Hence, it is not appropriate to determine which factor is superior to induce spermatogonial growth using the BGM system.
Using BGMs, although we found that FGF2 expanded GFRA1+ spermatogonia, their number and size were less than those of GDNF-expanded GFRA1+ spermatogonia. This result might be biased by two technical reasons. One might be undefined release kinetics of factors from BGMs as described above. Another is the choice of analytical method for large GFRA1+ clusters. Although we applied whole-mount immunofluorescence to analyze testicular tubules, synechia between seminiferous tubules and BGMs hampered analysis of colonies formed beneath BGMs. Simple observation of frozen sections could not render the whole architecture of colonies. The axis of a tubule section might affect the result of the size and number of large GFRA1+ clusters. However, we succeeded in obtaining colony images without collapsing their structure. Therefore, our data regarding the number and size of large GFRA1+ clusters might be underestimated. Based on these circumstances, our study was limited to comparison of the characteristics of resultant colonies induced by FGF2 or GDNF.
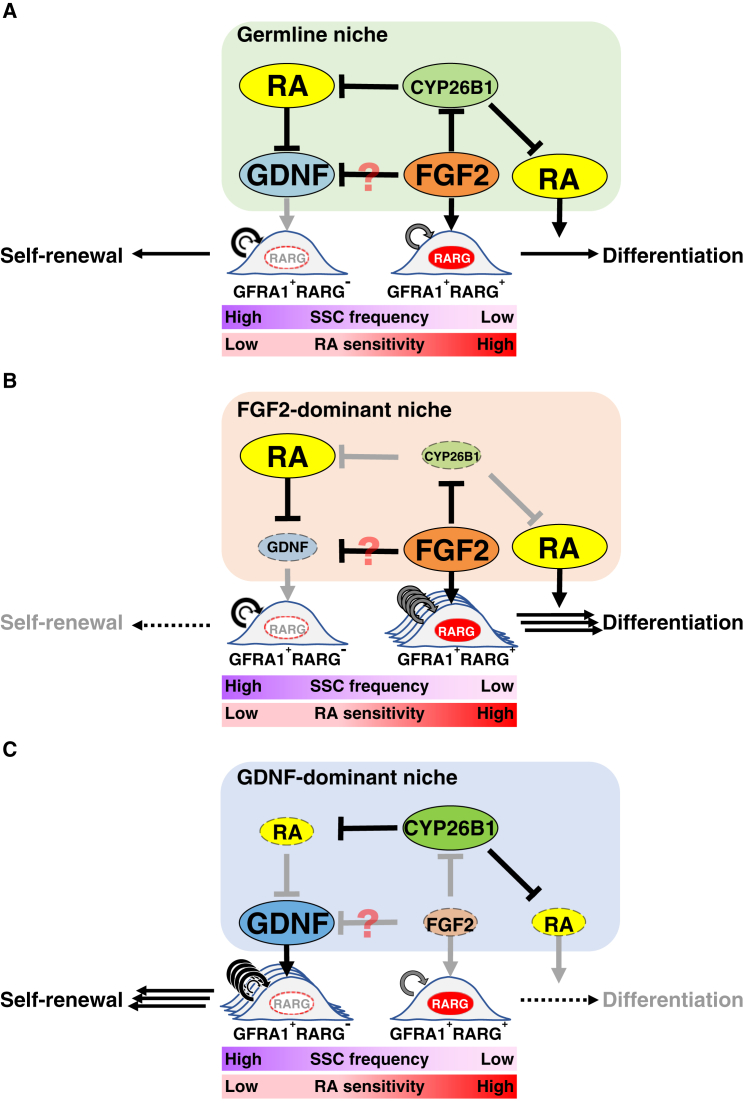
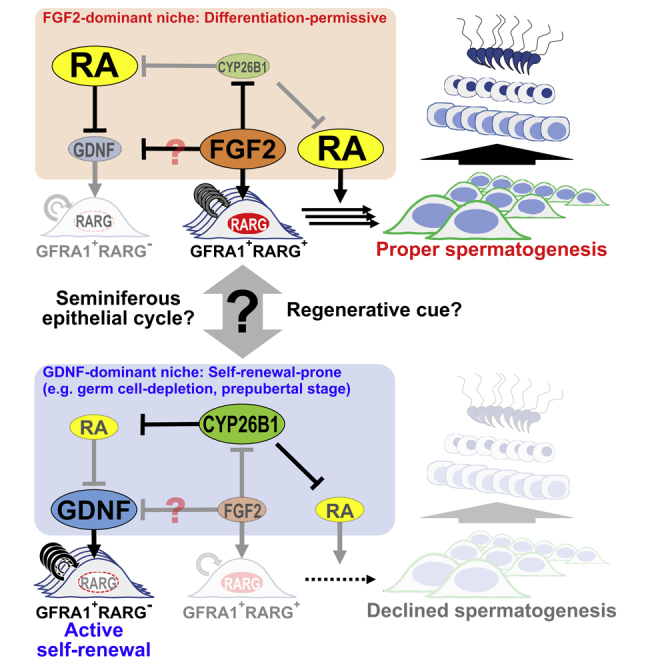
Both FGF2 and GDNF induced morphologically distinct large GFRA1+ clusters in vivo. GDNF-BGMs induced multilayered clusters that were morphologically similar to G-SPG, whereas FGF2-BGMs induced flat colonies on the basement membrane of seminiferous tubules, which were similar to F-SPG (Takashima et al., 2015). Our recent report revealed that F-SPG have a more differentiated phenotype without losing their SSC properties. These findings suggest that FGF2-expanded GFRA1+RARG+ spermatogonia in vivo might have a more differentiated phenotype. Indeed, Ikami et al. (2015) demonstrated the existence of GFRA+RARG+ spermatogonia, and that these cells are more susceptible than GFRA1+RARG− spermatogonia to RA-induced differentiation toward KIT+ differentiating spermatogonia. These observations support our speculation that FGF2 might facilitate spermatogonial differentiation by expanding differentiation-prone GFRA1+RARG+ spermatogonia (Figures 4A and 4B).
Figure 4.
Putative Role of FGF2 in the Mouse Germline Niche
(A) Molecular functions of FGF2 in the germline niche. GDNF expands GFRA1+RARG− spermatogonia, whereas FGF2 expands GFRA1+RARG+ spermatogonia that are a differentiation-prone subset (Ikami et al., 2015). Simultaneously, FGF2 also acts on the germline niche to facilitate RA actions via Cyp26b1 suppression. GDNF suppression might also contribute to expanding GFRA1+RARG+ spermatogonia.
(B and C) The FGF2-dominant niche is prone to differentiation because of permissiveness for expansion of GFRA1+RARG+ spermatogonia and RA actions (B), whereas the GDNF-dominant niche is prone to regeneration (C). Indeed, transplanted undifferentiated spermatogonia are prone to proliferation rather than differentiation in germ cell-depleted testes (Nagano et al., 1999, Nagai et al., 2012, Zohni et al., 2012). The present study also demonstrated that busulfan-mediated germ cell depletion increased the Gdnf/Fgf2 ratio in the testis (Figure S1).
See also Figure S1.
An important remaining issue is how FGF2 expands the GFRA1+RARG+ subset of undifferentiated spermatogonia. We determined whether FGF2 induced Rarg expression in GFRA1+ spermatogonia using cultured spermatogonial cell lines. In contrast to our expectation, although FGF2 suppressed Rarg in GS cells, FGF2 was relatively more permissive for Rarg expression than GDNF. These data strongly suggest that FGF2 does not induce Rarg in GFRA1+ spermatogonia in vivo, rejecting the hypothesis that FGF2 induces Rarg expression in GFRA1+ spermatogonia. Considering that FGF2 induces proliferation of undifferentiated spermatogonia, it is considered that at least some GFRA1+RARG+ spermatogonia are expanded by the proliferation of GFRA1+RARG+ spermatogonia themselves. Validation of the third hypothesis, that the GFRA1+RARG+ subset is derived from the GFRA1− subset via FGF2 stimuli, still remains to be clarified. Although how FGF2 suppresses GDNF in the germline niche (directly or through the CYP26B1-RA pathway) remains unknown, FGF2-mediated GDNF suppression might also contribute to expanding the GFRA1+RARG+ subset.
Which cells produce FGF2 in the testis has been controversial. Some reports demonstrated that Sertoli cells produce FGF2 (Smith et al., 1989, Mullaney and Skinner, 1992, Tadokoro et al., 2002), whereas others showed that germ cells produce FGF2 (Han et al., 1993, Zhang et al., 2012). Our results demonstrated that Sertoli cells are not the main origin of FGF2, at least under physiological conditions. Subsequent experiments revealed that germ cell depletion attenuated Fgf2 expression in the testis. Considering that germ cell-free Nanos3-knockout mice also show decreased Fgf8 expression in the testis (Hasegawa and Saga, 2014), Fgf8 and Fgf2 might be expressed in a similar manner. There are three possible explanations for this outcome. (1) Germ cells express Fgf2, and germ cell depletion causes loss of Fgf2. (2) The germline niche expresses Fgf2 depending on the germ cell-derived signal (JAG1-NOTCH2 pathway) (Garcia et al., 2014), and germ cell depletion attenuates Fgf2 expressed in the germline niche. (3) Busulfan treatment suppresses Fgf2 in the germline niche. Direct analysis of purified testis component cells is more likely to identify the Fgf2-expressing cells. However, busulfan-mediated expression changes of Fgf2 and Gdnf might be important in understanding the niche function. Germ cell depletion changes the Gdnf/Fgf2 balance to modify the functions of the germline niche from a physiological niche to a Gdnf-dominant niche appropriate for regeneration (Figures 4A–4C).
We also found that FGF2, but not RA, regulated Cyp26b1 in the germline niche. Hogarth et al. (2015a) demonstrated that Sertoli cell-expressed Cyp26b1 is indispensable for proper spermatogenesis, whereas Cyp26a1 deficiency in Sertoli cells does not cause any spermatogenic defects. Although it remains unclear whether Cyp26c1 deficiency affects fertility (Uehara et al., 2007), Cyp26b1 regulated by FGF2 might play an important role in regulating RA actions in the germline niche (Figures 4A and 4B). In this regard, Garcia et al. (2014) reported that germ cell-derived NOTCH signaling is required for proper spermatogenesis, which regulates Cyp26b1 and Gdnf in Sertoli cells. They proposed a model in which the population size of germ cells expressing NOTCH ligands affects the functions of Sertoli cells expressing Gdnf and Cyp26b1. It is intriguing how germ cells themselves regulate their differentiation dynamics via FGF2 and NOTCH pathways. Additionally, it is well known that RA is the master regulator of the seminiferous epithelial cycle (Hogarth et al., 2013). The RA concentration in testes is determined by the balance between RA production and metabolism (Hogarth et al., 2011). Furthermore, the length of the seminiferous epithelial cycle varies depending on the animal species and is determined by the germ cell genotype (França et al., 1998, Sugimoto et al., 2012). Therefore, it is also intriguing to speculate that germ cell-derived FGF2/NOTCH signals might define the length of the seminiferous epithelial cycle by regulating RA actions in the germline niche.
Our results can explain the functional differences between FGF2 and GDNF. In fact, FGF2 possesses a molecular function that permits RA-mediated differentiation by inducing differentiation-prone GFRA1+RARG+ spermatogonia. FGF2 also acts on the germline niche to facilitate RA actions for spermatogonial differentiation (Figure 4A). Previous reports suggest cyclical fluctuation of GDNF and RA in testicular tubules throughout the seminiferous epithelial cycle under physiological conditions (Grasso et al., 2012, Hogarth et al., 2015b). Furthermore, germ cell-deficient conditions are reported to be appropriate for proliferation of undifferentiated spermatogonia rather than spermatogonial differentiation (Nagano et al., 1999, Nagai et al., 2012). Indeed, busulfan treatment upregulates Gdnf expression in the testis (Figure S1) (Zohni et al., 2012). These findings suggest the existence of GDNF- and FGF2-dominant niches. The former is considered to promote the proliferation of undifferentiated spermatogonia including SSCs (Figure 4C). Previous reports have demonstrated that transplanted SSCs appear to focus on expansion of their population without production of differentiating progeny in the germ cell-depleted recipient testis (Nagano et al., 1999, Nagai et al., 2012). Our present study also showed a change in the Gdnf/Fgf2 balance in the germline niche after germ cell depletion by busulfan (Figures S1D and S1E). Considering the phenotype of Gdnf-transgenic mice and GDNF-BGM-treated testes, a GDNF-dominant niche might not permit spermatogonial differentiation. In contrast, an FGF2-dominant niche is considered to permit production of GFRA1+RARG+ spermatogonia for spermatogenesis (Figure 4B). However, FGF2 signals are not sufficient, and GDNF signals are indispensable for SSC maintenance (Meng et al., 2000). Although FGF2 expression dynamics remain to be elucidated in seminiferous tubules, it is intriguing to speculate that the physiological germline niche shifts cyclically between FGF2- and GDNF-dominant states. For a complete overview of spermatogonial dynamics in the germline niche, it is indispensable to understand spatiotemporal regulation of FGF2 as well as GDNF and RA.
Experimental Procedures
The institutional animal care and use committee of Shinshu University (approval nos. 260013 and 280120) and Tokushima University (experimental number 14,108) approved all animal experimentation protocols. Human testis tissues without pathological lesions were obtained and subjected to the experiments in accordance with the institutional ethics review board of Shinshu University to use human-derived material (test no. 3039), and the institutional ethics review board of Nagano Red Cross Hospital to use human-derived material (Nagano-Byo-Ki approval no. 25). Written consent was obtained following the committee-approved protocol before tissue collection.
Growth Factor Adsorption of BGMs
BGMs were prepared according to the original report (Tabata et al., 1999). For single testis treatment, freeze-dried BGMs (1 mg) were reconstituted with 10 μL of 1 mg/mL recombinant murine FGF2 or GDNF (PeproTech, London, UK) in distilled-deionized-autoclaved water. In this manner, growth factor solutions were completely absorbed in BGMs, demonstrating that the growth factors were entirely contained within BGMs. After overnight adsorption of growth factors at 4°C, the resultant BGMs were suspended in 15 μL of autoclaved physiological saline for transplantation.
Statistical Analyses
Results are presented as means ± SEM. Significance of differences between means for single comparisons was determined by the Student's t test. Multiple comparison analyses were carried out using one-way ANOVA followed by Tukey's honest significant difference test. p < 0.05 was considered statistically significant.
Author Contributions
S.T. conceived the concept, designed and performed the experiments, analyzed the data, wrote the manuscript, and secured funding. K.M. performed experiments, analyzed the data, and secured funding. M.S. performed experiments and analyzed the data. S.K. performed experiments. J.-I.J., K.H., Y.F., K.O., T.A., T.Y., M.T., Y.T., T.S., O.I., and S.H. provided reagents.
Acknowledgments
We thank T. Shinohara (Kyoto University) for generously providing GS cells, F-SPG, and G-SPG, KEYENCE (Osaka, Japan) for use of the BZ-X700 fluorescence microscope, H. Ogawa, Y. Kawahara, H. Adachi, M. Tone, S. Aiba, A. Tsuchimoto, T. Abe, D. Takemasa, A. Miura, and A. Nakano of S.T.'s laboratory for technical assistance and helpful discussions, and Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was funded by JSPS KAKENHI (JP16H05046), The Sumitomo Foundations (no.140785), The Naito Foundation (no. 4342-110), The Ito Foundation (no. Ken 16), The Hokuto Foundation for Bioscience, the Japan Health Foundation (no. 2016-3-145), the Mochida Memorial Foundation for Medical and Pharmaceutical Research, The Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, The Uehara Memorial Foundation, the Suzuken Memorial Foundation, the Takeda Science Foundation (to S.T.), and The Foundation of Nagano Prefecture Science Promotion (to K.M.).
Published: April 19, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.03.016.
Supplemental Information
References
- Buaas F.W., Kirsh A.L., Sharma M., McLean D.J., Morris J.L., Griswold M.D., de Rooij D.G., Braun R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Coffin J.D., Florkiewicz R.Z., Neumann J., Mort-Hopkins T., Dorn G.W., Lightfoot P., German R., Howles P.N., Kier A., O'Toole B.A. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol. Biol. Cell. 1995;6:1861–1873. doi: 10.1091/mbc.6.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya J.A., Hobbs R.M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K.E., Wolgemuth D.J., Pandolfi P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- de Rooij D.G., Russell L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- França L.R., Ogawa T., Avarbock M.R., Brinster R.L., Russell L.D. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol. Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Garbuzov A., Pech M.F., Hasegawa K., Sukhwani M., Zhang R.J., Orwig K.E., Artandi S.E. Purification of GFRα1+ and GFRα1- spermatogonial stem cells reveals a niche-dependent mechanism for fate determination. Stem Cell Reports. 2018;10:553–567. doi: 10.1016/j.stemcr.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia T.X., Farmaha J.K., Kow S., Hofmann M.C. RBPJ in mouse Sertoli cells is required for proper regulation of the testis stem cell niche. Development. 2014;141:4468–4478. doi: 10.1242/dev.113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gely-Pernot A., Raverdeau M., Célébi C., Dennefeld C., Feret B., Klopfenstein M., Yoshida S., Ghyselinck N.B., Mark M. Spermatogonia differentiation requires retinoic acid receptor γ. Endocrinology. 2012;153:438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- Grasso M., Fuso A., Dovere L., de Rooij D.G., Stefanini M., Boitani C., Vicini E. Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reproduction. 2012;143:325–332. doi: 10.1530/REP-11-0385. [DOI] [PubMed] [Google Scholar]
- Han I.S., Sylvester S.R., Kim K.H., Schelling M.E., Venkateswaran S., Blanckaert V.D., McGuinness M.P., Griswold M.D. Basic fibroblast growth factor is a testicular germ cell product which may regulate Sertoli cell function. Mol. Endocrinol. 1993;7:889–897. doi: 10.1210/mend.7.7.8413313. [DOI] [PubMed] [Google Scholar]
- Hara K., Nakagawa T., Enomoto H., Suzuki M., Yamamoto M., Simons B.D., Yoshida S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–672. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Namekawa S.H., Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells. 2013;31:2517–2527. doi: 10.1002/stem.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Saga Y. FGF8-FGFR1 signaling acts as a niche factor for maintaining undifferentiated spermatogonia in the mouse. Biol. Reprod. 2014;91:145. doi: 10.1095/biolreprod.114.121012. [DOI] [PubMed] [Google Scholar]
- Hogarth C.A., Amory J.K., Griswold M.D. Inhibiting vitamin A metabolism as an approach to male contraception. Trends Endocrinol. Metab. 2011;22:136–144. doi: 10.1016/j.tem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C.A., Evanoff R., Mitchell D., Kent T., Small C., Amory J.K., Griswold M.D. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biol. Reprod. 2013;88:40. doi: 10.1095/biolreprod.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C.A., Evans E., Onken J., Kent T., Mitchell D., Petkovich M., Griswold M.D. CYP26 enzymes are necessary within the postnatal seminiferous epithelium for normal murine spermatogenesis. Biol. Reprod. 2015;93:19. doi: 10.1095/biolreprod.115.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C.A., Arnold S., Kent T., Mitchell D., Isoherranen N., Griswold M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015;92:37. doi: 10.1095/biolreprod.114.126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikami K., Tokue M., Sugimoto R., Noda C., Kobayashi S., Hara K., Yoshida S. Hierarchical differentiation competence in response to retinoic acid ensures stem cell maintenance during mouse spermatogenesis. Development. 2015;142:1582–1592. doi: 10.1242/dev.118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kuroki S., Akiyoshi M., Ideguchi K., Kitano S., Miyachi H., Hirose M., Mise N., Abe K., Ogura A., Tachibana M. Development of a general-purpose method for cell purification using Cre/loxP-mediated recombination. Genesis. 2015;53:387–393. doi: 10.1002/dvg.22863. [DOI] [PubMed] [Google Scholar]
- Marui A., Tabata Y., Kojima S., Yamamoto M., Tambara K., Nishina T., Saji Y., Inui K., Hashida T., Yokoyama S. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ. J. 2007;71:1181–1186. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- Meng X., Lindahl M., Hyvönen M.E., Parvinen M., de Rooij D.G., Hess M.W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Mullaney B.P., Skinner M.K. Basic fibroblast growth factor (bFGF) gene expression and protein production during pubertal development of the seminiferous tubule: follicle-stimulating hormone-induced Sertoli cell bFGF expression. Endocrinology. 1992;131:2928–2934. doi: 10.1210/endo.131.6.1446630. [DOI] [PubMed] [Google Scholar]
- Nagai R., Shinomura M., Kishi K., Aiyama Y., Harikae K., Sato T., Kanai-Azuma M., Kurohmaru M., Tsunekawa N., Kanai Y. Dynamics of GFRα1-positive spermatogonia at the early stages of colonization in the recipient testes of W/Wν male mice. Dev. Dyn. 2012;241:1374–1384. doi: 10.1002/dvdy.23824. [DOI] [PubMed] [Google Scholar]
- Nagano M., Avarbock M.R., Brinster R.L. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Sharma M., Nabeshima Y., Braun R.E., Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariola H., Saarma M. Novel functions and signalling pathways for GDNF. J. Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Smith E.P., Hall S.H., Monaco L., French F.S., Wilson E.M., Conti M. A rat Sertoli cell factor similar to basic fibroblast growth factor increases c-fos messenger ribonucleic acid in cultured Sertoli cells. Mol. Endocrinol. 1989;3:954–961. doi: 10.1210/mend-3-6-954. [DOI] [PubMed] [Google Scholar]
- Sugimoto R., Nabeshima Y., Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech. Dev. 2012;128:610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Sada A., Yoshida S., Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tabata Y., Hijikata S., Muniruzzaman M., Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J. Biomater. Sci. Polym. Ed. 1999;10:79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y., Yomogida K., Ohta H., Tohda A., Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Takashima S., Kanatsu-Shinohara M., Tanaka T., Morimoto H., Inoue K., Ogonuki N., Jijiwa M., Takahashi M., Ogura A., Shinohara T. Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports. 2015;4:489–502. doi: 10.1016/j.stemcr.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A., Kishi K., Aiyama Y., Miura K., Takase H.M., Suzuki H., Kanai-Azuma M., Iwamori T., Kurohmaru M., Tsunekawa N. In vivo dynamics of GFRα1-positive spermatogonia stimulated by GDNF signals using a bead transplantation assay. Biochem. Biophys. Res. Commun. 2016;476:546–552. doi: 10.1016/j.bbrc.2016.05.160. [DOI] [PubMed] [Google Scholar]
- Ueda H., Hong L., Yamamoto M., Shigeno K., Inoue M., Toba T., Yoshitani M., Nakamura T., Tabata Y., Shimizu Y. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23:1003–1010. doi: 10.1016/s0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- Uehara M., Yashiro K., Mamiya S., Nishino J., Chambon P., Dolle P., Sakai Y. CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev. Biol. 2007;302:399–411. doi: 10.1016/j.ydbio.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ikada Y., Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Takahashi Y., Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12:1305–1311. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang S., Wang X., Liao S., Wu Y., Han C. Endogenously produced FGF2 is essential for the survival and proliferation of cultured mouse spermatogonial stem cells. Cell Res. 2012;22:773–776. doi: 10.1038/cr.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Sutliff R.L., Paul R.J., Lorenz J.N., Hoying J.B., Haudenschild C.C., Yin M., Coffin J.D., Kong L., Kranias E.G. Fibroblast growth factor 2 control of vascular tone. Nat. Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohni K., Zhang X., Tan S.L., Chan P., Nagano M.C. The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum. Reprod. 2012;27:44–53. doi: 10.1093/humrep/der357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.