CKD due to diabetes is associated with elevated risk of progressive loss of kidney function, cardiovascular disease, and premature mortality, even when treated with guideline-recommended approaches (1). The increasing prevalence coupled with the high risk of end organ damage highlights the need to search for new therapies to delay the progression of kidney and cardiovascular disease. However, the number of clinical trials undertaken to define novel therapies for patients with CKD is lacking behind other specialties. High costs, lack of global clinical trial networks, higher than average adverse event rates, and a relatively small total patient population may all contribute to this problem. Innovative approaches are thus needed to facilitate new trials in patients with CKD to improve the therapeutic armamentarium.
Some of these are already underway. Simplifying trials, reducing costs, growing networks, and developing and validating reliable earlier end points are all important (2–4). For example, large trials using extensive networks and routinely collected data and/or cluster-based approaches are being developed to test interventions in both CKD and dialysis, and they offer an opportunity to increase the efficiency of nephrology trials, allowing more to be undertaken (5).
Innovative approaches that support the simultaneous conduct of multiple trials in several related diseases with different interventions using the same infrastructure, while at the same time, assigning patients to therapies to which they mostly likely respond are an attractive future direction. To address this need, trial designs have been developed that evaluate more than one therapy (or combinations thereof) for a single disease or multiple related diseases, often targeting a particular biomarker-defined subgroup of patients. This type of design is also referred to as an umbrella or basket approach, or more broadly, it is referred to as a platform trial, describing an experimental platform for assessment of multiple interventions, where site networks continuously recruit participants to a number of ongoing trials using a master protocol with novel end points and statistical approaches that might use adaptive and/or Bayesian statistics.
Platform trials are increasingly used in the oncology area, and several are currently ongoing. The Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2 Trial is an exploratory, phase 2 platform in which patients are randomized to the most promising treatment on the basis of biomarker-defined subtypes (HER-2 status, hormone receptor status, and a genetic risk profile) of early-stage breast cancer (6). The Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination Trial is another example of a platform from the oncology area. On the basis of nonsmall cell lung cancer tumor markers on biopsy, patients were assigned to a treatment with the greatest potential for that individual using an adaptive randomization scheme (NCT00409968).
Various design elements of a platform trial are noteworthy. First, a shared master protocol is used for common elements of the multiple individual trials within the platform with relatively subtle trial design differences due to unique individual drug characteristics reflected in study-specific appendices, enabling sharing of clinical trial documents and procedures among trials. This facilitates clinically consistent trial conduct and increased efficiency. Second, the platform approach commonly involves some form of adaptive design to assign patients to the most promising drugs on the basis of new data accrued during the trial. In addition, the platform trial is not static, but it is flexible, which means that new promising drugs can enter the platform, while other drugs can be dropped due to lack of efficacy or adverse events. Declaring superiority or futility can be assessed continuously on the basis of data as they are accrued during the trial and is another adaptive design element.
Some challenges of platform trials should be mentioned. Developing a platform requires involvement of multiple parties who have to agree on the master protocol and clinical trial procedures and infrastructure, which involves more upfront planning and complexity than a single trial. In addition, when testing multiple therapies, one should always be aware of the possibility of chance findings. Rigorous prespecified testing procedures and appropriate robust statistical approaches should be in place to adequately address this. Finally, platform trials are an evolving area, but regulatory agencies are clearly exploring the use of this approach to accelerate the development of effective treatments (7).
The failure of at least some previous trials in nephrology may be explained by the substantial variation between individual patients in the factors driving disease progression and response to treatment as well as adverse events. The variation in drug response is not yet widely recognized, and therefore, another challenge for the future will be to understand whether it is possible to more efficiently test new interventions, simultaneously identifying those patients in whom the beneficial effects are maximal and side effects minimal (an approach commonly described as personalized medicine) (8).
Platform trials in oncology as well as other areas, such as Alzheimer disease, already use personalized medicine approaches, and they commonly allocate patients to interventions on the basis of genetic or biomarker data predicting the response to new therapies before a patient is exposed to the intervention. Given the paucity of predictive response biomarkers in CKD, it may be possible to assign patients to continue an intervention or switch to another arm on the basis of the response of a biomarker after a few weeks of treatment. This can be done using an efficacy marker, such as albuminuria, or a broader set of risk markers, because many drug have multiple effects (9)
An example of a clinical trial in which participants are selected on the basis of their response to the investigational therapy is the Study Of diabetic Nephropathy with AtRasentan (SONAR) Trial (10). The SONAR Trial aims to determine whether the endothelin receptor antagonist atrasentan is renoprotective in diabetic kidney disease. All participants were exposed to atrasentan for 6 weeks, and those in whom albuminuria is decreased >30% and in whom there are no clinical signs of sodium retention were randomized to double-blind atrasentan or placebo. The randomization of over 1000 patients in whom albuminuria did not fall (nonresponders) will help assess the value of this enrichment approach. The study has been discontinued due to a lower than expected event rate, and therefore, it remains to be seen what effect these design elements had in this outcome.
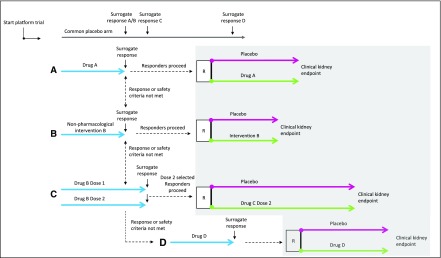
The platform approach might be a next step to advance enrichment designs. Within the platform, various phase 2 and 3 studies can be done, thereby providing an effective clinical trial infrastructure for identifying new therapies. Moreover, within the platform, individual patients may be able to move on to a new intervention depending whether they responded to the assigned therapy, although the statistical issues involved would need careful consideration (Figure 1). A future CKD platform could, therefore, incorporate the individual therapy response and selection of best available therapy for each patient, thereby paving the way for a more personalized approach to treatment of diabetic kidney disease.
Figure 1.
Design of a hypothesized platform in kidney disease. After the platform is launched, patients are screened and randomly assigned to treatment with intervention (A–C) or placebo. The intervention can be a lifestyle intervention or pharmacologic intervention. A common placebo arm is used in this example, which can increase clinical trial efficiency, although challenges with respect to blinding and multiple end point assessments have to be appropriately addressed. During the first phase of the hypothesized platform, the effect of the intervention is tested on a surrogate outcome to select promising therapies and/or optimal doses for a confirmatory clinical end point trial. Individuals who respond to the intervention according to a predefined surrogate response threshold and who tolerate the intervention will proceed to the confirmatory trial (gray shaded area); they will be randomly assigned to placebo or active therapy and followed for the remainder of the trial until the prespecified number of clinical end points is accrued. Individuals who do not show a predefined surrogate response (nonresponders) or in whom tolerability or safety issues arise will not be randomized to their first assigned intervention; they will rotate and be exposed to therapy with other interventions available in the platform. New drugs can enter the platform at any time (intervention D in the hypothesized platform), whereas futile therapies or doses considered to be inefficacious can be dropped from the platform. R, randomization.
Platform trials offer a potential solution to improve the number of trials in nephrology and may help to accelerate efforts to tailor effective therapies to particular subgroup of patients most likely to respond. This requires a global collaborative approach, and it is being explored through the International Society of Nephrology Advancing Clinical trials initiative (4), which is working with multiple parties willing to make the primary priority improvements in the care of patients with CKD.
Disclosures
H.J.L.H. is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck. He has a policy of all honoraria being paid to his employer. V.P. serves on steering committees for trials funded by AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer and serves on advisory boards and/or has spoken at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. He has a policy of all honoraria being paid to his employer.
Acknowledgments
The content of this article does not reflect the views or opinions of the American Society of Nephrology (ASN) or the Clinical Journal of the American Society of Nephrology (CJASN). Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.International Diabetes Federation: IDF Diabetes Atlas, 8th Ed., Brussels, Belgium, International Diabetes Federation, 2017 [Google Scholar]
- 2.Tong A, Craig JC, Nagler EV, Van Biesen W; SONG Executive Committee and the European Renal Best Practice Advisory Board; SONG Executive Committee and the European Renal Best Practice Advisory Board: Composing a new song for trials: The Standardized Outcomes in Nephrology (SONG) initiative. Nephrol Dial Transplant 32: 1963–1966, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ, Roy-Chaudhury P: Fostering innovation, advancing patient safety: The kidney health initiative. Clin J Am Soc Nephrol 8: 1609–1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Society of Nephrology: Advancing Clinical Trials, 2018. Available at: https://www.theisn.org/research/isn-act. Accessed March 23, 2018
- 5.Bond S, Payne R, Wilson E, Chowdry A, Caskey F, Wheeler D, Hiemstra T: Using the UK renal registry for a clinical trial in dialysis patients: The example of SIMPLIFIED. Trials 16[Suppl 2]: O15, 2015 [Google Scholar]
- 6.Park JW, Liu MC, Yee D, Yau C, van ’t Veer LJ, Symmans WF, Paoloni M, Perlmutter J, Hylton NM, Hogarth M, DeMichele A, Buxton MB, Chien AJ, Wallace AM, Boughey JC, Haddad TC, Chui SY, Kemmer KA, Kaplan HG, Isaacs C, Nanda R, Tripathy D, Albain KS, Edmiston KK, Elias AD, Northfelt DW, Pusztai L, Moulder SL, Lang JE, Viscusi RK, Euhus DM, Haley BB, Khan QJ, Wood WC, Melisko M, Schwab R, Helsten T, Lyandres J, Davis SE, Hirst GL, Sanil A, Esserman LJ, Berry DA; I-SPY 2 Investigators: Adaptive randomization of neratinib in early breast cancer. N Engl J Med 375: 11–22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodcock J, LaVange LM: Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 377: 62–70, 2017 [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Heerspink HJ: Unmet need in diabetic nephropathy: Failed drugs or trials? Lancet Diabetes Endocrinol 4: 638–640, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Heerspink HJ, Grobbee DE, de Zeeuw D: A novel approach for establishing cardiovascular drug efficacy. Nat Rev Drug Discov 13: 942, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Andress DL, Bakris G, Brennan JJ, Correa-Rotter R, Dey J, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray J, Perkovic V, Tobe S, Wigderson M, Parving HH, de Zeeuw D: Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: A clinical trial design novel to diabetic nephropathy [published online ahead of print February 6, 2018]. Diabetes Obes Metab doi:10.1111/dom.13245 [DOI] [PMC free article] [PubMed] [Google Scholar]