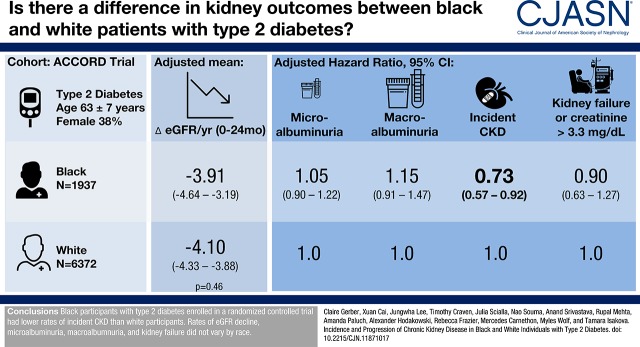
Abstract
Background and objectives
Type 2 diabetes and associated CKD disproportionately affect blacks. It is uncertain if racial disparities in type 2 diabetes-associated CKD are driven by biologic factors that influence propensity to CKD or by differences in type 2 diabetes care.
Design, setting, participants, & measurements
We conducted a post hoc analysis of 1937 black and 6372 white participants of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial to examine associations of black race with change in eGFR and risks of developing microalbuminuria, macroalbuminuria, incident CKD (eGFR<60 ml/min per 1.73m2, ≥25% decrease from baseline eGFR, and eGFR slope <−1.6 ml/min per 1.73 m2 per year), and kidney failure or serum creatinine >3.3 mg/dl.
Results
During a median follow-up that ranged between 4.4 and 4.7 years, 278 black participants (58 per 1000 person-years) and 981 white participants (55 per 1000 person-years) developed microalbuminuria, 122 black participants (16 per 1000 person-years) and 374 white participants (14 per 1000 person-years) developed macroalbuminuria, 111 black participants (21 per 1000 person-years) and 499 white participants (28 per 1000 person-years) developed incident CKD, and 59 black participants (seven per 1000 person-years) and 178 white participants (six per 1000 person-years) developed kidney failure or serum creatinine >3.3 mg/dl. Compared with white participants, black participants had lower risks of incident CKD (hazard ratio, 0.73; 95% confidence intervals, 0.57 to 0.92). There were no significant differences by race in eGFR decline or in risks of microalbuminuria, macroalbuminuria, and kidney failure or of serum creatinine >3.3 mg/dl.
Conclusions
Black participants enrolled in a randomized controlled trial had lower rates of incident CKD compared with white participants. Rates of eGFR decline, microalbuminuria, macroalbuminuria, and kidney failure did not vary by race.
Keywords: Biological Factors; Cardiovascular Diseases; chronic kidney disease; Confidence Intervals; creatinine; diabetes; Diabetes Mellitus, Type 2; Disease Progression; Follow-up Studies; glomerular filtration rate; Humans; Incidence; Renal Insufficiency; Renal Insufficiency, Chronic; risk factors
Introduction
Type 2 diabetes mellitus is a major public health problem and the leading cause of blindness, amputations and ESKD (1). Glycemic control, multifactorial risk factor modification, and self-management are the major corner stones of comprehensive treatment approaches to reduce the risks of long-term complications in patients with type 2 diabetes (2). Despite significant progress in type 2 diabetes care, retinopathy, neuropathy, nephropathy, and cardiovascular disease continue to impose a significant burden on affected individuals, their families, and the health care system (3).
The prevalence of type 2 diabetes is almost twice as high in non-Hispanic blacks as compared with non-Hispanic whites (1), and blacks have a disproportionate burden of diabetes-related complications (4). After development of CKD, blacks with type 2 diabetes have a risk of progression to ESKD that is approximately two to three-fold fold higher compared with whites (4–9). Accelerated deterioration of kidney function in black individuals with type 2 diabetes may be influenced by biologic factors that influence propensity to CKD and its severity or by differences in type 2 diabetes care (10,11). Previous studies identified genetic predisposition, low birth weight, hypertension, obesity, low socioeconomic status, poor access to high-quality health care, and high-risk health behaviors as contributors to CKD progression (4,12–15).
Randomized clinical trials provide protocol-driven care that may eliminate or reduce differences in care delivery and quality across groups, and may provide an opportunity to study racial differences in CKD development and progression. To our knowledge, an evaluation of racial differences in kidney outcomes in a type 2 diabetes population with low prevalence of CKD at baseline and within the context of a randomized controlled trial has not been performed. We conducted a post hoc analysis on a subset of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial population that self-identified as non-Hispanic white or non-Hispanic black. We examined the associations of black race with longitudinal change in eGFR and with risks of developing microalbuminuria, macroalbuminuria, incident CKD, and kidney failure or serum creatinine >3.3 mg/dl. We hypothesized that compared with white participants, black participants with type 2 diabetes who received standardized multifactorial type 2 diabetes care within the context of a randomized controlled trial would have faster eGFR decline and be at greater risk of development and progression of CKD during follow-up in the ACCORD trial.
Materials and Methods
Brief Description of the ACCORD Trial
The ACCORD trial was a randomized, multicenter, double 2×2 factorial, parallel treatment trial that enrolled 10,251 high-risk participants with type 2 diabetes across seven clinical center networks. The trial evaluated the effects of intensive versus standard glycemic control, fibrates versus placebo, and intensive versus standard BP control on major cardiovascular disease events. The study design, inclusion and exclusion criteria, predefined microvascular outcomes and their frequency of assessment, and results have been previously reported (16–18). Major eligibility criteria were age between 40 and 79 years old, known type 2 diabetes, defined according to the 1997 American Diabetes Association criteria (19), glycated hemoglobin level of 7.5% or higher, type 2 diabetes duration of >3 months, and presence of at least two cardiovascular disease risk factors, high likelihood of cardiovascular disease or history of cardiovascular disease. Individuals with serum creatinine >1.5 mg/dl within 2 months before the screening visit were excluded. Randomization occurred from 2001 to 2005. Because of increased all-cause mortality, the glycemic intervention was terminated in 2008 (17). The lipid and BP interventions were completed in 2009. Written informed consent was collected from all ACCORD trial participants, and institutional review board approval was obtained at all sites.
Study Population
We used ACCORD trial research materials obtained from the National Heart, Lung, and Blood Institute to analyze data of 8309 ACCORD trial participants who self-identified as non-Hispanic blacks (n=1937) and non-Hispanic whites (n=6372).
Exposure and Outcomes
We examined associations of black race with longitudinal change in eGFR and with time-to-development of microalbuminuria, macroalbuminuria, incident CKD, and kidney failure or serum creatinine >3.3 mg/dl (18). Among individuals with eGFR≥60 ml/min per 1.73 m2 and no microalbuminuria (<30 mg/g creatinine) at the baseline visit, we defined incident CKD as the new onset of eGFR<60 ml/min per 1.73 m2 after the baseline visit, ≥25% decrease from baseline, and an eGFR slope of <−1.6 ml/min per 1.73 m2 per year, which was the median yearly change in eGFR calculated from baseline and end of follow-up eGFR values.
Measurements and Assessment of Baseline Covariates
Standardized questionnaires at the baseline visit evaluated demographic characteristics, type 2 diabetes duration, smoking status, and medical and medication history. Height, weight, and BP were collected following a standardized protocol. eGFR was estimated from serum creatinine, measured by the Roche Creatinine Plus enzymatic method (Roche Diagnostics, Basel, Switzerland), using the CKD Epidemiology Collaboration equation (20). Urine creatinine was measured enzymatically on a Roche Double Modular P Analytics automated analyzer. Urinary albumin was measured by immunonephelometry on a Siemens BN II nephelometer. Hemoglobin A1c (HbA1c) was determined by automated high-performance liquid chromatography. Microalbuminuria and macroalbuminuria were defined as urinary albumin-to-creatinine ratio (UACR) ≥30 and ≥300 mg/g, respectively. To account for missing covariate data (smoking, 13% and retinopathy, 12%), we added a category of missing for these two covariates. The remaining covariates had minimal missing data (<5%).
Statistical Analyses
We compared baseline characteristics of the study population according to race. Statistical significance was determined using t test for continuous variables with normal distribution and chi-squared tests for categorical variables. To ascertain racial differences in delivered care within the context of the ACCORD trial, we compared achieved HbA1c according to glycemia intervention arm, achieved systolic BP according to BP intervention arm, and use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. To determine whether there were racial differences in outcome assessments either due to missed visits or differences in survival, we compared the number of serum creatinine measurements during follow-up and the incidence rate of mortality by race.
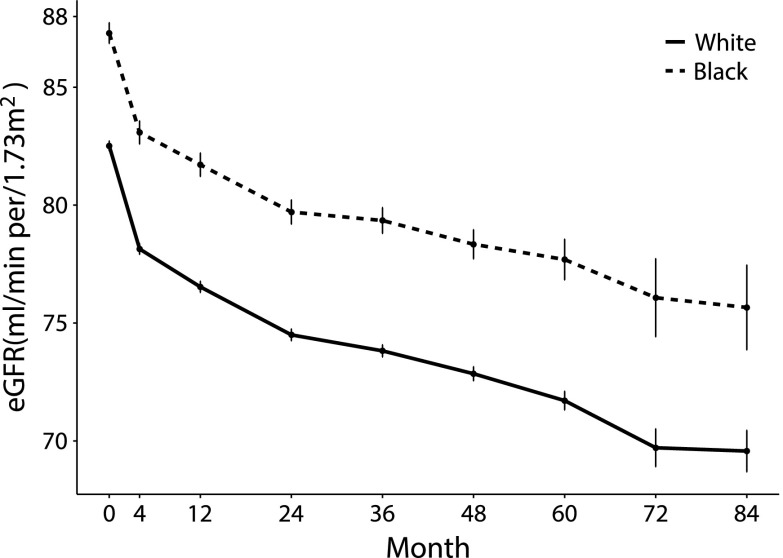
Because examination of mean eGFR during follow-up revealed that the eGFR slope varied with time (Figure 1), we used linear mixed models (21) with separate slopes for the period from baseline to 24 months and the period of 24 months to end of follow-up to examine differences between black versus white participants in longitudinal change in eGFR. To account for early hemodynamic changes in eGFR, we derived adjusted mean annualized change in eGFR during entire follow-up from a piecewise linear mixed model (21) with two knots. The two knots were positioned at month 4 and month 24, which allowed for different slopes before and after the knots. We included race×time, race×first spline for time and race×second spline for time interaction terms in the piecewise linear mixed model. All models included a random intercept for each participant and a random slope for time as a continuous variable to account for within-participant correlation. In model 1, we adjusted for trial interventions, including randomized glycemia, BP, and lipid arms, and the seven clinical center networks. In model 2, we adjusted for factors in model 1 and for demographics, including age, sex, education, and health insurance. In model 3, we adjusted for factors in model 2 and for kidney-specific factors, including baseline eGFR and presence of microalbuminuria and macroalbuminuria at baseline. In model 4, we adjusted for factors in model 3 and for other baseline clinical factors, including systolic BP, body mass index, HbA1c, smoking status, type 2 diabetes duration, history of heart failure, history of cardiovascular disease (myocardial infarction, stroke, revascularization, or angina), history of retinopathy, and use of medications, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, insulin, and thiazolidinediones.
Figure 1.
The rate of change in eGFR over time was comparable in black and white ACCORD trial participants. Mean absolute follow-up values are shown for black (dashed line) and white (solid line) participants. Error bars indicate SEM.
We applied Cox proportional-hazards regression models to analyze the associations of race with risks of microalbuminuria, macroalbuminuria, incident CKD, and kidney failure or serum creatinine >3.3 mg/dl. Follow-up time for all outcomes started at the baseline visit. We stratified the Cox models by trial interventions, including randomized glycemia, BP, and lipid arms, and the seven clinical center networks. Then, we adjusted the Cox models for the same covariates included in the linear mixed models. We report hazard ratios (HRs) with 95% confidence intervals (95% CIs) with white participants as the reference category. There was no violation of the proportional hazards assumption, using the Schoenfeld residual, for the effect of race.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided, and P values <0.05 were considered statistically significant. Because our objective in this post hoc analysis was to obtain hypothesis-generating results, we did not adjust for multiple testing. Reported P values are of nominal significance and serve as guides for possible associations.
Results
Baseline Characteristics of ACCORD Trial Participants
Consistent with ACCORD trial eligibility criteria (17), the study population included middle-aged and older participants with type 2 diabetes and associated complications (Table 1). The mean age was 63±7 years, the mean duration of type 2 diabetes was 11±8 years, and 35% of participants had cardiovascular disease at baseline. Compared with white participants, black participants had significantly higher HbA1c levels (8.5±1.1 versus 8.2±0.9; P value <0.001) and higher prevalence of retinopathy (16% versus 10%; P value <0.001). History of cardiovascular disease was more common in white compared with black participants (37% versus 30%; P value <0.001), despite lower systolic BP in white participants (135±16 versus 139±17; P value <0.001). There were no significant differences by race in allocation to intensive glycemic control arm. Imbalances in other trial interventions were expected because of the 2×2 factorial design, which entailed that a nonrandom subset of participants was included in the lipid trial and the remaining participants were included in the BP trial.
Table 1.
Baseline characteristics of black and white ACCORD trial participants
| Variable | Black Participants n=1937 | White Participants n=6372 |
|---|---|---|
| Age, yr | 62±6 | 63±7 |
| Female, n (%) | 967 (50) | 2156 (34) |
| Education | ||
| Less than high school graduate, n (%) | 401 (21) | 631 (10) |
| High school graduate (or GED), n (%) | 591 (31) | 1635 (26) |
| Some college or technical school, n (%) | 615 (32) | 2286 (36) |
| College graduate or more, n (%) | 326 (17) | 1817 (29) |
| Insurance coverage, n (%) | 1622 (84) | 5746 (90) |
| Systolic BP, mm Hg | 139±17 | 135±16 |
| Body mass index, kg/m2 | 32±5 | 33±5 |
| Hemoglobin A1c, % | 8.5±1.1 | 8.2±0.9 |
| Current smoking, n (%) | 792 (48) | 3168 (57) |
| Duration of diabetes, yr | 11±8 | 11±7 |
| Heart failure, n (%) | 91 (5) | 329 (5) |
| Cardiovascular disease, n (%) | 580 (30) | 2357 (37) |
| Baseline retinopathy, n (%) | 260 (16) | 588 (10) |
| Baseline eGFR, ml/min per 1.73 m2 | 87±19 | 83±17 |
| Baseline serum creatinine, mg/dl | 1.0±0.2 | 0.9±0.2 |
| Microalbuminuria, n (%) | 525 (28) | 1541 (25) |
| Macroalbuminuria, n (%) | 151 (8) | 370 (6) |
| Urinary albumin-to-creatinine ratio, mg/g | 15 (7–59) | 14 (7–42) |
| Prevalent CKD, n (%) | 756 (39) | 2337 (37) |
| Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, n (%) | 1392 (72) | 4424 (70) |
| Insulin use, n (%) | 390 (20) | 1280 (20) |
| Thiazolidinedione use, n (%) | 348 (18) | 1511 (24) |
| Randomization to intensive glycemic control arm, n (%) | 989 (51) | 3184 (50) |
| Randomization to intensive BP arm, n (%) | 546 (28) | 1411 (22) |
| Randomization to fenofibrate arm, n (%) | 387 (20) | 1812 (28) |
Value presented as number (percentage), mean±SD, or median (interquartile range). ACCORD, Action to Control Cardiovascular Risk in Diabetes; GED, General Equivalency Diploma.
In the entire study population, the average baseline eGFR was 84±17 ml/min per 1.73 m2. Compared with white participants, black participants had higher baseline eGFR (87±19 ml/min per 1.73 m2 versus 83±17 ml/min per 1.73 m2; P value <0.001). In contrast, microalbuminuria and macroalbuminuria were more common in black compared with white participants (28% versus 25% and 8% versus 6%; P value =0.02 and 0.002, respectively). The prevalence of CKD, as defined by eGFR<60 ml/min per 1.73 m2 or UACR≥30 mg/g at the baseline visit, was similar in black and white participants (39% versus 37%; Table 1).
Achievement of HbA1c and Systolic BP Targets, Use of Renoprotective Medications, Number of Follow-Up Serum Creatinine Assessments, and Survival
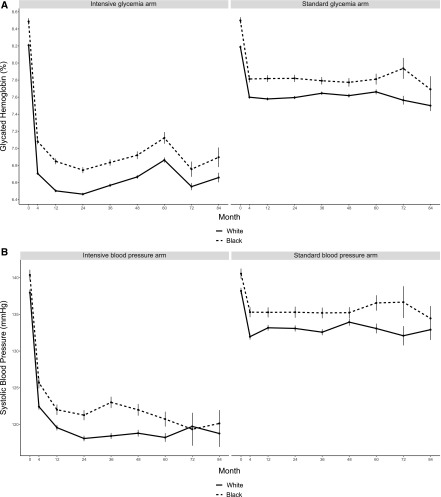
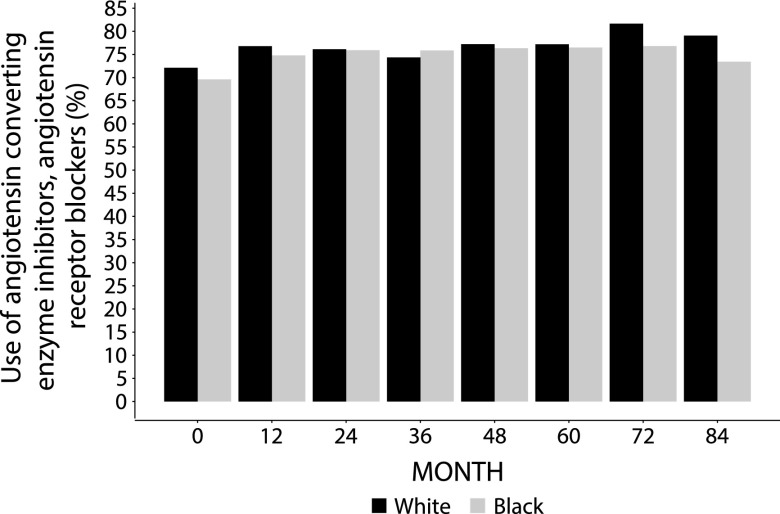
Although mean values were modestly higher among black participants compared with white participants, rapid achievement of HbA1c and systolic BP targets was accomplished in black and white participants, and these targets were maintained in both groups during follow-up (Figure 2). Throughout the duration of the trial, use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers was similar between white and black participants (Figure 3). The average number of serum creatinine levels obtained during follow-up was comparable in black and white participants (ten versus 11), and there was no difference between black participants and white participants in the incidence rate of mortality during the study (14 events per 1000 person-years follow-up; 95% CI, 12 to 17 versus 16 events per 1000 person-years follow-up; 95% CI, 14 to 17; P value =0.35).
Figure 2.
Intended reductions in mean HbA1C (A) and mean systolic BP (B) were attained in black and white ACCORD trial participants. Mean absolute follow-up values for HbA1c (A) and systolic BP (B) are shown for black (dashed line) and white (solid line) participants. Error bars indicate SEM.
Figure 3.
Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers at each study visit did not differ by race in ACCORD trial participants. Percentage of use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers by month in black (gray bars) and white (black bars) participants.
Change in eGFR over Time
Because the rate of change in eGFR was steeper between baseline and month 24 compared with the rest of follow-up, in addition to testing for differences in changes during the entire follow-up, we examined slopes before and after 24 months (Figure 1). Unadjusted and adjusted mean annualized changes in eGFR from baseline to month 24 and from month 24 through to the end of follow-up are summarized according to race in Table 2. During both time segments, unadjusted and adjusted eGFR slopes did not differ significantly between the groups. Similarly, adjusted mean annualized change in eGFR during entire follow-up, derived from a piecewise linear mixed model with two knots at month 4 and month 24, was comparable in black and white participants (−1.45 and −1.79 ml/min per 1.73 m2 per year, respectively; P value =0.18).
Table 2.
Mean annualized change in eGFR by time periods in black and white ACCORD trial participants
| Mean Annualized Change of eGFR, ml/min per 1.73 m2 per yr (95% Confidence Interval) | P Value | ||
|---|---|---|---|
| Model | Black, n=1937 | White, n=6372 | |
| Month 0–Month 24 | |||
| Unadjusted | −3.59 (−4.21 to −2.98) | −3.94 (−4.13 to −3.74) | 0.11 |
| Model 1 | −3.63 (−4.24 to −3.02) | −3.98 (−4.17 to −3.78) | 0.11 |
| Model 2 | −3.65 (−4.26 to −3.04) | −3.98 (−4.17 to −3.79) | 0.12 |
| Model 3 | −3.71 (−4.36 to −3.07) | −3.89 (−4.09 to −3.68) | 0.44 |
| Model 4 | −3.91 (−4.64 to −3.19) | −4.10 (−4.33 to −3.88) | 0.46 |
| Black, n=1862 | White, n=6158 | ||
| Month 24– End of Follow-Up | |||
| Unadjusted | −0.44 (−0.80 to −0.08) | −0.38 (−0.49 to −0.26) | 0.63 |
| Model 1 | −0.46 (−0.81 to −0.10) | −0.39 (−0.51 to −0.28) | 0.62 |
| Model 2 | −0.45 (−0.81 to −0.09) | −0.39 (−0.51 to −0.28) | 0.67 |
| Model 3 | −0.46 (−0.82 to −0.09) | −0.42 (−0.54 to −0.31) | 0.79 |
| Model 4 | −0.38 (−0.77 to 0.01) | −0.43 (−0.56 to −0.31) | 0.69 |
Model 1: adjusts for glycemia trial, intensive BP trial, standard BP trial, lipid fenofibrate trial, network. Model 2: adjusts for factors in model 1 and for demographics: age, female, education, and health insurance. Model 3: adjusts for factors in model 2 and for kidney-specific factors: baseline eGFR, microalbuminuria, and macroalbuminuria. Model 4: adjusts for factors in model 3 and for other baseline clinical factors: systolic BP, body mass index, hemoglobin A1c, smoking status, type 2 diabetes duration, history of heart failure, history of cardiovascular disease (myocardial infarction, stroke, revascularization, or angina), history of retinopathy, and baseline use of medications (angiotensin-converting enzymes, angiotensin receptor blockers, insulin, and thiazolidinediones). ACCORD, Action to Control Cardiovascular Risk in Diabetes.
Development and Progression of CKD
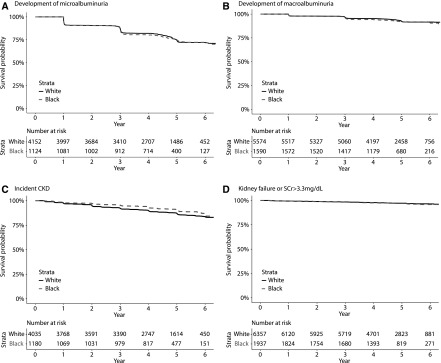
During a median follow-up that ranged between 4.4 and 4.7 years, 278 black participants (58 per 1000 person-years) and 981 white participants (55 per 1000 person-years) developed microalbuminuria, 122 black participants (16 per 1000 person-years) and 374 white participants (14 per 1000 person-years) developed macroalbuminuria, 111 black participants (21 per 1000 person-years) and 499 white participants (28 per 1000 person-years) developed incident CKD, and 59 black participants (seven per 1000 person-years) and 178 white participants (six per 1000 person-years) developed kidney failure or serum creatinine >3.3 mg/dl (Table 3). In unadjusted analyses, compared with white participants, black participants were not at increased risk of microalbuminuria, macroalbuminuria, and kidney failure or serum creatinine >3.3 mg/dl, and their risk for incident CKD was reduced (Figure 4, Table 3). After multivariable adjustment, risks of development of microalbuminuria, macroalbuminuria, and kidney failure or serum creatinine >3.3 mg/dl did not differ significantly between the two groups (Table 3). Adjusted HRs for incident CKD showed that black participants had lower risks of these events compared with white participants (Table 3).
Table 3.
Risks of kidney outcomes in black versus white ACCORD participants
| Outcomes | Development of Microalbuminuria (UAlb≥30 mg/g)a | Development of Macroalbuminuria (UAlb≥300 mg/g)b | Incident CKDa | Kidney Failure or SCr>3.3 mg/dl | ||||
|---|---|---|---|---|---|---|---|---|
| N events/total N | 1259/5276 | 496/7164 | 610/5215 | 237/8294 | ||||
| N events/total N, black | 278/1124 | 122/1590 | 111/1180 | 59/1937 | ||||
| N events/total N, white | 981/4152 | 374/5574 | 499/4035 | 178/6357 | ||||
| Median follow-up time, yr | 4.4 | 4.7 | 4.7 | 4.7 | ||||
| Incidence rate events per 1000 person-years follow-up (95% confidence interval) | Incidence Rate | P Value | Incidence Rate | P Value | Incidence Rate | P Value | Incidence Rate | P Value |
| Black | 58 (51 to 65) | 0.48 | 16 (14 to 20) | 0.18 | 21 (18 to 26) | 0.008 | 7 (5 to 9) | 0.48 |
| White | 55 (52 to 58) | 14 (13 to 16) | 28 (26 to 31) | 6 (5 to 7) | ||||
| Hazard Ratio | P Value | Hazard Ratio | P Value | Hazard Ratio | P Value | Hazard Ratio | P Value | |
| Unadjusted | 1.04 (0.91 to 1.19) | 0.53 | 1.15 (0.94 to 1.41) | 0.17 | 0.76 (0.62 to 0.93) | 0.009 | 1.11 (0.83 to 1.49) | 0.50 |
| Model 1 | 1.01 (0.87 to 1.17) | 0.91 | 1.08 (0.87 to 1.35) | 0.50 | 0.76 (0.60 to 0.95) | 0.02 | 0.94 (0.69 to 1.29) | 0.71 |
| Model 2 | 1.01 (0.87 to 1.17) | 0.92 | 1.13 (0.90 to 1.42) | 0.28 | 0.70 (0.56 to 0.88) | 0.002 | 0.94 (0.68 to 1.31) | 0.73 |
| Model 3 | 1.06 (0.91 to 1.23) | 0.48 | 1.15 (0.92 to 1.45) | 0.23 | 0.72 (0.57 to 0.90) | 0.005 | 0.93 (0.67 to 1.31) | 0.69 |
| Model 4 | 1.05 (0.90 to 1.22) | 0.57 | 1.15 (0.91 to 1.47) | 0.25 | 0.73 (0.57 to 0.92) | 0.009 | 0.90 (0.63 to 1.27) | 0.53 |
Model 1: stratified by glycemia trial, intensive BP trial, standard BP trial, lipid fenofibrate trial, lipid placebo trial, and network. Model 2: stratified by glycemia trial, intensive BP trial, standard BP trial, lipid fenofibrate trial, lipid placebo trial, and network, and adjusts for demographics: age, female, education, and health insurance. Model 3: stratified by glycemia trial, intensive BP trial, standard BP trial, lipid fenofibrate trial, lipid placebo trial, and network, and adjusts for factors in model 2 and for kidney-specific factors: baseline eGFR, microalbuminuria, and macroalbuminuria. Model 4: stratified by glycemia trial, intensive BP trial, standard BP trial, lipid fenofibrate trial, lipid placebo trial, and network, and adjusts for factors in model 3 and for other baseline clinical factors: systolic BP, body mass index, hemoglobin A1c, smoking status, type 2 diabetes duration, history of heart failure, history of cardiovascular disease (myocardial infarction, stroke, revascularization, or angina), history of retinopathy, and baseline use of medications (angiotensin-converting enzymes, angiotensin receptor blockers, insulin, and thiazolidinedione). ACCORD, Action to Control Cardiovascular Risk in Diabetes; UAlb, urinary albumin; SCr, serum creatinine.
No microalbuminuria, macroalbuminuria adjustment in model 3.
No macroalbuminuria adjustment in model 3.
Figure 4.
Compared to white ACCORD trial participants, blacks were not at increased risk of microalbuminuria, macroalbuminuria, and kidney failure, and their risk for incident CKD was reduced. Proportion of black (dashed line) and white (solid line) ACCORD trial participants free from (A) microalbuminuria, (B) macroalbuminuria, (C) incident CKD, and (D) kidney failure or serum creatinine >3.3 mg/dl.
Discussion
In this secondary analysis of the ACCORD trial, contrary to our hypothesis, we found that during a median follow-up period of 4–5 years, black race was not associated with accelerated eGFR decline, and that, compared with white participants, black participants had lower rates of incident CKD. Despite higher prevalence of microalbuminuria and macroalbuminuria in black compared with white participants at baseline, during follow-up there were no racial differences in the development of albuminuria. Although available follow-up time was limited, we did not identify any significant differences between groups in risk of progression of established CKD to kidney failure or serum creatinine >3.3 mg/dl. Our results suggest that delivery of standardized type 2 diabetes care before development of CKD may lead to similar short-term kidney outcomes in black and white individuals with type 2 diabetes. These findings have implications for treatment strategies aimed at prevention and management of CKD in type 2 diabetes.
The disproportionate burden of kidney disease in blacks with type 2 diabetes is well described (4–15). Epidemiologic studies have demonstrated that compared with whites, blacks are more likely to have albuminuria, experience early kidney function decline, and progress rapidly to ESKD (4–12). According to US Renal Data System, a national data registry on ESKD population in the United States, rates of ESKD due to diabetes are nearly three-fold greater among blacks compared with whites (22). Although many factors likely contribute to racial disparities in type 2 diabetes-associated CKD (4,12–15), recent discoveries of the association of kidney risk variants in the gene encoding apoL1 with accelerated progression of CKD in those with and without diabetes (23) have refocused attention on the effect of genetic background. Prior studies demonstrating persistent racial disparities in settings of comparable health care access (7,24–26) have also implicated the primacy of biologic factors over factors related to delivery of type 2 diabetes care.
By demonstrating that black and white ACCORD trial participants had comparable kidney outcomes, we now provide evidence in support of the beneficial effects of comprehensive type 2 diabetes care on eradicating racial disparities in development and progression of type 2 diabetes-associated CKD. Our findings differ from earlies studies (7,24–26), likely because we studied a population with low prevalence of CKD and within the context of a randomized clinical trial, which assured that existing standard of care was delivered to all participants. We found that, in spite of having more risk factors for adverse CKD outcomes (higher HbA1 and BP, higher prevalence of retinopathy and albuminuria), black participants had lower rates of eGFR-driven end points, and there were no racial differences in development of albuminuria-driven end points and of kidney failure or serum creatinine >3.3 mg/dl. Although higher eGFR in black participants at onset of follow-up may explain the reduced risks of eGFR-driven end points that we observed (27), the totality of our findings suggests that strategies that effectively deliver standardized type 2 diabetes care may reduce racial disparities in type 2 diabetes-associated CKD. This hypothesis is supported by 54% reduction in rate of ESKD in American Indians and Alaska Natives with type 2 diabetes after implementation of the Indian Health Service first Diabetes Standards of Care (28). By actively decreasing BP and HbA1c, implementing routine reporting of eGFR and yearly monitoring of UACR and prescription of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (29), in addition to providing clinical education programs and culturally relevant patient education materials (30), the program eradicated long-standing disparities in kidney outcomes in American Indians and Alaska Natives with type 2 diabetes. Another setting where equivalent access to health care was associated with absence of racial differences in the incidence of coronary heart disease and ischemic stroke is the US Veterans Health Administration (31). Reports of comparable compliance with achievement of quality of care indicators for black and white patients with stages 3 and 4 CKD receiving care in the Department of Defense health system are also encouraging (32), and suggest that delivery of standardized preventative care is achievable.
Strengths of our analysis include evaluation of multiple kidney outcomes, including change in eGFR, incident albuminuria, strictly defined incident CKD, and kidney failure, which we assessed during a median follow-up time of 4–5 years. Additionally, we were able to adjust for multiple covariates, including baseline retinopathy and type 2 diabetes duration. We acknowledge some limitations. Because we conducted a post hoc analysis, our results are hypothesis generating. Our findings are further limited by the low prevalence of CKD in the study population and by the short duration of follow-up. The ACCORD trial was terminated early because of the higher mortality in the group receiving intensive glycemic therapy, which resulted in limited total number of kidney failure events (17). However, we were able to evaluate other kidney outcomes. We were not able to assess differences in access to and quality of care before trial enrollment. Nevertheless, despite possible differences in antecedent care, we did not find racial differences in outcomes during the trial. To estimate kidney function, we used the CKD Epidemiology Collaboration equation. Although this method is likely imprecise for our study population (33,34), we relied on change in eGFR for our outcome definitions. Therefore, inherent error of cross-sectional eGFR assessments was likely minimized. Finally, this study is limited to middle-aged and older patients with type 2 diabetes who are at high risk of cardiovascular disease and who enrolled in a clinical trial. Our results may not be generalizable to a younger diabetic population, patients with nondiabetic kidney disease and to diabetic individuals in the community who are unlikely to volunteer to participate in a study.
We found that in a large racially diverse cohort of adults with type 2 diabetes and low prevalence of CKD who received protocol-driven type 2 diabetes care, black race was not associated with accelerated development and progression of CKD. The results suggest that delivery of standardized care for patients with type 2 diabetes may reduce racial disparities in type 2 diabetes-associated CKD. Future studies are needed to determine whether results similar to those achieved in the American Indians and Alaska Natives with type 2 diabetes could be achieved for the black race with delivery of standardized type 2 diabetes care.
Disclosures
T.I. received grant support from Shire. M.S.W. has served as a consultant or received honoraria from Akebia, Amag, Amgen, Ardelyx, Diasorin, Incyte, Keryx, Luitpold, Pfizer, Sanofi, and Ultragenyx and received grant support from Shire. R.M. has interest in Abbot Laboratories, AbbVie, Inc. and Teva Pharmaceuticals Industries Ltd.
Acknowledgments
This work was supported by grants R01DK102438 (T.I.), R01DK110087 (T.I.), R01DK081374 (M.S.W.), R01DK076116 (M.S.W.), R01DK094796 (M.S.W.), K24DK093723 (M.S.W.), U01DK099930 (M.S.W. and T.I.), and T32DK007169 (C.G.) from the National Institutes of Health, a Strategically Focused Research Network Center Grant on Health Disparities from the American Heart Association (M.R.C., M.S.W., and T.I.), and a Donald E. Wesson Research Fellowship from the American Society of Nephrology (C.G.).
This manuscript was prepared using Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial research materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ACCORD trial or the NHLBI. The funders had no role in the design, analysis, or interpretation of data, preparation and review of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Race in America: What Does It Mean for Diabetes and CKD?,” on pages 829–830.
References
- 1.US Department of Health and Human Services Centers for Disease Control and Prevention: National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2014. Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed July 19, 2017
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) : Management of hyperglycaemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55: 1577–1596, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention: Chronic disease prevention and health promotion, 2016. Available at: https://www.cdc.gov/chronicdisease/resources/publications/aag/diabetes.htm. Accessed July 19, 2017
- 4.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL: A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: The Atherosclerosis Risk in Communities study. Arch Intern Med 159: 1777–1783, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA 268: 3079–3084, 1992 [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Ding S, Guo H, Kats A, Lamb K, Li S, Li S, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US renal data system 2012 annual data report. Am J Kidney Dis 61(1 Suppl 1): A7, e1–e476, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Young BA, Katon WJ, Von Korff M, Simon GE, Lin EH, Ciechanowski PS, Bush T, Oliver M, Ludman EJ, Boyko EJ: Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: The pathways study. J Am Soc Nephrol 16: 219–228, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Nicholas SB, Kalantar-Zadeh K, Norris KC: Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 22: 6–15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Renal Data System: USRDS annual data report: Epidemiology of kidney disease in the United States, 2016. Available at https://www.usrds.org/2016/view/Default.aspx. Accessed July 19, 2017
- 10.Patzer RE, McClellan WM: Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol 8: 533–541, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, Bottinger EP, Kenny EE, Peter I: Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol 26: 1682–1692, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedewa SA, McClellan WM, Judd S, Gutiérrez OM, Crews DC: The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol 15: 136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans K, Coresh J, Bash LD, Gary-Webb T, Köttgen A, Carson K, Boulware LE: Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant 26: 899–908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyengar SK, Sedor JR, Freedman BI, Kao WH, Kretzler M, Keller BJ, Abboud HE, Adler SG, Best LG, Bowden DW, Burlock A, Chen YD, Cole SA, Comeau ME, Curtis JM, Divers J, Drechsler C, Duggirala R, Elston R, Guo X, Huang H, Hoffmann MM, Howard BV, Ipp E, Kimmel PL, Klag MJ, Knowler WC, Kohn OF, Leak TS, Leehey DJ, Li M, Malhotra A, März W, Nair V, Nelson RG, Nicholas SB, O’Brien SJ, Pahl MV, Parekh RS, Pezzolesi MG, Rasooly RS, Rotimi CN, Rotter JI, Schelling JR, Seldin MF, Shah VO, Smiles AM, Smith MW, Taylor KD, Thameem F3, Thornley-Brown DP, Truitt BJ, Wanner C, Weil EJ, Winkler CA, Zager PG, Igo RP Jr, Hanson RL, Langefeld CD; Family Investigation of Nephropathy and Diabetes (FIND) : Genome-wide association and trans-ethnic meta-snalysis for advanced diabetic kidney disease: Family Investigation of Nephropathy and Diabetes (FIND). PLoS Genet 11: e1005352, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crook ED, Patel SR: Diabetic nephropathy in African-American patients. Curr Diab Rep 4: 455–461, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD; ACCORD Study Group : Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am J Cardiol 99[12A]: 21i–33i, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT; Action to Control Cardiovascular Risk in Diabetes Study Group : Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD trial group : Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association: Clinical practice recommendations 1997. Diabetes Care 20[Suppl 1]: S1–S70, 1997 [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzmaurice G, Laird NM, Ware JH: Applied Longitudinal Analysis, New York, Wiley, 2004 [Google Scholar]
- 22.Ozieh MN, Dismuke CE, Lynch CP, Egede LE: Medical care expenditures associated with chronic kidney disease in adults with diabetes: United States 2011. Diabetes Res Clin Pract 109: 185–190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young BA, Maynard C, Boyko EJ: Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 26: 2392–2399, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Choi AI, Karter AJ, Liu JY, Young BA, Go AS, Schillinger D: Ethnic differences in the development of albuminuria: The DISTANCE study. Am J Manag Care 17: 737–745, 2011 [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJ, Solomon SD, Levey AS, Ivanovich P, Eckardt KU, Kewalramani R, Toto R, Pfeffer MA: Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. Am Heart J 170: 322–329, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Isakova T, Craven TE, Lee J, Scialla JJ, Xie H, Wahl P, Marcovina SM, Byington RP, Wolf M: Fibroblast growth factor 23 and incident CKD in type 2 diabetes. Clin J Am Soc Nephrol 10: 29–38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullock A, Burrows NR, Narva AS, Sheff K, Hora I, Lekiachvili A, Cain H, Espey D: Vital signs: Decrease in incidence of diabetes-related end-stage renal disease among American Indians/Alaska natives - United States, 1996-2013. MMWR Morb Mortal Wkly Rep 66: 26–32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narva AS: Reducing the burden of chronic kidney disease among American Indians. Adv Chronic Kidney Dis 15: 168–173, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Sequist TD, Cullen T, Acton KJ: Indian health service innovations have helped reduce health disparities affecting american Indian and alaska native people. Health Aff (Millwood) 30: 1965–1973, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K: Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 132: 1538–1548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao SW, Oliver DK, Das N, Hurst FP, Lentine KL, Agodoa LY, Sawyers ES, Abbott KC: Assessment of racial disparities in chronic kidney disease stage 3 and 4 care in the department of defense health system. Clin J Am Soc Nephrol 3: 442–449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delanaye P, Mariat C, Maillard N, Krzesinski JM, Cavalier E: Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol 6: 906–912, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, Nelson RG, Van Deventer M, Wang HY, Zuo L, Zhang YL, Levey AS: Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 79: 555–562, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]