Abstract
Background and objectives
Deficiency of essential trace elements and excess of potentially toxic trace elements are common in patients on hemodialysis. Whether these abnormalities are associated with poor outcomes is unknown but worth investigating, because they are potentially treatable.
Design, setting, participants, & measurements
We did a prospective longitudinal study of 1278 patients on incident hemodialysis, assessing blood concentrations of 25 trace elements at baseline. We used adjusted logistic regression to evaluate the association between trace element status and four outcomes (death, cardiovascular events, systemic infection, and hospitalization). A priori hypotheses concerned (1) deficiency of zinc, selenium, and manganese and (2) excess of lead, arsenic, and mercury. Concentrations of the other 19 elements were tested in hypothesis-generating analyses.
Results
Over 2 years of follow-up, 260 (20%) patients died, 285 (24%) experienced a cardiovascular event, 117 (10%) were hospitalized for systemic infection, and 928 (77%) were hospitalized for any cause. Lower concentrations of zinc or manganese and higher concentrations of lead, arsenic, or mercury were not independently associated with higher risk of clinical outcomes. Lower concentrations of selenium were strongly and independently associated with death (odds ratio, 0.86 per decile; 99.2% confidence interval, 0.80 to 0.93) and all-cause hospitalization (odds ratio, 0.92 per decile; 99.2% confidence interval, 0.86 to 0.98). In exploratory analyses, higher copper concentrations were significantly associated with higher risk of death (odds ratio, 1.07 per decile; 99.2% confidence interval, 1.00 to 1.15), and cadmium levels in the highest decile were associated with higher risk of death (odds ratio, 1.89; 99.2% confidence interval, 1.06 to 3.38).
Conclusions
Lower levels of zinc or manganese and higher concentrations of lead, arsenic, or mercury were not associated with higher risk of clinical outcomes, but lower concentrations of selenium were strongly and independently associated with the risks of death and hospitalization.
Keywords: Arsenic, Cadmium, Copper, Follow-up Studies, hemodialysis, hospitalization, Humans, Ions, Lead, Logistic Models, Longitudinal Studies, Manganese, Mercury, Prospective Studies, renal dialysis, Selenium, Trace Elements, Zinc
Introduction
Trace elements are present in parts per billion (micrograms per liter) or low parts per million (milligrams per liter) in biologic fluids or tissues (1). Patients on hemodialysis are at risk for deficiency of biologically essential trace elements, because such substances may be removed by diffusion and convection and also because these patients often have low dietary intake of these elements due to anorexia and dietary restrictions (1). For toxic elements, even small concentration gradients between blood and dialysate can lead to clinically relevant harm, which is exemplified by aluminum (2–6). Although concentrations of some trace elements are monitored in hemodialysis source water, body concentrations of these substances are rarely measured. Thus, patients on hemodialysis without significant residual kidney function are also at risk for accumulation of certain trace elements depending on dietary patterns, environmental exposures, and composition of the source water used for hemodialysis (7–9).
Multiple previous studies show that trace element status is frequently abnormal in patients on hemodialysis (10). However, relatively few have attempted to link trace element status with adverse outcomes in patients on hemodialysis (11–13). As a group, these studies have three key limitations. First, most such studies examine only a few trace elements or consider a limited number of clinical outcomes. Second, most of these studies enrolled only prevalent patients (usually at a single center), which introduces the possibility of selection and survivor biases. Third, most studies were small or had limited follow-up time, reducing their statistical power.
We did a prospective longitudinal study of 1278 patients on incident hemodialysis enrolled in a multicenter cohort study by assessing blood concentrations of 25 different trace elements at baseline. Our objective was to document the independent association between trace element status and four clinically relevant outcomes (death, cardiovascular events, systemic infection, and hospitalization). We aimed to use these data to identify potential targets for intervention in subsequent studies.
Materials and Methods
Participants
Eligible participants were recruited between March 2005 and November 2012 from the Northern and Southern Alberta Renal Programs (Edmonton, Red Deer, and Calgary). Written informed consent was obtained. The relevant research ethics boards approved the study. This study is reported according to the STrengthening Reporting of OBservational studies in Epidemiology guidelines (14). Adults (≥18 years old) initiating thrice weekly in-center hemodialysis were eligible for inclusion. Participants who were not willing or able to provide informed consent were excluded. In this analysis, participants were followed for up to 2 years or until death, migration outside the study region, or withdrawal of consent, whichever came sooner. Details of the Canadian Kidney Disease Cohort Study are presented elsewhere (15).
We collected demographic variables (age, sex, ethnicity [white, Indigenous, or otherwise], employment, and comorbidities [atrial fibrillation, myocardial infarction, heart failure, hypertension, peripheral vascular disease, dementia, cerebrovascular disease, diabetes mellitus, chronic obstructive pulmonary disease, cancers, liver diseases, obesity, psychiatric illness, and substance misuse, including smoking]) and clinical management variables (followed by a nephrologist for 3 months before dialysis initiation, year of dialysis initiation, and type of access).
Trace Elements and Laboratory Methods
Twenty-five trace elements were measured: aluminum, antimony, arsenic, barium, beryllium, cadmium, cesium, chromium, cobalt, copper, iron, lead, manganese, mercury, molybdenum, nickel, platinum, selenium, silver, strontium, thallium, tungsten, uranium, vanadium, and zinc. The reference values (fifth, 50th, and 95th percentiles) for these elements were taken from healthy populations as previously described (16). Concentrations of the trace elements of interest were measured in plasma using the Agilent 8800 Triple Quadrupole ICP-MS (Agilent Technologies, Santa Clara, CA), with helium and oxygen as the reaction gases. To evaluate assay precision and accuracy, blank serum was spiked at two levels. Three runs were set up on 3 different days. In each run, calibrators and two sources of Certified Reference Materials were run before sample injections. Ten aliquots of blank serum, low-spiked serum, and high-spiked serum samples were injected on each day. The percentage of recovery and the percentage of coefficient of variation were calculated from each run (intraday precision) and from all three runs (interday precision). Full details of the laboratory methods can be found elsewhere (16).
Outcomes
Clinical outcomes were all-cause death, cardiovascular events, hospitalizations for systemic infection (termed “infection”), and all-cause hospitalizations (all ascertained at 2 years). Death was ascertained by chart review, and the other outcomes were determined by linking with Alberta Health data (Supplemental Table 1 shows specific International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Canada codes). Participants from outside Alberta (British Columbia and Ontario) were excluded from all analyses of clinical outcomes except for death, because reliable follow-up data were not available. Cardiovascular events were defined by acute myocardial infarction, percutaneous coronary angioplasty, coronary artery bypass grafting, heart failure, and stroke or transient ischemic attacks using administrative algorithms (17). Infection was defined by hospitalization for bacteremia, sepsis, and pneumonia.
Design
This was a prospective cohort study. Data were collected via participant interviews, chart reviews, and clinical databases at baseline (start of hemodialysis), month 6, and years 1 and 2. Demographics, medical history, dialysis prescription, weight, and BPs were ascertained at baseline and updated at each ensuing visit. Access and modality histories were tracked throughout follow-up. Predialysis blood samples were taken by qualified dialysis unit personnel at baseline and stored in a −80°C freezer at the Canadian Biosample Repository.
Power Calculation
Our a priori hypotheses were that lower concentrations of zinc, selenium, and manganese as well as higher concentrations of lead, arsenic, and mercury would be associated with adverse clinical outcomes. Concentrations of other elements were evaluated as hypothesis-generating analyses. The power calculation was on the basis of a priori zinc deficiency, which was defined as in our prior work. Given a sample of 1300 patients and assuming that the prevalence of abnormality (deficiency or excess) is 45%, a Cox model would achieve 90% power at an adjusted α of 0.008 (a Bonferroni correction for six elements) to detect a hazard ratio of 1.4 of mortality associated with abnormality on the basis of cumulative mortality of 40% over 2 years. If multiple comparisons are neglected, a sample size of 1000 patients would have 80% power to detect a hazard ratio of 1.3 under the same assumptions. The power calculation was not inflated for loss to follow-up, because this is minimal for the outcome of mortality in this population.
Statistical Analyses
All analyses were done in Stata/MP 13.1 (www.stata.com). Descriptive statistics were reported as counts and percentages or medians and interquartile ranges as appropriate. Although we had originally planned to use Cox regression, given that our time period is fixed, our loss to follow-up was minimal, and because Cox regression introduces informative censoring, we selected unadjusted and adjusted logistic regression a priori in its place. Adjusted models were fit using the forward stepwise procedure (P<0.05 for inclusion and P<0.05 for removal). Candidate variables were the demographic and comorbidity covariates listed earlier (and in Table 1) and baseline trace elements (as ordinal equidistant decile bins in our primary analysis) (Supplemental Table 2) that were significant in the unadjusted analyses as linear terms. Missing candidate variable values were imputed using the most frequent categorical or median continuous value. In the forward stepwise procedure, a statistical model is built by considering one candidate variable at a time. The most significant candidate variable (if any) is retained in the model after cycling through the remaining candidate variables. A variable is dropped if it loses significance. P<0.01 was considered statistically significant for our six primary hypotheses (zinc, selenium, manganese, lead, arsenic, and mercury for death using trace element deciles). Odds ratios with 99.2% confidence intervals (99.2% CIs) were reported. In secondary analyses, (1) we compared the participants in the highest and lowest deciles with participants in deciles 2–9, and (2) we compared the participants above the 95th referent percentile and the participants below the fifth referent percentile with participants in percentiles 5–95.
Table 1.
Demographics and clinical characteristics at baseline
| Characteristics | All | Missing |
|---|---|---|
| N | 1278 | |
| Age, yr | 62 (51–72) | 0 (0) |
| 18–49.9 | 280 (22) | |
| 50–69.9 | 598 (47) | |
| 70+ | 400 (31) | |
| Men | 784 (61) | 0 (0) |
| Ethnicity | 0 (0) | |
| White | 983 (77) | |
| Indigenous | 93 (7) | |
| Other | 202 (16) | |
| Unemployed before dialysis start and retirementa | 74 (6) | 90 (7) |
| Initiated dialysis in 2009–2012 (versus 2005–2008) | 696 (54) | 0 (0) |
| Predialysis care >3 mo | 992 (78) | 1 (<1) |
| Arteriovenous access present at dialysis start | 295 (23) | 0 (0) |
| Prescribed duration, h | 4 (4–4) | 10 (1) |
| Comorbidities | ||
| Atrial fibrillation | 221 (17) | 0 (0) |
| Acute myocardial infarction | 282 (22) | 0 (0) |
| Body mass index, kg/m2 | 26 (23–31) | 24 (2) |
| Malnutrition (<18.5) | 63 (5) | |
| Obesity (≥30) | 365 (29) | |
| Cancer | 153 (12) | 0 (0) |
| Cerebrovascular disease | 180 (14) | 0 (0) |
| Chronic heart failure | 252 (20) | 0 (0) |
| Chronic lung disease | 156 (12) | 0 (0) |
| Diabetes mellitus | 675 (53) | 0 (0) |
| Dementia | 26 (2) | 0 (0) |
| Hypertension | 1120 (88) | 0 (0) |
| Liver disease | 83 (6) | 0 (0) |
| Peripheral vascular disease | 131 (10) | 0 (0) |
| Psychiatric disease | 177 (14) | 0 (0) |
| Substance misuse | 147 (12) | 0 (0) |
| Laboratory values | ||
| Albumin,b g/dl | 3.3 (3.0–3.7) | 5 (<1) |
| Creatinine, mg/dl | 5.96 (4.68–7.70) | 14 (1) |
Data are shown as N (%) or median (interquartile range) as appropriate.
This includes those who were on disability income and/or were homemakers.
Albumin values are only available from the Alberta cohort.
Results
Participants
Participant flow is shown in Supplemental Figure 1. Participant characteristics (Table 1) were similar to those of other Canadian patients on incident hemodialysis represented in the Canadian Organ Replacement Register (16). All participants were dialyzed using high-flux polysulfone membranes. Few demographic and clinical characteristics were missing (Table 1), and only one baseline trace element value was missing (Supplemental Table 2). Five percent of participants were from outside Alberta. Thirty-nine (3%) withdrew consent or migrated from Alberta before the end of the 2-year follow-up. Over 2 years of follow-up, 260 (20%) patients died, 285 (24%) experienced a cardiovascular event, 117 (10%) were hospitalized for systemic infection, and 928 (77%) were hospitalized for any cause.
The unadjusted risk of the clinical outcome for each element with significant associations is presented by decile in Supplemental Table 3.
Zinc, Selenium, and Manganese
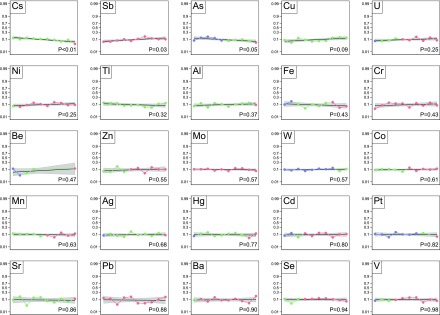
Figures 1–4 show the unadjusted correlation between concentrations of the 25 trace elements and the four clinical outcomes as well as the frequency of deficiency (less than the fifth percentile in the reference populations), referent concentrations (fifth to 95th percentiles), and elevated concentrations (>95th percentile).
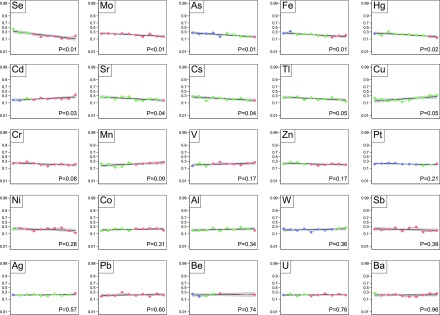
Figure 1.
Death at 2 years. Each plot represents one trace element. The markers within each plot represent the deciles of trace elements values of the study population: the first to the last decile from left to right. The blue markers indicate that all or some of the values are less than the fifth percentile of the referent population. Red markers indicate that all or some values are >95th percentile, and green markers indicate that all values are within the fifth to 95th percentiles. The logit of death at 2 years is regressed on each values’ decile as a linear transformation. The black curve is the line of regression. The gray area indicates the 95% confidence limits.
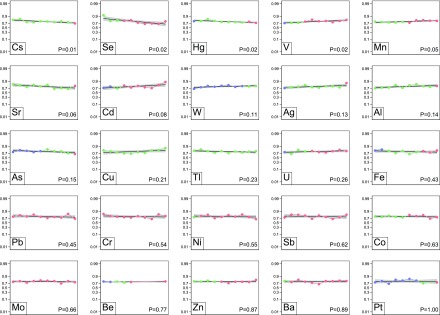
Figure 4.
Hospitalizations at 2 years. Each plot represents one trace element. The markers within each plot represent the deciles of trace elements values of the study population: the first to the last decile from left to right. The blue markers indicate that all or some of the values are less than the fifth percentile of the referent population. Red markers indicate that all or some values are >95th percentile, and green markers indicate that all values are within the fifth to 95th percentiles. The logit of death at 2 years is regressed on each values’ decile as a linear transformation. The black curve is the line of regression. The gray area indicates the 95% confidence limits.
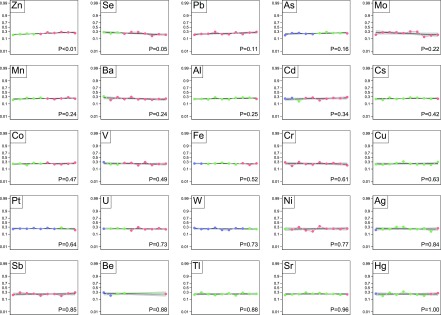
In unadjusted analyses, there was an association between zinc deciles and the risk of cardiovascular event (Figure 2), but there was not an association with death (Figure 1), infection (Figure 3), and all-cause hospitalization (Figure 4). After adjustment for potential confounders, there was no evidence of an association between zinc status and the risk of the adverse outcomes (Table 2).
Figure 2.
Cardiovascular events at 2 years. Each plot represents one trace element. The markers within each plot represent the deciles of trace elements values of the study population: the first to the last decile from left to right. The blue markers indicate that all or some of the values are less than the fifth percentile of the referent population. Red markers indicate that all or some values are >95th percentile, and green markers indicate that all values are within the fifth to 95th percentiles. The logit of death at 2 years is regressed on each values’ decile as a linear transformation. The black curve is the line of regression. The gray area indicates the 95% confidence limits.
Figure 3.
Infection events at 2 years. Each plot represents one trace element. The markers within each plot represent the deciles of trace elements values of the study population: the first to the last decile from left to right. The blue markers indicate that all or some of the values are less than the fifth percentile of the referent population. Red markers indicate that all or some values are >95th percentile, and green markers indicate that all values are within the fifth to 95th percentiles. The logit of death at 2 years is regressed on each values’ decile as a linear transformation. The black curve is the line of regression. The gray area indicates the 95% confidence limits.
Table 2.
Adjusted odds ratios of clinical outcomes after 2 years (99.2% confidence intervals)
| Trace Elements | Death | Cardiovascular Events | Infection Events | Hospitalizations |
|---|---|---|---|---|
| Participantsa | 1278 | 1211 | 1211 | 1211 |
| Events | 260 | 285 | 117 | 928 |
| Model 1—using all deciles as a linear term | ||||
| Per decile | ||||
| Arsenic | 0.92 (0.86 to 0.99) | |||
| Copper | 1.07 (1.00 to 1.15) | |||
| Selenium | 0.86 (0.80 to 0.93) | 0.92 (0.86 to 0.98) | ||
| Model 2—using the lowest and highest deciles versus remaining deciles | ||||
| Lowest decile | ||||
| Selenium | 3.43 (1.94 to 6.06) | 3.61 (1.46 to 8.92) | ||
| Highest decile | ||||
| Cadmium | 1.89 (1.06 to 3.38) | |||
| Model 3—using the fifth and 95th referent percentiles versus remaining percentiles | ||||
| >95th Referent percentile | ||||
| Copper | 3.68 (1.31 to 10.29) | |||
| Selenium | 0.48 (0.31 to 0.73) | 0.60 (0.38 to 0.95) |
All three models use the forward stepwise procedure, and they use all variables from Table 1 and Supplemental Table 3 as candidate variables. Missing values were imputed with the most frequent categorical or median continuous value. Albumin was not a candidate variable for cardiovascular events, infection events, and hospitalizations, because it was only available in Alberta participants. This table shows results from 12 models (three for each approach to modeling the trace elements times four for each type of outcome). Only the retained (due to significance at P<0.01) trace element variables are shown. Retained demographic and clinical characteristic variables are not shown.
Only Alberta participants were included in the modeling of cardiovascular events, infection events, and hospitalizations.
In unadjusted analyses, lower selenium deciles were significantly associated with risk of death (Figure 1), cardiovascular event (Figure 2), and all-cause hospitalization (Figure 4) but not infection (Figure 3). After adjustment, lower selenium deciles remained significantly associated with death (0.86 per decile; 99.2% CI, 0.80 to 0.93; P<0.001) (Table 2) and all-cause hospitalization (0.92 per decile; 99.2% CI, 0.86 to 0.98; P=0.001). The adjusted odds ratio of all-cause death associated with selenium concentration in the lowest decile was 3.43 (99.2% CI, 1.94 to 6.06; P<0.001) compared with those in deciles 2–9. Inspection of the absolute values for selenium concentrations seemed to suggest that excess mortality occurred even for values within the referent range; few participants had values below the fifth percentile as defined herein.
In unadjusted analyses, there was no association between manganese deciles and the risk of death (Figure 1), cardiovascular events (Figure 2), and infection (Figure 3), but lower deciles of manganese were associated with higher risk of all-cause hospitalization (Figure 4). After adjustment, lower deciles of manganese were no longer associated with all-cause hospitalization (Table 2).
Lead, Arsenic, and Mercury
In unadjusted (and adjusted) analyses, there was no association between lead deciles and the risk of death (Figure 1), cardiovascular events (Figure 2), infection (Figure 3), or all-cause hospitalization (Figure 4).
In contrast to our hypotheses, in unadjusted analyses, lower arsenic deciles were significantly associated with higher risk of death (Figure 1) and infection (Figure 3) but not all-cause hospitalization (Figure 4) and cardiovascular events (Figure 2). After adjustment for potential confounders, lower arsenic deciles remained associated with death (0.92 per decile; 99.2% CI, 0.86 to 0.99; P=0.002) but not infection (Table 2).
In unadjusted analyses, lower mercury concentrations were associated with higher risk of death (Figure 1) and all-cause hospitalization (Figure 4), but there was no association between mercury concentrations and the risk of cardiovascular events (Figure 2) or infection (Figure 3). After adjustment, lower deciles of mercury were no longer associated with adverse outcomes (Table 2).
Other Trace Elements
Higher copper concentrations were significantly associated with higher risk of death (1.07 per decile; 99.2% CI, 1.00 to 1.15) (Table 2). The adjusted odds ratio of all-cause death associated with copper concentration in the >95th referent percentile was 3.68 (99.2% CI, 1.31 to 10.29) compared with the fifth to 95th percentiles. Cadmium levels in the highest decile were associated with an increased risk of death (odds ratio, 1.89; 99.2% CI, 1.06 to 3.38).
Additional Exploratory Analyses
In additional exploratory analyses not corrected for multiple comparisons, cesium was significantly associated with death after adjustment (Supplemental Table 4): the adjusted odds ratio of all-cause death associated with cesium concentration was 0.93 per decile (95% CI, 0.89 to 0.99). In these analyses, beryllium was independently associated with infection events (1.08 per decile; 95% CI, 1.02 to 1.14), and vanadium was independently associated with hospitalizations (1.06 per decile; 95% CI, 1.01 to 1.11). The adjusted odds ratio of infections associated with beryllium concentration in the highest decile was 2.22 (95% CI, 1.34 to 3.67) compared with the intermediate deciles. The adjusted odds ratio of hospitalization associated with vanadium concentration in the >95th referent percentile was 1.57 (95% CI, 1.15 to 2.15) compared with percentiles 5–95. Other elements were independently associated with adverse events, although not linearly as deciles. The highest decile of barium was associated with infection events (2.71; 95% CI, 1.53 to 4.82). The >95th referent percentile of silver was associated with hospitalization (1.92; 95% CI, 1.12 to 3.27). The highest deciles of cadmium and nickel were associated with hospitalizations (1.94; 95% CI, 1.07 to 3.52 and 0.52; 95% CI, 0.33, 0.81, respectively).
Discussion
Abnormal concentrations of trace elements are common in patients on hemodialysis, and they represent a potentially treatable cause of adverse outcomes. In this large prospective cohort study of patients on incident hemodialysis, we examined the association between trace element status and several clinically relevant outcomes. Contrary to our a priori hypotheses, we found no convincing association between lower concentrations of zinc and manganese or higher concentrations of lead, arsenic, or mercury with adverse clinical outcomes. Among our a priori hypotheses, we did find an independent association between lower concentrations of selenium and the risk of death as well as a potential association with all-cause hospitalization. The magnitude of these associations seemed clinically relevant, because the odds of death and hospitalization were both approximately threefold higher in the lowest decile than in deciles 2–9.
Among the 19 other trace elements evaluated in hypothesis-generating analyses, only copper status was convincingly associated with an adverse outcome; higher concentrations of copper were associated with higher risks of death at the P<0.01 level. The magnitude of the association between higher concentrations of copper and the risk of death again seemed clinically relevant, with the adjusted odds of death more than threefold higher in the highest decile than in deciles 2–9 (Supplemental Table 4, Table 2).
There is a potential rationale for both of these observed associations. Selenium has multiple biologic functions, and lower concentrations of selenium have been associated with multiple adverse clinical and biochemical outcomes, including cardiovascular disease, diabetes, and malnutrition (18–22). Recent data from patients on hemodialysis suggest that low selenium concentrations are associated with markers of inflammation and oxidative stress and that supplementation can improve blood concentrations of such markers (23). Selenium supplementation is feasible and safe among patients on hemodialysis (24), and it may improve selenium concentrations (25). However, evidence that supplementation improves clinically relevant outcomes is lacking, and overt deficiency (values less than the fifth percentile from a referent population) was uncommon among participants in our study—which means that further investigation is required before supplementation could be recommended. Similarly, higher concentrations of copper in the general population have been linked with cardiovascular disease, anorexia (26), elevated blood concentrations of inflammatory markers (26,27), and perhaps, more rapid cognitive decline (28). However, most such data derive from toxicologic studies of acute or subacute exposure rather than the chronic low-concentration exposure that presumably occurs in patients on hemodialysis. A recent study done in Italy suggests that dietary intake of selenium is lower and intake of copper is higher in patients on hemodialysis than in the general population (29); whether these findings would apply to a Canadian hemodialysis population is unknown.
To our knowledge, no prior studies link abnormal copper status to adverse clinical outcomes in patients on hemodialysis. However, previous studies evaluating the link between copper status and clinical outcomes in patients on hemodialysis have tended to have low statistical power (30). For example, a recent study that found no association between copper status and the risk of death had a sample size of 111 patients on hemodialysis (11). Similar to our findings, a prior study of 1041 Japanese patients on prevalent hemodialysis found an independent association between lower concentrations of selenium and the risk of all-cause mortality and death from infection; concentrations of other trace elements were not assessed (12).
Although the biologic rationale and (for selenium) the consistent findings in previous studies both strengthen the likelihood that the observed associations are causal, we tested multiple hypotheses in our study, and thus, the nominal statistical significance of the findings must be viewed with caution. For selenium, our a priori hypothesis should increase confidence in our findings. In our opinion, the strong independent association between lower concentrations of selenium as well as the safety and availability of selenium supplements suggest that controlled trials of the latter power to assess clinical outcomes should be considered in patients on hemodialysis. In contrast, the exploratory nature of the analyses related to copper status suggests that the findings should be confirmed in additional studies. There is some evidence that copper is a biologic antagonist of selenium, but we did not find any evidence in adjusted analyses to suggest that the association between high levels of copper and adverse outcomes was stronger in people with lower levels of selenium or vice versa (data not shown).
Higher concentrations of cadmium have known adverse health effects (31), and therefore, in theory, strategies for reducing blood concentrations of cadmium could also be considered for further study. However, our findings are more consistent with a protective effect of low cadmium concentrations rather than a harmful effect of high cadmium concentrations, and thus, further investigation is required. The finding that higher levels of arsenic might be associated with better outcomes is not easily explained on clinical grounds.
Our study has important strengths, including its large sample size, rigorous analytical and laboratory methods, prospective design, multiple centers, and inclusion of only incident patients. However, our study also has some limitations, many of which are similar to those in our prior study of trace elements (16). First, we considered only plasma specimens as a measure of body burden, which may not be optimal for all trace elements. Second, we used reference concentrations derived from healthy populations without kidney failure (32–35), because referent concentrations for patients on hemodialysis have not been defined. This may account for some of the relatively unexpected findings, such as lower concentrations of selenium within the chosen referent range (as opposed to overt deficiency) being associated with adverse outcomes. Third, we did not collect data on the use of trace element supplementation in our participants, but trace element status is not routinely measured, and trace element supplements are infrequently prescribed in clinical practice. Similarly, we did not collect information on source water content of trace elements, residual kidney function, or extent of albuminuria, all of which could be associated with trace element status. Fourth, despite our best efforts, the possibility of residual confounding by unknown or unmeasured characteristics remains. Fifth, this is an observational study that correlates baseline measures of trace element status with clinical outcomes. The observed associations do not show causality. Sixth, we did not collect information on dietary intake, although all patients in the three participating centers are routinely advised to restrict their intake of protein, potassium, phosphate, sodium, and fluid.
In conclusion, we found an independent association between lower concentrations of selenium and the risk of death and all-cause hospitalization among patients on incident hemodialysis. We also found an independent association between higher concentrations of copper and the risk of death in this population. Future studies should evaluate the potential benefits of improving selenium concentrations among patients on hemodialysis. Further investigation is required to confirm the potential link between higher concentrations of copper and the risk of death among patients on hemodialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors of this report are grateful to the study coordinators Nancy Ruholl, Nasreen Ahmad, Coralea Bignell, Edita Delic, Sharon Gulewich, Julie Leidecker, Lorena McCoshen, Lisa McFaull, Mary Morgen, Rafael Sibrian, Sue Szigety, Charlynn Ursu, Gwen Winter, and Jessica Wagner; research assistants Lois Hannam, Rosie Hernandez, Bill Liu, Sandra Mackay, and Bev Vanderham; Ghenette Houston for administrative support; Len Hannam for database development; Sophanny Tiv for quality assurance; and Dawn Opgenorth for management. The authors also thank the patients on dialysis who participated in this research.
This work was supported by Canadian Institutes for Health Research grant MOP-84258, Canada Foundation for Innovation Foundation award FDN-143211 (to M.T.), and Canada Foundation for Innovation Leaders Opportunity Fund grant 26142.
The funders had no role in the design or analysis of this study or the drafting or approval of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11451017/-/DCSupplemental.
References
- 1.Rucker D, Thadhani R, Tonelli M: Trace element status in hemodialysis patients. Semin Dial 23: 389–395, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Wills MR, Savory J: Aluminum and chronic renal failure: Sources, absorption, transport, and toxicity. Crit Rev Clin Lab Sci 27: 59–107, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Kerr DN, Ward MK, Ellis HA, Simpson W, Parkinson IS: Aluminium intoxication in renal disease. Ciba Found Symp 169: 123–135, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Alfrey AC: Dialysis encephalopathy syndrome. Annu Rev Med 29: 93–98, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Altmann P, Al-Salihi F, Butter K, Cutler P, Blair J, Leeming R, Cunningham J, Marsh F: Serum aluminum levels and erythrocyte dihydropteridine reductase activity in patients on hemodialysis. N Engl J Med 317: 80–84, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Alfrey AC, LeGendre GR, Kaehny WD: The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med 294: 184–188, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Zima T, Mestek O, Nĕmecek K, Bártová V, Fialová J, Tesar V, Suchánek M: Trace elements in hemodialysis and continuous ambulatory peritoneal dialysis patients. Blood Purif 16: 253–260, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Zima T, Tesar V, Mestek O, Nemecek K: Trace elements in end-stage renal disease. 2. Clinical implication of trace elements. Blood Purif 17: 187–198, 1999 [DOI] [PubMed] [Google Scholar]
- 9.D’Haese PC, De Broe ME: Adequacy of dialysis: Trace elements in dialysis fluids. Nephrol Dial Transplant 11[Suppl 2]: 92–97, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, Thadhani R, Gill J; Alberta Kidney Disease Network : Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med 7: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CY, Wu ML, Chou YY, Li SY, Deng JF, Yang WC, Ng YY: Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin Nutr 31: 630–636, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Fujishima Y, Ohsawa M, Itai K, Kato K, Tanno K, Turin TC, Onoda T, Endo S, Okayama A, Fujioka T: Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrol Dial Transplant 26: 3331–3338, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Lin JL, Lin-Tan DT, Hsu CW, Yen TH, Chen KH, Hsu HH, Ho TC, Hsu KH: Association of blood lead levels with mortality in patients on maintenance hemodialysis. Am J Med 124: 350–358, 2011 [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bello AK, Thadhani R, Hemmelgarn B, Klarenbach S, Gill J, Chan C, Zimmerman D, Holmes D, Cembrowski G, Opgenorth D, Sibrian R, Karkhaneh M, Tiv S, Wiebe N, Tonelli M: Design and implementation of the Canadian Kidney Disease Cohort Study (CKDCS): A prospective observational study of incident hemodialysis patients. BMC Nephrol 12: 10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonelli M, Wiebe N, Bello A, Field CJ, Gill JS, Hemmelgarn BR, Holmes DT, Jindal K, Klarenbach SW, Manns BJ, Thadhani R, Kinniburgh D; Alberta Kidney Disease Network : Concentrations of trace elements in hemodialysis patients: A prospective cohort study. Am J Kidney Dis 70: 696–704, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H; Alberta Kidney Disease Network : Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak 15: 31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rayman MP: The importance of selenium to human health. Lancet 356: 233–241, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ryan-Harshman M, Aldoori W: The relevance of selenium to immunity, cancer, and infectious/inflammatory diseases. Can J Diet Pract Res 66: 98–102, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kurokawa S, Berry MJ: Selenium. Role of the essential metalloid in health. Met Ions Life Sci 13: 499–534, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph J, Loscalzo J: Selenistasis: Epistatic effects of selenium on cardiovascular phenotype. Nutrients 5: 340–358, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rij AM, Thomson CD, McKenzie JM, Robinson MF: Selenium deficiency in total parenteral nutrition. Am J Clin Nutr 32: 2076–2085, 1979 [DOI] [PubMed] [Google Scholar]
- 23.Salehi M, Sohrabi Z, Ekramzadeh M, Fallahzadeh MK, Ayatollahi M, Geramizadeh B, Hassanzadeh J, Sagheb MM: Selenium supplementation improves the nutritional status of hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant 28: 716–723, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Tonelli M, Wiebe N, Thompson S, Kinniburgh D, Klarenbach SW, Walsh M, Bello AK, Faruque L, Field C, Manns BJ, Hemmelgarn BR; Alberta Kidney Disease Network : Trace element supplementation in hemodialysis patients: A randomized controlled trial. BMC Nephrol 16: 52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omrani H, Golmohamadi S, Pasdar Y, Jasemi K, Almasi A: Effect of selenium supplementation on lipid profile in hemodialysis patients. J Renal Inj Prev 5: 179–182, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Toxicological Profile for Copper, Atlanta, GA, US Department of Health and Human Services, 2004
- 27.Ikee R, Tsunoda M, Sasaki N, Sato N, Hashimoto N: Clinical factors associated with serum copper levels and potential effect of sevelamer in hemodialysis patients. Int Urol Nephrol 45: 839–845, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Loef M, Walach H: Copper and iron in Alzheimer’s disease: A systematic review and its dietary implications. Br J Nutr 107: 7–19, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Bossola M, Di Stasio E, Viola A, Leo A, Carlomagno G, Monteburini T, Cenerelli S, Santarelli S, Boggi R, Miggiano G, Vulpio C, Mele C, Tazza L: Dietary intake of trace elements, minerals, and vitamins of patients on chronic hemodialysis. Int Urol Nephrol 46: 809–815, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Iglesias P, Selgas R, Romero S, Díez JJ: Selenium and kidney disease. J Nephrol 26: 266–272, 2013 [DOI] [PubMed] [Google Scholar]
- 31.US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Toxicological Profile for Cadmium, Atlanta, GA, US Department of Health and Human Services, 2012
- 32.Alimonti A, Bocca B, Mannella E, Petrucci F, Zennaro F, Cotichini R, D'Ippolito C, Agresti A, Caimi S, Forte G: Assessment of reference values for selected elements in a healthy urban population. Ann Ist Super Sanita 41: 181–187, 2005 [PubMed] [Google Scholar]
- 33.Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C: Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int 153: 39–44, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Cesbron A, Saussereau E, Mahieu L, Couland I, Guerbet M, Goullé JP: Metallic profile of whole blood and plasma in a series of 106 healthy volunteers. J Anal Toxicol 37: 401–405, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Minoia C, Sabbioni E, Apostoli P, Pietra R, Pozzoli L, Gallorini M, Nicolaou G, Alessio L, Capodaglio E: Trace element reference values in tissues from inhabitants of the European community. I. A study of 46 elements in urine, blood and serum of Italian subjects. Sci Total Environ 95: 89–105, 1990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.