Abstract
Secondary hyperparathyroidism develops in CKD due to a combination of vitamin D deficiency, hypocalcemia, and hyperphosphatemia, and it exists in nearly all patients at the time of dialysis initiation. There is insufficient data on whether to prefer vitamin D analogs compared with calcimimetics, but the available evidence suggests advantages with combination therapy. Calcium derangements, patient adherence, side effects, and cost limit the use of these agents. When parathyroid hormone level persists >800 pg/ml for >6 months, despite exhaustive medical interventions, monoclonal proliferation with nodular hyperplasia is likely present along with decreased expression of vitamin D and calcium-sensing receptors. Hence, surgical parathyroidectomy should be considered, especially if concomitant disorders exist, such as persistent hypercalcemia or hyperphosphatemia, tissue or vascular calcification including calciphylaxis, and/or worsening osteodystrophy. Parathyroidectomy is associated with 15%–57% greater survival in patients on dialysis, and it also improves hypercalcemia, hyperphosphatemia, tissue calcification, bone mineral density, and health-related quality of life. The parathyroidectomy rate in the United States declined to approximately seven per 1000 dialysis patient-years between 2002 and 2011 despite an increase in average parathyroid hormone levels, reflecting calcimimetics introduction and uncertainty regarding optimal parathyroid hormone targets. Hospitalization rates are 39% higher in the first postoperative year. Hungry bone syndrome occurs in approximately 25% of patients on dialysis, and profound hypocalcemia requires high doses of oral and intravenous calcium along with calcitriol supplementation. Total parathyroidectomy with autotransplantation carries a higher risk of permanent hypocalcemia, whereas risk of hyperparathyroidism recurrence is higher with subtotal parathyroidectomy. Given favorable long-term outcomes from observational parathyroidectomy cohorts, despite surgical risk and postoperative challenges, it is reasonable to consider parathyroidectomy in more patients with medically refractory secondary hyperparathyroidism.
Keywords: parathyroidectomy, Secondary hyperparathyroidism, CKD-osteodystrophy, Bone Density, Calciphylaxis, Calcitriol, Humans, Hypercalcemia, Hyperparathyroidism, Secondary, hyperphosphatemia, Hyperplasia, Hypocalcemia, Parathyroidectomy, quality of life, Receptors, Calcium-Sensing, renal dialysis, Uncertainty, Vitamin D
Introduction
Parathyroid hormone (PTH) levels start to increase with progression of CKD when the eGFR falls to approximately 45 ml/min per 1.73 m2. On transition to maintenance dialysis therapy, nearly all patients have secondary hyperparathyroidism defined as persistently high PTH level (normal PTH <65 pg/ml), and >80% of patients exhibit a serum PTH >150 pg/ml (1). Elevated PTH is considered both a consequence and perpetrator of mineral bone disorder, and it has been linked with CKD osteodystrophy, where high bone turnover results in higher risk of fractures, hyperphosphatemia, vascular and tissue calcification, anemia hyporesponsive to erythropoietin therapy, worse health-related quality of life, and increased mortality (2–4).
Causes of relatively high and low PTH in stage 5 CKD are summarized in Table 1. Proposed PTH targets in the dialysis population have varied widely across professional organizations in the absence of high-level evidence: from 60–240 pg/ml (Japanese Society for Dialysis Therapy 2012 guidelines) to two to nine times the upper normal range (130–600 pg/ml; Kidney Disease Improving Global Outcomes [KDIGO] guidelines 2009) (5). Given the lack of randomized, controlled trials to define the optimal PTH range, the most recent 2017 KDIGO guidelines for mineral bone disorder advise treatment of secondary hyperparathyroidism on the basis of the individual patient’s temporal PTH trends (6). In these guidelines, parathyroidectomy is suggested for patients with all stages of CKD ranging from early eGFR decline <60 ml/min per 1.73 m2 to dialysis who fail to respond to pharmacologic therapy. Here, we will briefly review secondary hyperparathyroidism pathophysiology and discuss medical therapies for hyperparathyroidism and their limitations, and then, we will focus on the role of parathyroidectomy and its implications.
Table 1.
Causes of low- and high-parathyroid hormone levels in CKD
| Causes of Relatively Low PTH in CKD Stage 5 (PTH<150 pg/ml)a | Causes of Relatively High PTH in CKD Stage 5 (PTH>300 pg/ml)a |
|---|---|
| Relative hypercalcemia | Hypocalcemia |
| Calcium-based bindersb | Vitamin D deficiency of progressive CKD |
| Calcium-rich diet | Urinary calcium loss (e.g., loop diuretics) |
| Higher calcium concentration in dialysate bathb | Inhibition of bone resorption (e.g., RANK ligand inhibitors, such as denosumab) |
| Elevated PTH–related peptide in malignancy | Elevated fibroblast growth factor-23 |
| Metabolic syndrome | Vitamin D deficiency |
| Diabetes mellitus | Hyperphosphatemia |
| Malnutrition-inflammation complexb | Black race |
| Oxidative stress | Resistant (tertiary) hyperparathyroidism |
| Peritoneal dialysisb | |
| Advanced age | |
| White race | |
| PTH assay errors | |
| High 1,25-dihydroxy or 25-hydroxy vitamin D levels | |
| Administration of nutritional D | |
| Active vitamin D analogs (D mimetics) | |
| Administration of calcimimeticsb | |
| Administration of recombinant PTH (teriparatide) | |
| Adynamic bone disease | |
| Postparathyroidectomy |
PTH, parathyroid hormone; RANK, receptor activator of nuclear factor κB. Modified from ref. 25, with permission.
Note that, in earlier stages of CKD, other ranges can be considered for “high” PTH (e.g., >70 pg/ml in stage 3 and >110 pg/ml in stage 4; see prior versions of Kidney Disease Outcomes Quality Initiative guidelines 2004).
Relevant to patients on dialysis.
Secondary Hyperparathyroidism Pathogenesis and Patterns of Parathyroid Hyperplasia
Fibroblast growth factor-23 exponentially increases as kidney function declines and downregulates 1α-hydroxylase expression in proximal tubular cells. This leads to marked reduction in 1α,25-dihydroxyvitamin D (calcitriol), the active form of vitamin D. Parathyroid cells express vitamin D receptors and calcium-sensing receptors, and these cells proliferate and ramp up PTH synthesis in the setting of 1α,25-dihydroxyvitamin D deficiency and low ionized calcium concentrations. Development of secondary hyperparathyroidism, in turn, mitigates these derangements by increasing 1α-hydroxylase expression in the kidney and mobilization of ionized calcium from bone. High extracellular phosphorus concentrations, which are commonly observed among patients with advanced CKD, can also increase PTH expression (7), although the phosphorus-sensing mechanisms remain unclear in humans. Although fibroblast growth factor-23 directly suppresses PTH gene expression, its inhibitory effect on secondary hyperparathyroidism may be relatively limited due to decreased klotho expression in parathyroid glands among patients with advanced stages of CKD (8).
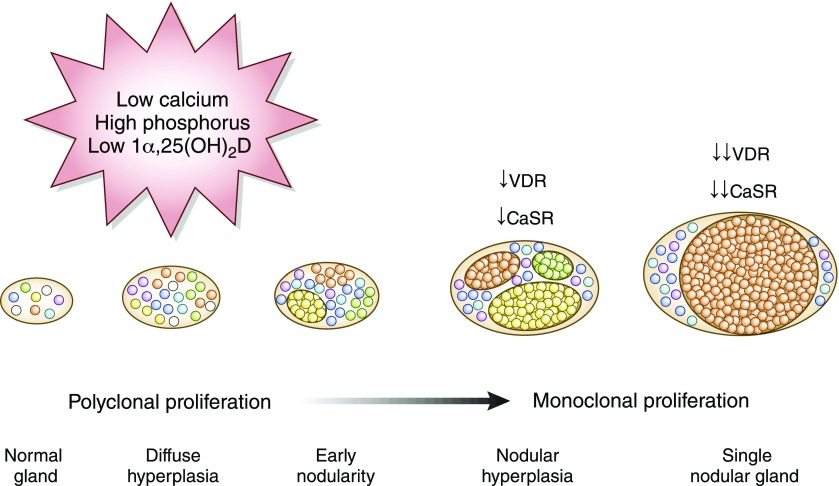
Patterns of parathyroid hyperplasia in secondary hyperparathyroidism are classified into four categories: diffuse hyperplasia, early nodularity in diffuse hyperplasia, nodular hyperplasia, and single nodular gland (9) (Figure 1). Secondary hyperparathyroidism initially presents with polyclonal parathyroid cell proliferation (i.e., diffuse hyperplasia), for which active vitamin D agents, calcimimetics, and phosphorus-lowering managements are effective in lowering PTH concentrations. However, if not managed appropriately, parathyroid glands develop progressive monoclonal expansion of adenomatous-like tissue (i.e., nodular hyperplasia) and become resistant to medical therapies due to reduced expression of vitamin D and calcium-sensing receptors (10).
Figure 1.
Pathophysiology of secondary hyperparathyroidism in CKD. Circulating fibroblast growth factor-23 increases early in CKD and suppresses 1α-hydroxylase in the kidney, leading to deficiency of active vitamin D [1α,25(OH)2D]. Hyperphosphatemia in CKD also stimulates parathyroid hormone (PTH) secretion. Vitamin D deficiency and chelation of calcium by phosphorus result in hypocalcemia, which further stimulates parathyroid proliferation. At the level of nodular hyperplasia, there is reduced expression of the vitamin D receptor (VDR) and the calcium-sensing receptor (CaSR), and the secondary hyperparathyroidism is refractory to medical therapies, such as vitamin D agents and calcimimetics.
Adverse Consequences of Elevated PTH
CKD Osteodystrophy and Fracture Risk
Proposed mechanisms for skeletal resistance to PTH in CKD include decrease in osteoblast PTH receptor expression, accumulation of the inactive 7–84 PTH fragment, and accumulation of osteoprotegerin (11). It was previously thought that blood PTH levels two to three times higher than normal are necessary to maintain normal bone turnover. However, bone biopsy studies have shown that PTH poorly predicts underlying bone turnover; about 15% of patients with PTH levels >600 pg/ml have normal or low bone turnover on biopsy (12). Alkaline phosphatase is re-emerging as a better predictor of high/low bone turnover, and it has a more linear association with mortality in patients on dialysis than PTH (13).
There is conflicting data from animal studies, where intermittent and continuous exposures to elevated PTH may induce opposite effects in bone turnover. Continuous infusion of supraphysiologic levels of PTH in 5/6-nephrectomized rats leads to high-turnover disease and an osteoporotic effect (14). Similar studies in animals with normal kidney function have shown that continuous PTH administration decreases bone mass; however, intermittent PTH has an opposite anabolic effect on bone (15). Adjusted risk of hip, vertebral, or distal radius wrist fractures is 31% lower after parathyroidectomy compared with in matched controls (16), likely via mitigating high-turnover bone disease (i.e., osteitis fibrosa) and increasing bone mineral density (4).
Vascular and Tissue Calcification
Preclinical studies suggest that PTH effects on the vasculature again differ on the basis of intermittent versus sustained elevation of circulating PTH. CKD rats subjected to 5/6 nephrectomy and continuous PTH infusion developed hypercalcemia and severe medial aortic calcification (14). In contrast, subcutaneous injections of teriparatide [PTH (1–34)] in diabetic male LDL receptor–null mice inhibited vascular calcification and aortic osteogenic transformation (17). These studies are not able to parse out the singular effects of PTH apart from hypercalcemia or hyperphosphatemia, important confounders that are known to independently and synergistically induce vascular calcification. Furthermore, secondary hyperparathyroidism severity is incrementally associated with higher levels of circulating alkaline phosphatase in patients on dialysis (18), and the latter is a strong predictor of coronary artery calcification in this population (19). Hence, the vascular calcification of secondary hyperparathyroidism may be mediated via alkaline phosphatase, and this biologically plausible hypothesis supports the survival association with parathyroidectomy. Calciphylaxis or calcific uremic arteriolopathy is often associated with skin necrosis and nonhealing ulcers with poor prognosis. Parathyroidectomy has been associated with lower mortality among patients with calciphylaxis (20); however, no large cohort studies have established whether parathyroidectomy results in regression of vascular or other soft tissue calcification, and PTH levels are inconsistently associated with presence of calciphylaxis (21).
Hyporesponsive Anemia
Resistant anemia with hyporesponsiveness to erythropoiesis stimulating agents has been defined as failure to achieve target hemoglobin in the presence of adequate iron stores with an erythropoietin-α equivalent dose of 450 U/kg per week intravenously (300 U/kg per week subcutaneously) for 4–6 months (22). Potential mechanisms by which elevated PTH may affect red blood cell production include direct toxicity of PTH on bone marrow erythroid progenitors and increased hemolysis, and an indirect effect is via induction of marrow fibrosis (23). Bone biopsy data suggest that improvement of anemia after parathyroidectomy depends on reversibility of bone marrow fibrosis (24).
Pharmacologic Therapies for Hyperparathyroidism and Their Limitations
Vitamin D Therapy
Calcitriol and vitamin D analogs, including vitamin D mimetics (such as paricalcitol and maxacalcitol), are effective in reducing PTH levels in CKD. Historically, large observational studies noted a survival advantage among patients on dialysis treated with injectable vitamin D compounds compared with placebo, prompting widespread use in the ESKD population (25). Routine use of vitamin D analogs in predialysis patients with CKD is discouraged in the KDIGO 2017 guidelines due to the 22.6%–43.3% rate of hypercalcemia that was observed with paricalcitol (compared with 0.9%–3.3% in placebo groups) in two randomized, controlled trials: the Paricalcitol Capsule Benefits in Renal Failure–Induced Cardiac Morbidity trial and the Effect of Paricalcitol on Left Ventricular Mass and Function in CKD trial (6). In these trials, left ventricular mass and cardiac function were similar in paricalcitol and placebo groups after 48–52 weeks of therapy. Although there is evidence that nutritional vitamin D (cholecalciferol and ergocalciferol) and the novel extended release 25-OH vitamin D analog calcifediol can effectively lower PTH in predialysis patients with CKD without incurring hypercalcemia, evidence is lacking on long-term mortality or cardiovascular effects.
The use of vitamin D analogs is constrained by a narrow therapeutic window, because pharmacologic doses increase gut mineral absorption, and the subsequent hypercalcemia and hyperphosphatemia may promote vascular calcification. This issue is compounded by resistance to vitamin D analogs in patients in whom severe secondary hyperparathyroidism has progressed to nodular hyperplasia with decreased vitamin D receptor expression (10). After a point is reached where escalation of vitamin D analog dose is not feasible due to hypercalcemia, hyperphosphatemia, and/or parathyroid gland resistance, despite concurrent use of a calcimimetic (see below), parathyroidectomy is a reasonable next step.
Calcimimetics
Calcimimetics and vitamin D analogs have become the mainstay of pharmacologic therapy for secondary hyperparathyroidism in patients on dialysis in the United States. Calcimimetics are positive allosteric modulators of the calcium-sensing receptor, inducing a conformational change that increases sensitivity of the parathyroid glands to circulating calcium. The Randomized Trial of Cinacalcet versus Vitamin D Analogs as Monotherapy in Secondary Hyperparathyroidism trial showed that cinacalcet was as effective as vitamin D analogs in lowering PTH (26). Calcimimetics increase vitamin D receptor expression and often lower serum calcium and phosphorus in patients on dialysis, providing a rationale for calcimimetics and vitamin D therapy to be used in combination to control secondary hyperparathyroidism. Whether calcimimetics affect mortality, major cardiovascular events, or fracture rate remains controversial. The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events trial evaluated cinacalcet versus placebo in 3883 patients on hemodialysis and noted a nonsignificant reduction in the primary composite end point of all-cause mortality, nonfatal myocardial infarction, hospitalization for unstable angina, congestive heart failure, and peripheral vascular events (27). However, prespecified subanalyses showed a significant reduction in the primary composite end point for those age >65 years old (hazard ratio, 0.70; 95% confidence interval, 0.60 to 0.81; P≤0.001) and for all-cause mortality (hazard ratio, 0.68; 95% confidence interval, 0.58 to 0.81; P≤0.001). The intravenous calcimimetic etelcalcetide was Food and Drug Administration approved in 2017, but the intravenous route is still associated with an approximately 10% rate of gastrointestinal side effects (28). Cost-effectiveness is another consideration; addition of cinacalcet incurs an additional United States $3000–4000 per year on top of the costs of vitamin D and phosphorus binders (29). If calcimimetic side effects are intolerable or if out-of-pocket costs to the patient become prohibitive, parathyroidectomy is a reasonable option.
Phosphorus Binders
Although hyperphosphatemia is an important mediator in the pathogenesis of early secondary hyperparathyroidism, it can also happen as a consequence of secondary hyperparathyroidism as evidenced by a downtrend in serum phosphorus that parallels the PTH drop in the calcimimetics clinical trials (27,28). Management of secondary hyperparathyroidism can significantly improve phosphorus control by reversing high-turnover bone disease and restoring the capacity of the skeleton to act as a reservoir for calcium and phosphorus mineral. Phosphorus binders have limited use as a therapy in established secondary hyperparathyroidism. Phosphorus binders are not approved for use in the predialysis population, and the use of calcium-containing salts, such as calcium carbonate or acetate, to correct hyperphosphatemia in patients on dialysis increases the risk of hypercalcemia and vascular calcification (30). Calcium-free binders, such as sevelamer and lanthanum carbonate, as well as iron-based binders may be associated with less arterial calcification, but the effect on survival is unclear (31). Serum calcium and phosphorus control is improved after parathyroidectomy (4) and may remove the need for phosphorus binders.
Indications for Parathyroidectomy
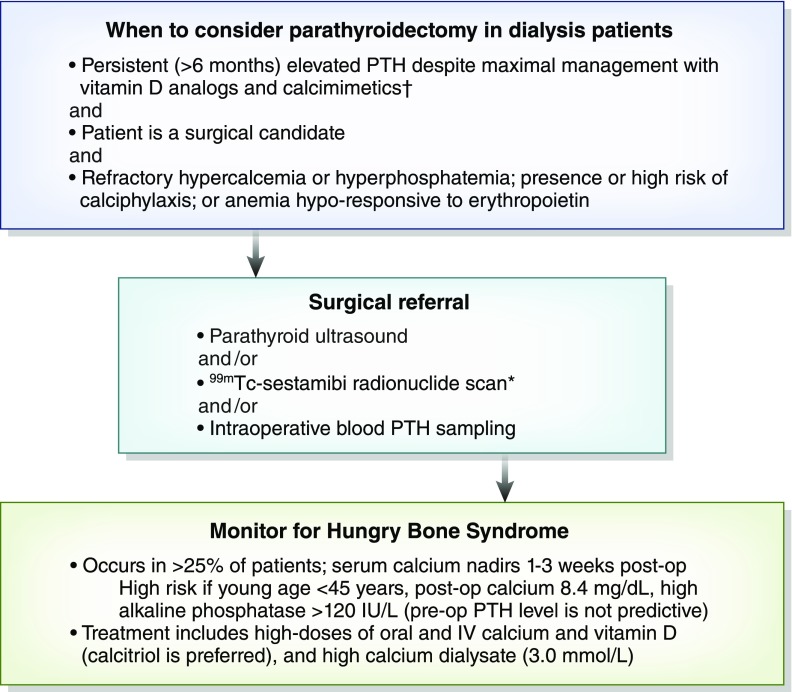
Parathyroidectomy is required in about 15% of patients after 10 years and 38% of patients after 20 years of ongoing dialysis therapy (32). Parathyroidectomy is indicated for patients with secondary hyperparathyroidism that is refractory to medical therapy (i.e., calcimimetics and vitamin D analogs). Although typically asymptomatic, severe secondary hyperparathyroidism can manifest as bone and joint pain, muscle weakness, or refractory pruritus, leading to worse health-related quality of life. Associated complications may include hypercalcemia, uncontrolled hyperphosphatemia, anemia hyporesponsive to erythropoietin therapy, and increased mortality risk through accelerating vascular and tissue calcification (2,3). We would emphasize that there is insufficient evidence on whether vitamin D analogs versus calcimimetics should be the first-line therapy for secondary hyperparathyroidism. From a pathophysiologic standpoint (Figure 1), an argument could be made for both agents to be used early in the disease when the vitamin D and calcium-sensing receptors are intact. There is evidence from clinical trials that more patients were able to achieve PTH targets when cinacalcet was added onto standard therapy with active vitamin D agents and phosphorus binders, and the combination therapy allowed for downtitration of vitamin D doses (33). As noted above, combination therapy may also avoid blood calcium derangements, because vitamin D therapy can lead to hypercalcemia, whereas calcimimetics can induce hypocalcemia; no trials have been done to specifically address this calcium balance issue. Because of cheaper cost and prescriber familiarity, the vitamin D analogs are usually the first agents prescribed for PTH control. Persistently elevated PTH values >800 pg/ml (>6 months) that remain nonresponsive to pharmacologic therapy, including maximally tolerated doses of vitamin D analogs and calcimimetics, are generally accepted as a criterion for parathyroidectomy (34) given the association with improved survival from observational studies (see below), especially if nodular hyperplasia is confirmed on imaging. Hyperplastic parathyroid gland volume >500 mm3 or glands >1 cm in long diameter strongly suggest nodular transformation, which is often refractory to medical therapy (Figure 1). It is our opinion that there is no definitive lower PTH threshold and that parathyroidectomy in patients on dialysis is reasonable when levels are in the 600- to 800-pg/ml range if there is (1) persistent hypercalcemia or hyperphosphatemia (corrected serum calcium >10.2 mg/dl [>2.5 mmol/L] or phosphorus >5.5 mg/dl [>1.8 mmol/L]) despite patient compliance to diet and optimized vitamin D analog/calcimimetic doses, (2) elevated risk or presence of calciphylaxis, or (3) erythropoietin-resistant anemia when other modifiable factors, such as iron deficiency or gastrointestinal bleeding, have been ruled out (Figure 2). Of note, although parathyroidectomy has been associated with lower mortality among patients with calciphylaxis (20), the significance of elevated PTH in calciphylaxis pathogenesis was recently challenged in a report from a national German registry, where only 6% of the 253 patients had a PTH of >600 pg/ml (21).
Figure 2.
Perioperative considerations for parathyroidectomy in patients on dialysis. Optimal parathyroid hormone (PTH) level remains unknown; although most would agree that persistent levels >800 pg/ml warrant parathyroidectomy, a lower threshold may be reasonable. *Preoperative (pre-op) ultrasound and 99mTc-sestamibi scintigraphy may help reduce the risk of recurrent disease by (1) detecting ectopic glands and (2) identifying which parathyroid gland has the lowest sestamibi uptake and can be used as the remnant tissue. †Used together to maintain serum calcium in normal range, because vitamin D analogs raise calcium, whereas calcimimetics lower calcium. Use of calcimimetics may be limited by gastrointestinal side effects and high cost. *Preoperative (pre-op) ultrasound and 99mTc-sestamibi scintigraphy may help reduce the risk of recurrent disease by (1) detecting ectopic glands and (2) identifying which parathyroid gland has the lowest sestamibi uptake and can be used as the remnant tissue. IV, intravenous; post-op, postoperation.
Total versus Subtotal Parathyroidectomy
Most patients on dialysis with secondary hyperparathyroidism have multiple enlarged parathyroid glands, for which the surgical options are subtotal or total parathyroidectomy. When total parathyroidectomy with autotransplantation is done, a fragment of parathyroid tissue is placed into the sternocleidomastoid or forearm muscle or the subcutaneous abdominal adipose tissue. There are no significant differences in surgical outcomes between subtotal and total parathyroidectomy (35). However, subtotal parathyroidectomy preserves a remnant parathyroid gland with its original blood supply, and hence, it has a lower risk of postoperative permanent hypocalcemia (36). Subtotal (targeted) parathyroidectomy is preferred if there is a single or double adenoma causing tertiary hyperparathyroidism after kidney transplantation, for which the incidence of recurrent secondary hyperparathyroidism is low (37). Conversely, total parathyroidectomy with autotransplantation is preferred for patients with compelling reasons to avoid reoperative neck surgery (i.e., thyroid conditions that potentially require additional surgical procedures, prior history of repeated neck surgery, known recurrent laryngeal nerve injury, significant medical comorbidities, or intolerance of general anesthesia) (38). For patients with longer life expectancy or those with little or no likelihood of undergoing kidney transplantation, a total parathyroidectomy alone (without autotransplantation) is superior in terms of preventing recurrent refractory secondary hyperparathyroidism (39). The long-term effect of excessively low PTH levels remains unclear, although there are concerns for a shift from high- to low-bone turnover disease with potential adverse vascular outcomes after parathyroidectomy (12).
Subtotal parathyroidectomy is more commonly performed in the United States for secondary hyperparathyroidism (36), whereas total parathyroidectomy with autotransplantation is more prevalent in Japan (40). The preference for type of parathyroidectomy procedure likely reflects variability in dialysis practices; Japanese patients with ESKD have longer 5-year survival rates than their American counterparts (60% versus 42%, respectively), but also, they have exceptionally low rates of kidney transplantation (five versus 36 per 1000 dialysis patient-years, respectively) (41,42).
Hospitalization costs associated with parathyroidectomy average United States $14,000 (43). Surgical techniques have improved through advancement of minimally invasive surgery guided by preoperative localization via ultrasonography, highly sensitive 99mTc-sestamibi radionuclide scans, and intraoperative blood PTH sampling. Unadjusted rates of in-hospital mortality after parathyroidectomy in the United States declined significantly from 1.7% in 2002 to 0.8% in 2011 (43).
International Trends in Parathyroidectomy Rates
Analysis of United States parathyroidectomy rates between 2002 and 2011 among all patients on dialysis and kidney transplant recipients in the US Renal Data System showed that the rate fell from 7.9 per 1000 patients in 2003 to 3.3 per 1000 patients in 2004 (43). This coincided with the commercial launch of cinacalcet in 2004. Subsequently, parathyroidectomy rates increased through 2006 and then remained constant around 5.0 per 1000 patients through 2011 (43), despite an overall uptrend in average PTH levels in the United States dialysis population.
In contrast, the Dialysis Outcomes and Practice Patterns Study noted a consistent decline in parathyroidectomy rates in North America (the United States and Canada) between 2002 and 2011 to a rate of approximately 7.5 per 1000 patients (5). Parathyroidectomy rates similarly declined in Europe, Australia, and New Zealand in the same period. Median PTH values have been consistently increasing (approximately 300 pg/ml as of 2011) in North America, Europe, Australia, and New Zealand. The disparity between decreasing parathyroidectomy rates and increasing PTH levels may be explained in part by limitations in cinacalcet dose titration due to gastrointestinal side effects, and it reflects the prevailing uncertainty surrounding optimal PTH targets.
Parathyroidectomy rates in Japan fell abruptly after the advent of cinacalcet to approximately two per 1000 patients (5). Median PTH in Japan has remained around 150 pg/ml, in keeping with the Japanese Society for Dialysis Therapy’s 2012 recommendation to target a PTH range of 60–240 pg/ml (note that the prior Japanese Society for Dialysis Therapy guidelines targeted an even lower PTH range of 60–180 pg/ml) (5). The Japanese hemodialysis population has better survival rates compared with European and United States cohorts (41,42), and although certain practice patterns have been proposed to affect mortality outcomes, such as use of highly purified dialysate and lower ultrafiltration rates, it is possible that targeting much lower PTH levels in Japan has contributed to this survival superiority. PTH gene polymorphisms may influence PTH levels in patients on dialysis (44), but this has not been explored across different ethnic groups.
Associations with Improved Survival after Parathyroidectomy: Limitations in Observational Studies
Successful parathyroidectomy that achieves sustained decrease in PTH levels can ameliorate many secondary hyperparathyroidism–related symptoms, improve management of serum calcium and phosphorus, decrease risk of bone fracture (16), increase bone mineral density (4), and improve health-related quality of life (45). Parathyroidectomy has consistently been associated with improved survival in large observational dialysis cohorts, with a reported 15%–57% reduction in all-cause mortality (Table 2). Therefore, parathyroidectomy is reasonable in the presence of persistent hypercalcemia or hyperphosphatemia without need for a defined PTH threshold, especially when medical therapies have been maximized or cannot be tolerated due to side effects.
Table 2.
Observational studies examining outcomes after parathyroidectomy in patients on dialysis
| Study | Country | Sample Size, PTx/Medical Treatment | Mean Age, yr | Women, % | Dialysis Duration, mo | Mean Duration of Follow-Up, mo | Study Period | Mean Serum iPTH, pg/ml | Mortality Difference with PTx |
|---|---|---|---|---|---|---|---|---|---|
| Kestenbaum et al., 2004 (56) | United States | 4558/4558 | 47.6 | 57.5 | 40.2 | 36 | 1994–2001 | Not reported | Short-term ↑ death <90 d after PTx; long-term ↓ all-cause death 15% |
| Sharma et al., 2012 (57) | United States | 150/1044 | 42.0 | 53.0 | 86.4 | 43.2 | 1993–2009 | 1770.0 | ↓ All-cause death 37%, ↓ CV death 33% |
| Goldenstein et al., 2013 (58) | Brazil | 123/128 | 48.0 | 45.7 | 72.0 | 23 | 2005–2012 | 1455.1 | ↓ All-cause death 57% |
| Ivarsson et al., 2015 (59) | Sweden | 423/1234 | 55.8 | 50.4 | Not reported | 56.3 | 1991–2009 | Not reported | ↓ All-cause death 20% |
| Komaba et al., 2015 (51) | Japan | 4428/4428 | 59.2 | 44.3 | 150.5 | 12 | 2004–2005 | 382.5 | ↓ All-cause death 34%, ↓ CV death 41% |
A consistent association with improved survival was observed in the dialysis population across studies. PTx, parathyroidectomy; iPTH, intact parathyroid hormone level; CV, cardiovascular.
A recent meta-analysis by Chen et al. (46) extracted data from 13 cohort studies involving 22,053 patients, of whom approximately 10,000 underwent parathyroidectomy, where parathyroidectomy was associated with an overall 28% reduction in all-cause mortality and a 37% reduction in cardiovascular mortality. Table 2 summarizes the largest studies in patients on dialysis (where the parathyroidectomy group included >100 patients). The association with improved survival after parathyroidectomy is not observed in kidney transplant recipients (Table 3), suggesting that improvement of kidney-regulated mineral metabolism post-transplant may have an important effect on mortality.
Table 3.
Observational studies examining outcomes after parathyroidectomy in patients with kidney transplants
| Study | Country | Sample Size, PTx/Medical Treatment | Mean Age, yr | Women, % | Dialysis Duration before Kidney Transplant, mo | Median Time to PTx after Transplant, mo | Mean Duration of Follow-Up, mo | Study Period | Mean Serum iPTH, pg/ml | Mortality and Allograft Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Evenepoel et al., 2007 (60) | Belgium | 90/1653 | 50.9 | 50.0 | 47.7 | 11.0 | 62.6 (From time of transplant) | 1989–2004 | 107.1 | Mortality difference; ↑ Cr 1.76–1.91 in first month after PTx; no long-term Cr difference |
| Ivarsson et al., 2015 (59) | Sweden | 156/892 | 51.9 | 50.0 | 61.2 | Not reported | 76.9 (From time of PTx) | 1991–2009 | Not reported | No mortality difference |
A consistent association with improved survival was observed in the dialysis population across studies. PTx, parathyroidectomy; iPTH, intact parathyroid hormone level; Cr, creatinine.
Although the observational data postparathyroidectomy are compelling and show potential survival and clinical benefits, several considerations are to be acknowledged. First, it is unlikely that there ever will be a randomized, controlled trial to examine outcomes after parathyroidectomy given the potential surgical morbidity, hospitalization costs, increasing age and comorbidities among patients on dialysis, and the directed agenda from pharmaceutical companies to develop novel drug therapies. Therefore, data on parathyroidectomy outcomes are limited to observational studies with their intrinsic limitations, such as confounding by indication, which is especially pertinent with a surgical intervention. In this case, when nephrologists refer patients for parathyroidectomy, they have already assessed the patient to be an adequate operative candidate and expect potential benefits to outweigh risks and costs.
Second, secondary hyperparathyroidism–related symptoms, such as pain, weakness, and pruritus, are frequently observed among patients on dialysis irrespective of PTH values, and hence, they may or may not improve after parathyroidectomy. Third, parathyroidectomy is associated with significant postoperative morbidity, with hospitalization rates that are 39% higher in the first year after parathyroidectomy compared with the preceding year (47). Fourth, medical compliance needs to be ascertained before labeling a patient as having refractory secondary hyperparathyroidism; adherence to medications is notoriously difficult in patients on dialysis. Fifth, among patients on dialysis with greater residual kidney function, parathyroidectomy may be less effective in decreasing serum phosphorus levels (48). This is because PTH-induced urinary phosphorus excretion can contribute substantially to solute clearance, even at low levels of eGFR.
Prior concerns that abrupt lowering of PTH after parathyroidectomy would lead to adverse outcomes seem to be unfounded. Earlier studies in the dialysis population described a J-shaped relationship between PTH and survival (49,50), where both extremes of very low (<60 pg/ml) and high (>300 mg/ml) PTH levels were each associated with significantly higher mortality risk. However, recent data showed that the higher risk of mortality associated with very low PTH was most evident among patients who had not received any treatment for secondary hyperparathyroidism (5). Furthermore, analysis of >4000 Japanese patients on dialysis observed the association between increased mortality and low PTH to hold true only among patients who had not undergone parathyroidectomy (51). In contrast, among patients who had previously undergone parathyroidectomy, the lowest PTH levels were associated with the best clinical outcomes (51). Adding to the complexity of PTH interpretation, biochemical detection of a low PTH can be misleading, because protein-energy wasting and inflammation in CKD suppress PTH secretion (Table 1). Obesity and race can also affect PTH levels, and there is interfacility variability due to the use of different commercial assays (25). In summary, observational cohort studies suggest that parathyroidectomy is associated with improved survival, although exact mechanisms remain unclear.
Hungry Bone Syndrome
Hungry bone syndrome is defined by a decrease in serum total calcium to <8.4 mg/dl (2.1 mmol/L) and/or prolonged hypocalcemia for >4 days postparathyroidectomy (52) due to unopposed osteoblast uptake of mineral after acute drop in PTH levels. Concomitant hypophosphatemia, hypomagnesemia, and hyperkalemia can be seen. In recent retrospective analyses of 144 patients on dialysis who underwent parathyroidectomy, approximately 27% of patients developed hungry bone syndrome (53,54). Risk factors included younger age (47.5 versus 54.5 years old in one series; ≤45 years old in a separate cohort), higher body weight (60.7 versus 49.8 kg), higher preoperative serum alkaline phosphatase (415 versus 221 IU/L), lower preoperative serum calcium level (9.76 versus 10.4 mg/dl), and lower postoperative serum calcium (7.1 versus 8.3 mg/dl) (53,54). PTH and use of calcimimetics or vitamin D analogs did not predict hungry bone syndrome (54). Hungry bone syndrome often requires intravenous calcium repletion for an average of 7 days postoperatively followed by high doses of oral calcium and vitamin D analog supplementation; calcitriol is the agent of choice given its marked calcemic effects. Serum calcium levels decline to a minimum 3 weeks after surgery (55), and the use of high-calcium dialysate can be considered during this period. Figure 2 summarizes perioperative considerations for patients with ESKD undergoing parathyroidectomy for medically refractory hyperparathyroidism.
Conclusions
Secondary hyperparathyroidism is common in advanced CKD, and first-line medical therapy includes the use of vitamin D agents and calcimimetics. Vitamin D agents may incur hypercalcemia, whereas calcimimetics lower serum calcium, and combined use of these drugs may diminish the risk of calcium derangements. About 15% of patients will need parathyroidectomy for medically refractory secondary hyperparathyroidism after 5–10 years on dialysis. Rates of parathyroidectomy have been stable despite an overall uptrend in PTH levels in the United States dialysis population, reflecting uncertainty in the nephrology community regarding the optimal PTH range to target. Randomized clinical trials of parathyroidectomy are lacking, and risks of increased hospitalization rates in the first postoperative year and the high likelihood of hungry bone syndrome must be assessed on an individual basis. However, given that large observational dialysis cohorts have shown multiple positive associations after parathyroidectomy, including decreased all-cause mortality, normalization of calcium and phosphorus, decreased fracture rate, and improved health-related quality of life, parathyroidectomy is a reasonable option in patients with CKD with medically refractory secondary hyperparathyroidism.
Disclosures
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, the American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, the National Institutes of Health, the National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma. The other authors declare no conflicts of interest.
Acknowledgments
W.L.L. has received research funding from the American Heart Association. Y.O. has been supported by the Uehara Memorial Foundation Research Fellowship. K.K.-Z. is supported by National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health research grants R01-DK95668, K24-DK091419, and R01-DK078106 and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee, and AVEO.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Komaba H, Nakamura M, Fukagawa M: Resurgence of parathyroidectomy: Evidence and outcomes. Curr Opin Nephrol Hypertens 26: 243–249, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, Andreucci VE, Fukagawa M, Frimat L, Mendelssohn DC, Port FK, Pisoni RL, Robinson BM: Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: The DOPPS study. Clin J Am Soc Nephrol 10: 98–109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB: Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: What’s changed and why it matters. Kidney Int 92: 26–36, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ: Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komaba H, Fukagawa M: FGF23-parathyroid interaction: Implications in chronic kidney disease. Kidney Int 77: 292–298, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Goto S, Komaba H, Fukagawa M: Pathophysiology of parathyroid hyperplasia in chronic kidney disease: Preclinical and clinical basis for parathyroid intervention. NDT Plus 1[Suppl 3]: iii2–iii8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki Y, Yamato H, Nii-Kono T, Fujieda A, Uchida M, Hosokawa A, Motojima M, Fukagawa M: Insufficiency of PTH action on bone in uremia. Kidney Int Suppl 70: S34–S36, 2006 [DOI] [PubMed] [Google Scholar]
- 12.El-Husseini A, Wang K, Edon AA, Sawaya BP: Parathyroidectomy-A last resort for hyperparathyroidism in dialysis patients. Semin Dial 30: 385–389, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Lau WL, Kalantar-Zadeh K, Kovesdy CP, Mehrotra R: Alkaline phosphatase: Better than PTH as a marker of cardiovascular and bone disease? Hemodial Int 18: 720–724, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhães AO, Custódio MR, Batista DG, Jorgetti V, Moysés RM: Vascular calcification: Contribution of parathyroid hormone in renal failure. Kidney Int 71: 1262–1270, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Uzawa T, Hori M, Ejiri S, Ozawa H: Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone 16: 477–484, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B: Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 18: 2401–2407, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA: Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem 278: 50195–50202, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Li J, Molnar MZ, Zaritsky JJ, Sim JJ, Streja E, Kovesdy CP, Salusky I, Kalantar-Zadeh K: Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant 28: 1516–1525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K: Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol 4: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, Bridges AD, Claus PL, Davis MD, Dillon JJ, El-Zoghby ZM, Hickson LJ, Kumar R, McBane RD, McCarthy-Fruin KA, McEvoy MT, Pittelkow MR, Wetter DA, Williams AW: Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc 91: 1384–1394, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Brandenburg VM, Kramann R, Rothe H, Kaesler N, Korbiel J, Specht P, Schmitz S, Krüger T, Floege J, Ketteler M: Calcific uraemic arteriolopathy (calciphylaxis): Data from a large nationwide registry. Nephrol Dial Transplant 32: 126–132, 2017 [DOI] [PubMed] [Google Scholar]
- 22.IV: IV. NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: Update 2000. Am J Kidney Dis 37[1 Suppl 1]: S182–S238, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Gallieni M, Corsi C, Brancaccio D: Hyperparathyroidism and anemia in renal failure. Am J Nephrol 20: 89–96, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Mandolfo S, Malberti F, Farina M, Villa G, Scanziani R, Surian M, Imbasciati E: Parathyroidectomy and response to erythropoietin therapy in anaemic patients with chronic renal failure. Nephrol Dial Transplant 13: 2708–2709, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP: Kidney bone disease and mortality in CKD: Revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl 117: S10–S21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wetmore JB, Gurevich K, Sprague S, Da Roza G, Buerkert J, Reiner M, Goodman W, Cooper K: A randomized trial of cinacalcet versus vitamin D analogs as monotherapy in secondary hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol 10: 1031–1040, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Bushinsky DA, Cunningham J, Drueke TB, Ketteler M, Kewalramani R, Martin KJ, Mix TC, Moe SM, Patel UD, Silver J, Spiegel DM, Sterling L, Walsh L, Chertow GM: Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: Two randomized clinical trials. JAMA 317: 146–155, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Shireman TI, Almehmi A, Wetmore JB, Lu J, Pregenzer M, Quarles LD: Economic analysis of cinacalcet in combination with low-dose vitamin D versus flexible-dose vitamin D in treating secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis 56: 1108–1116, 2010 [DOI] [PubMed] [Google Scholar]
- 30.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Schneider R, Slater EP, Karakas E, Bartsch DK, Schlosser K: Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World J Surg 36: 318–326, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Drüeke TB, Ritz E: Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin J Am Soc Nephrol 4: 234–241, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, Drüeke TB, Cunningham J, Sherrard DJ, McCary LC, Olson KA, Turner SA, Martin KJ: Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67: 760–771, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Jia X, Kong X, Wang Z, Cui M, Xu D: Total parathyroidectomy with autotransplantation versus subtotal parathyroidectomy for renal hyperparathyroidism: A systematic review and meta-analysis. Nephrology (Carlton) 22: 388–396, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Anderson K Jr., Ruel E, Adam MA, Thomas S, Youngwirth L, Stang MT, Scheri RP, Roman SA, Sosa JA: Subtotal vs. total parathyroidectomy with autotransplantation for patients with renal hyperparathyroidism have similar outcomes. Am J Surg 214: 914–919, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Nichol PF, Starling JR, Mack E, Klovning JJ, Becker BN, Chen H: Long-term follow-up of patients with tertiary hyperparathyroidism treated by resection of a single or double adenoma. Ann Surg 235: 673–678, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milas M: Parathyroidectomy in end stage renal disease. In: UpToDate, edited by Carty SE, Chen W, Waltham, MA, UpToDate Inc. Available at https://www.uptodate.com/contents/parathyroidectomy-in-end-stage-renal-disease. Accessed October 25, 2017 [Google Scholar]
- 39.Liu ME, Qiu NC, Zha SL, Du ZP, Wang YF, Wang Q, Chen Q, Cen XX, Jiang Y, Luo Q, Shan CX, Qiu M: To assess the effects of parathyroidectomy (TPTX versus TPTX+AT) for secondary hyperparathyroidism in chronic renal failure: A systematic review and meta-analysis. Int J Surg 44: 353–362, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Tominaga Y, Kakuta T, Yasunaga C, Nakamura M, Kadokura Y, Tahara H: Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the parathyroid surgeons’ society of Japan. Ther Apher Dial 20: 6–11, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, Wakai K, Wada A, Nitta K: Annual Dialysis Data Report 2014, JSDT Renal Data Registry (JRDR). Renal Replacement Therapy 3: 18, 2017 [Google Scholar]
- 42.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JL, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert H, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V: US renal data system 2016 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 69[Suppl 1]: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SM, Long J, Montez-Rath ME, Leonard MB, Norton JA, Chertow GM: Rates and outcomes of parathyroidectomy for secondary hyperparathyroidism in the United States. Clin J Am Soc Nephrol 11: 1260–1267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gohda T, Shou I, Fukui M, Funabiki K, Horikoshi S, Shirato I, Tomino Y: Parathyroid hormone gene polymorphism and secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis 39: 1255–1260, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Cheng SP, Lee JJ, Liu TP, Yang TL, Chen HH, Wu CJ, Liu CL: Parathyroidectomy improves symptomatology and quality of life in patients with secondary hyperparathyroidism. Surgery 155: 320–328, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Wang K, Yu S, Lai L, Zhang X, Yuan J, Duan W: Long-term mortality after parathyroidectomy among chronic kidney disease patients with secondary hyperparathyroidism: A systematic review and meta-analysis. Ren Fail 38: 1050–1058, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Ishani A, Liu J, Wetmore JB, Lowe KA, Do T, Bradbury BD, Collins AJ: Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol 10: 90–97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montenegro J, Cornago I, Gallardo I, García-Ledesma P, Hernando A, Martinez I, Muñoz RI, Romero MA: Efficacy and safety of cinacalcet for the treatment of secondary hyperparathyroidism in patients with advanced chronic kidney disease before initiation of regular dialysis. Nephrology (Carlton) 17: 26–31, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Dukkipati R, Kovesdy CP, Colman S, Budoff MJ, Nissenson AR, Sprague SM, Kopple JD, Kalantar-Zadeh K: Association of relatively low serum parathyroid hormone with malnutrition-inflammation complex and survival in maintenance hemodialysis patients. J Ren Nutr 20: 243–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC; ARO Investigators: Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M: Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 88: 350–359, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Jain N, Reilly RF: Hungry bone syndrome. Curr Opin Nephrol Hypertens 26: 250–255, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Goldfarb M, Gondek SS, Lim SM, Farra JC, Nose V, Lew JI: Postoperative hungry bone syndrome in patients with secondary hyperparathyroidism of renal origin. World J Surg 36: 1314–1319, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Ho LY, Wong PN, Sin HK, Wong YY, Lo KC, Chan SF, Lo MW, Lo KY, Mak SK, Wong AK: Risk factors and clinical course of hungry bone syndrome after total parathyroidectomy in dialysis patients with secondary hyperparathyroidism. BMC Nephrol 18: 12, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latus J, Roesel M, Fritz P, Braun N, Ulmer C, Steurer W, Biegger D, Alscher MD, Kimmel M: Incidence of and risk factors for hungry bone syndrome in 84 patients with secondary hyperparathyroidism. Int J Nephrol Renovasc Dis 6: 131–137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]