Abstract
Background and objectives
Autosomal dominant polycystic kidney disease is the most common inheritable kidney disease, frequently thought to become symptomatic in adulthood. However, patients with autosomal dominant polycystic kidney disease may develop signs or symptoms during childhood, in particular hypertension. Although ambulatory BP monitoring is the preferred method to diagnose hypertension in pediatrics, data in children with autosomal dominant polycystic kidney disease are limited.
Design, setting, participants, & measurements
Our retrospective multicenter study was conducted to collect ambulatory BP monitoring recordings from patients with autosomal dominant polycystic kidney disease age <18 years old. Basic anthropometric parameters as well as data on kidney function, BP treatment, and kidney ultrasound were also collected.
Results
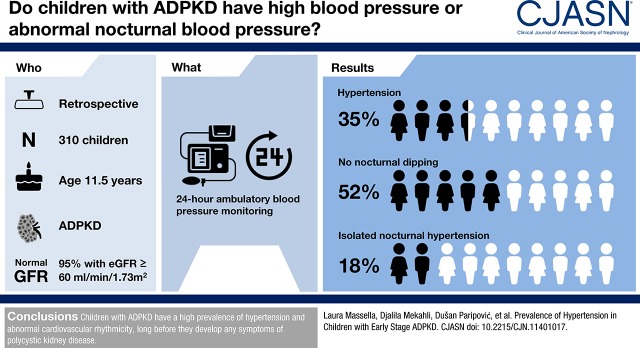
Data from 310 children with autosomal dominant polycystic kidney disease with a mean age of 11.5±4.1 years old were collected at 22 European centers. At the time when ambulatory BP monitoring was performed, 95% of children had normal kidney function. Reference data for ambulatory BP monitoring were available for 292 patients. The prevalence rates of children with hypertension and/or those who were treated with antihypertensive drugs were 31%, 42%, and 35% during daytime, nighttime, or the entire 24-hour cycle, respectively. In addition, 52% of participants lacked a physiologic nocturnal BP dipping, and 18% had isolated nocturnal hypertension. Logistic regression analysis showed a significant association between a categorical cyst score that was calculated on the basis of the number of cysts >1 cm per kidney and daytime hypertension (odds ratio, 1.70; 95% confidence interval, 1.21 to 2.4; P=0.002), nighttime hypertension (odds ratio, 1.31; 95% confidence interval, 1.05 to 1.63; P=0.02), or 24-hour hypertension (odds ratio, 1.39; 95% confidence interval, 1.08 to 1.81; P=0.01). Kidney length, expressed as SD score, was also significantly associated with nighttime hypertension (odds ratio, 1.23; 95% confidence interval, 1.06 to 1.42; P=0.10).
Conclusions
These data indicate high prevalence of hypertension in children with autosomal dominant polycystic kidney disease starting at young ages.
Keywords: ABPM; Ambulatory Blood Pressure Monitoring; Antihypertensive Agents; Autosomal Dominant Polycystic Kidney Disease; blood pressure; Blood Pressure Determination; Blood Pressure Monitoring, Ambulatory; Child; Cysts; Humans; hypertension; kidney; Logistic Models; pediatrics; Polycystic Kidney, Autosomal Dominant; Prevalence; Retrospective Studies; Rhythm Analysis
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease (1), and it is usually considered to be an adult condition. In recent years, however, observational and prospective studies have shown that a significant proportion of patients already have hypertension during childhood (2–7). Less frequently, children show hematuria, proteinuria, or early impairment of kidney function (3,6,8,9). Additional signs include impaired urine concentration ability, glomerular hyperfiltration, and increased left ventricular mass (4,10–12). The severity of symptoms is associated with early development of cysts, nephromegaly, and from a genetic standpoint, truncating mutations in the PKD1 gene (2,5,8,13,14).
Children with hypertension are more likely to experience rapid decline in kidney function (2), an association that is usually not observed in the adult population, because hypertension is nearly always present. Although ambulatory BP monitoring (ABPM) is the preferred method to diagnose hypertension in children (15,16), ABPM data in children with ADPKD remain scant (5).
With the development of new therapies aimed at reducing cyst growth, early detection of hypertension with ABPM in asymptomatic children with ADPKD may be useful in selecting candidates for early treatment (17–19).
On these premises, we have established the ADPKiDs study, a European multicenter retrospective cohort study of children with ADPKD who had undergone ABPM. The goal was to better analyze the prevalence of BP abnormalities in this population and their relationship with age and the severity of kidney involvement.
Materials and Methods
Overview
The study was coordinated by the Bambino Gesù Children’s Hospital in Rome, Italy, and it was approved by its Internal Ethical Review Committee. It was performed in adherence to the Declaration of Helsinki and included 22 centers from 14 European countries, namely Belgium, Czech Republic, France, Germany, Italy, Lithuania, Poland, Portugal, Romania, Russia, Serbia, Slovakia, Spain, and Turkey. Referring physicians of each collaborating center obtained parental consent to transfer anonymized data to the coordinating center.
A web-based data collection form was developed, allowing participating centers to provide patient data and upload ABPM files. In each center, patients <18 years old with an established diagnosis of ADPKD who had undergone an ABPM recording were recruited. ADPKD was diagnosed if patients had at least one kidney cyst and a positive family history or a proven mutation in the PKD1 or PKD2 genes.
Mandatory data were limited to age, sex, height, weight, and BP medications. GFR was estimated (eGFR) using recently published quadratic formulas on the basis of height, sex, age, and serum creatinine (20). If available, centers were asked to provide kidney ultrasound data, including kidney length and the number of cysts. In the interests of simplicity and consistency, cyst burden was stratified on the basis of the number of large cysts. Five classes were defined according to the number of cysts >1 cm in each kidney as follows: none, one to two, three to five, six to ten, and more than ten cysts. Each kidney was scored separately. To evaluate the global cyst burden, a “cyst score” was created by giving incremental points (from zero to four) to each cyst class and adding the scores from each kidney.
Total kidney volume could not be assessed retrospectively. The magnitude of kidney enlargement was approximated using kidney length measured by ultrasonography. Nephromegaly was defined as a mean kidney length >2 SD scores using previously reported reference values (21).
Of the 310 participants with ABPM, eGFR and ultrasound data were available in 257 (83%), ultrasound data alone were available in 13 (4%), eGFR data alone were available in 21 (7%), and no eGFR or ultrasound data were available in 19 participants (6%).
ABPM and Rhythm Analyses
ABPMs were performed between 2008 and 2015, and results were collected in any available format. Most files (229 of 310) included the complete ABPM dataset. In a minority of patients (81 of 310), results were restricted to mean BP and heart rate (HR) values during daytime, nighttime, and 24 hours. These latter records could not be used for rhythm analysis, which also required at least 22 hours of recording with no gaps exceeding 65 minutes in duration. These conditions were fulfilled in 137 ABPM files. The procedure has been described elsewhere (22,23) and uses partial Fourier analyses to fit a cosine function on a circadian (24 hours) rhythm or ultradian rhythms (12, 8, or 6 hours). In addition, the software estimates rhythm amplitudes and acrophases (Supplemental Figure 1).
ABPM values were expressed as SD scores on the basis of reference values calculated on 1254 healthy white children (24), and they were converted to percentiles. For 18 children <5 years old and <120 cm, the SD scores could not be calculated due to lack of reference values. ABPM outcome measures were on the basis of European and North American guidelines for the interpretation of BP values in children (15,25). Hypertension was diagnosed in the presence of a mean systolic BP (SBP) and/or diastolic BP (DBP) >95th percentile. Isolated nocturnal hypertension was defined as the presence of nocturnal hypertension in the absence of abnormal BP values during daytime. High-normal BP was defined as a mean SBP and/or DBP ≥90th and <95th percentile. Optimal BP was defined on the basis of the European Society of Hypertension guidelines for children with nonproteinuric CKDs, which recommend a target BP <75th percentile (15). Consequently, suboptimal BP was defined as a mean SBP and/or DBP ≥75th and <90th percentile. The absence of nocturnal dipping (“nondipping”) was defined as a nocturnal BP fall of <10% of daytime values (night-to-day BP ratio >0.9) (15). For the analysis of rhythm prevalence and characteristics, data were used from already collected analyses conducted in 938 children without kidney disease who participated in a multicenter trial performed by the German Working Group on Pediatric Hypertension (22).
Statistical Analyses
Data were analyzed using IBM SPSS Statistics 21.0.0.2. All data are two sided and considered significant for P values <0.05. Normal data are expressed as mean±SD. Data that do not follow a normal distribution are expressed as median value and interquartile range. Variables included in the multivariable logistic analysis were restricted to those that had a P value <0.10 at the univariable level. Cyst score was used in the model as a categorical variable. Odds ratios were adjusted for age, sex, center effect, and the use of BP medications. The analysis of center effect is provided in Supplemental Table 1, showing that only patients from one center were at significantly higher risk of being hypertensive.
Results
Overall, 310 patients, equally distributed between sexes, were included in the study (Table 1). The mean age was 11.5±4.1 years old, and the age distribution was homogeneous between the ages of 4 and 18 years old (Supplemental Figure 2). The diagnosis of ADPKD was on the basis of clinical and ultrasonographic findings in the vast majority of participants. Family history was negative in 9% of patients. In these patients, the diagnosis was established by genetic analysis documenting PKD1 gene mutations. No PKD2 mutation was reported.
Table 1.
Participant characteristics of 310 children with autosomal dominant polycystic kidney disease
| Patient characteristics | Number (percent) Mean±SD |
|---|---|
| No. of patients | 310 |
| Age, yr | 11.5±4.1 |
| Sex, boy-to-girl ratio | 162:148 (52:48) |
| Birth weight, ga | 3291±620 |
| Family history | |
| Paternal inheritance | 130 (42) |
| Maternal inheritance | 128 (41) |
| Absence of familial history | 27 (9) |
| Details not available | 25 (8) |
| Genetic diagnosis | |
| PKD1 gene mutation | 38 (12) |
| PKD2 gene mutation | 0 (0) |
| Not tested | 272 (88) |
| Kidney functionb | |
| eGFR, ml/min per 1.73 m2 | 120±18 |
| Decreased eGFR, <90 ml/min per 1.73 m2 | 15/278 (5) |
| No. of BP medications | |
| None | 248/310 (80) |
| Anyc | 62/310 (20) |
| 1 | 48/310 (165) |
| 2 | 12/310 (4) |
| 3 | 2/310 (0.6) |
Main patient characteristics. Numbers in parentheses indicate percentage values. In 27 patients without family history, the diagnosis was on the basis of genetic testing. Only pathogenic mutations in the PKD1 gene were reported.
Birth weight was available for 113 patients (four patients with premature birth were excluded).
eGFR data were available for 278 patients (90%).
Medications included angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in 90% of patients, calcium channel blockers in 27% of patients, β- or α- and β-blockers in 9% of patients, and diuretics in 7% of patients.
CKD was observed in 5% of participants. Twelve patients had an eGFR between 60 and 90 ml/min per 1.73 m2, and three had an eGFR of 45–59 ml/min per 1.73 m2. One fifth of the children were treated with BP medications at the time of the ABPM recording. The majority were taking one drug, usually an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker; <5% of patients received two or three BP medications.
Kidney Ultrasound
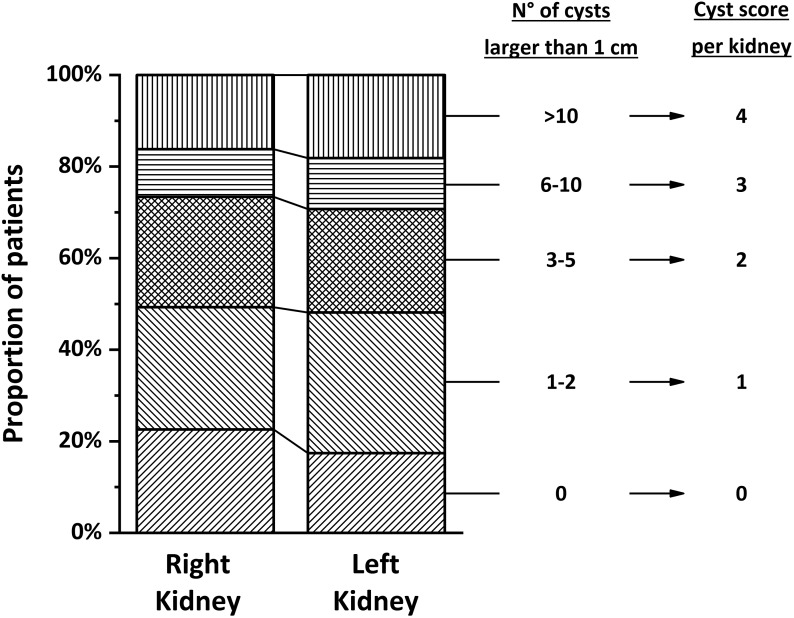
Ultrasonographic data were available in 270 patients (87%). Approximately one half of the cohort had zero to two cysts >1 cm per kidney, whereas <20% had more than ten cysts >1 cm per kidney (Figure 1). The prevalence of nephromegaly was 36% in children ages <11 years old as opposed to 50% in older children (P=0.04) (Supplemental Figure 3).
Figure 1.
Cystic changes evaluated by renal ultrasound in 270 participants. The figure shows the relative distribution of cyst classes for each kidney (details are in the text). This classification is on the basis of the number of cysts >1 cm per kidney, and it is used to calculate a “cyst score,” which corresponds to the sum of scores of each kidney.
ABPM Results
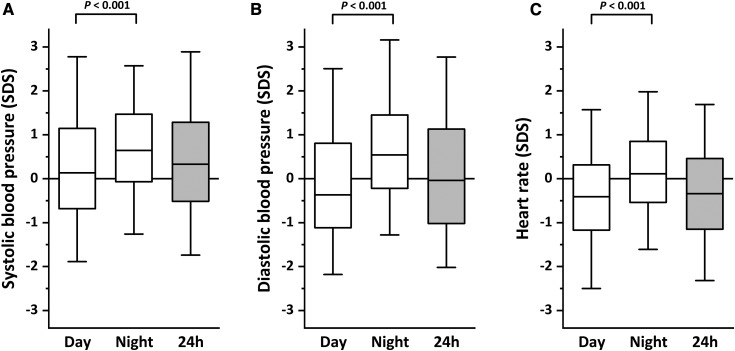
On average, most BP values were within the normal range during the day, whereas a significant shift toward higher BP levels was observed at night, indicating lack of the nocturnal dipping in a large proportion of patients (Figure 2, Supplemental Figure 4). Conversely, HR was, on average, low during daytime but not at night.
Figure 2.
Distribution of daytime, nighttime, and 24-hour ambulatory BP monitoring results. Box plots show the medians and interquartile values for BP (A and B) and heart rate (C) expressed as SD score (SDS). Whiskers indicate the 5th and 95th percentiles.
ABPM results are summarized in Table 2. Approximately one fifth of patients (21%) had BP higher than the 95th percentile during the entire day/night cycle. When considering patients receiving BP medications at the time of the ABPM recording, approximately one third of the cohort (35%) had either hypertension or were under treatment for hypertension. Less than one half of the cohort had BP values within the optimal range (<75th percentile) without taking BP medications. In addition, one half of the cohort lacked a significant BP dipping at night (52%), and nearly one fifth (18%) had isolated nocturnal hypertension.
Table 2.
Stratification of ambulatory BP monitoring results by diagnosis
| Diagnosis | Day, % | Night, % | 24 h,% |
|---|---|---|---|
| Hypertension (SBP and/or DBP ≥p95) | 15 | 30 | 21 |
| High-normal BP (SBP and/or DBP ≥p90 to <p95) | 10 | 10 | 11 |
| Suboptimal BP (SBP and/or DBP ≥p75 to <p90) | 14 | 19 | 16 |
| Optimal BP (SBP and DBP <p75) | 62 | 41 | 53 |
| Nondippers (SBP or DBP) | 52 | ||
| Isolated nocturnal hypertension | 18 | ||
| Hypertension (SBP and/or DBP ≥p95) and/or BP medication | 31 | 42 | 35 |
| Optimal BP (SBP and DBP <p75) without BP medication | 52 | 35 | 46 |
The table summarizes the ambulatory BP monitoring diagnoses during daytime, nighttime, and 24 hours. BP values were expressed as SD scores using available reference data for ambulatory BP monitoring in children (24) and converted into percentile values. BP outcome definitions were derived from published European and North American guidelines (15,25) (details are in the text). SBP, systolic BP; DBP, diastolic BP; p95, 95th percentile; p90, 90th percentile; p75, 75th percentile.
Risk Factor Analyses
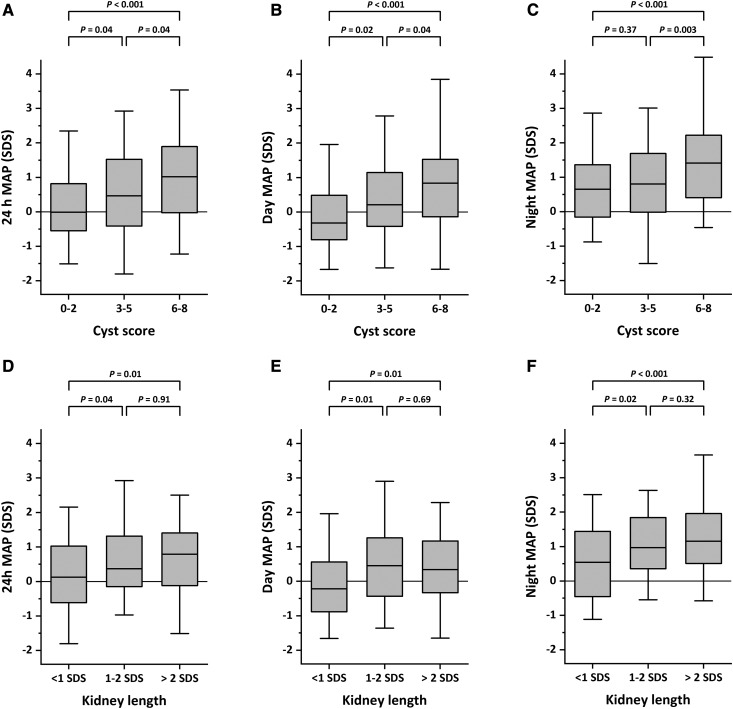
Table 3 shows the results of a logistic regression analysis aimed at identifying risk factors for different hypertension outcomes. High cyst score was a significant risk factor for hypertension. Kidney length was only significantly associated with nighttime and isolated nocturnal hypertension. These results are apparent in Figure 3, which shows the distribution of mean arterial pressure for different subclasses of cyst score or kidney length. Conversely, cyst score and kidney length did not affect nocturnal dipping. Thirty patients did not have cysts >1 cm (cyst score =0). In these patients, the prevalence of 24-hour hypertension was 14% compared with 24% for the remainder of the cohort; differences did not reach statistical significance (P=0.27). Low birth weight and eGFR were not associated with hypertension (Supplemental Table 2).
Table 3.
Analysis of risk factors for different ambulatory BP monitoring outcomes
| Diagnosis | Units | Adjusted OR | 95% CI | P Value |
|---|---|---|---|---|
| 24-h Hypertension | ||||
| Variable | ||||
| Cyst score (categorical 0–8) | 1 Point | 1.39 | 1.08 to 1.81 | 0.01 |
| Kidney length | 1 SD score | 1.14 | 0.99 to 1.30 | 0.06 |
| Daytime hypertension | ||||
| Variable | ||||
| Cyst score (categorical 0–8) | 1 Point | 1.70 | 1.21 to 2.40 | 0.002 |
| Kidney length | 1 SD score | 1.00 | 0.84 to 1.20 | 0.94 |
| Nighttime hypertension | ||||
| Variable | ||||
| Cyst score (categorical 0–8) | 1 Point | 1.31 | 1.05 to 1.63 | 0.02 |
| Kidney length | 1 SD score | 1.23 | 1.06 to 1.42 | 0.01 |
| Isolated nocturnal hypertension | ||||
| Variable | ||||
| Cyst score (categorical 0–8) | 1 Point | 1.15 | 0.92 to 1.43 | 0.22 |
| Kidney length | 1 SD score | 1.17 | 1.01 to 1.34 | 0.03 |
| Nondipping | ||||
| Variable | ||||
| Cyst score (categorical 0–8) | 1 Point | 0.90 | 0.77 to 1.05 | 0.17 |
| Kidney length | 1 SD score | 1.07 | 0.95 to 1.21 | 0.25 |
The table shows the results of a multivariable logistic regression that analyzes risk factors for different ambulatory BP monitoring outcomes. ORs are adjusted for age, sex, use of BP medications, and center effect, and they are indicated with their corresponding 95% CIs. OR, odds ratio; 95% CI, 95% confidence interval.
Figure 3.
Influence of the cyst score and kidney length SD score (SDS) on mean arterial pressure (MAP). Box plots show the medians and interquartile values for daytime, nighttime, and 24-hour MAP values for different classes of cyst score (A–C) and kidney length (D–F). Data are expressed as SDS. Whiskers indicate the 5th and 95th percentiles.
Rhythm Analyses
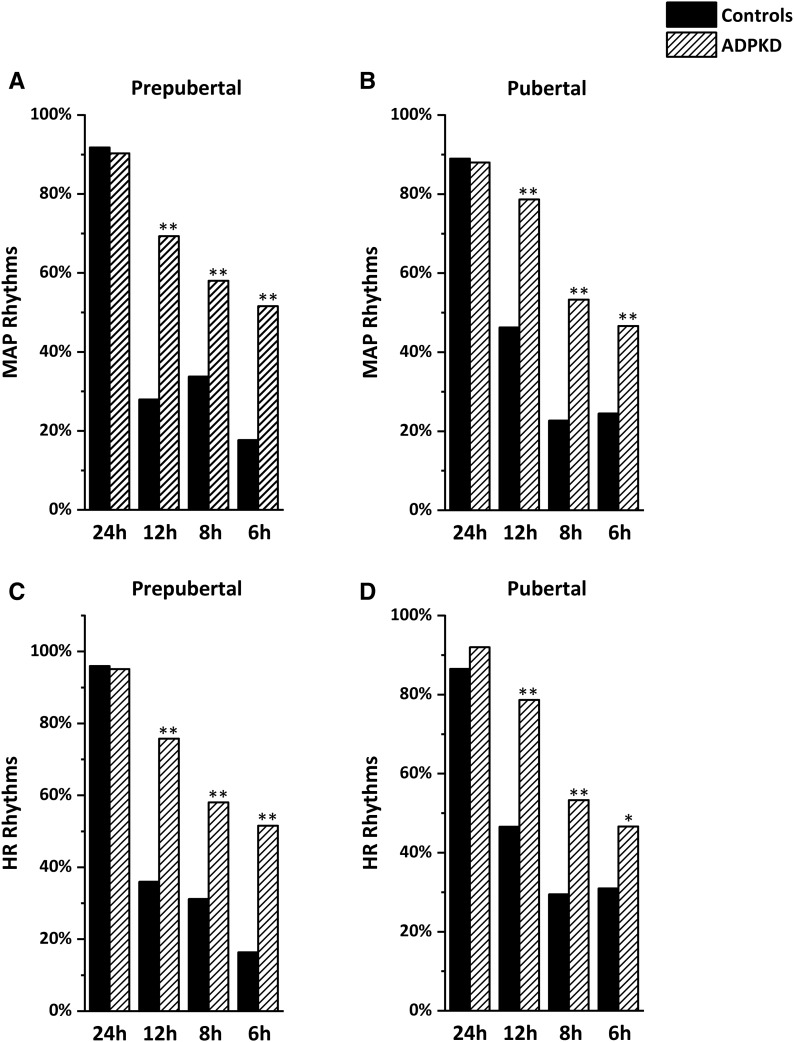
Typically, most children have a circadian rhythm of their BP and HR, with values being lower at night, whereas ultradian rhythms are only present in a minority of participants. Figure 4 shows the prevalence of circadian and ultradian rhythms in children with ADPKD compared with normal prepubertal and pubertal children. A marked increase in the prevalence of ultradian rhythms was observed. In addition, rhythms in children with ADPKD were characterized by lower amplitudes and higher acrophases compared with controls (Supplemental Table 3). No association was observed between age and the prevalence of ultradian rhythms, which for the most part, were not associated with hypertension or the absence of nocturnal dipping (Supplemental Table 3).
Figure 4.
Prevalence of circadian and ultradian rhythms in 137 participants. The figure shows the prevalence of significant rhythms obtained by partial Fourier analysis on 24-hour mean arterial pressure (MAP; A and B) and heart rate (HR; C and D) data. Data are calculated separately for prepubertal (n=62) and pubertal (n=75) children, and they are compared with control children (22). ADPKD, autosomal dominant polycystic kidney disease. *P<0.01; **P<0.001.
Sensitivity Analyses
Referring centers were asked to specify the indications for performing ABPM and the total number of patients with ADPKD that they were following at the time of the study. It was estimated that 410 patients ages 4–18 years old were followed in referring centers during the study period. Of these, 76% underwent ABPM. Overall, 66% performed ABPM as part of a systematic clinical evaluation of children with ADPKD, whereas 27% underwent ABPM in the suspicion of hypertension or because hypertension had been established. For the remaining 7% of patients, no specific protocol was followed.
To study the effect of variables on results, sensitivity analyses were performed. After excluding patients from centers that did not have ABPM as a systematic test and patients taking BP medications, no significant effect on ABPM findings was observed (Table 4). In addition, no difference was observed after removing patients from one center, in which a higher prevalence of hypertension was observed (Supplemental Table 4). Because antihypertensive drugs can generate ultradian rhythms, the analysis was repeated after removing patients taking BP medications. No significant changes in the prevalence of circadian and ultradian rhythms were observed (Supplemental Table 5).
Table 4.
Assessment of potential confounding factors by sensitivity analysis
| Diagnosis | All Patients, n=292, % | Only ABPM Performed as a Screening Test, n=230 | Only Patients Not Taking BP Medications, n=193 | ||
|---|---|---|---|---|---|
| % | P Value | % | P Value | ||
| 24 h | |||||
| Hypertension | 21 | 18 | 0.55 | 18 | 0.49 |
| Hypertension and/or BP medications | 35 | 35 | 0.92 | ||
| Day | |||||
| Hypertension | 15 | 12 | 0.40 | 14 | 0.70 |
| Hypertension and/or BP medications | 31 | 32 | 0.92 | ||
| Night | |||||
| Hypertension | 30 | 28 | 0.57 | 27 | 0.42 |
| Hypertension and/or BP medications | 42 | 41 | 0.97 | ||
| Nondipping | 52 | 51 | 0.85 | 52 | 0.95 |
| Isolated nocturnal hypertension | 18 | 19 | 0.83 | 15 | 0.36 |
The table compares the prevalence of selected diagnoses that are reported in Table 1 in the entire cohort and after removing patients who did not perform ABPM as a routine screening test or patients who were taking BP medications. The effect of removing subsets of patients was evaluated by chi-squared tests; results are expressed using the P value. All tests failed to show significant changes in both subanalyses. ABPM, ambulatory BP monitoring.
Discussion
Typically, ADPKD becomes symptomatic in time, because the cyst burden and kidney volume increase (17,26,27). Although hypertension can develop early in the course of the disease, its prevalence during childhood is not clearly established (2–7,9,28,29). This study shows that a significant proportion of children with ADPKD has high BP, particularly at night.
The analyses of published data are limited by differences among studies in sample size, age, methods for defining hypertension, and recruitment biases. One of the first studies (140 children with a mean age of 8.7 years old) reported a prevalence of hypertension of 27% (7), which correlated with higher left ventricular mass (12). Other single-center cohort analyses have reported incidences ranging from 6% to 35% (3,5,6). Cadnapaphornchai et al. (2) observed a prevalence of 32% in 85 children and young adults (age range, 4–21 years old) included in a prospective trial testing enalapril in ADPKD. Hypertension was defined in that study by home BP monitoring, a method that correlates better with ABPM than office BP (30,31), although correlation with end organ damage in children has been established mostly with ABPM (32). The same investigators have also shown that children with hypertension and ADPKD have larger kidneys, have higher left ventricular mass index, and experience faster decline in kidney function, especially if glomerular hyperfiltration is present (2,4,11,33).
The results of the ADPKiDs Study have substantial clinical effect, because they indicate that ABPM allows detection of hypertension, including isolated nocturnal hypertension, in a significant number of children and that subjects with high BP have more cysts, a future that is known to correlate with faster progression (1,8,26,27,34). These findings suggest that ABPM should be performed in all children with an established diagnosis of ADPKD.
A large body of evidence shows that ABPM represents a more accurate method to diagnose hypertension, particularly in children, by avoiding white coat hypertension and allowing detection of masked and nocturnal hypertension (15,16). ABPM has also been shown to correlate better with target organ damage in the pediatric population (32,35–38). Remarkably, ABPM data in children with ADPKD are very limited. Seeman et al. (5) have analyzed ABPM recordings from 62 children and found that 22 patients (35%) had hypertension, larger kidney volumes, and more cysts. In a subsequent study, they also observed that children with hypertension had more frequently decreased kidney concentrating capacity (10).
Overall, the ADPKiDs Study confirms in a very large cohort of pediatric patients a strong association between BP values and kidney enlargement. Unfortunately, height-adjusted total kidney volume, which represents the gold standard to measure kidney enlargement (8,26,39), could not be assessed. Nephromegaly was only estimated using kidney length, which nonetheless, has been shown to correlate well with kidney volume and be a good predictor of evolution in adult patients with ADPKD (39). In the routine clinical practice, kidney length is usually measured by ultrasounds in pediatric patients with ADPKD, because kidneys are smaller and because magnetic resonance imaging requires anesthesia in young children. As expected, cyst burden and kidney length were associated with higher BP. Notably, however, hypertension was not associated with older age, indicating that it may develop very early, although often not in a severe form. In this regard, the analysis was well powered, because the age distribution was homogeneous across the entire age range of the cohort.
For the first time, our analysis reveals increased prevalence of ultradian rhythms with blunted variability in ADPKD. A number of pathologic conditions have been shown to alter physiologic rhythmicity in children (23,40–42). In particular, high prevalence of ultradian rhythms with lower amplitudes and delayed acrophases has been observed in children with hypertension or CKD (40,42), very similar to what we observed in children with ADPKD. The exact interpretation of these findings remains uncertain. Altered rhythmicity in patients with hypertension correlates with loss of nocturnal dipping, which is an established risk factor for cardiovascular disease (43). Cardiovascular rhythm anomalies could also represent an early sign of altered sympathetic activity (23,43,44), preceding the development of vascular damage. This would be consistent with data showing altered endothelial-dependent dilation and increased stiffness of large arteries in children and young adults with ADPKD, despite normal BP and normal body mass index (45). In addition, impaired cardiac handling of intracellular calcium has been observed in experimental models reproducing Pkd2 mutations (46,47). Regardless of the exact nature of the rhythmic changes observed in children with ADPKD, it is remarkable that their prevalence failed to correlate with age, hypertension, or the absence of nocturnal dipping, suggesting that they represent a very early dysfunction.
The ADPKiDs Study cohort has selection biases and carries the risk of overestimating the prevalence of hypertension and other BP abnormalities. Among possible biases, tertiary care hospitals that participated in the study may have recruited more severe patients, symptomatic patients may have been more likely to undergo ABPM, and families with more severe forms of ADPKD may have been more prone to consult. Nonetheless, two thirds of patients underwent ABPM as part of a routine evaluation of children with ADPKD, and sensitivity analyses failed to show significant changes after excluding patients at higher risk of hypertension. Altogether and considering that most pediatric studies, including the ADPKiDs Study, have selection biases, the prevalence of hypertension in children with ADPKD can be estimated between 20% and 35%.
Much debate exists as to whether it is appropriate to screen children at risk of ADPKD (34). Systematic screening was not recommended until recently (34). However, recent studies suggest the efficacy of targeted therapies in slowing disease progression (19,29), providing the rationale for early diagnosis. Theoretically, these treatments could be more efficient if started during childhood, in particular in patients who are at risk of being fast progressors (28), if side effects are acceptable. The negative psychologic consequences of a diagnosis during childhood could be outweighed by benefits derived from early changes in lifestyle and BP control (28). In this respect, it should be noted that treating hypertension in adults with ADPKD improves cardiac hypertrophy, but benefits on kidney function are less pronounced compared with other CKDs (48,49). This has also been observed in children with ADPKD treated with angiotensin-converting enzyme inhibitors (2). The effect of very early BP control in patients with ADPKD, who are known to be at high risk of cardiovascular events later in life, is not well established. It seems, however, reasonable to expect that it would be significant. ABPM helps to identify children who are likely to progress faster and may be candidates for newer treatments.
Disclosures
F.S. received honoraria for consulting services from Otsuka Pharmaceutical, and F.E. serves on the Otsuka Pharmaceutical safety board.
Supplementary Material
Acknowledgments
This study was supported by an impulsion grant provided by the Working Group on Inherited Kidney Disease of the European Renal Association–European Dialysis and Transplant Association.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11401017/-/DCSupplemental.
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4: 820–829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selistre L, de Souza V, Ranchin B, Hadj-Aissa A, Cochat P, Dubourg L: Early renal abnormalities in children with postnatally diagnosed autosomal dominant polycystic kidney disease. Pediatr Nephrol 27: 1589–1593, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Helal I, Reed B, McFann K, Yan XD, Fick-Brosnahan GM, Cadnapaphornchai M, Schrier RW: Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 2439–2443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeman T, Dusek J, Vondrichová H, Kyncl M, John U, Misselwitz J, Janda J: Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit 8: 107–110, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Mekahli D, Woolf AS, Bockenhauer D: Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol 25: 2275–2282, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA: The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 4: 1654–1660, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chapman AB, Guay-Woodford LM: Renal volume in children with ADPKD: Size matters. Clin J Am Soc Nephrol 4: 698–699, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Reddy BV, Chapman AB: The spectrum of autosomal dominant polycystic kidney disease in children and adolescents. Pediatr Nephrol 32: 31–42, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Seeman T, Dusek J, Vondrák K, Bláhová K, Simková E, Kreisinger J, Dvorák P, Kyncl M, Hríbal Z, Janda J: Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 53: 629–634, 2004 [PubMed] [Google Scholar]
- 11.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 74: 1192–1196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeier M, Geberth S, Schmidt KG, Mandelbaum A, Ritz E: Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1451–1457, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney WE Jr, Avner ED: Diagnosis and management of childhood polycystic kidney disease. Pediatr Nephrol 26: 675–692, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A: 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34: 1887–1920, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Flynn JT, Urbina EM: Pediatric ambulatory blood pressure monitoring: Indications and interpretations. J Clin Hypertens (Greenwich) 14: 372–382, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grantham JJ, Torres VE: The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 12: 667–677, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, Devuyst O, Drechsler C, Eckardt KU, Emma F, Knebelmann B, Le Meur Y, Massy ZA, Ong AC, Ortiz A, Schaefer F, Torra R, Vanholder R, Więcek A, Zoccali C, Van Biesen W: Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: A position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31: 337–348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW: Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 889–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao A, Cachat F, Faouzi M, Bardy D, Mosig D, Meyrat BJ, Girardin E, Chehade H: Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int 83: 524–530, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Di Zazzo G, Stringini G, Matteucci MC, Muraca M, Malena S, Emma F: Serum creatinine levels are significantly influenced by renal size in the normal pediatric population. Clin J Am Soc Nephrol 6: 107–113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadtstein C, Wühl E, Soergel M, Witte K, Schaefer F; German Study Group for Pediatric Hypertension : Normative values for circadian and ultradian cardiovascular rhythms in childhood. Hypertension 43: 547–554, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Saner C, Simonetti GD, Wühl E, Mullis PE, Janner M: Circadian and ultradian cardiovascular rhythmicity in obese children. Eur J Pediatr 175: 1031–1038, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F; German Working Group on Pediatric Hypertension : Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee on Screening and Management of High Blood Pressure In Children : Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140: e20171904, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Grantham JJ: Rationale for early treatment of polycystic kidney disease. Pediatr Nephrol 30: 1053–1062, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Cadnapaphornchai MA: Clinical trials in pediatric autosomal dominant polycystic kidney disease. Front Pediatr 5: 53, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wühl E, Hadtstein C, Mehls O, Schaefer F; Escape Trial Group : Home, clinic, and ambulatory blood pressure monitoring in children with chronic renal failure. Pediatr Res 55: 492–497, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Stergiou GS, Alamara CV, Salgami EV, Vaindirlis IN, Dacou-Voutetakis C, Mountokalakis TD: Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press Monit 10: 143–147, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee : Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW: Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6: 369–376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants : Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 88: 17–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K: Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens 21: 443–450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gellermann J, Kraft S, Ehrich JH: Twenty-four-hour ambulatory blood pressure monitoring in young children. Pediatr Nephrol 11: 707–710, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Gimpel C, Wühl E, Arbeiter K, Drozdz D, Trivelli A, Charbit M, Gellermann J, Dusek J, Jankauskiene A, Emre S, Schaefer F; ESCAPE Trial Group : Superior consistency of ambulatory blood pressure monitoring in children: Implications for clinical trials. J Hypertens 27: 1568–1574, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Páll D, Juhász M, Lengyel S, Molnár C, Paragh G, Fülesdi B, Katona E: Assessment of target-organ damage in adolescent white-coat and sustained hypertensives. J Hypertens 28: 2139–2144, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Bhutani H, Smith V, Rahbari-Oskoui F, Mittal A, Grantham JJ, Torres VE, Mrug M, Bae KT, Wu Z, Ge Y, Landslittel D, Gibbs P, O'Neill WC, Chapman AB; CRISP Investigators : A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 88: 146–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litwin M, Simonetti GD, Niemirska A, Ruzicka M, Wühl E, Schaefer F, Feber J: Altered cardiovascular rhythmicity in children with white coat and ambulatory hypertension. Pediatr Res 67: 419–423, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Wolfenstetter A, Simonetti GD, Pöschl J, Schaefer F, Wühl E: Altered cardiovascular rhythmicity in children born small for gestational age. Hypertension 60: 865–870, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Wühl E, Hadtstein C, Mehls O, Schaefer F; ESCAPE Trial Group : Ultradian but not circadian blood pressure rhythms correlate with renal dysfunction in children with chronic renal failure. J Am Soc Nephrol 16: 746–754, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y: Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama study. J Hypertens 20: 2183–2189, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Kooman JP, Usvyat L, van der Sande FM, Thijssen S, Levin N, Leunissen KM, Kotanko P: ‘Time and time again’: Oscillatory and longitudinal time patterns in dialysis patients. Kidney Blood Press Res 35: 534–548, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Nowak KL, Farmer H, Cadnapaphornchai MA, Gitomer B, Chonchol M: Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 32: 342–347, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paavola J, Schliffke S, Rossetti S, Kuo IY, Yuan S, Sun Z, Harris PC, Torres VE, Ehrlich BE: Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol 58: 199–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo IY, Kwaczala AT, Nguyen L, Russell KS, Campbell SG, Ehrlich BE: Decreased polycystin 2 expression alters calcium-contraction coupling and changes β-adrenergic signaling pathways. Proc Natl Acad Sci U S A 111: 16604–16609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB; HALT-PKD Trial Investigators : Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371: 2255–2266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Moore CG, Perrone RD; HALT-PKD Trial Investigators : Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med 371: 2267–2276, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.