Summary
Trophic factor delivery to the brain using stem cell-derived neural progenitors is a powerful way to bypass the blood-brain barrier. Protection of diseased neurons using this technology is a promising therapy for neurodegenerative diseases. Glial cell line-derived neurotrophic factor (GDNF) has provided benefits to Parkinsonian patients and is being used in a clinical trial for amyotrophic lateral sclerosis. However, chronic trophic factor delivery prohibits dose adjustment or cessation if side effects develop. To address this, we engineered a doxycycline-regulated vector, allowing inducible and reversible expression of a therapeutic molecule. Human induced pluripotent stem cell (iPSC)-derived neural progenitors were stably transfected with the vector and transplanted into the adult mouse brain. Doxycycline can penetrate the graft, with addition and withdrawal providing inducible and reversible GDNF expression in vivo, over multiple cycles. Our findings provide proof of concept for combining gene and stem cell therapy for effective modulation of ectopic protein expression in transplanted cells.
Keywords: induced pluripotent stem cell, gene therapy, inducible reversible transgene, tetracycline, glial cell line-derived neurotrophic factor, doxycycline, piggyBac, luciferase, non-invasive imaging, cell therapy
Graphical Abstract
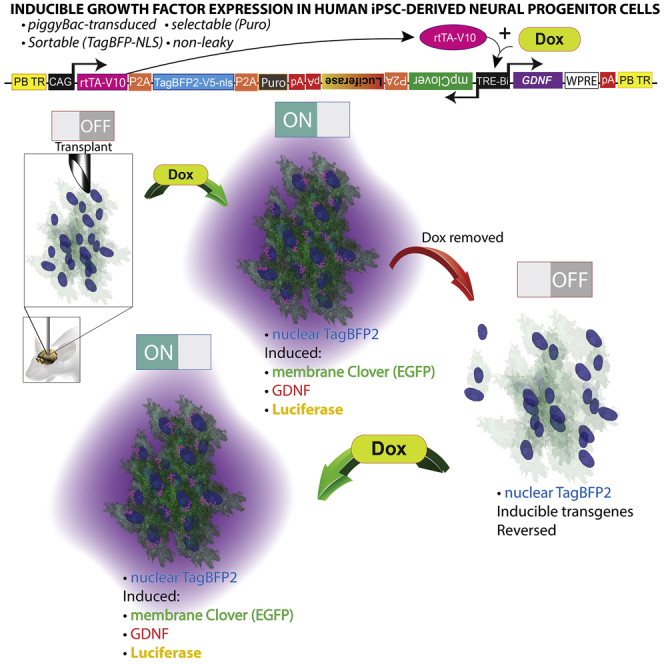
Highlights
-
•
Created plasmid with tetracycline transactivator along with dual reporters and GDNF
-
•
Efficient, stable transduction of human iPSC-derived neural progenitor cells
-
•
Inducible and reversible in vivo expression of GDNF, reporter protein, and luciferase
-
•
Promising stem cell and gene therapy strategy for neurodegenerative diseases
Svendsen, Breunig, and colleagues generated a plasmid containing a tetracycline transactivator and a response element expressing dual reporters and GDNF. Stably transduced human iPSC-derived neural progenitors show inducible, reversible expression of GDNF, a fluorescent reporter protein, and luciferase in vitro and following transplantation into the mouse brain. This combined cell and gene therapy advancement is promising for neurodegenerative diseases.
Introduction
Combined cell and gene therapy approaches to both rejuvenate cellular niches and provide therapeutic molecules to diseased host cells is a promising treatment approach for neurological disorders (Gowing et al., 2017). The delivery of various growth factors to the site of damage using ex vivo genetically modified cells has been shown to support host neurons in disease models of amyotrophic lateral sclerosis (ALS) and Parkinson's, Huntington's, and Alzheimer's diseases (Behrstock et al., 2006, Blesch and Tuszynski, 1995, Deng et al., 2016, Ebert et al., 2008, Ebert et al., 2010, Gowing et al., 2017, Klein et al., 2005). Our group has shown that human neural progenitor cells genetically engineered to stably produce glial cell line-derived neurotrophic factor (GDNF) can survive, migrate, release GDNF, and protect degenerating neurons (Behrstock et al., 2006, Klein et al., 2005). Critically, these cells have been confirmed to be safe and non-tumorigenic and are being used in the first-ever US Food and Drug Administration-approved phase 1/2a clinical cell and gene therapy trial to protect motor neurons in ALS (NCT02943850, 2017).
ALS is a rapidly progressing disease, hence constitutive trophic factor release may be satisfactory without considering long-term effects of continual drug administration. In contrast, other neurological diseases with a longer life span may not require sustained growth factor secretion. Postmortem analysis of a Parkinsonian patient only temporarily receiving GDNF in the caudate putamen showed persistent neuronal sprouting that may explain maintained effects after GDNF delivery cessation (Love et al., 2005, Patel et al., 2013). Furthermore, constitutive growth factor release does not allow for cessation in the event of a contraindication. For instance, the development of Lhermitte's symptoms reported by some patients receiving GDNF (Nutt et al., 2003). In addition, sustained GDNF expression can cause aberrant sprouting and desensitization of the targeted neurons, which importantly were reversed upon GDNF cessation using a tetracycline-regulated system (Georgievska et al., 2002, Georgievska et al., 2004). Overall, there is a clear rationale for growth factor regulation in these types of clinical trial.
External factors such as mifepristone, rapamycin, or tetracycline ([Tet], and its analog doxycycline [dox]) can be used to regulate gene expression (Akhtar et al., 2015, Espadas-Alvarez et al., 2017, Tereshchenko et al., 2014). GDNF delivered by direct gene transfer in the rodent has been regulated (Chtarto et al., 2016), but this has yet to be accomplished for engrafted human neural cells engineered to release GDNF. Finally, the need for repeated on-off flexibility may be required, yet the current standard in the field is to only induce or repress a gene over a single cycle.
In the current study we developed a dox-mediated method for inducing and reversing GDNF expression in human induced pluripotent stem cell (iPSC)-derived neural progenitor cells (iNPCs) transplanted to the adult mouse brain. We show that dox administration can inducibly and reversibly modulate GDNF secretion in vivo. As such, we demonstrate that the powerful technologies of iPSCs, ex vivo cell engineering, and gene regulation can be combined as a unique approach to treat disorders where regulated protein delivery may be desired.
Results
A Vector to Provide Regulated Expression of Reporter Genes and GDNF
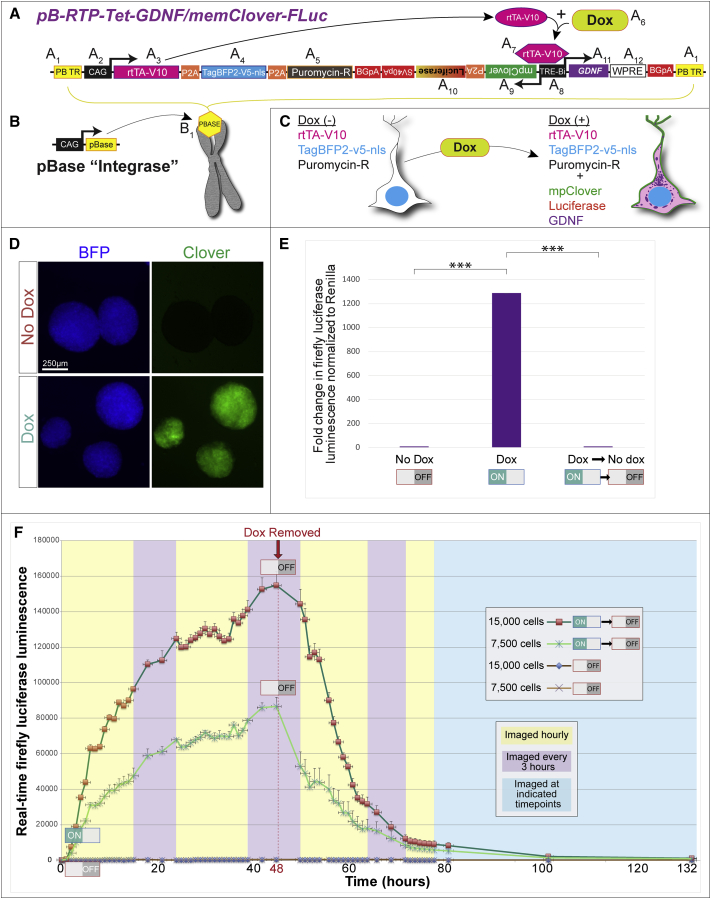
Tet-ON systems rely on constitutive expression of the rtTA-V10 transactivator and a dox-responsive TRE-Bi (Tet-response element bidirectional) promoter (Akhtar et al., 2015). We observed that if the transactivator and inducible promoter are expressed in different plasmids and co-transfected into cells, the cells drifted during expansion and selected against doubly transgenic populations, resulting in cells that are non-inducible (data not shown). Incorporating these elements as well as reporter genes in a single system requires a large vector, which was accomplished with our created vector named “pB-RTP-Tet-GDNF/memClover-FLuc” (piggyBac-reverse transactivator/TagBFP2nls/PacR-Tet-inducible-GDNF/membrane Clover-firefly luciferase) (Figure 1A). The vector is flanked by piggyBac terminal repeats (PB TR) (Figure 1A1), which allows for its stable genomic integration when the pBase enzyme is transiently expressed (Chen and LoTurco, 2012). The vector has two promoters––a constitutively active CMV/Chick β-actin (also known as CAG) promoter and an inducible, bidirectional TRE-Bi promoter. The CAG promoter (Figure 1A2) drives constitutive expression of the rtTA-V10 (also known as tet-ON) transactivator (Figure 1A3), TagBFP2-V5-nls (enhanced blue fluorescent protein with a V5 tag and nuclear localization sequence) (Figure 1A4), and the puromycin resistance gene (Figure 1A5). Transgenes in tandem are separated by self-cleaving peptide linkers (P2A). Dox addition (Figure 1A6) causes the rtTA-V10 transactivator to undergo a conformational change (Figure 1A7), allowing it to bind to the TRE-Bi promoter (Figure 1A8) and catalyze transcription of downstream transgenes; and dox withdrawal reverses the induced gene expression. The first cistron of the TRE-Bi promoter harbors a myristoylated and palmitoylated (MyrPalm) Clover reporter (mpClover) (Figure 1A9) followed by destabilized firefly luciferase (Luc2P) (Figure 1A10). Clover is a GFP, and the MyrPalm sequences of which localize this protein to the cell membrane (Breunig et al., 2015). A membrane fluorescent reporter was chosen in order to avoid visual overlap with perinuclear GDNF localization. Luc2P has a half-life several-fold less than wild-type luciferase and was incorporated for gene expression analysis in live animals over time. The second cistron downstream of the inducible TRE-Bi promoter harbors GDNF (Figure 1A11) followed by the woodchuck hepatitis virus post-transcriptional element for increased gene expression (Figure 1A12). Well-characterized rabbit β-globin poly(A)s were placed downstream of the respective elements to terminate transcription and prevent spurious transgene expression. The pB-RTP-Tet-GDNF/memClover-FLuc vector is transfected alongside a pBase plasmid (Figure 1B) to promote stable genomic integration (Figure 1B1). In summary, the presence or absence of dox dictates the expression of the inducible transgenes (Clover, luciferase, and GDNF), while the rtTA-v10 transactivator, TagBFP-v5-nls, and puromycin resistance genes are constitutively expressed (Figure 1C).
Figure 1.
Description of pB-RTP-Tet-GDNF/memClover-FLuc Vector
(A) pB-RTP-Tet-GDNF/memClover-FLuc plasmid that is designed to stably integrate into genome when transfected in combination with pBase plasmid.
(B) pBase plasmid.
(C) Transgenes constitutively expressed or expressed only in the presence of doxycycline.
(D) Live unstained fluorescent imaging of human iNPCs nucleofected with pB-RTP-Tet-GDNF/memClover-FLuc and grown as neurospheres.
(E) Firefly luciferase activity normalized to renilla luciferase in iNPCs. N = 4 independent experiments.
(F) Real-time firefly luciferase activity in live culture of iNPCs. N = 3 independent experiments. Error bars represent ± SEM.
∗∗∗p < 0.001.
Reporter Protein Production Is Reversibly Regulated In Vitro
To assess the dox-regulated system in therapeutically relevant cells, human iNPCs were nucleofected with the pB-RTP-Tet-GDNF/memClover-FLuc and pBase vectors. Nucleofected cells were grown as free-floating spheres (termed EZ spheres) that could be easily maintained and rapidly expanded (Ebert et al., 2013). In the absence of dox, spheres constitutively expressed BFP, and within 24 hr of adding dox, spheres expressed both BFP and GFP, indicating that the TRE-Bi promoter was responding to dox (Figure 1D).
To quantify reporter expression from the TRE-Bi promoter, iNPCs were nucleofected as above, along with an Ef1-renilla plasmid for renilla luciferase constitutive expression to normalize protein. A dual-luciferase assay revealed that cells expressed a ∼1,300-fold increase in firefly luciferase activity (normalized to renilla) after dox treatment, and this returned to near basal levels upon dox removal (Figure 1E). To assess the kinetics of the TRE-Bi-inducible promoter, firefly luciferase activity was quantified in a live culture of the nucleofected iNPCs grown in the presence of luciferin (Figure 1F). Bioluminescence rapidly increased after dox addition at time 0, and slowly decreased after dox removal (at 48 hr). In order to assess if the TRE-Bi promoter could be reversibly induced, the IncuCyte S3 Live-Cell Analysis System was used to take hourly images of the nucleofected iNPCs. Clover expression could be induced, reversed, and re-induced by adding, removing, and re-adding dox to the culture (Video S1). As a clinically-relevant cell product would need to be expanded and banked, we assessed if the TRE-Bi promoter would remain dox responsive with an extensive cell expansion and freeze. Flow analysis revealed that 83% of the constitutively BFP+ cells were induced to express GFP (Figure S1), demonstrating that the TRE-Bi promoter remained functional.
GDNF Production Is Reversibly Regulated In Vitro
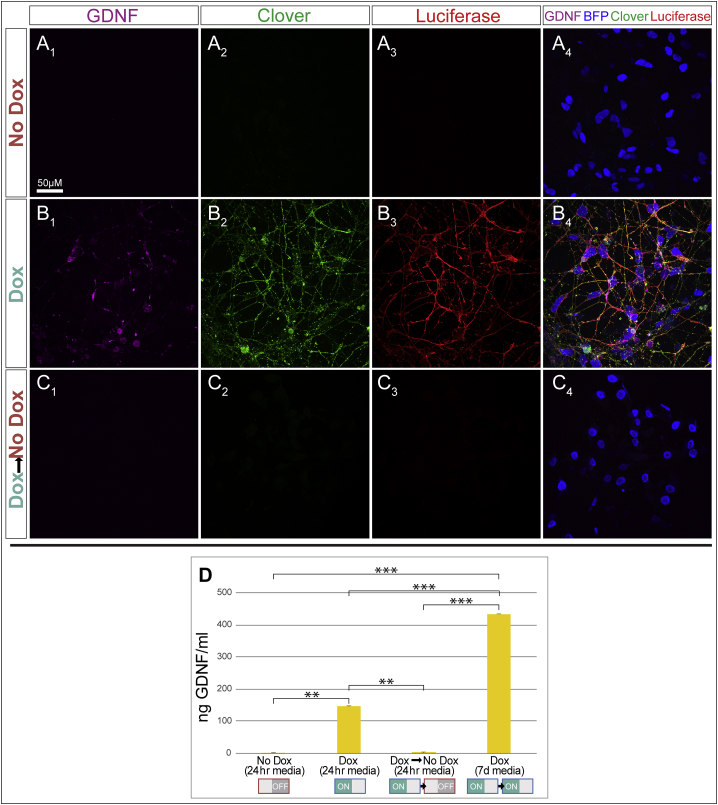
To ensure that our system could regulate a therapeutically relevant molecule, GDNF production was assessed in nucleofected iNPCs. Immunofluorescence revealed that GDNF was not visible in cells in the absence of dox, which confirms our previous report that wild-type cells do not produce GDNF (Ebert et al., 2008). The reporter proteins Clover and luciferase were also not produced in the absence of dox, whereas BFP was produced under the constitutive promoter (Figure 2A). In the presence of dox, GDNF as well as Clover and luciferase were detected, along with maintained BFP production (Figure 2B). When dox was subsequently removed, GDNF and reporter gene production all ceased, while BFP was maintained (Figure 2C).
Figure 2.
Doxycycline Regulates GDNF Expression from pB-RTP-Tet-GDNF/memClover-FLuc Nucleofected iPSC-Derived NPCs
(A and B) Nucleofected iNPCs grown in the (A) absence or (B) presence of dox.
(C) Nucleofected iNPCs grown in the absence of dox after dox was added and removed.
(D) ELISA of 24 hr incubated medium from cells in (A–C), as well as in a culture with GDNF protein accumulation for 7 days. N = 3 independent experiments. Error bars represent ±SEM. ∗∗p < 0.01, ∗∗∗p < 0.001.
In order to quantify the regulation of GDNF, ELISA was used on cells grown in the presence of dox (ON), absence of dox (OFF), in the presence then absence of dox (ON→OFF), and in the presence of dox for an extended period of time (ON→ON) wherein the medium was not changed (Figure 2D). Once again, cells did not produce appreciable levels of GDNF in the absence of dox. In contrast, cells produced robust levels of GDNF in the presence of dox after only 24 hr and this accumulated over 7 days of continuous dox. Critically, GDNF could be reversibly turned on and returned to baseline levels based on the presence/absence of dox. Normalizing ELISA results with stereological counts of GDNF+ cells demonstrated that dox-treated cultures secreted 7.34 × 10−4 pg of GDNF/GDNF+ cells/hr. The demonstration that proteins can be successfully induced and reversed in vitro sets the stage for protein regulation in cells following transplantation.
Reporter Transgene Expression Is Inducible and Reversible in iNPC Transplants in the Adult Brain
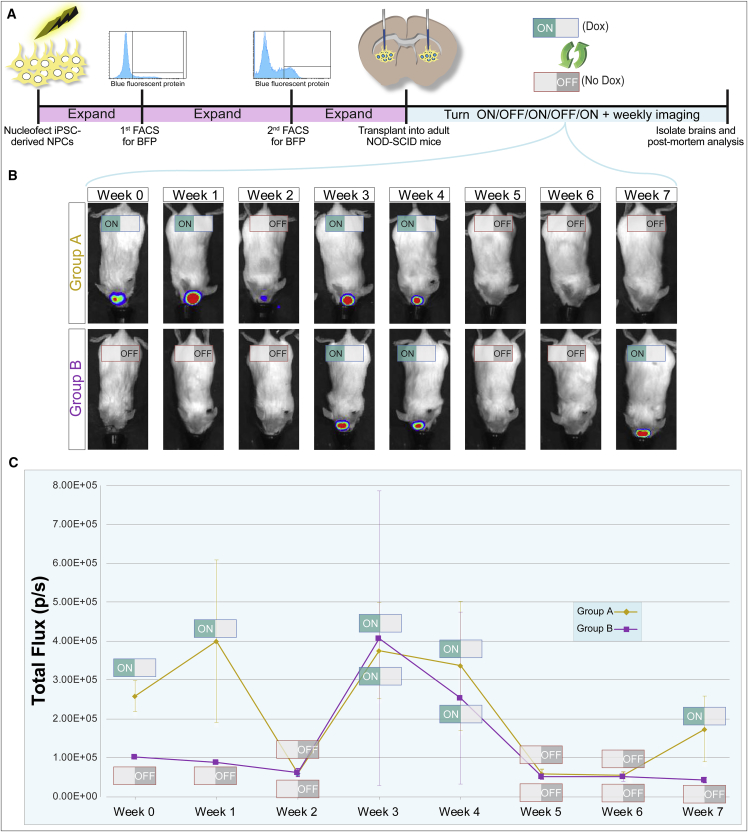
To investigate the ability of this system to regulate protein expression in vivo, nucleofected iNPCs were expanded and transplanted to the adult non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mouse brain (Figure 3A). During in vitro expansion, cells underwent two rounds of fluorescence-activated cell sorting (FACS) for nuclear BFP. The parent cell population contained 15.6% BFP+, which were then expanded as neurospheres for 7 weeks and sorted a second time for BFP, which yielded 32.4% BFP+ cells. Three weeks after the second sort, cells were divided into two groups, with one group receiving dox in culture for 3 days prior to transplantation and the other group receiving no dox. NOD-SCID mice (n = 14) were transplanted with 200,000 iNPCs into each striatal hemisphere, with 10 animals receiving cells pre-treated with dox and 4 animals receiving untreated cells. Animals did not receive dox before the transplant.
Figure 3.
Reporter Transgene Expression Is Inducible and Reversible in iPSC-Derived NPC Transplants
(A) Experimental design for nucleofection, expansion, FACS, transplant, treatment, and post-mortem analysis.
(B) Weekly bioluminescence imaging of one animal from each group (animal no. 9 for group A and animal no. 3 for group B) over a 7-week in vivo experimental period. ON/OFF buttons indicate if animal received dox during the week prior to imaging.
(C) Summary of weekly bioluminescence activity for all animals (N = 14). One animal (no. 13) was excluded from this analysis due an abnormally high bioluminescence signal observed and no inducible transgene signal observed by post-mortem immunohistochemistry. Error bars represent ± SEM.
One day after transplantation, all ten animals transplanted with the dox-treated cells emitted luciferase signal above background, which was calculated based on the signal from the animal's hind region (Figures 3B and 3C; week 0). To determine if the transplanted cells could be subsequently turned “ON” and “OFF” multiple times, animals were separated into two groups and switched ON→OFF→ON→OFF→ON (group A) or OFF→ON→OFF→ON (group B). Weekly bioluminescence imaging demonstrated that dox effectively regulated the luciferase protein in vivo, both in representative animals that started the trial on dox and ended off dox as well as started off dox and ended on dox (Figure 3B). Luciferase activity quantification at the weekly imaging session confirmed both the increase in luciferase activity in dox-treated animals and the inducibility of gene expression over time (Figure 3C). Collectively, this provides in vivo proof-of-principle for dox-inducible gene expression.
GDNF Expression Is Inducible and Reversible in iNPC Transplants
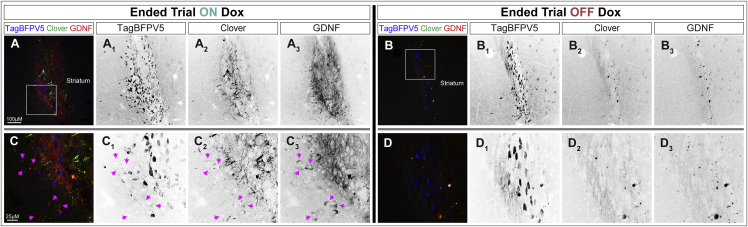
To confirm regulated GDNF expression in transplanted cells, animals were euthanized after the final imaging session and fluorescent immunohistochemistry assessed GDNF, Clover, and BFP production (Figure 4). Transplants in an animal ending the trial on dox (Figure 4A) and its littermate ending the trial off dox (Figure 4B) demonstrated BFP+ cells in the striatal region, yet Clover and GDNF were only detected in the dox-treated animal. High magnification showed that host cells adjacent to the graft site might be taking up GDNF in the dox-treated animal (Figure 4C, pink arrows), whereas this was not observed in the off-dox animal (Figure 4D). Collectively, these data reveal that GDNF secretion can be induced and reversed in iNPCs months after transplantation.
Figure 4.
Doxycycline Mediates GDNF Expression in Transplanted iPSC-Derived NPCs
(A and B) Striatal region of (A) animal no. 1 at experimental endpoint [Started OFF→ON→OFF→Ended ON] and (B) Animal no. 7 at experimental endpoint [Started ON→OFF→ON→Ended OFF].
(C) High magnification image of squared region in (A). Pink arrows indicate GDNF expression in areas distant from TagBFP+ cells, suggesting that host cells uptake secreted GDNF.
(D) High magnification image of squared region in (B). Note: Clover signal and GDNF signal are likely background given the overlap (B2/B3 and D2/D3).
See also Figure S2.
Finally, 3,3′-diaminobenzidine (DAB) staining, which provides high sensitivity immunohistochemical detection and has effectively tracked human GDNF-expressing NPC transplants, was used to determine the production and location of GDNF protein (Klein et al., 2005) (Figure S2 shows DAB staining of littermate animals from Figure 4). A blinded analysis revealed that all animals (except one, animal no. 8) that ended the trial on dox exhibited increased GDNF signal, which was not observed in any animal that ended the trial off dox (Table S1). This analysis also revealed that variations in bioluminescence intensity might be due to varying graft location. Specifically, some animals that exhibited remarkably high bioluminescence signal had grafted cells in the meninges, which is likely due to cell reflux into the injection tract during needle retraction (Figures S3 and S4). Overall, the extensive analysis of all study animals confirmed a strong correlation between bioluminescence signal and GDNF expression.
Discussion
This report provides proof-of-concept that GDNF produced by engineered human neural progenitor cells can be tightly regulated across multiple cycles in vivo. This technology can be applied to other growth factors, providing a valuable means to protect neurons damaged in different diseases. Using pB-RTP-Tet-GDNF/memClover-FLuc, we observed that dox penetrates the parenchymal transplant site to permit transgene activation from the inducible TRE-Bi promoter. We also report that transgene activity can be induced and reversed multiple cycles. Thus, GDNF administration can be attenuated or stopped if patients develop side effects, desensitization, or other transgene-related phenomena during disease progression. Alternatively, the drug regimen could be varied to allow for re-sensitization of host receptors and signaling pathways. This is in stark contrast to ex vivo gene therapy with constitutive gene expression. Importantly, the amount of dox concentration required for gene control (15 μg dox/g weight) is below antimicrobial doses and does not increase the presence of dox-resistant bacteria or negatively affect the gut flora (Chtarto et al., 2016).
Interestingly, in comparison with the lentiviral transduction of human NPCs used for the ongoing ALS clinical trial (NCT02943850, 2017), the dox-catalyzed system described herein provides nearly 2-fold higher GDNF secretion (Capowski et al., 2007). This increase may be attributed to piggyBac transposition that can mediate more copies of plasmid genomic insertion than lentiviral transduction. Furthermore, GDNF transcription with previous lentiviral experiments is dependent on the PGK promoter that is activated by endogenous transcription factors. In the present study, GDNF transcription is initiated by binding of the tet-ON transactivator to the TRE-Bi promoter that is catalyzed by a conformational change initiated by dox administration. Thus, transcription is not limited by a naturally occurring transcription factor, but rather the amount of transactivator and dox present.
It is important to note that transplanting ex vivo genetically engineered neural progenitors adds a level of safety compared with direct viral transduction of host cells with pB-RTP-Tet-GDNF/memClover-Fluc. Specifically, direct viral transduction to the brain may not only target healthy cells that neighbor diseased cells but also may further compromise diseased host cells. In addition, simply introducing healthy neural progenitor/stem cells, in it of itself, may have beneficial effects on the diseased host milieu in various diseases (Marsh and Blurton-Jones, 2017, McBride et al., 2004, Nichols et al., 2013). Therefore, the synergistic impact of both cell and inducible gene therapy may surpass gene therapy alone. Especially when the neural cells are derived from a human iPSC source, critically providing the promise of autologous transplantation.
No animal exhibited abnormal behavior or any overt damage to the transplanted striatum based on examination of the brain. Importantly, no tumors or ectopic growths were observed in any of the transplant animals in this study or our previous iPSC transplant studies (Sareen et al., 2014). However, translating this therapy to the clinic will require further safety and efficacy testing. First, the long-term efficacy, toxicity, and potential antigenicity of the constitutively expressed transactivator (rtTA-V10) must be tested. Second, pBase in our study was used as a means to stably integrate pB-RTP-Tet-GDNF/memClover-FLuc due to its ease of use. Although pBase mediates random integration, we have not seen tumors in over 500 mice where pBase was used (Breunig et al., 2015). However, a clinical-grade product may necessitate single-copy site-specific integration. Toward this end, targeting the AAVS1 safe landing may allow for long-term stable integration and methylation-resistant expression of transgenes (Hatada et al., 2015, Oceguera-Yanez et al., 2016). Importantly, we have observed marked dox-mediated transgene expression at the single-copy level using the same TRE-Bi promoter used in this study (Kim et al., 2016). Finally, a limitation to translate this strategy into humans may be the large area of the brain that needs to be covered for a therapeutic effect. However, we predict that the neural progenitor cells will migrate into areas of damage, and it is possible to target larger regions of the striatum using multiple injections and convection-enhanced delivery (Fiandaca et al., 2008). We are now investigating this in a primate model of Parkinson's disease. The next stage for this approach would be to develop a cGMP-grade viral construct and to expand and bank cGMP iPSC-derived neural progenitor cells for use in human clinical trials.
In conclusion, this proof-of-principle study lays the foundation for combined inducible gene and cell therapies that function to provide protection for the treatment of neurodegenerative diseases.
Experimental Procedures
All laboratory animal procedures were approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center. Detailed methods are available in Supplemental Information.
Dox Addition to Cell Culture or to Animals
Dox solution was maintained in light protection and at 4°C. For cell culture, dox (Clontech 631311) was added to culture medium at 100 ng/mL. For in vivo work, animals were administered dox (15 μg dox/g weight) every 3–4 days by oral gavage (e.g., 60 μL for a 20-g animal), using a soft-tipped feeding needle (Instech FTP-20-30; Plymouth Meeting, PA) attached to a 1-mL syringe (BD 309659; Franklin Lakes, NJ).
Transplantation
Nucleofected iNPCs, which underwent two rounds of FACS for BFP, were transplanted into the striatum (2 μL of cells at 100,000 cells/μL into each hemisphere) of 6-month old NOD-SCID mice (Nod.cb17-Prkdcscid/J, Jackson Laboratory, no. 1303; Bar Harbor, ME). Three days before transplantation, a subset of cells was treated with dox. For transplantation, cells were dissociated, diluted to a final concentration of 100,000 cells/μL in conditioned medium, and maintained on ice. Animals were anesthetized with isoflurane and placed in a stereotaxic frame. Cells were injected bilaterally relative to bregma at 0.7 mm rostral and ±2.0 mm lateral. A 5-μL Hamilton microsyringe backfilled with PBS was loaded with 2 μL of cell solution and inserted to −4.00 mm. The microsyringe was then raised to −3.50 mm and the cell solution was injected at 1 μL/min. After cell injection, the microsyringe was raised to −3.00 mm, held for 2 min, and slowly withdrawn. Animals were checked daily.
Author Contributions
A.A.A., C.N.S., and J.J.B. designed the study and wrote and edited the manuscript. A.A.A. performed the experiments with assistance from G.G., N.C., C.T., and V.B.M. for animal studies, and N.K., V.B.M., S.S., L.G., D.S., G.K., H.P., G.L., and B.S. for in vitro studies.
Acknowledgments
We thank Dr. Soshana Svendsen from Cedars-Sinai for critical editing and review of this manuscript and Dr. J. Loturco for providing original piggyBac plasmid. J.J.B. was supported by NIH R33-CA202900 and ACS-RSG-16-217 grants and to C.N.S. by the Board of Governor's Regenerative Medicine Institute. N.K. was funded by the California Institute of Regenerative Medicine Bridges fellowship (EDUC2-08381).
Published: April 26, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and one video and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.03.024.
Contributor Information
Joshua J. Breunig, Email: joshua.breunig@cshs.org.
Clive N. Svendsen, Email: clive.svendsen@cshs.org.
Supplemental Information
This video demonstrates the ability to tightly regulate transgenic EGFP by dox in vitro.
References
- Akhtar A.A., Molina J., Dutra-Clarke M., Kim G.B., Levy R., Schreiber-Stainthorp W., Danielpour M., Breunig J.J. A transposon-mediated system for flexible control of transgene expression in stem and progenitor-derived lineages. Stem Cell Reports. 2015;4:323–331. doi: 10.1016/j.stemcr.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrstock S., Ebert A., McHugh J., Vosberg S., Moore J., Schneider B., Capowski E., Hei D., Kordower J., Aebischer P. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13:379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- Blesch A., Tuszynski M. Ex vivo gene therapy for Alzheimer's disease and spinal cord injury. Clin. Neurosci. 1995;3:268–274. [PubMed] [Google Scholar]
- Breunig J.J., Levy R., Antonuk C.D., Molina J., Dutra-Clarke M., Park H., Akhtar A.A., Kim G.B., Hu X., Bannykh S.I. Ets factors regulate neural stem cell depletion and gliogenesis in Ras pathway glioma. Cell Rep. 2015;12:258–271. doi: 10.1016/j.celrep.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Capowski E.E., Schneider B.L., Ebert A.D., Seehus C.R., Szulc J., Zufferey R., Aebischer P., Svendsen C.N. Lentiviral vector-mediated genetic modification of human neural progenitor cells for ex vivo gene therapy. J. Neurosci. Methods. 2007;163:338–349. doi: 10.1016/j.jneumeth.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Chen F., LoTurco J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J. Neurosci. Methods. 2012;207:172–180. doi: 10.1016/j.jneumeth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtarto A., Humbert-Claude M., Bockstael O., Das A.T., Boutry S., Breger L.S., Klaver B., Melas C., Barroso-Chinea P., Gonzalez-Hernandez T. A regulatable AAV vector mediating GDNF biological effects at clinically-approved sub-antimicrobial doxycycline doses. Mol. Ther. Methods Clin. Dev. 2016;5:16027. doi: 10.1038/mtm.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Torrest A., Pollock K., Dahlenburg H., Annett G., Nolta J.A., Fink K.D. Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells. Neural Regen. Res. 2016;11:702–705. doi: 10.4103/1673-5374.182682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A.D., Barber A.E., Heins B.M., Svendsen C.N. Ex vivo delivery of GDNF maintains motor function and prevents neuronal loss in a transgenic mouse model of Huntington's disease. Exp. Neurol. 2010;224:155–162. doi: 10.1016/j.expneurol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Ebert A.D., Beres A.J., Barber A.E., Svendsen C.N. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp. Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Ebert A.D., Shelley B.C., Hurley A.M., Onorati M., Castiglioni V., Patitucci T.N., Svendsen S.P., Mattis V.B., McGivern J.V., Schwab A.J. EZ spheres: a stable and expandable culture system for the generation of pre-rosette multipotent stem cells from human ESCs and iPSCs. Stem Cell Res. 2013;10:417–427. doi: 10.1016/j.scr.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadas-Alvarez A.J., Bannon M.J., Orozco-Barrios C.E., Escobedo-Sanchez L., Ayala-Davila J., Reyes-Corona D., Soto-Rodriguez G., Escamilla-Rivera V., De Vizcaya-Ruiz A., Eugenia Gutierrez-Castillo M. Regulation of human GDNF gene expression in nigral dopaminergic neurons using a new doxycycline-regulated NTS-polyplex nanoparticle system. Nanomedicine. 2017;13:1363–1375. doi: 10.1016/j.nano.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Fiandaca M.S., Forsayeth J.R., Dickinson P.J., Bankiewicz K.S. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B., Jakobsson J., Persson E., Ericson C., Kirik D., Lundberg C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum. Gene Ther. 2004;15:934–944. doi: 10.1089/hum.2004.15.934. [DOI] [PubMed] [Google Scholar]
- Georgievska B., Kirik D., Björklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp. Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- Gowing G., Svendsen S., Svendsen C.N. Ex vivo gene therapy for the treatment of neurological disorders. Prog. Brain Res. 2017;230:99–132. doi: 10.1016/bs.pbr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Hatada S., Subramanian A., Mandefro B., Ren S., Kim H.W., Tang J., Funari V., Baloh R.H., Sareen D., Arumugaswami V. Low-dose irradiation enhances gene targeting in human pluripotent stem cells. Stem Cells Transl. Med. 2015;4:998–1010. doi: 10.5966/sctm.2015-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.B., Dutra-Clarke M., Levy R., Park H., Sabet S., Molina J., Akhtar A.A., Bannykh S., Danielpour M., Breunig J.J. Generating in vivo somatic mouse mosaics with locus-specific, stably-integrated transgenic elements. bioRχiv. 2016 [Google Scholar]
- Klein S.M., Behrstock S., McHugh J., Hoffmann K., Wallace K., Suzuki M., Aebischer P., Svendsen C.N. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum. Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- Love S., Plaha P., Patel N.K., Hotton G.R., Brooks D.J., Gill S.S. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- Marsh S.E., Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: the role of neurotrophic support. Neurochem. Int. 2017;106:94–100. doi: 10.1016/j.neuint.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J.L., Behrstock S.P., Chen E.Y., Jakel R.J., Siegel I., Svendsen C.N., Kordower J.H. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J. Comp. Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- Nichols N.L., Gowing G., Satriotomo I., Nashold L.J., Dale E.A., Suzuki M., Avalos P., Mulcrone P.L., McHugh J., Svendsen C.N. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med. 2013;187:535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr., Lozano A.M., Penn R.D., Simpson R.K., Jr., Stacy M. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Oceguera-Yanez F., Kim S.I., Matsumoto T., Tan G.W., Xiang L., Hatani T., Kondo T., Ikeya M., Yoshida Y., Inoue H. Engineering the AAVS1 locus for consistent and scalable transgene expression in human iPSCs and their differentiated derivatives. Methods. 2016;101:43–55. doi: 10.1016/j.ymeth.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Patel N.K., Pavese N., Javed S., Hotton G.R., Brooks D.J., Gill S.S. Benefits of putaminal GDNF infusion in Parkinson disease are maintained after GDNF cessation. Neurology. 2013;81:1176–1178. doi: 10.1212/WNL.0b013e3182a55ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D., Gowing G., Sahabian A., Staggenborg K., Paradis R., Avalos P., Latter J., Ornelas L., Garcia L., Svendsen C.N. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J. Comp. Neurol. 2014;522:2707–2728. doi: 10.1002/cne.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshchenko J., Maddalena A., Bahr M., Kugler S. Pharmacologically controlled, discontinuous GDNF gene therapy restores motor function in a rat model of Parkinson's disease. Neurobiol. Dis. 2014;65:35–42. doi: 10.1016/j.nbd.2014.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates the ability to tightly regulate transgenic EGFP by dox in vitro.