Abstract

Syringaresinol was synthesized in a one-pot conversion containing eugenol oxidase (EUGO) and horseradish peroxidase (HRP) using the relatively cheap 2,6-dimethoxy-4-allylphenol as a substrate. This conversion is fully coupled as the hydrogen peroxide generated from the reaction of EUGO with the substrate is utilized by the HRP to convert the formed sinapyl alcohol into syringaresinol. To improve the performance of EUGO on 2,6-dimethoxy-4-allylphenol, structure-inspired enzyme engineering was performed. This yielded the I427A EUGO mutant that is significantly more efficient with 2,6-dimethoxy-4-allylphenol. The I427A EUGO mutant together with HRP were capable of efficiently producing syringaresinol as a major product. After optimization and upscaling the conversion to a semipreparative scale (1 gr), syringaresinol was obtained in 81% yield.
Keywords: syringaresinol, (per)oxidase, one-pot, enzyme engineering, enzyme cascade
One-pot enzymatic cascades have received an increasing attention over recent years.1,2 Such approaches are attractive as they save time, money, and energy needed to make or isolate compounds with a high degree of purity and quality.3 These cascades also allow conversions in which labile intermediates can be used. One-pot cascades have involved a wide range of enzymes ranging from but not exclusive to dehydrogenases, transaminases, Baeyer–Villiger monooxygenases, and oxidases.2−5 Recently, we published a one-pot two-enzyme cascade conversion utilizing an oxidase and a peroxidase to convert eugenol into lignin-like oligomers.6 The diversity of enzymes used in one-pot reactions has added to the value of this approach to synthesize various chiral compounds and other compounds which are difficult to be produced by conventional chemical means.
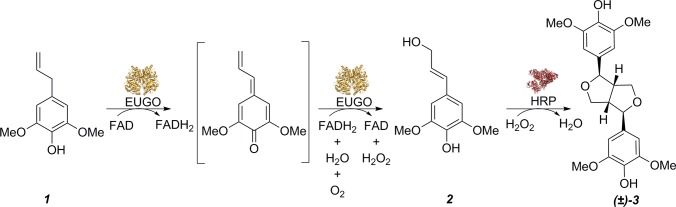
In this paper, the synthesis of syringaresinol (3) in a one-pot two-enzyme conversion is described (see Scheme 1). Syringaresinol (3) is a lignan formed from two sinapyl alcohol (2) units linked via a β–β linkage. The lignan is known to display various interesting bioactivity properties. It inhibits the motility of Helicobacter pylori in the stomach thus playing a role in protection from gastric cancer.7 It has also been found to help in the healing of wounds when present as a glucoside.8,9 Chung and co-workers displayed the effect of syringaresinol (3) in increasing the production of nitric oxide from nitric oxide synthase and its complementary effect on vasorelaxation of blood vessels.10 Syringaresinol is a very rigid compound due to the presence of a cis-fused bis-furanic moiety in its structure. This makes it an attractive candidate for use as a substitute for bisphenol A in industrial resins.11,12
Scheme 1. Conversion of 2,6-Dimethoxy-4-allylphenol (1) to Sinapyl Alcohol (2) Using EUGO and Sinapyl Alcohol to Syringaresinol (3) Using HRP.
Despite being available naturally in plants, syringaresinol is isolated from natural sources in a very low yield.8 Chemical synthesis of syringaresinol has achieved yields reaching 67% through copper catalysis. However, these methods are problematic with regard to the toxicity of the chemicals used and the costs of the various purification steps involved.10 The biocatalytic synthesis of syringaresinol from sinapyl alcohol has been described recently using a laccase from Trametes versicolor producing the lignan in a yield of 93% in one step.8 This method is very efficient in a multigram scale, yet, concern should be taken to the price of the starting material, sinapyl alcohol.
Inspired by the approach in which sinapyl alcohol is converted into syringaresinol, we designed a two-step one-pot conversion starting from the relatively cheap 2,6-dimethoxy-4-allylphenol. This allylphenol was shown to be a substrate for eugenol oxidase (EUGO) by which it is converted into sinapyl alcohol. Except for sinapyl alcohol, hydrogen peroxide is also formed upon conversion by EUGO. This prompted us to choose a peroxidase to perform the oxidative coupling of the formed sinapyl alcohol. The advantage of this system is that the hydrogen peroxide generated by EUGO is utilized directly by the peroxidase. While EUGO is the only known oxidase capable to convert 2,6-dimethoxy-4-allylphenol into sinapyl alcohol, the activity is relatively low when compared with other substrates, such as eugenol, vanillyl alcohol and 4-(hydroxy-1-ethyl)-2-methoxyphenol.13,14 Analysis of the structure of EUGO suggests that the active site of the enzyme has limited space for accommodating the two methoxy groups in the substrate.
In order to optimize the efficiency of the cascade conversion, the activity of EUGO on 2,6-dimethoxy-4-allylphenol was optimized by performing structure-inspired mutagenesis to create a larger active site. The generated mutant EUGO was used with horseradish peroxidase (HRP) for the efficient conversion of 2,6-dimethoxy-4-allylphenol into syringaresinol in high yield.
EUGO was previously shown to exhibit activity with dimethoxy-substituted phenolic substrates as opposed to other enzymes having a similar structure.13 The main reason for this unique property seems to come from the fact that EUGO has a glycine at position 392, making sufficient room for a second methoxy moiety on the phenolic substrate. Still, EUGO displays a higher activity on phenolic substrates that carry only one or no methoxy substituent. Inspection of the isoeugenol-complexed crystal structure of EUGO (PDB: 5FXD) suggested several residues that may hamper binding of the second methoxy moiety: Ile427 and Val166. Other residues that are in the same area (Tyr91, Tyr471, and Arg472) were not considered as targets for mutagenesis because they have a role in arranging a proper interaction with the 4-hydroxy group of the substrate. In fact, these latter three amino acids are involved in the stabilization of the phenolate form of the substrate and therefore play a role in forming the quinone methide intermediate (see Figure 1).13 In order to accommodate two methoxy groups in the substrate binding pocket, several Ile427 and Val166 mutants were prepared: I427V, I427A, I427G, and V166A. By introducing relatively small residues, more efficient binding of the target substrate may be achieved.
Figure 1.

Active site of EUGO with the flavin cofactor (yellow), a docked 2,6-dimethoxy-4-allylphenol (green) and highlighting residues (cyan): Tyr91, Tyr471, and Arg472, essential for stabilization of the phenolate form of the bound substrate, and Ile427 and Val166 that were mutated to improve the activity with 2,6-dimethoxy-4-allylphenol.
The steady state kinetics for the conversion of 2,6-dimethoxy-4-allylphenol (1) for each of the three different Ile427 mutants revealed that the most active mutant was the I427A (kcat = 0.72 s–1, Km = 0.46 μM). This mutant enzyme displays a more than 2-fold higher kcat when compared with the wild type enzyme while also the apparent affinity (Km) decreased (wt-EUGO: kcat = 0.37 s–1, Km = 0.77 μM). The other two mutants also displayed improved kinetic parameters (I427V: kcat = 0.59 s–1, Km = 0.34 μM, I427G: kcat = 0.59 s–1, Km = 0.39 μM). Yet, the I427G mutant suffered from substrate inhibition at elevated substrate concentrations. The V166A mutant displayed a somewhat lower Km but also a reduced activity when compared with wild type EUGO (V166A: kcat = 0.124 s–1, Km = 0.39 μM). The steady-state kinetic data for all above-mentioned enzymes are shown in Figure S1. Due to its superior kinetic properties, EUGO I427A was used to explore its use as biocatalyst for producing sinapyl alcohol.
Sinapyl alcohol is a relatively expensive compound, whereas 2,6-dimethoxy-4-allylphenol (1), its precursor in this study, is several orders of magnitude cheaper. This is probably due to the fact that (1) and other 4-allylphenols can be readily isolated from herbs and spices. This makes it attractive to explore the use of 2,6-dimethoxy-4-allylphenol as a starting material to prepare high-value compounds, such as sinapyl alcohol. We first studied the conversion of 2,6-dimethoxy-4-allylphenol into sinapyl alcohol using EUGO I427A. When 0.5 μM of EUGO I427A was used in the conversion, incomplete conversion of 2,6-dimethoxy-4-allylphenol to sinapyl alcohol was seen (data not shown). It was found that EUGO I427A was capable of completely converting 2,6-dimethoxy-4-allylphenol into sinapyl alcohol within 22 h when 5 μM of the enzyme was added to a solution containing 10 mM of the substrate. No other products could be detected (see Figure S2).
After having established that EUGO I427A can be used to produce sinapyl alcohol, a cascade reaction that included the oxidase and HRP was investigated. Incubation of 2,6-dimethoxy-4-allylphenol with EUGO I427A and HRP in one-pot resulted in formation of one major soluble product: syringaresinol, a dimer of two sinapyl alcohol monomers linked to each other through a β–β linkage. When EUGO I427A (5 μM) and HRP (0.65 μM) were added to a reaction containing 10 mM 2,6-dimethoxy-4-allylphenol, complete conversion of 2,6-dimethoxy-4-allylphenol to racemic syringaresinol as a major product was achieved after 25 h (see Figure S3). The reaction proceeds through the formation of sinapyl alcohol by oxidation of 2,6-dimethoxy-4-allylphenol by EUGO, whereas, in a second step, HRP utilizes the hydrogen peroxide that is generated to form a free radical from the formed sinapyl alcohol. Through dimerization of the formed radicals, syringaresinol is formed. Though it is known that HRP can be inactivated by hydrogen peroxide, the in situ production of the cosubstrate prevented accumulation of excessive hydrogen peroxide limiting this inactivation effect. 2,6-Dimethoxy-4-allylphenol is a unique substrate as both the 2- and 6-positions are blocked by methoxy-groups for alternative coupling reactions. This hinders the ability to form other linkages that are characteristic of lignin polymers, as found when using eugenol as a precursor in a EUGO/HRP-catalyzed reaction.8 The two methoxy moieties direct the reaction toward formation of the dimerized oxidation product from sinapyl alcohol.
Some insoluble product was also formed in the reaction above, which was isolated and analyzed separately. GPC analysis of the material showed that the insoluble product consisted of higher molecular weight material, in the range of oligomers with a length of 2–10 aromatic units (see Figure S4, Mn = 600, Mw = 750, d = 1.24). Analysis by 2D HSQC and 2D HMBC nuclear magnetic resonance (NMR) revealed major signals that can be ascribed to syringaresinol as well as to 2,6-dimethoxy-4-allylphenol units (Figures S5 and S6). Unlike our previously reported work on eugenol, no clear lignin type linkages were detected using 2,6-dimethoxy-4-allylphenol as a substrate apart from the β–β linkages from syringaresinol. A signal for a β-O-4 linkage that has been modified on the α-position could be observed; however, the exact nature of this β-O-4 type linkage or other connectivity of the aromatic units remains unclear. Overall, it seems that the conversion of 2,6-dimethoxy-4-allylphenol by this enzyme cascade has a high preference for the formation of β–β dimers over typical β-O-4 type linkages.
After testing the conversion of 2,6-dimethoxy-4-alllylphenol to syringaresinol on a small scale, a one gram conversion was performed. The conversion was done in a 250 mL reaction volume using 5% dimethylsulfoxide (DMSO, v/v) as a cosolvent. After high-performance liquid chromatography (HPLC) analysis confirmed complete conversion of the starting material, the product was isolated from the solution by ethyl acetate extraction and purified by column chromatography. The whole conversion was completed in 22 h and resulted in a yield of 870 mg (81%) syringaresinol from 1 g of the starting material. The isolated syringaresinol appeared as pale yellow crystals. Figure S7 shows the 1H NMR and 13C NMR spectra for the isolated lignan. In addition to the syringaresinol, ≈80 mg of insoluble product was formed.
We have successfully been able to produce syringaresinol in a high yield from a relatively cheap precursor in a facile one-pot two-enzyme conversion. For this, first the performance of the required oxidase was successfully optimized through site-directed mutagenesis of EUGO to improve its acceptance of 2,6-dimethoxy-4-allylphenol as a substrate. The combined use of EUGO I427A and HRP resulted in full conversion of 2,6-dimethoxy-4-allylphenol and formation of syringaresinol as major product. The lignan was purified (81% yield) after a 1 g scale conversion and analyzed using both 1H NMR and 13C NMR to confirm its purity. This newly developed biocatalytic system for synthesis of syringaresinol could serve as a good source for producing this medically and industrially important compound on a large scale.
Acknowledgments
We would like to thank Douwe Zijlstra, Peter J. Deuss and Nikola Lončar for their assistance in the analysis of the synthesized product and fruitful discussions. Maximillian Fürst helped in the design of the enzyme mutants. Mohamed Habib received funding from the Cultural Affairs and Missions Sector, Ministry of Higher Education, Egypt.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.8b01235.
Experimental details; primers used for the quickchange mutagenesis of isoleucine at 427 position in EUGO; buffer compositions for the purification of wild type and mutant EUGO; recipe and conditions for the Quickchange mutagenesis of isoleucine at position 427 into alanine, valine, and glycine and valine at position 166 into alanine in EUGO; steady state kinetic curves; HPLC chromatograms; GPC analysis; 2D 1H 13C HSQC NMR analysis (600 MHz, DMSO-d6) of the insoluble product; 2D 1H 13C HMBC NMR analysis (600 MHz, DMSO-d6) of the insoluble product; and 1H NMR and 13C NMR of syringaresinol purified from 1 g conversion of 2,6-dimethoxy-4-allylphenol using EUGO I427A and HRP (PDF)
Author Contributions
§ M.H. and M.W.F. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Riva S.; Fessner W.-D.. Cascade Biocatalysis: Integrating Stereoselective and Environmentally Friendly Reactions; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Sperl J. M.; Sieber V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. 10.1021/acscatal.7b03440. [DOI] [Google Scholar]
- Bornadel A.; Hatti-Kaul R.; Hollmann F.; Kara S. A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate’. ChemCatChem 2015, 7, 2442–2445. 10.1002/cctc.201500511. [DOI] [Google Scholar]
- Tavanti M.; Mangas-Sanchez J.; Montgomery S. L.; Thompson M. P.; Turner N. J. A Biocatalytic Cascade for the Amination of Unfunctionalised Cycloalkanes. Org. Biomol. Chem. 2017, 15, 9790–9793. 10.1039/C7OB02569F. [DOI] [PubMed] [Google Scholar]
- Colpa D. I.; Lončar N.; Schmidt M.; Fraaije M. W. Creating Oxidase-Peroxidase Fusion Enzymes as a Toolbox for Cascade Reactions. ChemBioChem 2017, 18, 2226–2230. 10.1002/cbic.201700478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M. H. M.; Deuss P. J.; Lončar N.; Trajkovic M.; Fraaije M. W. A Biocatalytic One-Pot Approach for the Preparation of Lignin Oligomers using an Oxidase/Peroxidase Cascade Enzyme System. Adv. Synth. Catal. 2017, 359, 3354–3361. 10.1002/adsc.201700650. [DOI] [Google Scholar]
- Fujikawa T.; Yamaguchi A.; Morita I.; Takeda H.; Nishibe S. Protective Effects of Acanthopanax senticosus HARMS from Hokkaido and Its Components on Gastric Ulcer in Restrained Cold Water Stressed Rats. Biol. Pharm. Bull. 1996, 19, 1227–1230. 10.1248/bpb.19.1227. [DOI] [PubMed] [Google Scholar]
- Jaufurally A. S.; Teixeira A. R. S.; Hollande L.; Allais F.; Ducrot P.-H. Optimization of the Laccase-Catalyzed Synthesis of (±)-Syringaresinol and Study of Its Thermal and Antiradical Activities. ChemistrySelect 2016, 1, 5165–5171. 10.1002/slct.201600543. [DOI] [Google Scholar]
- Semenov A. A.; Enikeev A. G.; Khobrakova V. B.; Razuvayeva Y. G.; Nikolayev S. M. Study on the Healing Effect of Syringaresinol β-D - Monoglucoside. Int. J. Biomed. 2013, 3, 287–290. [Google Scholar]
- Chung B. H.; Kim S.; Kim J. D.; Lee J. J.; Baek Y. Y.; Jeoung D.; Lee H.; Choe J.; Ha K. S.; Won M. H.; Kwon Y. G.; Kim Y. M. Syringaresinol Causes Vasorelaxation by Elevating Nitric Oxide Production through the Phosphorylation and Dimerization of Endothelial Nitric Oxide Synthase. Exp. Mol. Med. 2012, 44, 191–201. 10.3858/emm.2012.44.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier M.; Hollande L.; Jaufurally A. S.; Pernes M.; Ménard R.; Grimaldi M.; Beaugrand J.; Balaguer P.; Ducrot P. H.; Allais F. Syringaresinol: A Renewable and Safer Alternative to Bisphenol A for Epoxy-Amine Resins. ChemSusChem 2017, 10, 738–746. 10.1002/cssc.201601595. [DOI] [PubMed] [Google Scholar]
- Janvier M.; Ducrot P. H.; Allais F. Isocyanate-Free Synthesis and Characterization of Renewable Poly(hydroxy)urethanes from Syringaresinol. ACS Sustainable Chem. Eng. 2017, 5, 8648–8656. 10.1021/acssuschemeng.7b01271. [DOI] [Google Scholar]
- Nguyen Q.-T.; de Gonzalo G.; Binda C.; Rioz-Martínez A.; Mattevi A.; Fraaije M. W. Biocatalytic Properties and Structural Analysis of Eugenol Oxidase from Rhodococcus Jostii RHA1: A Versatile Oxidative Biocatalyst. ChemBioChem 2016, 17, 1359–1366. 10.1002/cbic.201600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; Mazon H.; van den Heuvel R. H. H.; Janssen D. B.; Fraaije M. W. Discovery of a Eugenol Oxidase from Rhodococcus Sp. Strain RHA1. FEBS J. 2007, 274, 2311–2321. 10.1111/j.1742-4658.2007.05767.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.