Abstract
Objective
To evaluate factors associated with delayed surgical treatment among women with endometrial cancer.
Methods
Using the National Cancer Database (NCDB), we analyzed time to first surgery for epithelial endometrial cancer patients who underwent surgical treatment from 2003–2011. Poisson regression was used to examine delays > 6 weeks between diagnosis and surgery, controlled for patients’ sociodemographic and clinical characteristics. Survival for women diagnosed between 2003–2006 with timely versus delayed surgery was compared using Cox proportional hazards regression.
Results
The study included 112,041 women diagnosed at 1108 continuously reporting NCDB hospitals. Survival through 2011 was available for 40,184 women. All patients underwent hysterectomy. Twenty-eight percent of patients underwent surgery > 6 weeks after diagnosis. Poisson regression estimates indicated that being younger than 40 years old, being black or Hispanic, having Medicaid or being uninsured, or being from the lowest education quartile were associated with a significantly higher likelihood of surgical wait time > 6 weeks. Patients diagnosed in 2010–2011 were more likely (IRR 1.32, 95% CI 1.24–1.40) to undergo surgery > 6 weeks after diagnosis compared to patients treated in 2003. Survival for women with surgical wait times > 6 weeks was worse than those treated within 6 weeks of diagnosis (HR 1.14, 95% CI 1.09–1.20).
Conclusions
Being a minority patient and having lower socioeconomic status or poor insurance coverage were associated with an increased likelihood of delayed surgical treatment. Wait times > 6 weeks from diagnosis of endometrial cancer to definitive surgery may have a negative impact on survival.
Endometrial cancer is the most common gynecologic malignancy in the United States [1]. Endometrial cancer typically presents as a low-grade tumor and early-stage disease, making it an often curable disease with timely surgical-based treatment [2].
Diagnosis of endometrial cancer is commonly made with an endometrial biopsy. After diagnosis, standard of care consists of definitive surgical treatment with hysterectomy [3]. The time period between diagnosis of cancer and definitive surgical treatment is defined as the surgical wait time. Long surgical wait times can be influenced by numerous factors, such as patient preference or surgeon availability, and can reflect structural problems within a health care system. Several studies have demonstrated that longer surgical wait times are linked with poor access to services, geographic and socioeconomic barriers, inefficiency, and poor quality of care [4–6]. Long wait times may affect outcomes, including delays to adjuvant therapy, increased patient anxiety, and worse overall survival [7–8].
In 2008, the National Comprehensive Cancer Network (NCCN), the National Quality Forum, and the Institute of Medicine recommended timely care as quality measures for cancer patients [9–10]. However, there are few data to guide providers, institutions, and accreditation bodies in setting parameters for wait times [11]. The effect of wait times on survival has been studied in a variety of cancers, including breast cancer; yet few large-scale multicenter studies have examined wait times for uterine cancer surgery.
Using the National Cancer Data Base (NCDB), the aims of this study were to analyze 1) trends in the incidence of delayed treatment (defined as surgery greater than six weeks from diagnosis) and 2) the association of patients’ sociodemographic and clinical characteristics with the likelihood of delayed treatment; and 3) analyze whether delayed treatment is associated with all-cause mortality.
Methods
Data Source
The National Cancer Data Base (NCDB), a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons, is a nationwide, facility-based oncology registry that collects data from more than 1,500 CoC-accredited facilities [12–13]. Zip code-based indicators of socioeconomic status and facility level characteristics are also available through the NCDB.
Data are coded and reported according to a nationally established protocol coordinated under the auspices of the North American Association of Central Cancer Registries [14]. All data within the NCDB are compliant with the privacy requirements of the Health Insurance Portability and Accountability Act (HIPAA). Institutional Review Board approval was not required for this study because no patient, provider, or hospital identifiers were examined.
Study Population
The study population was limited to women in whom endometrial cancer was their first cancer diagnosis, who received all or part of their care at the reporting hospital, had a biopsy-proven endometrial cancer treated with surgery, and were diagnosed with AJCC 0-IV uterine cancer between 2003–2011. Patients who received neoadjuvant treatment or hormonal treatment or were diagnosed with non-epithelial uterine cancers were excluded.
Patient Characteristics, Time Periods and Definition of Delayed Treatment
Patients’ ages were categorized as 39 and under, 40 to 49, 50 to 69, and 70 years or greater. Race/ethnicity was classified into non-Hispanic white, non-Hispanic black, Hispanic, Asian, and other. Insurance status was categorized as Medicaid/uninsured or other insurance (Medicare or private). Income and education levels were categorized based on zip-code quartiles of census-based median income and educational attainment at the time of diagnosis. For comorbid disease, we examined the effect of the Charlson-Deyo comorbidity score, which is based on ICD-9 codes for chronic diseases, trichotomized as 0, 1, or 2 or greater [15]. Tumor stage was categorized according to the American Joint Committee on Cancer (AJCC) 7th edition guidelines [16]. Tumor histology was defined according to the International classification of disease for oncology (ICD-O) and categorized as either Type I or Type II endometrial cancers [17]. We created four time periods (2003, 2004–2006, and 2007–2009, and 2010–2011) to control for trends in diagnosis and treatment over the study period. Finally, we examined the time from diagnosis to first and definitive surgery. We defined wait times a priori as a dichotomous variable on the basis of whether surgery occurred within six weeks (≤42 days) of diagnosis. The six-week time point was identified because this is the benchmark wait time for surgical treatment in Canada as specified by Cancer Care Ontario (CCO) [5]. To the best of our knowledge, no benchmark currently exists for timely surgery in endometrial cancer in the United States.
Statistical Analysis
All analyses were performed using STATA statistical software (STATA, version 14: College Station, TX). Chi-square tests were used to test the significance of univariate associations with delayed treatment. Poisson regression, which provides an incidence rate ratio (IRR) that is closer to relative risk than an odds ratio [18–19] was used to test the significance of delays greater than six weeks between diagnosis and surgery controlled for patient sociodemographic and clinical characteristics, hospital characteristics, and time period. Survival for women diagnosed between 2003 and 2006 with timely versus delayed surgery was compared using the log rank test and Cox proportional hazards regression which controlled for patient and hospital covariates.
Results
The NCDB identified 441,863 women as having a diagnosis of endometrial cancer between 1998 and 2011. Of these, 329,822 were excluded (Figure 1), leaving 112,041 in the final cohort. Patients were excluded primarily because of non-epithelial histology (n = 132,122), diagnosis was made at the time of hysterectomy (n = 62,089), non-primary cancer diagnosis (n = 35,976), stage was missing (n = 29,492), patient received neoadjuvant treatment (n = 14,150), or patient was diagnosed and before 2003 (n = 30,539).
Figure 1.
Patient characteristics are presented in Table 1. Mean age was 61.8 +/− 11.4 years, 81.5% (n = 91,323) of women were non-Hispanic white, and 91.9% (n = 102,982) had some form of private or Medicare insurance. The majority of women were diagnosed with Stage I disease (n = 85,646, 76.4%) and endometrioid adenocarcinoma (n = 93,207, 83.2%) on final pathology. Twenty eight percent of women (n = 31,903) underwent surgery greater than six weeks after their initial diagnosis. The mean time from diagnosis to first surgery was 38 +/− 30 days.
Table 1.
Descriptive Statistics
| Wait Time | ||||
|---|---|---|---|---|
| All patients n, (%) |
< 6 weeks n, (%) |
> 6 weeks n, (%) |
p value | |
| N = 112,041 | 80,138 (71.5) | 31,903 (28.5) | ||
| Age (mean +/− SD, years) | 61.8 +/− 11.4 | <0.001 | ||
| < 40 yo | 3,409 (3.0) | 2,101 (61.6) | 1,308 (38.4) | |
| 40–49 yo | 10,421 (9.3) | 7,520 (72.2) | 2,901 (27.8) | |
| 50–59 yo | 34,741 (31.0) | 25,299 (72.8) | 9,442 (27.2) | |
| 60–69 yo | 36,250 (32.4) | 25,967 (71.6) | 10,283 (28.4) | |
| > 70 yo | 27,220 (24.3) | 19,251 (70.7) | 7,969 (29.3) | |
| Race/Ethnicity | <0.001 | |||
| Non-Hispanic White | 91,323 (81.5) | 67,084 (73.5) | 24,239 (26.5) | |
| Non-Hispanic Black | 9,601 (8.6) | 5,872 (62.2) | 3,729 (38.8) | |
| Hispanic | 5,748 (5.1) | 3,413 (59.4) | 2,335 (40.6) | |
| Asian | 2,544 (2.3) | 1,748 (68.7) | 796 (31.3) | |
| Other/Unknown | 2,825 (2.5) | 2,021 (71.5) | 804 (28.5) | |
| Insurance Status | <0.001 | |||
| Private Insurance | 102,982 (91.9) | 74,970 (72.8) | 28,012 (27.2) | |
| Medicaid/Uninsured | 9,059 (8.1) | 5,168 (57.1) | 3,891 (42.9) | |
| Income Quartile | ||||
| Lowest quartile | 13,517 (12.1) | 8,870 (65.5) | 4,647 (34.4) | <0.001 |
| Second quartile | 18,740 (16.7) | 13,154 (70.2) | 5,586 (29.2) | |
| Third quartile | 29,936 (26.7) | 21,474 (71.7) | 8,462 (28.3) | |
| Highest quartile | 43,822 (39.1) | 32,309 (73.3) | 11,513 (28.2) | |
| Unknown | 6,026 (5.4) | 4,331 (71.9) | 1,695 (28.1) | |
| Education Quartile | ||||
| Lowest quartile | 16,636 (14.8) | 10,728 (64.5) | 5,908 (35.5) | <0.001 |
| Second quartile | 24,051 (21.5) | 16,663 (69.3) | 7,388 (30.7) | |
| Third quartile | 26,096 (23.3) | 18,763 (72.0) | 7,313 (28.0) | |
| Highest quartile | 39,222 (35.0) | 29,625 (75.5) | 9,597 (24.5) | |
| Unknown | 6,036 (5.4) | 4,339 (71.9) | 1,697 (28.1) | |
| CoMorbidity | <0.001 | |||
| CDS 0 | 83,490 (74.5) | 61,138 (73.2) | 22,352 (26.8) | |
| CDS 1 | 9,059 (8.0) | 15,814 (67.8) | 7,502 (32.2) | |
| CDS 2 | 5,235 (4.5) | 3,186 (60.9) | 2,049 (39.1) | |
| AJCC Stage | <0.001 | |||
| Stage 0 | 526 (0.5) | 386 (73.4) | 140 (26.6) | |
| Stage 1 | 85,646 (76.4) | 61,128 (71.4) | 24,518 (28.6) | |
| Stage 2 | 10,212 (9.1) | 7,023 (68.8) | 3,189 (31.2) | |
| Stage 3 | 15,113 (13.5) | 11,184 (74.0) | 3,929 (26.0) | |
| Stage 4 | 544 (0.5) | 417 (76.7) | 127 (23.3) | |
| Grade | <0.001 | |||
| 1 | 45,422 (40.5) | 31,752 (69.9) | 13,670 (30.1) | |
| 2 | 34,425 (30.7) | 24,797 (72.0) | 9,628 (28.0) | |
| 3 | 22,622 (20.2) | 16,941 (74.9) | 5,681 (25.1) | |
| Unknown | 9,572 (8.6) | 6,648 (69.5) | 2,924 (30.5) | |
| Nodes | <0.001 | |||
| Negative | 75,359 (67.3) | 54,756 (72.7) | 20,603 (27.3) | |
| Positive | 8,852 (7.9) | 6,507 (73.5) | 2,345 (26.5) | |
| Not done | 27,402 (24.5) | 18,570 (67.8) | 8,832 (32.2) | |
| Unknown | 428 (0.3) | 305 (71.3) | 123 (28.7) | |
| Year of Treatment | <0.001 | |||
| 2003 | 8,915 (8.0) | 6,822 (76.5) | 2,093 (23.5) | |
| 2004–2006 | 31,270 (27.9) | 23,475 (75.1) | 7,795 (24.9) | |
| 2007–2009 | 39,548 (35.3) | 27,713 (70.1) | 11,835 (29.9) | |
| 2010–2011 | 32,308 (28.8) | 22,128 (68.5) | 10,180 (31.5) | |
| Type of Endometrial Cancer | <0.001 | |||
| Type I | 93,207 (83.2) | 66,315 (71.2) | 26,892 (28.8) | |
| Type II | 18,834 (16.8) | 13,823 (73.4) | 5,011 (26.6) | |
Poisson regression indicated the following were all significantly associated with delay in surgery greater than six weeks from diagnosis (Table 2): being younger than 40 years of age compared to age > 70 years (IRR 1.08, CI 1.01–1.13, p = 0.014), being non-Hispanic Black (IRR 1.35, CI 1.28–1.42, p < 0.001) or Hispanic (IRR 1.31, CI 1.20–1.43, p < 0.001) compared to non-Hispanic White, having Medicaid or no insurance (IRR 1.43, CI 1.37–1.50, p < 0.001) compared to other insurance, being from the lowest education zip-code quartile compared to the highest (IRR 1.28, CI 1.17–1.40, p < 0.001), and having co-morbid disease as designated by Charlson-Deyo index > 0 (CDS 1: IRR 1.14, CI 1.10–1.18, p < 0.001; CDS 2: IRR 1.36, CI 1.29–1.42, p < 0.001). Patients diagnosed in 2010–2011 were 32.5% more likely (IRR 1.32, 95% CI 1.24–1.40, p < 0.001) to undergo surgery greater than six weeks after diagnosis compared to patients treated in 2003.
Table 2. Poisson Regression – Likelihood of Late Treatment.
Poisson regression estimates for likelihood of treatment > 6 weeks (n=112,041); women from 1,081 National Cancer Data Base-reporting hospitals diagnosed between 2003 and 2011.
IRR = Incident Rate Ratio
95% CI = 95% Confidence Interval
| Late Treatment | IRR (CI 95%) | p value |
|---|---|---|
| Age | ||
| < 40 yo | 1.07 (1.01–1.13) | 0.014 |
| 40–49 | 0.86 (0.83–0.89) | <0.001 |
| 50–59 | 0.87 (0.84–0.90) | <0.001 |
| 60–69 | 0.92 (0.90–0.94) | <0.001 |
| > 70 yo | Reference | |
| Race/Ethnicity | ||
| Non-Hispanic White | Reference | |
| Non-Hispanic Black | 1.35 (1.28–1.42) | <0.001 |
| Hispanic | 1.31 (1.20–1.43) | <0.001 |
| Asian | 1.15 (1.01–1.30) | 0.029 |
| Other/Unknown | 1.05 (0.97–1.15) | 0.229 |
| Insurance Status | ||
| Private Insurance | Reference | |
| Medicaid/Uninsured | 1.43 (1.37–1.50) | <0.001 |
| Income | ||
| Lowest quartile | 0.95 (0.87–1.05) | 0.347 |
| Second quartile | 0.93 (0.86–1.01) | 0.067 |
| Third quartile | 0.95 (0.89–1.0) | 0.046 |
| Fourth quartile | Reference | |
| Unknown | 0.592 | |
| Education | ||
| Lowest quartile | 1.28 (1.17–1.40) | <0.001 |
| Second quartile | 1.21(1.14–1.28) | <0.001 |
| Third quartile | 1.14 (1.09–1.19) | <0.001 |
| Fourth quartile | Reference | |
| Unknown | 0.75 (0.20–2.87) | 0.677 |
| CoMorbidity | ||
| Charlson-Deyo Score 0 | Reference | |
| Charlson-Deyo Score 1 | 1.14 (1.10–1.18) | <0.001 |
| Charlson-Deyo Score 2 | 1.36 (1.29–1.42) | <0.001 |
| AJCC Stage | ||
| Stage 0 | 1.10 (0.90–1.36) | 0.357 |
| Stage 1 | 1.21(1.04–1.41) | 0.012 |
| Stage 2 | 1.36 (1.16–1.59) | <0.001 |
| Stage 3 | 1.16 (1.00–1.34) | 0.045 |
| Grade | ||
| 1 | Reference | |
| 2 | 0.93 (0.90–0.96) | <0.001 |
| 3 | 0.82 (1.01–1.11) | <0.001 |
| Unknown | 1.00 (0.94–1.06) | 0.914 |
| Nodes | ||
| Negative | Reference | |
| Positive | 1.06 (1.01–1.11) | 0.020 |
| Not done | 1.14 (1.10–1.19) | <0.001 |
| Unknown | 1.04 (0.83–1.29) | 0.754 |
| Year of Treatment | ||
| 2003 | Reference | |
| 2004–2006 | 1.06 (1.01–1.12) | 0.013 |
| 2007–2009 | 1.28 (1.21–1.35) | <0.001 |
| 2010–2011 | 1.33 (1.25–1.41) | <0.001 |
| Type of Endometrial Cancer | ||
| Type I | 1.08 (1.03–1.13) | 0.002 |
| Type II | Reference |
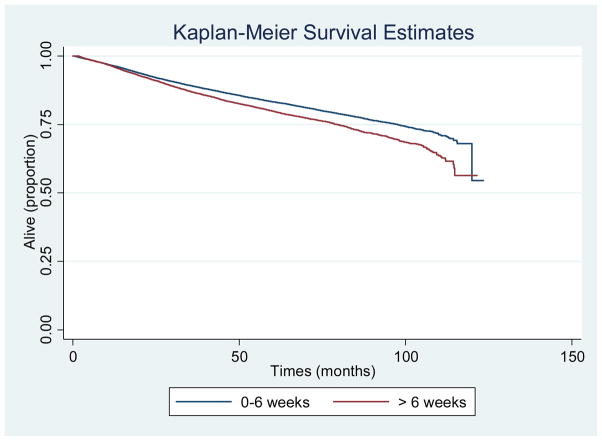
Survival through 2011 was available for 40,184 women diagnosed between 2003 and 2006. Cox regression analysis demonstrated the following characteristics were associated with for poorer survival (Table 3): non-Hispanic black race/ethnicity (HR 1.17, CI 1.08–1.27, p < 0.001), Medicaid or uninsured status (HR 1.40, CI 1.28–1.53, p < 0.001), lowest quartile of zip-code based income (HR 1.14, CI 1.02–1.26, p = 0.016) and education (HR 1.16, CI 1.05–1.29, p = 0.003), presence of co-morbidity (CDS1: HR 1.37, CI 1.30–1.45, p < 0.001; CDS2: 2.18, CI 1.97–2.41, p < 0.001), and grade 3 tumor (HR 2.40, CI 2.24–2.57, p < 0.001). Survival for women with surgical wait times greater than six weeks was significantly worse compared to patients who were surgically treated within six weeks of diagnosis (HR 1.14, 95% CI 1.09–1.20) (Figure 2).
Table 3. Cox Regression – Factors Associated with Survival.
Cox regression results for all-cause mortality for patients diagnosed with uterine cancer (n=40,184); women from 1,081 National Cancer Data Base-reporting hospitals diagnosed between 2003 and 2006, with follow-up through 2011.
| Risk Factor | Hazard Ratio (95% CI) | p value |
|---|---|---|
| Treatment | ||
| < 6 weeks | Reference | |
| > 6 weeks | 1.15 (1.10–1.21) | <0.001 |
| Age | ||
| < 40 yo | 0.18 (0.14–0.22) | <0.001 |
| 40–49 | 0.19 (0.17–0.21) | <0.001 |
| 50–59 | 0.28 (0.26–0.30) | <0.001 |
| 60–69 | 0.47 (0.44–0.50) | <0.001 |
| > 70 yo | Reference | |
| Race/Ethnicity | ||
| Non-Hispanic White | Reference | |
| Non-Hispanic Black | 1.17 (1.08–1.27) | <0.001 |
| Hispanic | 0.80 (0.71–0.90) | <0.001 |
| Asian | 0.63 (0.52–0.76) | <0.001 |
| Other/Unknown | 0.97 (0.83–1.14) | 0.712 |
| Insurance Status | ||
| Private Insurance | Reference | |
| Medicaid/Uninsured | 1.40 (1.28–1.53) | <0.001 |
| Income | ||
| Lowest quartile | 1.14 (1.02–1.26) | 0.016 |
| Second quartile | 1.09 (1.01–1.19) | 0.036 |
| Third quartile | 1.07 (1.00–1.15) | 0.05 |
| Fourth quartile | Reference | |
| Unknown | 0.15 | 0.114 |
| Education | ||
| Lowest quartile | 1.16 (1.05–1.29) | 0.003 |
| Second quartile | 1.07 (0.99–1.17) | 0.074 |
| Third quartile | 1.08 (1.01–1.17) | 0.020 |
| Fourth quartile | Reference | |
| Unknown | 8.02 (0.75–85.4) | 0.084 |
| CoMorbidity | ||
| CDS 0 | Reference | |
| CDS 1 | 1.37 (1.30–1.45) | <0.001 |
| CDS 2 | 2.18 (1.97–2.41) | <0.001 |
| AJCC Stage | ||
| Stage 0 | 0.85 (0.05–0.14) | <0.001 |
| Stage 1 | 0.11 (0.08–0.14) | <0.001 |
| Stage 2 | 0.20 (0.15–0.26) | <0.001 |
| Stage 3 | 0.30 (0.23–0.39) | <0.001 |
| Stage 4 | Reference | |
| Grade | ||
| 1 | Reference | |
| 2 | 1.48 (1.39–1.58) | <0.001 |
| 3 | 2.40 (2.24–2.57) | <0.001 |
| Unknown | 2.15 (1.95–2.38) | <0.001 |
| Nodes | ||
| Negative | Reference | |
| Positive | 1.38 (1.27–1.50) | <0.001 |
| Not done | 1.50 (1.42–1.59) | <0.001 |
| Unknown | 1.24 (0.89–1.73) | 0.195 |
| Year of Treatment | ||
| 2003 | Reference | |
| 2004–2006 | 0.99 (0.94–1.04) | 0.646 |
| 2007–2009 | N/A | |
| 2010–2011 | N/A | |
| Type of Endometrial Cancer | ||
| Type I | 0.72 (0.68–0.76) | <0.001 |
| Type II | Reference |
Figure 2.
Discussion
To the best of our knowledge, this is the first study to examine the impact of surgical wait times on survival in women with epithelial endometrial cancer using a large national database sample in the United States. Furthermore, it is the first study to investigate disparities in surgical wait time in women with endometrial cancer.
The findings of this study demonstrate that race and ethnicity, socioeconomic factors, and insurance coverage are all associated with increased likelihood of delayed surgical treatment, suggesting that disparities exist in timing of surgery after diagnosis. Furthermore, surgical wait times greater than six weeks from diagnosis to definitive surgery may have a negative impact on overall survival in women diagnosed with epithelial endometrial cancer. Although a causal relationship between wait times and overall survival cannot be established based on this data alone, these results suggest that it is important to consider time from diagnosis to surgery as a potential factor in overall outcomes in women diagnosed with epithelial endometrial cancer.
While few studies have examined the influence of surgical wait times on outcomes in women with endometrial cancer, the studies that do exist provide conflicting results. One study by Menczer et al [20], evaluated 181 women with endometrial cancer from a single Israeli cancer center between 1970 and 1986. This study concluded that a treatment delay of less than 4 months did not affect survival of women with endometrial cancer. Similarly, Matsuo et al [6], assessed 485 women with Type I endometrial cancer from a single United States medical center from 2000 to 2013. This study also found that wait time for surgical staging was not associated with decreased survival outcome in patients with Type I endometrial cancer.
In contrast, Elit et al [5] performed a large population based study of over 9,000 women with uterine cancer in Canada utilizing the Ontario Cancer Registry and found that longer wait times from diagnosis of uterine cancer to definitive surgery had a negative impact on overall survival. Our sample, which also used a significantly larger sample size than previous studies and included a broader range of endometrial cancers (Type I and Type II), also demonstrated a decrease in survival with surgical wait times greater than 6 weeks, independent of type of endometrial cancer.
The results of this study are consistent with previously published data in women with endometrial cancer using large cancer registries. These findings are also consistent with studies published in other cancers, including breast cancer [21], rectal cancer [4], and melanoma [22]. These findings, as in other cancers, highlight the importance of establishing national benchmarks for surgical wait times in order to maximize overall outcomes in women diagnosed with cancer.
In addition to finding differences in overall survival related to surgical wait time, this study also identified sociodemographic differences in surgical wait times in women diagnosed with endometrial cancer. It is well established in the literature that women with lower socioeconomic status and racial or ethnic minorities are consistently diagnosed with higher-stage cancer and disproportionately receive substandard care, resulting in higher mortality rates [23–24]. A recent review by Collins et al. on gynecologic cancer disparities reported that endometrial cancer mortality in black women is twice that of white women. The report further states that the etiology of this disparity is multifaceted; however, much of the evidence suggests that “equal care leads to equal outcomes” for black women diagnosed with gynecologic cancers [25]. The findings of this study continue to support differences in treatment related to sociodemographic factors. Specifically, we found that black women were 35% more likely to have surgery greater than six weeks after diagnosis compared to white women, while Hispanic women were 31% more likely to experience surgical delay greater than six weeks compared to white women. Furthermore, women with Medicaid or no insurance were 43% more likely to experience delay greater than six weeks and those women from the lowest education zip-code quartile were 28% more likely to experience surgical delay greater than six weeks. Given our findings that surgical delay greater than six weeks was associated with worse survival in this cohort of women, the findings of this study suggest that disparities regarding access to timely surgical management may be a contributing factor to the overall disparities that exist in gynecologic cancer outcomes.
Finally, our study evaluated trends in surgical treatment times over the study time period and found that women diagnosed in 2010–2011 were 32.5% more likely to undergo surgery greater than six weeks after diagnosis compared to patients treated in 2003. These results are consistent with findings from other studies, and may reflect an increase in patients with endometrial cancer being referred to a smaller number of higher-volume centers, resulting in hospital crowding and longer wait times for surgery [4].
There are several limitations to this study. First, because the NCDB only contains data from CoC-accredited hospitals, these findings may not be generalizable to the larger population, specifically minority or low income patients who may be treated at non-accredited hospitals. Furthermore, this study is limited by using cancer registry data such that there is no information regarding specific causes of longer wait times (ie: patient preference, unavailability of surgical time, etc). Specifically, there is no information regarding causes of delay in surgery due to preoperative medical testing or imaging needed prior to surgery. Given that those patients with more medical co-morbidities (higher Charlson Deyo score) had longer wait time for surgery, it is possible that surgical delays were related to required preoperative medical clearance or workup. Future studies should focus on specific causes for surgical delay in order to identify possible barriers or bottle-necks that delay or prevent timely surgical management of patients diagnosed with endometrial cancer.
In addition to the above limitations, while the results of this study demonstrate that treatment delays greater than six weeks were associated with decreased survival, we must take caution in concluding that the delay in surgery was the causal factor influencing survival. Instead, we must consider that a delay in surgery may represent a proxy for a more complex sociodemographic influences that may contribute to worse overall survival. This is likely in so far as information on disease recurrence or cause of death was not available; thus, non-cancer deaths reflecting social determinants of health heavily contribute to survival times.
In conclusion, this study demonstrates that surgical wait times greater than 6 weeks from diagnosis of endometrial cancer to definitive surgery may have a negative impact on overall survival. Furthermore, patient characteristics such as race and ethnicity, socioeconomic factors, and insurance coverage are all associated with increased likelihood of delayed surgical treatment. As a result of these findings, attention should be placed on minimizing surgical wait times in women diagnosed with endometrial cancer and increased focus should be placed on those women most likely to experience surgical delay in order to reduce potential disparities in access to the highest standard of care.
Acknowledgments
This study was supported by the Surgical Outcomes and Quality Improvement Center of Northwestern University
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, et al. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. [Accessed 10/1/2015];Uterine Neoplasms. Version 1.2016. http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 4.Yun YH, Kim YA, Min YH, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731–2737. doi: 10.1093/annonc/mds101. [DOI] [PubMed] [Google Scholar]
- 5.Elit LM, O’Leary EM, Pond GR, et al. Impact of wait times on survival for women with uterine cancer. J Clin Oncol. 2014;32:27–33. doi: 10.1200/JCO.2013.51.3671. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo K, Opper NR, Ciccone MA, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer. Obstet Gynecol. 2015;125:424–433. doi: 10.1097/AOG.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 7.Wait Times Alliance Canada. Reducing Wait Times and Achieving Benchmarks. Ottawa, ON, Canada: Canadian Medial Association; Nov, 2006. [Google Scholar]
- 8.Liederbach E, Sisco M, Wang C, et al. Wait times for breast surgical operations, 2003–2011: a report from the National Cancer Data Base. Ann Surg Oncol. 2015;22:899–907. doi: 10.1245/s10434-014-4086-7. [DOI] [PubMed] [Google Scholar]
- 9.Desch CE, McNiff KK, Schneider EC, et al. American Society of clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol. 2008;26:3631–37. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 11.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253:779–85. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Stewart AK, Winchest DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Data Base. The American College of Surgeons; Chicago, IL: Oct, 2015. https://www.facs.org/quality%20programs/cancer/ncdb. [Google Scholar]
- 14.Bilimoria KT, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and –nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–81. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 8. New York: 2010. [Google Scholar]
- 17.International Classification of Disease for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Zhang J, Yu K. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 19.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Menczer J, Krissi H, Chetrit A, et al. The effect of diagnosis and treatment delay on prognostic factors and survival in endometrial carcinoma. Am J Obstet Gynecol. 1995;173:774–778. doi: 10.1016/0002-9378(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 21.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 22.Pacifico MD, Pearl RA, Grover R. The UK Government two-week rule and its impact on melanoma prognosis: an evidence-based study. Ann R Coll Surg Engl. 2007;89:609–615. doi: 10.1308/003588407X205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Caontrol. 2009;20:417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSantis C, Jemal A, Ward E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Causes Control. 2010;219:1445–50. doi: 10.1007/s10552-010-9572-z. [DOI] [PubMed] [Google Scholar]
- 25.Collins Y, Holcombe K, Chapman-Davis E, et al. A report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133:353–61. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]