Abstract
Baicalein is a natural flavone that exhibits anticancer properties. Using microarrays we found that DDIT4 was the highest transcript induced by baicalein in cancer cells. We confirmed in multiple cancer cell lines large, dose-related expression of DDIT4 by quantitative RT-PCR and immunoblot, which correlates with growth inhibition. Time course experiments demonstrate that DDIT4 is rapidly inducible, with high expression maintained for several days in vitro. Induction of DDIT4 expression is p53 independent based on evaluation of p53 knockout cells. Since DDIT4 is known to inhibit mTORC1 activity we confirmed that baicalein suppresses phosphorylation of mTORC1 targets. Using RNA interference we demonstrate that mTORC1 activity and growth inhibition by baicalein is attenuated by knockdown of DDIT4. We furthermore demonstrate suppression of established tumors by baicalein in a mouse model of breast cancer with increased DDIT4 expression in the tumors. Finally, we demonstrate that baicalein upregulates DDIT4 and causes mTORC1 and growth inhibition in platinum resistant cancer cells in marked contrast to platinum chemotherapy treatment. These studies demonstrate that baicalein inhibits mTORC1 through DDIT4 expression, and may be useful in cancer chemotherapy and chemoprevention.
Keywords: DDIT4, baicalein, mTOR, IRF1, cellular stress
1. Introduction
Baicalein is a flavonoid which is an active component of the Traditional Asian Medicine (TAM) extract Huang Qin, which comes from the root of Scutellaria baicalensis. In TAM, Huang Qin is used to reduce fever, protect the liver, and treat cancer [1,2]. Interestingly, baicalein is present in the common herb thyme, and is the active component in the killing of MRSA bacteria by thyme extract [3]. As a component of Huang Qin, baicalein is found in many herbal concoctions, including Sho-saiko-to [4], and a drug based on TAM extracts, PHY906 [5], both of which have been evaluated in clinical trials. Baicalein causes tumor suppression of cancer cells in vitro and in vivo. Baicalein has numerous biological effects, such as the inhibition of 12-lipoxygenase [6–8], anti-oxidant effects [9], cell-cycle inhibition [10], apoptosis of cancer cells [6,7], and inhibition of invasion and metastases [11].
DNA-Damage-Inducible Transcript 4 (DDIT4), also known as REDD1 and RTP801, is a cytoplasmic protein originally identified to be transcriptionally upregulated in the setting of DNA damage [12], but has since been found to be upregulated by multiple forms of cellular stress, including oxidative stress [12], endoplasmic reticulum stress [13], hypoxia [14], and starvation [15]. DDIT4 is known to inhibit mTORC1 activity through activation of the TSC1/2 complex [15], although the precise mechanism for this has not been completely delineated. Inhibition of mTORC1 decreases activation of downstream targets by phosphorylation, including the ribosomal S6 protein kinases (S6K1 and S6K2) which phosphorylates ribosomal S6, which is believed to be involved in regulating translation and cell proliferation [15].
We originally identified baicalein as an activator of Interferon Regulatory Factor 1 (IRF1) using a cell-based screening assay, which is able to cause growth inhibition and tumor suppression in cancer cells in vitro and in vivo [16]. In this current study, we performed microarray analysis of baicalein-treated cancer cells to identify potential transcriptional targets for baicalein. We found that only one gene, DDIT4, was expressed over 2-fold at 12h, and DDIT4 was by far the top transcript at 24h. We further confirmed the marked induction of DDIT4 transcript and protein in multiple cancer cell lines. We then demonstrated that DDIT4 expression leads to inhibition of mTORC1 activity as seen by decreases in the phosphorylation of the mTORC1 targets S6K1 and S6, using RNA interference to further confirm the role of DDIT4 in baicalein-mediated mTOR and growth inhibition. We then demonstrated that baicalein is able to suppress tumor growth in a xenogeneic model of breast cancer with marked increase in DDIT4 protein in tumors by immunohistochemistry. Finally, we found that baicalein is able to inhibit growth of chemotherapy resistant cancer cells in correlation with DDIT4 induction and mTOR inhibition.
2. Material and Methods
For microarray analysis, quantitative RT-PCR (qRT-PCR), overexpression of DDIT4, and knockdown of DDIT4 expression using RNA interference, see Supplementary Methods.
Mice
SCID-Beige mice were from Charles River and maintained in accordance with the Institutional Animal Care and Use Committee at City of Hope. Tumors were generated as previously described [17] with further details including treatment and immunohistochemistry provided in Supplementary Methods.
Cell lines and culture
The human cancer cell lines OVCAR8, BT549, Hs578T, AGS, MDA468, and SKBR3 were from ATCC. HCT116 wild type and HCT116 p53 −/− were provided by L. Zhang with the permission of B. Vogelstein [18,19]. A2780 and A2780R (isogenic platinum resistant, 20) were provided by Dr. R. Jove. All cell lines were cultured as described in Supplementary Methods.
Chemicals and reagents
Baicalein was from Cayman Chemical (Ann Arbor, MI). Baicalin, genistein, cisplatin, and carboplatin were from Sigma. Antibodies anti-pS6K1, anti-S6K1, anti-pS6, and anti-S6 were from Cell Signaling; anti-DDIT4 was from Protein Technology; and anti-β-actin from Millipore. FITC–conjugated goat anti-mouse and anti-rabbit IgG were from Pierce Biotechnology. The enhanced chemiluminescence substrates were from Thermo Scientific. Dimethyl sulfoxide (DMSO) was from ATCC.
Western blotting
Cells were treated, lysed, cleared by centrifugation, and immunoblotted as previously described [16,18].
Cell proliferation assay
Cell proliferation was measured by MTT assay as previously described. MTT conversion to formazan dye correlates with the number of living cells [16,18].
Statistical analyses
Experiments were carried out in triplicate or more, with in vitro data presented as mean±SD and in vivo data presented as mean±SEM. Statistical comparison of values were made using 2-tailed Student's t test, and statistical significance was considered to be present when p<0.05.
3. Results
Microarray analysis demonstrates significant upregulation of DDIT4 transcript in AGS cells
Considering our original studies were performed in the STAT1-deficient cell line AGS [16], we used the same cell line to perform microarray gene expression profiling to identify the candidate genes induced by baicalein treatment. We treated AGS cells with baicalein or carrier control at 20µM in triplicate for 12 or 24h. Total RNA was extracted from each sample, and mRNA gene expression profiling was performed using the Affymetrix Human Genome U133 Plus 2.0 array. Microarray data analysis was carried out using Partek Genomics Suite. The significant differential expressed genes (DEGs, 2-fold plus p≤0.05) at 12 and 24h are shown in Table I. DDIT4 is the top transcript at 24h, and is the only gene with greater than 2-fold expression at 12h.
Table I.
Microarray Analysis of Baicalein Treated Cancer Cells at 12 and 24 Hours
| Symbol | Fold Change |
p-value | Entrez Gene Name |
|---|---|---|---|
| 24 Hours | |||
| DDIT4 | 5.397 | 4.42E-14 | DNA-damage-inducible transcript 4 |
| INHBE | 3.958 | 4.92E-09 | inhibin, beta E |
| S100P | 3.134 | 1.98E-13 | S100 calcium binding protein P |
| CHAC1 | 3.077 | 2.54E-12 | ChaC, cation transport regulator homolog 1 (E. coli) |
| ASNS | 2.986 | 5.54E-15 | asparagine synthetase (glutamine-hydrolyzing) |
| SLC7A11 | 2.929 | 2.69E-14 | solute carrier family 7, member 11 |
| ECM2 | 2.909 | 1.33E-08 | extracellular matrix protein 2, female organ/adipocyte specific |
| CTH | 2.738 | 4.21E-11 | cystathionase (cystathionine gamma-lyase) |
| PCK2 | 2.618 | 1.43E-11 | phosphoenolpyruvate carboxykinase 2 (mitochondrial) |
| IFI44L | 2.580 | 4.46E-08 | interferon-induced protein 44-like |
| XAF1 | 2.298 | 4.96E-08 | XIAP associated factor 1 |
| ANKRD1 | 2.298 | 5.87E-09 | ankyrin repeat domain 1 (cardiac muscle) |
| KIF21B | 2.278 | 7.28E-10 | kinesin family member 21B |
| SERPINB2 | 2.173 | 3.36E-07 | serpin peptidase inhibitor, clade B (ovalbumin), member 2 |
| IL8 | 2.110 | 2.73E-08 | interleukin 8 |
| SLC1A4 | 2.098 | 3.19E-10 | solute carrier family 1, member 4 |
| RIMKLB | 2.095 | 1.71E-05 | ribosomal modification protein rimK-like family member B |
| HUS1 | 2.063 | 2.54E-07 | HUS1 checkpoint homolog (S. pombe) |
| C1ORF116 | 2.036 | 9.05E-10 | chromosome 1 open reading frame 116 |
| NT5DC4 | 2.026 | 3.55E-07 | 5'-nucleotidase domain containing 4 |
| IFI44 | 2.010 | 1.42E-06 | interferon-induced protein 44 |
| 12 Hours | |||
| DDIT4 | 3.222 | 3.38E-12 | DNA-damage-inducible transcript 4 |
AGS cells were treated with DMSO or baicalein at 20µM in triplicate for 12 or 24h. Total RNA was isolated and ultimately evaluated by microarray using the Affymetrix Human Genome U133 Plus 2.0 arrays. Significant differentially expressed genes were determined using Partek® software.
Confirmation of DDIT4 expression in AGS cells by quantitative RT-PCR and Western blotting
We then confirmed that baicalein increases DDIT4 mRNA and protein in AGS cells in a dose related fashion using quantitative RT-PCR (qRT-PCR) and immunoblotting. We first confirmed that baicalein can eliminate AGS cells in a dose-related fashion using MTT assay (Figure 1A). By 72h at 80µM the cells are essentially eliminated. We then showed that baicalein can increase DDIT4 expression as high as 20-fold at 80µM, while the isoflavone genistein, which has the same molecular formula as baicalein, but a different structure, shows minimal increase in DDIT4 mRNA expression (Figure 1B). This was also seen by immunoblotting (Figure 1C).
Figure 1.
Confirmation of DDIT4 expression by quantitative RT-PCR (qRT-PCR) and Western blot. A, Baicalein can eliminate AGS cells in a dose-dependent manner assessed by MTT assay. Cells were treated with doses indicated for 72h (n=6, mean±SD). B, qRT-PCR for DDIT4 expression in AGS cells. AGS cells were treated with baicalein or genistein at indicated concentrations or carrier control for 24h. Total RNA was isolated and subjected to qRT-PCR for DDIT4. Data was normalized to β-Actin. C, Western blotting for DDIT4 expression in cell lysates. β-Actin was used as loading control.
Baicalein induces DDIT4 expression in breast cancer cell lines
We then evaluated a range of cell lines to include breast cancer cells, including the more aggressive triple negative and HER2 overexpressing cancers. As seen in Figure 2, baicalein starts to induce expression of DDIT4 as low as 10µM or lower. The growth inhibitory dose response of baicalein on MDA468 and SKBR3 have already been published [16], and show that growth inhibition can be seen with doses of baicalein as low as 1µM over 72h. At 24h, MDA468 (Figure 2A) and SKBR3 (Figure 2B) clearly increase DDIT4 expression at 10µM and higher in a dose related fashion, with genistein, the isomeric control, showing no activity at all even at the 80µM. We further confirmed the increase in DDIT4 expression in another triple negative breast cancer cell line Hs578T (Figure 2C), for which the glucuronidated control of baicalein, baicalin, shows no increased expression of DDIT4 by immunoblot and no inhibition of growth, while once again baicalein shows marked increase in DDIT4 mRNA and protein expression that correlates with the growth suppression. The triple negative breast cancer cell line BT549 showed DDIT4 expression and growth suppression by baicalein in the single digit µM range (Figure 2D).
Figure 2.
Baicalein induces DDIT4 expression in different breast cancer cell lines. A, MDA468 cells were treated with baicalein or genistein at indicated concentrations or carrier control for 24h. Total protein was obtained for Western blotting for DDIT4. Densitometry values normalized to β-actin are below each band. Total RNA was isolated for qRT-PCR for DDIT4. B, SKBR3 cells. C, Hs578T cells were treated with baicalein or baicalin at indicated concentrations or carrier control. Growth inhibition at 72h was measured by MTT assay in third panel. D, BT549 cells were treated with baicalein at indicated concentrations or carrier control for 24h and total protein was obtained for Western blotting for DDIT4 (inset), with MTT assay for growth inhibition at 72h. All data are means±SD, n=6.
Baicalein induces DDIT4 as early as two hours which is sustained for over two days in vitro
We then performed a time course evaluation of DDIT4 mRNA and protein expression in MDA468. As seen in Figure 3A, baicalein induced DDIT4 expression as early as 2h after treatment which appeared to be sustained over control for over 2d. As time passed during the growth of the cells in culture it appears that DDIT4 expression also increased over time, but clearly baicalein treatment was markedly increased despite this. This was clearly seen both by qRT-PCR and immunoblotting.
Figure 3.
Baicalein induction of DDIT4 is sustained and is p53-independent. A, MDA468 cells were treated with baicalein at 40µM or carrier control for time course as indicated. Total RNA was isolated and qRT-PCR performed for DDIT4. Total protein was obtained and Western blotting performed for DDIT4. B, HCT116 wildtype cells were treated with baicalein or genistein at indicated concentrations or carrier control for 24h. Total RNA isolated and qRT-PCR for DDIT4 performed. Total protein obtained for Western blotting for DDIT4, and MTT assay at 72h performed. Densitometry values normalized to β-actin are shown. C, HCT116 p53−/− cells were treated with baicalein or genistein at indicated concentrations or carrier control for 24h. Total RNA isolated and qRT-PCR for DDIT4 performed. Total protein isolated and Western blotting for DDIT4 performed, with MTT assay of HCT116 P53−/− cells. qRT-PCR and MTT assay data are means±SD, n=6.
Baicalein induction of DDIT4 is p53-independent
DDIT4 expression has been reported to be p53 dependent [12]. However, we have previously found that baicalein can induce other p53-dependent proteins in the absence or mutation of p53, such as p21 and PUMA [16], as we have seen with IRF1 expression [18]. Furthermore, most of the breast cancer cell lines involved in this study are p53 null/mutant. In order to study this we used the well-studied diploid colon cancer cell line HCT116 and its associated p53-null derivative HCT116 p53 −/−, for which p53 has been deleted by homologous recombination [19]. HCT116 seemed more resistant to the growth inhibitory properties of baicalein as seen with the MTT assays (lower panels, Figure 3B and 3C). Nevertheless, DDIT4 expression was clearly increased in both cell lines by immunoblot and qRT-PCR in a dose related fashion, with similar increases in the p53-null cells (upper panels, Figure 3B and 3C).
Baicalein-induced DDIT4 suppresses mTORC1 activity
DDIT4 is known to inhibit mTORC1 activity through activation of the TSC1/2 complex, although the precise mechanism for this has not been completely delineated [15]. Inhibition of mTORC1 decreases activation of downstream targets by phosphorylation, including the ribosomal S6 protein kinases (S6K1 and S6K2) which phosphorylates ribosomal S6, which is believed to be involved in regulating translation and cell proliferation [15]. We first confirmed that DDIT4 in and of itself was able to suppress mTORC1, based on suppression of phosphorylation of the mTORC1 target S6K1, and further downstream S6. As seen in Figures 4A and 4B, there was inhibition of phosphorylation with the increase in DDIT4 by transfection of a vector with constitutive overexpression of tagged DDIT4 in both Hs578T (Figure 4A) and HCT116 (Figure 4B). Furthermore, DDIT4 overexpression markedly inhibited the growth of these cells, but this was minimally or not enhanced with baicalein treatment (right panels Figures 4A and 4B).
Figure 4.
Baicalein-induced DDIT4 suppresses mTORC1 activity. A, DDIT4 overexpression in Hs578T cells suppresses mTORC1. Hs578T cells with empty vector or DDIT4 overexpression vector were lysed and total protein isolated for Western blotting for DDIT4, PS6K1, S6K1, PS6, S6, and β-Actin. Results of MTT assay of plated cells for 72h is in right panel with indicated treatment (means±SD, n=6). B, HCT116 wildtype cells as in A. C, HCT116 cells were treated with baicalein, baicalin or carrier control for 24h, and were collected for Western blotting as in A. Densitometry values normalized to β-actin are shown. D, HCT116 cells were transfected with Control siRNA or DDIT4 siRNA, then treated with baicalein at 40µM for 24h with Western blotting as in A. E, MDA468 cells were treated with baicalein, baicalin or carrier control (C) for 24h, with Western blotting as in A. F, MDA468 cells were transfected with Control siRNA or DDIT4 siRNA, then treated with baicalein at 20µM for 24h with Western blotting as in A.
We then evaluated the effect of baicalein on phosphorylation of the mTORC1 target S6K1 and the downstream target S6 including the setting of suppression of DDIT4 expression using DDIT4 siRNA. In order to study this, we used HCT116 and MDA468 cell lines because these seemed to have the best response in terms of suppression of DDIT4 expression using DDIT4 siRNA. As seen in Figure 4C, baicalein, but not the baicalin control, increased DDIT4 expression in a dose-related fashion, with suppression of phosphorylation of both S6K1 and S6 in HCT116 cells. There was no significant change in the S6K1 or S6 protein which further supports that this was due to suppression of mTORC1 activity. Furthermore, we used DDIT4 siRNA to knock down DDIT4 expression, and as seen in Figure 4D, we were able to both knock down the baseline expression of DDIT4 and prevent an increase in DDIT4 expression in the presence of baicalein, and in so doing, we found no mTORC1 inhibition by baicalein when DDIT4 was knocked down. This further supports that the inhibition of mTOR was due to the increase in DDIT4 expression. We found identical results in MDA468 (Figures 4E and 4F).
DDIT4 significantly mediates the inhibition of growth by baicalein in MDA468 cells
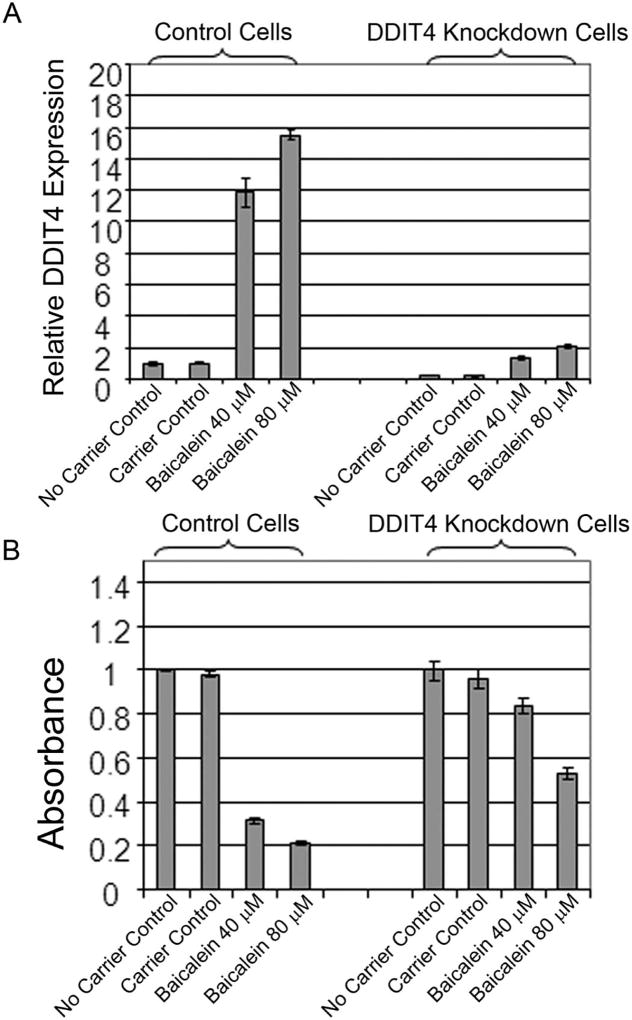
We then evaluated the role of the baicalein-induced DDIT4 expression in the growth inhibition of MDA468 cells. We found that siRNA transfection in the appropriate controlled setting increased the resistance of cancer cells to baicalein-induced growth suppression over 72h, requiring increased doses of baicalein (that is 40 to 80µM) to achieve significant growth suppression in control cells (Figure 5B). In order to further knock down the baicalein-induced DDIT4 expression at high concentrations of baicalein over 72h we performed siRNA transfection in bulk selected shRNA expressing MDA468 cells so that even in the presence of 80µM of baicalein DDIT4 mRNA was suppressed by more than 80% (Figure 5A). In this setting, over 72h, growth suppression was significantly attenuated in the DDIT4 knockdown cells (p<0.0001), implicating DDIT4 as a significant mediator of growth inhibition by baicalein (Figure 5B).
Figure 5.
DDIT4 mediates growth inhibition by baicalein in MDA468 cells. A. MDA468 cells expressing shRNA against DDIT4 (DDIT4 Knockdown Cells) and Control cells were further treated with DDIT4 siRNA or control siRNA as in Fig. 4 for further eradication of DDIT4 expression in the presence of high concentrations of baicalein (40 and 80µM) and subjected to qRT-PCR for DDIT4. B, MDA468 cells treated as in 5A were assessed by MTT assay at 72h (n=6, mean±SD).
Baicalein suppresses tumor growth of MDA468 cancer cells without toxicity to the host and increases DDIT4
We evaluated the ability of baicalein to suppress tumor growth of the triple negative breast cancer MDA468 tumors in an orthotopic xenogeneic model in SCID-Bg mice. Female SCID-Bg mice with existing tumors received baicalein injections intraperitoneally (i.p.) 20mg/kg for 5d/wk, or carrier control injections at the same schedule, or carrier control injections and one dose of cisplatin 5mg/kg i.p. on day 5. As seen in Figure 6A, baicalein caused suppression of tumor growth similar to cisplatin alone. However, baicalein was injected 5 times per week with no visible toxicity, while one cisplatin injected mouse died and most cisplatin mice showed asthenia and piloerection. We furthermore performed studies of the tumors in baicalein injected mice versus carrier control and found that DDIT4 protein was markedly increased in mice injected with baicalein, both by tumor immunoblotting, and by immunofluorescent staining (Figures 6B, 6C). Furthermore we performed immunohistochemistry for the proliferation marker Ki67 of tumors from carrier control and baicalein treated mice and found that the large majority of tumors from mice treated with carrier control showed markedly increased Ki67 (in the 70% range, Figure 6D) versus baicalein treated mice (in the 30% range, Figure 6D). Finally, we also performed immunohistochemistry for apoptosis by TUNEL staining and found almost no TUNEL staining in the large majority of control treated tumors while TUNEL staining was found in most baicalein treated tumors (Figure 6D).
Figure 6.
Baicalein inhibits MDA468 cancer cell xenografts in vivo with increased DDIT4 expression in tumors. A, Baicalein suppresses MDA468 tumors in SCID-Beige mice (n=5 or 6, mean±SEM). Female SCID-Beige mice with orthotopically injected MDA468 tumors were injected with baicalein intraperitoneally (i.p.) at 20mg/kg for 5d/wk, or carrier at the same schedule. One dose of cisplatin was injected on d5 (arrow) at 5mg/kg i.p. *p<0.05 from Day 12 on for baicalein versus control. B, Western blot of total protein from tumors in baicalein treated mice versus carrier control. C, Immunofluorescent staining of tumor sections from baicalein versus carrier treated mice. Blue, DAPI; green, DDIT4. D, Immunohistochemistry of tumor sections from baicalein versus carrier treated mice. Blue, hematoxylin; brown, DAB
Baicalein suppresses the growth of ovarian cancer cells in correlation with DDIT4 expression and mTOR inhibition, including chemotherapy resistant cells versus platinum treatment in vitro
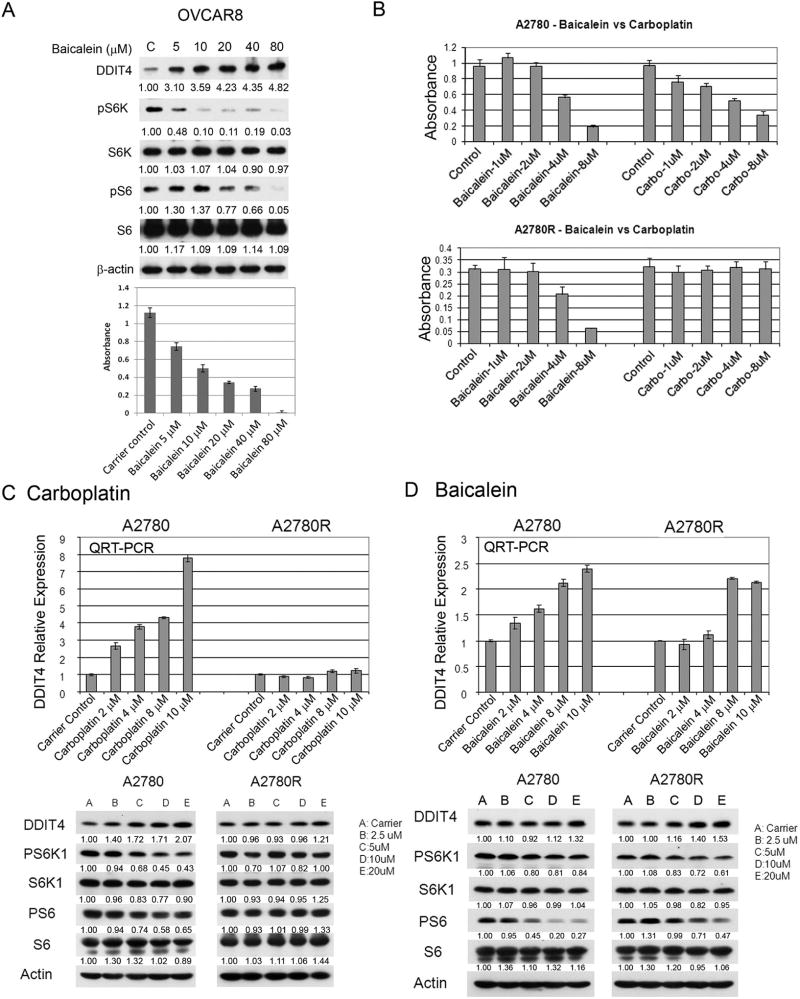
We also evaluated the ovarian cancer cell line OVCAR8 in vitro and found that ovarian cancer cells showed a marked response to baicalein with outstanding correlation of DDIT4 expression and mTOR inhibition as reflected by pS6K and pS6 immunoblotting with growth inhibition by MTT assay (Figure 7A). To further compare baicalein with chemotherapy treatment we used the in vitro platinum resistant ovarian cancer cell model, A2780, which is platinum chemotherapy sensitive, and the isogenic cell line A2780R, which was derived from A2780 to be platinum chemotherapy resistant [20]. As seen in Figure 7B, baicalein and carboplatin both demonstrated marked growth inhibition of the platinum sensitive cancer cell line A2780 in a dose responsive fashion, but only baicalein was able to markedly inhibit the platinum resistant cancer cell line A2780R, with carboplatin having no effect at all at equivalent doses. As seen in Figure 7C baicalein significantly increases DDIT4 expression and inhibits activation of the mTOR targets S6K1 and S6 in both platinum sensitive and platinum resistant cell lines. The more terminal mTOR target S6 seems to be particularly inhibited by baicalein. However, as seen in Figure 7D carboplatin, while markedly increasing DDIT4 mRNA expression in platinum sensitive cells, does not appreciably increase DDIT4 mRNA expression in the platinum resistant cells. As seen in the Western blots DDIT4 protein expression is also markedly increased with inhibition of the mTOR targets in the platinum sensitive cell line, which is markedly attenuated in the platinum resistant cells.
Figure 7.
Baicalein suppresses ovarian cancer cells in correlation with DDIT4 expression and mTOR inhibition, including chemotherapy resistant ovarian cancer cellsversus platinum chemotherapy treatment. A, Ovarian cancer cell line OVCAR8 treated with baicalein or control at indicated concentrations were harvested at 24h for immunoblotting for indicated proteins in upper panel. MTT assay with same treatment at 72h in lower panel (n=6, mean±SD). B, MTT assay of A2780 and A2780R cells treated with baicalein or carboplatin at indicated concentrations or carrier control (n=6, mean±SD). C, A2780 and A2780R cells were treated with baicalein or carrier control for 24h. Total RNA was isolated for qRT-PCR and total protein was obtained for Western blot. Densitometry values normalized to actin are shown. D, A2780 and A2780R cells were treated with carboplatin at indicated concentrations or carrier control for 24h. Total RNA was isolated for qRT-PCR and total protein lysate was obtained for Western blot.
4. Discussion
In this study, we have demonstrated that the natural flavone baicalein, an active component of the TAM root extract, Huang Qin, from Scutellaria baicalensis, causes a marked increase in DDIT4 expression, and this causes DDIT4 associated inhibition of mTOR activity. This is seen in a whole range of cancer cells. DDIT4 plays a major role in inhibition of mTOR activity but probably plays a partial role in growth inhibition by baicalein on cancer cells, since near complete inhibition of DDIT4 expression did not completely block the growth inhibitory effect of baicalein in our RNA interference breast cancer cell line model (Fig. 5B). We have furthermore demonstrated effective tumor suppression by injection of baicalein 5 days/week, equivalent to a single dose of platinum chemotherapy injection, but without toxicity. Furthermore, we were able to confirm that DDIT4 expression was high in the tumors of mice given baicalein. Finally, we have found in a platinum chemotherapy resistance model in vitro that not only baicalein, but also carboplatin, increases DDIT4 expression in platinum sensitive ovarian cancer cells, but in platinum resistant ovarian cancer cells only baicalein significantly increases DDIT4 expression and causes inhibition of growth.
Recently, it has been reported that DDIT4−/− cells have increased sensitivity to DNA damage in terms of apoptotic response with respect to doxorubicin and radiation, both in vitro and in vivo, in the setting of abnormally increased p53 protein and activity [21]. This could imply that upregulation of DDIT4 would decrease p53 and make cells less sensitive to DNA damage and thus chemotherapy and/or radiation. In our cancer cells DDIT4 upregulation clearly appears to result in an anti-tumor response. Our studies were performed on p53-deficient or mutated cells for which there should not be any significant p53-mediated responses and may therefore be due to other factors. Clearly DDIT4 expression and mTOR inhibition results in anti-tumor effects which are multifactorial for which the presence of wild-type p53 is unlikely to be a factor.
Significant DDIT4 expression by baicalein is seen at concentrations in the low micromolar range, with dose-dependent increase in DDIT4 transcript and protein and cell growth inhibition. We purposely limited our study to concentration levels in the double-digit µM range or lower, since these concentrations should be pharmacologically achievable in vivo based on multiple studies of the pharmacokinetics of flavonoids, including baicalein. Studies of flavonoids in humans have demonstrated that dietary consumption of foods rich in flavonoids can result in plasma levels of individual flavonoids in the single-digit µM range. For example, women who consume varying amounts of flavonoids from soy milk have plasma genistein levels of 0.8–2.2µM [22]. After repeated injections of baicalein intraperitoneally into mice at 20mg/kg for five days/week, there was no evidence of toxicity. Furthermore, baicalein as a component in herbal extracts has been ingested by humans in multiple human studies and in TAM [1,3,4]. In fact, baicalein is present in thyme and may be the active component in thyme’s antibacterial effect in herbal concoctions [2]. These reports support the hypothesis that baicalein may be well-tolerated in humans with plasma levels comparable to those used in our study if given in pharmacologic doses. Recently, DDIT4 was reported to mediate mTOR inhibition and cell cycle arrest by metformin in cancer cells [23]. In that study, while DDIT4 expression and inhibition of mTOR targets was impressive, the dose given in vitro was 1 to 10 mmol/L (i.e. 1,000–10,000µM) or higher. Metformin is currently being assessed in phase III clinical trials against cancer. We would anticipate similar impressive upregulation of DDIT4 protein and complete suppression of mTOR targets with baicalein, but the doses used in our study are much more likely to be pharmacologically achievable and tolerable.
The determination of the mechanism of enhancement of DDIT4 by baicalein requires further study. We believe that DDIT4 is upregulated as a stress response induced by baicalein. As our laboratory originally identified baicalein by HTS for IRF1 activity, it is notable that IRF1 is well known to be induced as part of the DNA damage response. Baicalein has been recently reported to induce a DNA damage response which activates polEta, interestingly along with other known antioxidants such as reservatrol and genistein [24]. Furthermore, it was reported that there were no significant increase in mutations compared to other chemotherapeutic agents and it was therefore speculated that these antioxidants could be used in lieu of chemotherapy for cancer [24]. Treatments such as ionizing radiation have already been found to increase steady state levels of the IRF1 protein through a concerted mechanism that includes a decrease in the rate of degradation [25]. For DDIT4, the mechanism appears to be primarily transcriptional since over 20-fold increases in mRNA can be seen in certain settings (Fig. 1). Reactive oxygen species (ROS) mediated stress may play a role since numerous studies have demonstrated the potential role of ROS in baicalein-induced cellular effects, including apoptosis [26]; however, we have not found an increase in ROS in baicalein treated cells (Supplementary Data).
We have linked baicalein with inhibition of the PI3K pathway, in particular mTOR. Recently, another study has also demonstrated mTOR inhibition by baicalein in cancer cells via activation of AMPKα [27]. mTOR has been an attractive target for cancer treatment; in fact the mTOR inhibitor everolimus has recently been FDA approved for use in the treatment of metastatic breast cancer. Everolimus dosage is limited by oral sores and stomatitis. It is possible that baicalein, which is present in nature and is even present in the herb thyme [3], may be able to inhibit mTOR activity with less toxicity, although it is likely to be less specific than everolimus. There is currently no baicalein available for humans in its pure form, although it is available as an ingredient in Scutellaria baicalensis, thyme, Oryxilum indicum, and other herbal extracts, including the drug, PHY906 [4].
Regardless, there are likely to be multiple off-target effects of baicalein not related to DDIT4. In our laboratory, baicalein was identified by cell based screening of enhancers of IRF1 activity [16]. Baicalein has been found to inhibit 12-LOX activity [5–7] as well as increase the TRAIL receptor DR5 [26]. While DDIT4 clearly stood out by microarray analysis of baicalein-treated cells, DDIT4 and mTOR inhibition are not likely to be the sole mediators of in vitro and in vivo growth inhibition. Multiple transcriptional targets of IRF1, such as XAF1, IFI44, and IFI44L are also increased by microarray and may also play a separate role.
In conclusion, we have demonstrated that baicalein causes a marked induction of DDIT4 that mediates mTOR inhibition and growth inhibition in cancer cells. As a natural product already found in a common herb (thyme) and TAM, baicalein can clearly be tolerated in humans, and significant doses do not appear to be toxic in mice. Because of this baicalein should be an excellent candidate for a clinical trial, not only because it appears to be non-toxic, but also because as a single component of a TAM, clear mechanistic and outcome studies should be achievable. While DDIT4 expression is clearly involved in the mechanism of baicalein’s effects, it may furthermore be used as a biomarker of efficacy in clinical trials as well.
Supplementary Material
Highlights.
Baicalein, a natural compound, causes tumor suppression of cancer cells both in vitro and in vivo.
Baicalein upregulates DDIT4 which inhibits mTOR and mediates growth inhibition.
Baicalein is effective in chemotherapy resistant cancer cells.
Acknowledgments
JHY was supported by NIH K08CA098403, Komen BCTR0708040, and donor support from the Markel-Friedman Fund, Don and Susan Schwarz, the City of Hope Board of Governors, and the Panda Charitable Foundation – Cherng Program in Natural Therapies. The authors thank Dr. Michael Weiss for writing assistance, and Dr. Richard Jove for advice on the research plan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interests: No potential conflicts of interests were disclosed.
Conflict of interest
The authors declare no competing financial interest.
References
- 1.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita M, Shiota S, Kuroda T, Hatano T, Yoshida T, Mizushima T, et al. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005;49:391–6. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 4.Deng G, Kurtz RC, Vickers A, Lau N, Yeung KS, Shia J, et al. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J. Ethnopharmacol. 2011;136:83–7. doi: 10.1016/j.jep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Kummar S, Copur MS, Rose M, Wadler S, Stephenson J, O'Rourke M, et al. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer. 2011;10:85–96. doi: 10.1016/j.clcc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Ding XZ, Kuszynski CA, El-Metwally TH, Adrian TE. Lipoxygenase inhibition induced apoptosis, morphological changes, and carbonic anhydrase expression in human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 1999;266:392–9. doi: 10.1006/bbrc.1999.1824. [DOI] [PubMed] [Google Scholar]
- 7.Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol. Cancer Ther. 2002;1:929–35. [PubMed] [Google Scholar]
- 8.Sekiya K, Okuda H. Selective inhibition of platelet lipoxygenase by baicalein. Biochem Biophys. Res. Commun. 1982;105:1090–5. doi: 10.1016/0006-291x(82)91081-6. [DOI] [PubMed] [Google Scholar]
- 9.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–5. [PubMed] [Google Scholar]
- 10.Hsu SL, Hsieh YC, Hsieh WC, Chou CJ. Baicalein induces a dual growth arrest by modulating multiple cell cycle regulatory molecules. Eur. J. Pharmacol. 2001;425:165–71. doi: 10.1016/s0014-2999(01)01144-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Zhang S, Ji Y, Li J, An P, Ren H, et al. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 13.Whitney ML, Jefferson LS, Kimball SR, Savitsky D, Tamura T, Yanai H, et al. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem. Biophys. Res. Commun. 2009;379:451–5. doi: 10.1016/j.bbrc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Wang Y, Xing Q, Yan J, Senthil M, Akmal Y, et al. Identification of a natural compound by cell-based screening that enhances interferon regulatory factor-1 activity and causes tumor suppression. Mol. Cancer Ther. 2011;10:1774–83. doi: 10.1158/1535-7163.MCT-11-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzoferrato E, Liu Y, Gambotto A, Armstrong MJ, Stang MT, Gooding WE, et al. Ectopic expression of interferon regulatory factor-1 promotes human breast cancer cell death and results in reduced expression of surviving. Cancer Res. 2004;64:8381–8. doi: 10.1158/0008-5472.CAN-04-2223. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Senthil M, Ren B, Yan J, Xing Q, Yu J, et al. IRF-1 transcriptionally upregulates PUMA, which mediates the mitochondrial apoptotic pathway in IRF-1-induced apoptosis in cancer cells. Cell Death Differ. 2010;17:699–709. doi: 10.1038/cdd.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt W, Chaney SG. Role of carrier ligand in platinum resistance of human carcinoma cell lines. Cancer Res. 1993;53:799–805. [PubMed] [Google Scholar]
- 21.Vadysirisack DD DD, Baenke F, Ory B, Lei K, Ellisen LW. Feedback control of p53 translation by REDD1 and mTORC1 limits the p53-dependent DNA damage response. Mol. Cell Biol. 2011;31:4356–65. doi: 10.1128/MCB.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J. Nutr. 1994;124:825–32. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 23.Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–72. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 24.Fox JT, Sakamuru S, Huang R, Teneva N, Simmons SO, Xia M, et al. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. U.S.A. 2012;109:5423–8. doi: 10.1073/pnas.1114278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamment J, Ramsay E, Kelleher M, Dornan D, Ball KL. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene. 2002;21:7776–85. doi: 10.1038/sj.onc.1205981. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi H, Yoshida T, Horinaka M, Yasuda T, Goda AE, Konishi M, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–27. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- 27.Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014 doi: 10.1111/febs.12969. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.