Abstract
Asymmetric hydrogenation of unprotected N-H imines catalyzed by Rh/bisphosphine-thiourea provided chiral amines with up to 97% yield and 95% ee. 1H NMR studies, coupled with control experiments, implied that catalytic chloride-bound intermediates were involved in the mechanism via the dual hydrogen-bonding interaction. Deuteration experiments proved that the hydrogenation proceeded through a pathway consistent with the imine.
Keywords: asymmetric catalysis, hydrogenation, ion pairs, ketimine, rhodium
Graphical abstract
Bisphosphine-thiourea was successfully used in the Rh catalyzed asymmetric hydrogenation of unprotected iminium salts (up to 97% yield and 95% ee). Even if 0.2 mol % catalyst was used, 95% conversion and 94% ee was obtained. The control experiments and 1H NMR studies implied that the anion binding between the thiourea and chloride ions was involved in the mechanism. Deuteration experiments proved that the hydrogenation proceeded through a pathway consistent with the imine.

Chiral amines are powerful pharmacophores of biologically active molecules for pharmaceuticals and agrochemicals, such as the elastase inhibitor DMP 777, calcimimetic agent Sensipar (Cinacalcet) and type II calcimimetics NPS R-568. (Figure 1).[1] As one of the most efficient synthetic approaches, metal-catalyzed asymmetric hydrogenation deserves special attention. Many successful catalytic systems have been developed, including asymmetric reductive amination and asymmetric hydrogenation of enamines and imines.[1–7] However, owing to the complex interactions between catalysts and substrates/products, the imine-enamine tautomerization and the E/Z interconversion of imines, asymmetric hydrogenation of enamines and imines is still a largely underdeveloped area in contrast to the advances of olefins and ketones.[1b] Unprotected N-H imines and enamines are attractive but challenging substrates.[3–5] Asymmetric hydrogenation of them eliminates the use of N-protecting groups and has broad potential applications in industry. To date, although there are several successful examples of unprotected enamines in academic research and industrial utilization,[3] the unprotected imines are rarely studied.[4,5] Our group reported the first example of iminium salts[5] involved in the substrate activation strategy[1e,3–7] with well-studied Ir-based catalysts. We aimed to develop a novel and efficient Rh-based catalytic system for this transformation.
Figure 1.
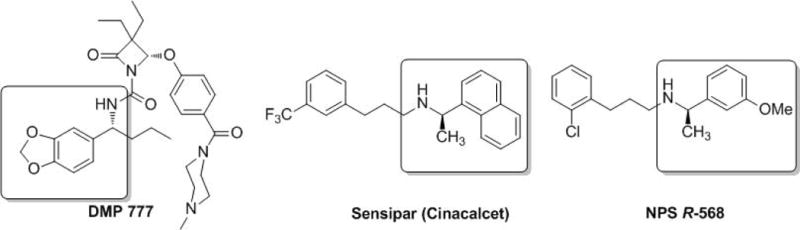
Related drugs (candidates) containing chiral amines.
Thiourea has been widely used as a hydrogen-bond donor in organocatalysis.[8] Most research focuses on the direct activation of neutral substrates by hydrogen bonding while recent studies take advantage of the anion binding of ion-pairing intermediates.[9] Inspired by the strategies developed in organocatalysis[10–12] and our previous research on the Rh/bisphosphine catalyzed asymmetric hydrogenation of nitroalkenes assisted by thiourea,[13] we sought to extend anion-binding catalysis to the transition metal-catalyzed asymmetric hydrogenation. We envisioned that thiourea could interact with a counterion in the catalytic pathway (Figure 2).
Figure 2.

Extension of our Rh/Bisphosphine-Thiourea catalytic system.
Herein, we describe the first example of Rh/bisphosphine catalyzed asymmetric hydrogenation of unprotected N-H imines assisted by thiourea with up to 97% yield and 95% ee.
As we reported previously,[13] the catalytic system was solvent dependent and poor results were given using Ir, Pd and Ru.[14] In addition, pressures, temperatures and additives were examined to study the turnover number (TON) limit of this transformation.[14] 99% conversion and 94% ee was observed with 1 mol % catalyst at 25 °C under 10 atm H2 (Scheme 1). Even if 0.2 mol % catalyst was used, 95% conversion and 94% ee was obtained. This system provided remarkably higher TON than our previous Ir-f-Binaphane catalytic system (5 mol % catalyst).[5]
Scheme 1.

[a] 1a (0.1 mmol) and a Rh/L ratio of 1/1.1 in 1.0 mL solvent. [b] ee and conversion was determined by chiral GC of the corresponding acetamides.
Under the optimized conditions, a variety of N-H imines were tested (Scheme 2). Most substrates with meta and para substitutions on the phenyl ring afforded high yields and enantioselectivities (92-97% yield and 90-95% ee). However, the chloro group and methoxy group resulted in an obvious decrease of the yields. The ortho-methoxy group on the phenyl ring resulted in 34% yield and 84% ee (2i). 1-naphthyl and 2-naphthyl amines were obtained with 95% ee and 92% ee respectively (2j and 2k). Changing the R2 group had a significant effect on the outcome. When R2 was ethyl group, both lower conversion and enantioselectivity were observed (2l). As the R2 was changed to bulkier butyl group, further loss of conversion and enantioselectivity was observed (2m). The catalytic system displayed low activity and enantioselectivity for dialkyl ketimine (2n) and diaryl ketimine.[15] Otherwise, 2-methyl quinolinium chloride was hydrogenated in 97% yield with 82% ee at 25 °C under 5 atm H2 with 1 mol % catalyst (2o).
Scheme 2.
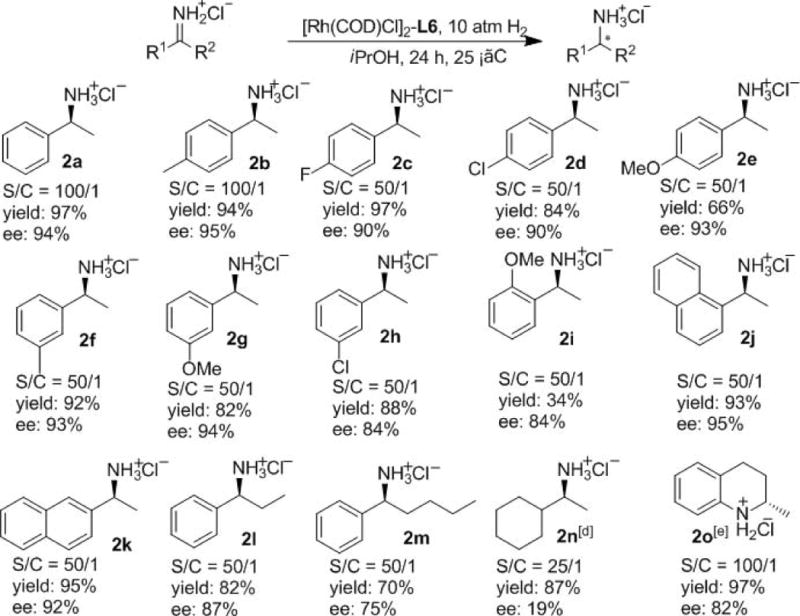
[a] Reaction conditions: 1 (0.2 mmol) and a Rh/L ratio of 1/1.1 in 2.0 mL solvent. [b] Isolated yield. [c] ee was determined by chiral GC of the corresponding acetamides. [d] 45 °C, 50 atm H2. [e] 25 °C, 5 atm H2; ee was determined by chiral GC of the free amines.
To obtain insight into this catalytic system, a series of chiral ligands were prepared and control experiments were undertaken (Table 1). Consistent with our recent report,[13] the Rh-bisphosphine complex without (thio)urea motif (L1) showed very low activity and enantioselectivity (Table 1, entry 1). Urea L2 provided 22% conversion and 66% ee in sharp contrast with the thiourea L6 (Table 1, entry 2 vs. 6). The CF3 group on the 3,5-bis(trifluoromethyl)phenyl moiety remained important in the catalytic system (Table 1, entries 3-5).[16] The N-methylation of L6 led to a dramatic decrease of the conversion and enantioselectivity (Table 1, entry 7), which suggested that the NH was involved in the activation of iminium salts and the stereoselectivity of hydrogenation. Furthermore, the low conversion and enantioselectivity obtained with monodentate phosphorus ligands implied that the bisphosphine moiety was essential (Table 1, entry 9). Importantly, neither the combination of the chiral phosphine with the thiourea, nor the combination of the chiral thiourea with the simple phosphine improved this reaction (Table 1, entry 1 vs. 11, entry 8 vs. 10), which pointed to the importance of the covalent linker for high activity and enantioselectivity.
Table 1.
Ligands study and control experiments. [a]

| |||
|---|---|---|---|
| Entry | Ligand | Conv.[b] [%] | ee[b] [%] |
| 1 | L1 | 2 | 55 |
| 2 | L2 | 22 | 66 |
| 3 | L3 | 6 | 11 |
| 4 | L4 | 72 | 87 |
| 5 | L5 | 76 | 90 |
| 6 | L6 | 99 | 94 |
| 7 | L7 | 26 | 38 |
| 8 | L8 | 2 | 11 |
| 9 | L9 | 9 | 84 |
| 10[c] | L8 | 5 | 8 |
| 11[d] | L1 | 9 | 57 |
Reaction conditions: 1a (0.1 mmol) and a Rh/L/1a ratio of 1/1.1/100 in 1.0 mL solvent.
Determined by chiral GC of the corresponding acetamides.
Rh/L/1a/Ph3P = 1/1.1/100/2.2.
Rh/L/1a/thiourea = 1/1.1/100/1.1.
Different counterions and additives were also investigated (Table 2). When the chloride counterion in 1a was replaced with trifluoromethanesulfonate, only 20% conversion and 53% ee was observed (Table 2, entry 1). Interestingly, the addition of chloride counterion from LiCl and tetrabutylammonium chloride (TBAC) increased the conversions and enantioselectivities (Table 2, entries 2 and 3). However, the addition of bromide from tetrabutylammonium bromide (TBAB) and iodide counterions from tetrabutylammonium iodide (TBAI) decreased the conversions and enantioselectivities (Table 2, entries 4-6). This phenomenon implied that the chloride ion played a crucial role in the catalytic system.
Table 2.
Substrates study and control experiments. [a]
| ||||
|---|---|---|---|---|
| Entry | 1 | Additive | Conv.[b][%] | ee[b][%] |
| 1 | 1p | – | 20 | 53 |
| 2 | 1p | TBAC | 86 | 94 |
| 3 | 1p | LiCl | 71 | 93 |
| 4 | 1a | – | 99 | 94 |
| 5 | 1a | TBAB | 77 | 90 |
| 6 | 1a | TBAI | 32 | 89 |
1a (0.1 mmol) and a Rh/L/1a/Additive ratio of 1/1.1/100/100 in 1.0 mL solvent.
Determined by chiral GC of the corresponding acetamides.
Further information about the reaction was provided by 1H NMR studies of mixtures generated from ligands and TBAC (Figure 3). The addition of varying amounts of TBAC to L6 in CDCl3 resulted in downfield shifts of the NH proton signals. However, no change was observed for the NH proton signal of L7. Analogous experiments employing a series of different ligands gave similar results.[14] This finding was consistent with a dual hydrogen-bonding interaction between the catalyst’s thiourea and chloride ions.[10c,10f,12b] This observation, coupled with the fact that optimal yields and ee values involve chloride ions, led us to propose that catalytic chloride-bound intermediates are involved in the mechanism via the dual hydrogen-bonding interaction (Figure 2).
Figure 3.

a) 1H NMR spectra of L6 with TBAC; b) 1H NMR spectra of L7 with TBAC. The NH were marked.
To gain further insight into this transformation, the asymmetric hydrogenation was performed under D2. 1H NMR analysis of the crude product showed that 2a had incorporated deuterium in the α-position (Scheme 3), suggesting that the hydrogenation proceeded through a pathway consistent with the imine.[3c,5a,14]
Scheme 3.

Deuteration experiment.
With the ultimate goal being the fast screening of reaction conditions in the future, we initiated collaborative work with the Anslyn group and verified their method to measure ee values of chiral amines based on circular dichroism (CD) spectroscopy.[17] Although the accuracy is not ideal (an average absolute error of 9%),[14] it is much faster and being transitioned to aid in our research.
In conclusion, we report the first Rh/bisphosphine-thiourea catalyzed asymmetric hydrogenation of unprotected N-H imines. The chiral amines were obtained in high yields and enantioselectivities. Based on the control experiments and 1H NMR studies, we propose that the anion binding interaction between thiourea and chloride counterion plays an important role in the catalytic system. Deuteration experiments suggested that the hydrogenation proceeded through a pathway consistent with the imine. Further research on the mechanism of catalysis and other applications is currently under way, as is the use of the HTS methodology.
Supplementary Material
Footnotes
This work was supported by the National Science Foundation (NSF CHE 0956784), the China Scholarship Council, the National S&T Major Project of China (2012ZX09504001-003) and the National Institutes of Health (NIH R01GM077437).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
Contributor Information
Qingyang Zhao, Key Laboratory of Preclinical Study for New Drugs of Gansu Province, Lanzhou University, Lanzhou 730000, China; Department of Chemistry &Chemical Biology, Department of Medicinal Chemistry, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, New Jersey 08854, USA.
Jialin Wen, Department of Chemistry &Chemical Biology, Department of Medicinal Chemistry, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, New Jersey 08854, USA.
Renchang Tan, Department of Chemistry &Chemical Biology, Department of Medicinal Chemistry, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, New Jersey 08854, USA.
Kexuan Huang, Department of Chemistry &Chemical Biology, Department of Medicinal Chemistry, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, New Jersey 08854, USA.
Pedro Metola, Department of Chemistry, The University of Texas at Austin 1 University Station A5300, Austin, TX 78712-0165.
Rui Wang, Key Laboratory of Preclinical Study for New Drugs of Gansu Province, Lanzhou University, Lanzhou 730000, China.
Eric V. Anslyn, Department of Chemistry, The University of Texas at Austin 1 University Station A5300, Austin, TX 78712-0165
Xumu Zhang, Department of Chemistry &Chemical Biology, Department of Medicinal Chemistry, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, New Jersey 08854, USA.
References
- 1.For reviews:; a) Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley-VCH; Weinheim: 2010. [Google Scholar]; b) Zhang W, Zhang X. In: Comprehensive Chirality. Carreira EM, Yamamoto H, editors. Elsevier; Amsterdam: 2012. pp. 301–317. [Google Scholar]; c) Xie JH, Zhu SF, Zhou QL. Chem Rev. 2010;111:1713–1760. doi: 10.1021/cr100218m. [DOI] [PubMed] [Google Scholar]; d) Nugent TC, El-Shazly M. Adv Synth Catal. 2010;352:753–819. [Google Scholar]; e) Yu Z, Jin W, Jiang Q. Angew Chem. 2012;124:6164. doi: 10.1002/anie.201200963. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:6060–6072. doi: 10.1002/anie.201200963. [DOI] [PubMed] [Google Scholar]
- 2.For examples of asymmetric reductive amination:; a) Blaser HU, Buser HP, Jalett HP, Pugin B, Spindler F. Synlett. 1999:867–868. [Google Scholar]; b) Chi Y, Zhou YG, Zhang X. J Org Chem. 2003;68:4120–4122. doi: 10.1021/jo026856z. [DOI] [PubMed] [Google Scholar]; c) Kadyrov R, Riermeier TH. Angew Chem. 2003;115:5630. doi: 10.1002/anie.200352503. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:5472–5474. doi: 10.1002/anie.200352503. [DOI] [PubMed] [Google Scholar]; d) Williams GD, Pike RA, Wade CE, Wills M. Org Lett. 2003;5:4227–4230. doi: 10.1021/ol035746r. [DOI] [PubMed] [Google Scholar]; e) Steinhuebel D, Sun Y, Matsumura K, Sayo N, Saito T. J Am Chem Soc. 2009;131:11316–11317. doi: 10.1021/ja905143m. [DOI] [PubMed] [Google Scholar]; f) Li C, Villa-Marcos B, Xiao J. J Am Chem Soc. 2009;131:6967–6969. doi: 10.1021/ja9021683. [DOI] [PubMed] [Google Scholar]; g) Mattei P, Moine Gr, Püntener K, Schmid R. Org Process Res Dev. 2011;15:353–359. [Google Scholar]; h) Strotman NA, Baxter CA, Brands KMJ, Cleator E, Krska SW, Reamer RA, Wallace DJ, Wright TJ. J Am Chem Soc. 2011;133:8362–837. doi: 10.1021/ja202358f. [DOI] [PubMed] [Google Scholar]; i) Chang M, Liu S, Huang K, Zhang X. Org Lett. 2013;15:4354–4357. doi: 10.1021/ol401851c. [DOI] [PubMed] [Google Scholar]; j) Talwar D, Salguero NP, Robertson CM, Xiao J. Chem Eur J. 2014;20:245–252. doi: 10.1002/chem.201303541. [DOI] [PubMed] [Google Scholar]
- 3.For examples:; a) Hsiao Y, Rivera NR, Rosner T, Krska SW, Njolito E, Wang F, Sun Y, Armstrong JD, Grabowski EJJ, Tillyer RD, Spindler F, Malan C. J Am Chem Soc. 2004;126:9918–9919. doi: 10.1021/ja047901i. [DOI] [PubMed] [Google Scholar]; b) Clausen AM, Dziadul B, Cappuccio KL, Kaba M, Starbuck C, Hsiao Y, Dowling TM. Org Process Res Dev. 2006;10:723–726. [Google Scholar]; c) Hansen KB, Hsiao Y, Xu F, Rivera N, Clausen A, Kubryk M, Krska S, Rosner T, Simmons B, Balsells J, Ikemoto N, Sun Y, Spindler F, Malan C, Grabowski EJJ, Armstrong JD. J Am Chem Soc. 2009;131:8798–8804. doi: 10.1021/ja902462q. [DOI] [PubMed] [Google Scholar]; d) Hou G, Li W, Ma M, Zhang X, Zhang X. J Am Chem Soc. 2010;132:12844–12846. doi: 10.1021/ja105674y. [DOI] [PubMed] [Google Scholar]; e) Birch M, Challenger S, Crochard JP, Fradet D, Jackman H, Luan A, Madigan E, Mathew JS, McDowall N, Meldrum K, Gordon CM, Peach P, Yeo S. Org Process Res Dev. 2011;15:1358–1364. [Google Scholar]; f) Matsumura K, Zhang X, Hori K, Murayama T, Ohmiya T, Shimizu H, Saito T, Sayo N. Org Process Res Dev. 2011;15:1130–1137. [Google Scholar]
- 4.For examples of asymmetric transfer hydrogenation:; a) Gosselin F, O’Shea PD, Roy S, Reamer RA, Chen C-y, Volante RP. Org Lett. 2004;7:355–358. doi: 10.1021/ol047431x. [DOI] [PubMed] [Google Scholar]; b) Nguyen TB, Bousserouel H, Wang Q, Guéritte Fo. Org Lett. 2010;12:4705–4707. doi: 10.1021/ol102043x. [DOI] [PubMed] [Google Scholar]; c) Nguyen TB, Wang Q, Guéritte F. Chem Eur J. 2011;17:9576–9580. doi: 10.1002/chem.201101694. [DOI] [PubMed] [Google Scholar]
- 5.a) Hou G, Gosselin F, Li W, McWilliams JC, Sun Y, Weisel M, O’Shea PD, Chen C-y, Davies IW, Zhang X. J Am Chem Soc. 2009;131:9882–9883. doi: 10.1021/ja903319r. [DOI] [PubMed] [Google Scholar]; b) Hou G, Tao R, Sun Y, Zhang X, Gosselin F. J Am Chem Soc. 2010;132:2124–2125. doi: 10.1021/ja909583s. [DOI] [PubMed] [Google Scholar]
- 6.Pyrrolidinium salts:; a) Magee MP, Norton JR. J Am Chem Soc. 2001;123:1778–1779. doi: 10.1021/ja003630+. [DOI] [PubMed] [Google Scholar]; b) Guan H, Iimura M, Magee MP, Norton JR, Janak KE. Organometallics. 2003;22:4084–4089. [Google Scholar]; c) Guan H, Iimura M, Magee MP, Norton JR, Zhu G. J Am Chem Soc. 2005;127:7805–7814. doi: 10.1021/ja0506861. [DOI] [PubMed] [Google Scholar]; Quinolinium salts:; d) Tadaoka H, Cartigny D, Nagano T, Gosavi T, Ayad T, Genêt JP, Ohshima T, Ratovelomanana-Vidal V, Mashima K. Chem Eur J. 2009;15:9990–9994. doi: 10.1002/chem.200901477. [DOI] [PubMed] [Google Scholar]; Isoquinolinium salts:; e) Iimuro A, Yamaji K, Kandula S, Nagano T, Kita Y, Mashima K. Angew Chem. 2013;125:2100. doi: 10.1002/anie.201207748. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:2046–2050. doi: 10.1002/anie.201207748. [DOI] [PubMed] [Google Scholar]
- 7.a) Zhou S, Fleischer S, Junge K, Beller M. Angew Chem. 2011;123:5226–5230. doi: 10.1002/anie.201100878. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:5120–5124. doi: 10.1002/anie.201100878. [DOI] [PubMed] [Google Scholar]; b) Tang W, Johnston S, Iggo JA, Berry NG, Phelan M, Lian L, Bacsa J, Xiao J. Angew Chem. 2013;125:1712–1716. doi: 10.1002/anie.201208774. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:1668–1672. doi: 10.1002/anie.201208774. [DOI] [PubMed] [Google Scholar]
- 8.For reviews:; a) Pihko PM. Hydrogen Bonding in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2009. [Google Scholar]; b) Zhang Z, Schreiner PR. Chem Soc Rev. 2009;38:1187–1198. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]; c) Knowles RR, Jacobsen EN. Proc Natl Acad Sci U S A. 2010;107:20678–20685. doi: 10.1073/pnas.1006402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For reviews:; a) Phipps RJ, Hamilton GL, Toste FD. Nat Chem. 2012;4:603–614. doi: 10.1038/nchem.1405. [DOI] [PubMed] [Google Scholar]; b) Brak K, Jacobsen EN. Angew Chem. 2013;125:558–588. doi: 10.1002/anie.201205449. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:534–561. doi: 10.1002/anie.201205449. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mahlau M, List B. Angew Chem. 2013;125:540–556. doi: 10.1002/anie.201205343. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:518–533. doi: 10.1002/anie.201205343. [DOI] [PubMed] [Google Scholar]
- 10.a) Taylor MS, Jacobsen EN. J Am Chem Soc. 2004;126:10558–10559. doi: 10.1021/ja046259p. [DOI] [PubMed] [Google Scholar]; b) Taylor MS, Tokunaga N, Jacobsen EN. Angew Chem. 2005;117:6858. doi: 10.1002/anie.200502277. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:6700–6704. doi: 10.1002/anie.200502277. [DOI] [PubMed] [Google Scholar]; c) Raheem IT, Thiara PS, Peterson EA, Jacobsen EN. J Am Chem Soc. 2007;129:13404–13405. doi: 10.1021/ja076179w. [DOI] [PubMed] [Google Scholar]; d) Zuend SJ, Jacobsen EN. J Am Chem Soc. 2009;131:15358–15374. doi: 10.1021/ja9058958. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Knowles RR, Lin S, Jacobsen EN. J Am Chem Soc. 2010;132:5030–5032. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN. Science. 2010;327:986–990. doi: 10.1126/science.1182826. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Birrell JA, Desrosiers JN, Jacobsen EN. J Am Chem Soc. 2011;133:13872–13875. doi: 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Burns NZ, Witten MR, Jacobsen EN. J Am Chem Soc. 2011;133:14578–14581. doi: 10.1021/ja206997e. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Lin S, Jacobsen EN. Nat Chem. 2012;4:817–824. doi: 10.1038/nchem.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Lalonde MP, McGowan MA, Rajapaksa NS, Jacobsen EN. J Am Chem Soc. 2013;135:1891–1894. doi: 10.1021/ja310718f. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Bergonzini G, Schindler CS, Wallentin CJ, Jacobsen EN, Stephenson CR. Chem Sci. 2014;5:112–116. doi: 10.1039/C3SC52265B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) De CK, Klauber EG, Seidel D. J Am Chem Soc. 2009;131:17060–17061. doi: 10.1021/ja9079435. [DOI] [PubMed] [Google Scholar]; b) Klauber EG, De CK, Shah TK, Seidel D. J Am Chem Soc. 2010;132:13624–13626. doi: 10.1021/ja105337h. [DOI] [PubMed] [Google Scholar]; c) De CK, Seidel D. J Am Chem Soc. 2011;133:14538–14541. doi: 10.1021/ja2060462. [DOI] [PubMed] [Google Scholar]; d) Min C, Mittal N, Sun DX, Seidel D. Angew Chem. 2013;125:14334–14338. doi: 10.1002/anie.201308196. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:14084–14088. doi: 10.1002/anie.201308196. [DOI] [PubMed] [Google Scholar]
- 12.a) Lee YS, Alam MM, Keri RS. Chem Asian J. 2013;8:2906–2919. doi: 10.1002/asia.201300814. [DOI] [PubMed] [Google Scholar]; b) Schafer AG, Wieting JM, Fisher TJ, Mattson AE. Angew Chem. 2013;125:11531–11534. doi: 10.1002/anie.201305496. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:11321–11324. doi: 10.1002/anie.201305496. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Li S, Huang K, Wang R, Zhang X. Org Lett. 2013;15:4014–4017. doi: 10.1021/ol401816y. [DOI] [PubMed] [Google Scholar]
- 14.For more details, see supplementary information.
- 15.Phenyl(p-tolyl)methaniminium chloride and (2-Chlorophenyl)(phenyl)methaniminium chloride were used while no desired product was obtained under the optimized conditions.
- 16.Lippert KM, Hof K, Gerbig D, Ley D, Hausmann H, Guenther S, Schreiner PR. Eur J Org Chem. 2012;2012:5919–5927. [Google Scholar]
- 17.Dragna JM, Pescitelli G, Tran L, Lynch VM, Anslyn EV, Di Bari L. J Am Chem Soc. 2012;134:4398–4407. doi: 10.1021/ja211768v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


