Abstract
Precision medicine is poised to have an impact on patients, health care delivery systems and research participants in ways that were only imagined 15 years ago when the human genome was first sequenced. While discovery using genome-based technologies has accelerated, these have only begun to be adopted into clinical medicine. Here we define precision medicine and the stakeholder ecosystem required to enable its integration into research and health care. We explore the intersection of data science, analytics and precision medicine in creating a learning health system that carries out research in the context of clinical care and at the same time optimizes the tools and information used to delivery improved patient outcomes. We provide examples of real world impact, and conclude with a policy and economic agenda that will be necessary for the adoption of this new paradigm of health care both in the United States and globally.
Clay Christiansen has described precision medicine as disruptive in its ability to drive down health care costs without compromising quality or outcomes (1). While precision medicine as a health care strategy continues to evolve with early areas of impact on “the triple aim,” significant challenges stand in the way of its fully disruptive potential. In this review, we explore the current state of precision medicine, its growing ecosystem, and the opportunities that lie ahead for health care and research.
Precision Medicine – A National Research Agenda
In 2015, President Barack Obama announced that the United States would embark on a government funded precision medicine initiative that will enroll over 1 million people. In what is now called “All of US” (2), participants are expected to share the data generated or captured over more than 10 years from sequencing, electronic medical records, personal reported information, and digital health technologies. These data will be the subject of analyses to drive both a completely novel scientific agenda for our understanding of disease biology and pathogenesis, as well as an agenda for data- and precision- driven health care for individuals and for populations. Both would contribute to a novel paradigm of healthcare expected to impact health and decisions across the lifespan [see below] and to enhance the already emergent examples of precision health care being used, from reproductive counseling and prenatal testing at conception to healthy aging and molecular autopsies at death.
Influencing the launch of the United States Precision Medicine Initiative is the dramatic decline in cost and increase in throughput of DNA sequencing (3), the near ubiquitous adoption of electronic medical records (EMRs) across the United states (4), and growth of digital health as a source of continuous and rich personal data (5). Other genome-based technology platforms [for example, assays for RNA, proteins, metabolites] are also increasingly being used to classify disease states (as diagnostic tests) and to predict future clinical outcomes (as prognostic tests). Together, these approaches form the basis for 1) a new molecular taxonomy of disease, 2) provide more precise ways to screen for and to detect disease at its earliest molecular manifestations, often pre-clinically, and 3) allow the selection of certain drugs guided by a patient’s underlying genetic makeup. Given that a disease’s evolution from baseline risk to clinical signs and symptoms often occurs over many years, it is likely, in the future, periodic molecular and digital profiling will shift health care strategies from acute intervention and disease management to a focus on assessing health and proactive management of disease risks and prevention.
What Is Precision Medicine?
The National Research Council’s Toward Precision Medicine (6) adopted the definition of precision medicine from the President’s Council of Advisors on Science and Technology in 2008 as: “The tailoring of medical treatment to the individual characteristics of each patient…to classify individuals into subpopulations that differ in their susceptibility to a particular disease or their response to a specific treatment. Preventative or therapeutic interventions can then be concentrated on those who will benefit, sparing expense and side effects for those who will not”. As the definition suggests, the power of precision medicine lies in its ability to guide health care decisions toward the most effective treatment for a given patient, and thus, improve care quality, while reducing the need for unnecessary diagnostic testing and therapies.
The conceptual distinction between personalized and precision medicine
Personalized medicine refers to an approach to patients that considers their genetic make-up but with attention to their preferences, beliefs, attitudes, knowledge and social context, whereas precision medicine describes a model for health care delivery that relies heavily on data, analytics, and information. This model goes beyond genomics and has vast implications for our nation’s research agenda and for its implementation and adoption into health care. Precision medicine – and the ecosystem that supports it -- must embrace patient centered-ness and engagement, digital health, genomics and other molecular technologies, data sharing and data science to be successful.
Components of the Precision Medicine Ecosystem
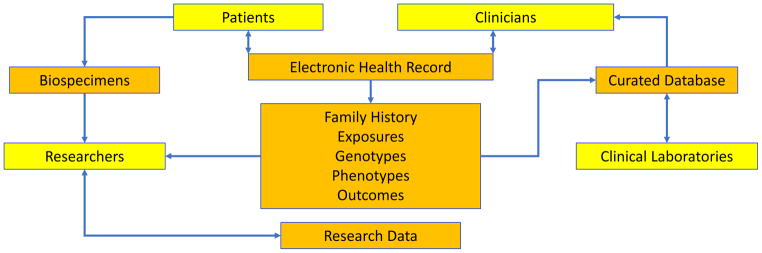
A precision medicine ecosystem ideally links patients, providers, clinical laboratories and researchers (see figure 1). With the advent of EMRs and robust IT systems supporting both research and health care delivery, patients (and research participants) who agree to provide biospecimens and share their clinical and research data are at the epicenter of contributions to the research enterprise. Researchers generate new findings from the data derived from samples linked to digital phenotypes, family history and environmental exposures all captured as part of clinical care. Clinicians utilize a growing knowledge base curated from clinical laboratories (8–10). This assembly of data from a variety of sources sets the stage for a powerful precision medicine ecosystem which, when coupled to others, leads to a dissemination of knowledge that enables other systems to benefit. The extended precision medicine ecosystem includes government as sponsors of precision medicine research and regulators of precision medicine products, industry as partners in development and commercialization of precision medicine products, professional societies as enablers of the training of the next generation of researchers, providers, and policy analysts, and payers who evaluate the appropriateness of precision medicine interventions and the financing that support their use in health care.
Exhibit 1.
The Precision Medicine Ecosystem
Source: Adapted from reference 7
The Learning health system and precision medicine
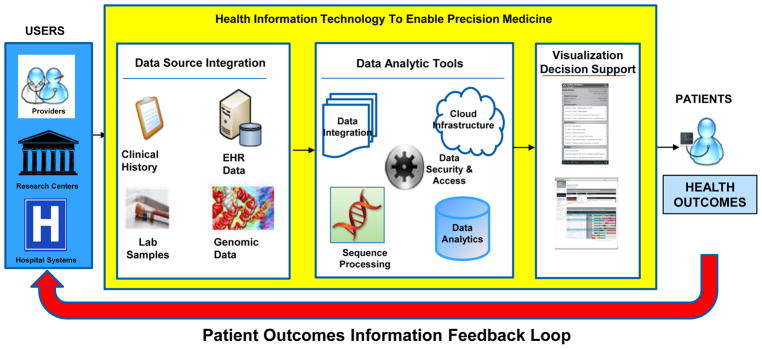
The inclusion of genomic data in a knowledge-generating health care system infrastructure is a way to harness the full potential of that information to optimize patient care (11, see figure 2). In such a system, clinical practice and research inform each other with the goal of improving the efficiency and effectiveness of disease prevention, diagnosis, and treatment (12).
Exhibit 2.
The Genomics Enabled Learning Health System
Source: Adapted from reference 11
All stakeholders in the precision medicine ecosystem are involved in shaping that system, and deciding how to use the data. In particular, providers will need information at the point of decision so that they are able to use it in the context of their clinical workflow and patients will need to define preferences about the use and sharing of their genomic and other information with researchers and others beyond the delivery system in which they receive care. Researchers will need to identify and adopt best practices for research using EMR-linked genomic information. Health systems will need to offer providers tools and systems that will enable them to make more informed decisions. The health information technology community will need to design secure and interoperable genomics-enabled systems for actionable use in both health care and community settings. Policy makers will need to address the return of results, privacy, confidentiality, and education while developing regulations and economic incentives that can align all stakeholders toward the same outcomes. Patients stand to benefit with optimized health outcomes in such a genomics and data enabled learning precision health system.
Data science, digital health and precision medicine
Three platforms are converging in health care: digital health, data science, and precision medicine. Large-scale collection of biological, radiological, and translational bioinformatics datasets are being formed from digital-sensing devices and multi-omic information with both research and clinical-decision support applications. Making full use of these multidimensional data streams necessitates the development of standardized methods of data aggregation and analysis and cross-disciplinary translation of emerging computational techniques, such as machine learning, natural-language processing, and artificial intelligence. The application of these new analytical methods to health care may enable us to define the dynamic patterns of health and disease and to create more efficient and sustainable models of care that are driven by data and technology.
Data sharing is a high-payoff strategy
Integrating high-quality data into a health care system must be a priority for ensuring that the best possible information is available for patient care and research (12). In fact, several ongoing efforts within government and the private sector are aimed at establishing data repositories for large-scale genomic information (2, 13). However, they are currently not readily accessible for use in the EMR. Data that are standardized, comparable, and consistent, facilitate the reuse of those data for discovery in multiple contexts beyond their original use. For example, the EMR coupled with gene sequencing information has been a powerful discovery tool for identifying genetic variants associated with disease and for understanding individual response to therapeutics (14). The economics of data sharing might be obvious: if a health system shares 100 genomes and patient records with 10 others who agree to do the same, each gains 900 for the cost of 100. The power of genomics enabled research and health care is proportional to the amount of data that can be accessed and analyzed.
Health care systems supporting complete learning cycles that encompass both the analysis of data to produce results and the use of those results to develop changes in clinical practices are systems that will allow for optimal learning. Summed across all individuals in such systems, genomic data could inform strategies to improve population health and contribute to care management. Just-in-time information, guidelines for clinical action, and more information on the clinical utility of genetic testing would help physicians make effective use of genomic information and integrate it in their practices similar to other medical test information.
Precision Medicine – Where Are We Today?
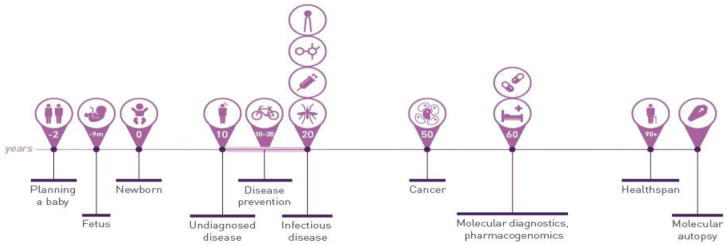
A number of applications of precision medicine contribute to healthcare at many points in an individual’s lifespan (Figure 3). Genetic screening can be used prior to conception to predict the risk of passing on genetic disorders to offspring (15, 16). At 8–12 weeks of pregnancy, an expectant mother can have genetic testing to assess chromosomal abnormalities of the fetus, and even have whole genome sequencing of the fetus performed (17). At birth, sequencing can be used to rapidly diagnose many critical conditions for which there may be actionable results leading to reduced morbidity and mortality (18). Later in life, these types of approaches can be applied to diagnose and treat a variety of diseases, most notably in more precisely diagnosing cancer and guiding therapy for chronic diseases (19–21)
Exhibit 3.
Timeline Of Precision Medicine Applications In Across The Lifespan
Source: From reference 22, with permission
Over the past decade a growing number of genomic markers of efficacy, adverse events and dosing of therapeutics have been discovered and recommended for clinical use (see Table 1), but their uptake into clinical practice has been variable, even when their actionability has been supported by evidence. In some cases, such as with the HLA-B*5701 genotype for the HIV drug abacavir, and HLA-B*1502 for the anti-seizure drug carbamazepine, carriers of these genotypes should avoid the drug entirely to eliminate a serious adverse event. In other cases, such as variants in TPMT for mercaptopurine or in CYP2C9/VKORC1 for warfarin, adjusting the dose of drug based on genotype can help to avoid toxicity and improve efficacy. However, actionability is not enough to ensure uptake of pharmacogenomics testing as exemplified with the anti-platelet drug clopidogrel, where despite having an FDA ‘black box warning’ for efficacy in individuals carrying CYP2C19 genetic variants, there is often no clear consensus among physicians on its use. Genetic markers that predict reduced therapeutic efficacy may face a high hurdle for established drugs, unless there is strong evidence of the test’s clinical validity and utility (see below)
TABLE 1.
Examples of Precision Medicine Testing in Use Today
| Clinical Question: | Susceptibility | Screening | Diagnosis | Prognosis | Drug Response | Monitoring | |
|---|---|---|---|---|---|---|---|
| Cancer | Indication: | Breast Colon Sarcomas |
Cervical | Cancer of unknown primary Ovarian Cancer |
Breast Cancer | Imatinib (CML) Crizotinib (NSCL) |
Tumor recurrence or progression |
| Test: |
BRCA1, BRCA2 HNPCC TP53, PTEN |
HPV genotypes | Cancer Origin™ OVA1® |
Oncotype DX (21-gene assay) MammaPrint (70-gene assay) HER2/neu, ER, PR |
BCR-ABL EML4-ALK |
CTCs | |
| Cardiovascular Disease | Indication: | CAD | Familial Hypercholesterolemia |
1. Long QT Syndrome 2. ACS 3. CAD |
ACS | 1. Clopidogrel 2. Statins 3. Warfarin |
Transplant rejection |
| Test: | 9p21 | APOB, LDLR, LDLRAP1, PCSK9 |
1. KVLQT1, HERG, SCN5A, ANKB, Mink, MiRP1, KCNJ2, CACNA1C, CAV3, SCN4- beta 2. TnI, BNP, CRP 3. CorusCAD® |
cTnI |
1. CYP2C19 2. SLCO1B1 3. CYP2C9 VKORC1 |
AlloMap® |
ACS = acute coronary syndromes; BNP = brain natriuretic peptide; CAD = coronary artery disease; CRP = C-reactive protein; CTCs = circulating tumor cells; ER = estrogen receptor; HPV = human papillomavirus; LQTS = long QT syndrome; PR = progesterone receptor; TnI = troponin I.
Translational Precision Medicine Research: New Initiatives
The translation of precision medicine into clinical care and health policy has lagged behind the pace of basic science discoveries. For example, Roberts et al concluded from a literature review that “although genomic discovery provides the potential for population health benefit, the current knowledge base around implementation to turn this promise into a reality is severely limited” (23). Another example is our findings on comparative effectiveness research studies of precision medicine, based on a structured literature review and expert assessment of gaps (24). We concluded that there is a limited body of high quality evidence about the effect of using genomic tests on health outcomes and that there are many evidence gaps for comparative effectiveness research to address. Similarly, it is our impression that many published studies to date have been case studies of single institutions, with few studies providing empirical evidence on a broad scale.
Although there is a relative lack of research to date on the implementation of precision medicine, new initiatives are starting to address the need for evidence generation. We describe below several ongoing initiatives that provide new evidence on translation of precision medicine into clinical care and health policy.
Clinical Sequencing Evidence-Generating Research (CSER2)
CSER2 builds upon the initial CSER Consortium, initiated in 2010 and funded by the National Human Genome Research Institute (NHGRI) and the National Cancer Institute (NCI). The new grants totaling $18.9 million will support the development of methods needed to integrate genome sequencing into the practice of medicine, improve the discovery and interpretation of genomic variants, and investigate the impact of genome sequencing on healthcare outcomes. The goal is to accelerate the use of genome sequencing in clinical care by generating innovative approaches and best practices to ensure that the effectiveness of genomic medicine can be applied to all individuals and groups, including diverse and underserved populations, and in healthcare settings that extend beyond academic medical centers. CSER2 includes six clinical sites and one Coordinating Center who will work together to: 1) define, generate and analyze evidence regarding the clinical utility of genome sequencing; 2) research the critical interactions among patients, family members, health practitioners, and clinical laboratories that influence implementation of clinical genome sequencing; and 3) identify and address real-world barriers to integrating genomic, clinical, and healthcare utilization data within a healthcare system to build a shared evidence base for clinical decision-making.
The initial CSER grants made substantial progress in building the evidence base for clinical sequencing but much remains to be done (25). The new CSER2 grants move beyond exploratory studies (to test whether genome sequencing could actually be implemented into clinical care) to studies that directly implement such testing. The CSER2 grants focus particularly on recruiting diverse racial and ethnic groups and historically underrepresented groups in genomics research (26,27). There is also an emphasis on studying clinical healthcare settings outside of academic medical centers. Other areas of emphasis include greater integration of stakeholder perspectives such as payers.
Implementing GeNomics In practice (IGNITE)
To address the challenges to widespread clinical implementation of genomic medicine, a prerequisite for developing evidence of its real-world utility, the NHGRI and NCI-funded IGNITE Network (28), comprised of six projects and a coordinating center, was established in 2013 with nearly $30M in funding to support the development, investigation and dissemination of genomic medicine practice models that seamlessly integrate genomic data into the electronic health record and that deploy tools for point of care decision making. IGNITE projects vary in scope and design, including exploring genetic markers for disease risk prediction and prevention, developing tools for using family history data, incorporating pharmacogenomic data into clinical care, refining disease diagnosis using sequence based mutation discovery, and creating novel educational approaches. A second round of IGNITE projects aimed at evidence generation and targeting underserved populations and minorities will be launched in 2018 (29).
The IGNITE Network’s innovative series of pilot demonstration projects aim to enhance translation of validated actionable genomic information into clinical settings and develop and use measures of outcome in response to genome-based clinical interventions. The network has defined and overcome a series of challenges to genomic medicine implementation: 1) Implementation science requires both a transdisciplinary team and an implementation framework. Thus to enable genomic medicine implementation teams with the right expertise need to be assembled. Implementation frameworks should be established that guide intervention deployment, assessment, and analyses. IGNITE adopted and adapted the consolidated framework for implementation research (30) in creating a network focused on developing lessons for the larger community. 2) It is important to optimize the setting and personnel to carry out the implementation research in the clinic. Pre-implementation research is often overlooked as a critical element to ensure that researchers understand and take into account the priorities, concerns and educational needs of these key stakeholders before implementation begins. 3) Genomic medicine research is information technology (IT) intensive. Broad implementation of genomic medicine requires that IT solutions work with an EMR to either incorporate genomic information into it extract phenotypic data from it. Thus IT leadership at the implementing institution needs to prioritize its incorporation.
Global Efforts to Develop Precision Medicine as a Science and Health Care Strategy
Worldwide, many efforts and initiatives are underway to create national implementation strategies for genomic medicine (Table 2); however, many of these efforts are being carried out in the absence of external collaboration, risking the duplication of efforts and slowing the pace of discovery and translation (31). Globally, key barriers exist to implementing and integrating precision medicine technologies into health care practice include the absence of supporting IT infrastructure, lack of data standards and interoperability, insufficient decision support technology, and insufficient funding for translational health research. Policies to support progress in these areas will be critical to the adoption and integration of PM technologies into health care worldwide.
Table 2.
Selected Global Precision Medicine-Implementation Programs
| Country (name of project, web site) | Goals of programs |
|---|---|
| Australian Genomics Health Alliance https://www.australiangenomics.org.au/ |
Develop national framework for translating –omics discoveries into clinical research and practice, including advice on return of results from genomics research and clinical testing |
| Belgium (Belgian Medical Genomics Initiative, BeMGI) http://www.bemgi.be/ |
Predict clinical outcome from genomic information and fulfil a pilot role towards concerted integration of genomic information in clinical care in Belgium. |
| Canada (Genome Canada) https://www.genomecanada.ca/ |
Large-scale research projects focused on the application of genomics in the area of precision health. Precision health can be seen as a more evidence-based approach to decision making with regards to health care and public health. |
| Estonia (Estonian Program for Personal Medicine) https://en.wikipedia.org/wiki/Estonian_Genome_Project |
Sequence 5K individuals, develop Estonian genotyping array, pilot of 50K Estonian Biobank members, offer to all 35–65 yo (~500K) and link to EMR |
| France (Genomic Medicine 2025) https://aviesan.fr/fr/aviesan/accueil/toute-l-actualite/plan-francemedecine-genomique-2025 |
Deploy the instruments of the genomic care pathway and to allow access to genomic medicine for all concerned (patients and their families as indicated) in the territory |
| Israel (Bench To Beside Project) https://www.weizmann.ac.il/WeizmannCompass/sections/features/the-bench-to-bedside-project |
Weizmann Institute and Clalit project aiming to sequence 100,000 Israeli genomes from selected patients |
| Japan (Implementation of Genomic Medicine Project, IGMP) http://www.src.riken.jp/english/project/person/ |
Use genomics for optimized diagnosis, treatment and prevention |
| Korea (Genome Technology to Business Translation Program) http://www.cdc.go.kr/NIH/eng/main.jsp |
Use genomics to develop early diagnosis and treatment approaches for personalized and preventive medicine |
| Luxembourg (Centre for Systems Biomedicine) https://wwwfr.uni.lu/recherche/priorites_de_recherche/luxembourg_centre_for_systems_biomedicine_lcsb |
National Centre of Excellence in Early Diagnosis and Stratification of Parkinson’s Disease |
| Singapore (POLARIS) https://www.a-star.edu.sg/polaris/ |
Pilot TGFBI testing for disease diagnosis and family risk assessment in stromal corneal dystrophies, then implement 90-gene panel for gastrointestinal cancers |
| Thailand (Pharmacogenomics and Personalized Medicine) http://www.thailandpg.org/ |
Implement pharmacogenomics card to identify risk for top ten drugs with risk for Stevens Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN), integrated with nationwide pharmacovigilance program |
| United Kingdom (Genomics England) http://www.genomicsengland.co.uk/ |
Sequence 100K whole genomes and link to National Health Service records to treat individual patients and better understand cancer, rare and infectious diseases |
| United States (All of Us) https://allofus.nih.gov/ |
Recruit one million participants representative of the population and share data from EMRs, digital health and genomics to enhance scientific discovery and clinical care |
Source: Adapted from reference 30
A Policy Agenda for Precision Medicine
It is clear that precision medicine represents a paradigm shift in health care that is both maturing and is here to stay. There has been a rapid increase in the availability and use of genomic tests and this growth is expected to continue. A particularly strong trend is the increased use of multigene tests, including gene panels, whole exome sequencing, and whole genome sequencing. To fully realize the integration of PM into medicine, a policy agenda must be implemented (32–34). With the growth in PM come both policy opportunities and challenges. A report by the National Academy of Medicine (35) notes the following challenges that we use to structure our discussion
(1) Evidence Generation
There continues to be a need for high quality evidence that precision medicine actually improves patient outcomes if it is to be widely adopted. Although progress has been made in some areas and the initiatives described earlier will continue to provide new evidence, a major challenge remains with the acute and rapid evolution of the field – not only growth in the availability and use of novel technologies broadly but also the rapid growth of multigene panels that use sequencing technologies. There are currently more than 70,000 unique genetic testing products on the market and an average of 10 new products are added each day (36). The market for clinical sequencing - which encompasses the use of sequencing tests for diagnosis, risk prediction, therapy selection and monitoring, and screening - is growing at a compound annual rate of 28% (37).
The policy challenge, therefore, is how to obtain the needed evidence when the field is growing and changing so rapidly that the “gold standard” of large randomized clinical trials may be infeasible. Various alternatives have been proposed that offer creative approaches to generating evidence such as new models of risk-sharing and evidence development between technology developers, health care systems, and payers (38, 39). Unfortunately, there will be no one-size-fits-all approach for evidence generation since the evidentiary threshold will vary with the risk of the test and with the financial impact on the stakeholders.
(2) Data Sharing and Infrastructure Needs
The implementation of PM will require access to large-scale, detailed, and highly integrated patient data. Thus, many initiatives are focused on increasing inter-operability of patient data and enhancing data systems that enable the use of PM data at the point of care (35). Although great strides have been in recent years towards achieving a “paperless health care system” that is based on EMRs, much more needs to be done to integrate data across systems and to mine data that already exist but remain in silos.
Under the rubric of “infrastructure” is the regulatory landscape. A key topic for the future will be the evolving regulatory landscape for precision medicine tests. There continues to be uncertainty about how the FDA will regulate PM tests and the extent to which they will increase their oversight over “laboratory-developed tests” (LDTs), which do not require FDA approval. Most precision medicine tests are currently LDTs. A related challenge for the FDA will be to develop their approach to regulating diagnostics that incorporate sequencing technologies. These tests will require flexible and evolving regulatory approaches (34).
(3) Incorporating Genomic and Other Molecular Data into Clinical Care and Research
Given that new health care innovations typically take years to be adopted, it is not surprising that the integration of PM into clinical care has been slower than some observers have predicted. Many different sectors and activities have to coalesce in order to promote adoption including the appropriate education, data systems, coverage and reimbursement, health system processes, and health policies.
These issues are relevant not only to the US but also to other countries and regions that are implementing PM or likely to do so in the near future (see table 2). Initiatives such as the All of Us Research Program in the US and the Genomics England 100,000 Genomes Project have been widely publicized, but there has been less attention to other global initiatives and opportunities that are occurring outside the “epicenters”. For example, France recently committed $700 million to fund sequencing centers, and China has committed up to $10 billion to fund its PM initiative (36). Particularly rapid growth of clinical sequencing is expected not only in China but also other Asia-Pacific countries such as India. However, our impression is that there are few published studies of PM implementation outside of the well-known leading countries.
(4) Diagnostics, Drug Discovery, and the Economics of Precision Medicine
As with all new health care interventions, implementation will be stymied if such interventions do not provide demonstrated value or if payers and consumers are unwilling to pay for them. There remain many challenges to determining “if and when” PM provides sufficient value relative to its costs, and whether payers should reimburse for PM testing. Our recent analyses of private payer coverage policies for genetic tests measuring multiple genes (such as panel or whole exome sequencing tests) indicate that there is limited and variable coverage of such tests (40).
Several structured reviews of economic evaluations of PM have been published (41–53). Many of these reviews have focused on evaluations of pharmacogenetic testing while others have focused on PM more broadly and one study focused on sequencing (52). In general, these reviews have noted that the number of economic evaluations of PM is increasing, although there is still a relatively limited evidence base on the economic value of PM and many gaps in the topics covered. The number of studies included in the reviews ranged from five (52) to 128 (42). Studies found that PM interventions are generally similar in cost-effectiveness as other types of health care interventions, with a majority of interventions found to be cost-effective relative to standard practice but only a minority of studies finding PM to be cost-saving. Reviews noted a number of methodological challenges that should be addressed in order to better assess the economic value of PM. Other challenges include identifying relevant studies, dealing with heterogeneity across studies, and broadening the focus of studies to other conditions.
(5) Participant Engagement and Trust
Last but not least, patients and consumers must be participants in PM for it to achieve its potential. The adoption of precision medicine raises many questions about patient engagement and trust, including: What constitutes truly informed consent? Who owns genetic information and should make decisions about what results are returned and how they are used? How can privacy be assured? What outcomes matter most to patients?
One focus of future work should be the increasing emphasis on the ability of precision medicine to impact not only individuals but also populations – what has been termed “precision public health” (54,55). The initial drive toward precision public health is occurring, but much more work lies ahead to develop a robust evidentiary foundation for use (54). Another area of emphasis should be understanding how precision medicine could increase or decrease historical disparities in access to care. Do historically unserved populations have access to precision medicine and what policies could ensure appropriate access? These questions will need to be addressed within the larger and shifting context of health reform and proposed revisions to Medicaid programs.
An Action Plan for Precision Medicine
The full realization of precision medicine’s disruptive potential will require a multipronged scientific, clinical and policy agenda. Democratization of data underpins both the scientific advances that enable not only precision medicine but medicine itself. A culture with proper incentives for sharing of data will be required. The precision medicine ecosystem’s stakeholders - participants, patients, providers, payers and regulators – each will require evidence of value in terms of quality of life, quality of medical care and efficiency and effectiveness optimized for cost. If successful, more care will occur before disease is apparent – a shift from disease treatment to disease prevention and early detection. Precision medicine is not uniquely American – it is a global agenda (31) - and requires global leadership and perseverance to see it through to its rightful place in health and society.
Contributor Information
Geoffrey S Ginsburg, Duke Center for Applied Genomics & Precision Medicine, Duke University, Durham, NC 27708, Ph: 919 668 6210.
Kathryn A Phillips, Center for Translational and Policy Research on Personalized Medicine, UCSF School of Pharmacy, San Francisco, CA 94118, Ph: 415 502 8271.
References
- 1.Christenson CM, Groopman JN, Hwang J. The innovators prescription. New York, NY: McGraw-Hill Education; 2009. [Google Scholar]
- 2. [accessed December 6, 2017]; https://allofus.nih.gov/
- 3. [accessed December 3, 2017]; https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/
- 4. [accessed December 3, 2017]; https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php.
- 5. [accessed December 3, 2017]; https://globenewswire.com/news-release/2017/09/22/1131466/0/en/Digital-Health-Market-will-Reach-USD-536-6-Billion-by-2025-Transparency-Market-Research.html.
- 6.National Research Council, Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 7.Aronson SJ, Rehm HL. Building the foundation for genomics in precision medicine. Nature. 2015 Oct 15;526(7573):336–42. doi: 10.1038/nature15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016 Jan 4;44(Database issue):D862–D868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehm HL, PhD, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, et al. ClinGen — The Clinical Genome Resource. N Eng J Med. 2015;372:2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed December 6, 2017]; http://exac.broadinstitute.org/about.
- 11.Roundtable on Translating Genomic-Based Research for Health; Board on Health Sciences Policy; Institute of Medicine. Genomics-Enabled Learning Health Care Systems: Gathering and Using Genomic Information to Improve Patient Care and Research: Workshop Summary. National Academies Press; Washington, DC: 2015. [PubMed] [Google Scholar]
- 12.Ginsburg GS. Medical Genomics: Gather and Use Genetic Data in Health Care. Nature. 2014 Apr 24;508:451–453. doi: 10.1038/508451a. [DOI] [PubMed] [Google Scholar]
- 13.https://www.regeneron.com/sites/all/themes/regeneron_corporate/files/science/RGC_FactSheet_GHSCollaboration_Final.pdf
- 14.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, Almoguera B, Basford MA, Bielinski SJ, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014 Oct;96(4):482–9. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaback MM. Population-based genetic screening for reproductive counseling: The Tay-Sachs disease model. Eur J Pediatr. 2000;159(Suppl 3):S192–S195. doi: 10.1007/pl00014401. [DOI] [PubMed] [Google Scholar]
- 16.Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3:65ra4. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitzman JO, Snyder MW, Ventura M, Lewis AP, Qiu R, Simmons LE, et al. Non-invasive whole genome sequencing of a human fetus. Sci Transl Med. 2012 Jun 6;4(137):137ra76. doi: 10.1126/scitranslmed.3004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid Whole-Genome Sequencing for Genetic Disease Diagnosis in Neonatal Intensive Care Units. Sci Transl Med. 2012;4(154):154ra135. doi: 10.1126/scitranslmed.3004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topol EJ. Individualized medicine from pre-womb to tomb. Cell. 2014 Mar 27;157(1):241–253. doi: 10.1016/j.cell.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013 Jun 12;5(189):189sr4. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 21.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015 Jun 4;372(23):2229–34. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg GS, Finkelman E, Balatbat C, Flott K, Prestt J, Dzau V. Precision Medicine: A Global Action Plan for Impact. Doha, Qatar: World Innovation Summit for Health; 2016. [Google Scholar]
- 23.Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19(8):858–63. doi: 10.1038/gim.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips KA, Deverka PA, Sox HC, Khoury MJ, Sandy LG, Ginsburg GS, et al. Making genomic medicine evidence-based and patient-centered: a structured review and landscape analysis of comparative effectiveness research. Genetics in medicine: official journal of the American College of Medical Genetics. 2017;19(10):1081–91. doi: 10.1038/gim.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Integrating Genomic Sequencing into Clinical Care. CSER and Beyond. 2015 [cited 2017 October 31]. Available from: https://www.genome.gov/Multimedia/Slides/CSER_Beyond_2015/CSER_Beyond_WorkshopReport.pdf.
- 26.NIH accelerates the use of genomics in clinical care: New fundingawards focus on diverse and underserved populations. 2017 [Available from: https://www.nih.gov/news-events/news-releases/nih-accelerates-use-genomics-clinical-care.
- 27.Clinical Sequencing Evidence-Generating Research (CSER2) 2017 [Available from: https://cser-consortium.org/
- 28.Implementing Genomics in Practice. www.ignite-genomics.org.
- 29.https://grants.nih.gov/grants/guide/rfa-files/RFA-HG-17-010.html
- 30.http://www.cfirguide.org/
- 31.Manolio TA, Abramowicz M, Al-Mulia F, Anderson W, Balling R, Berger AC, et al. Global implementation of genomic medicine: We are not alone. Sci Transl Med. 2015 Jun 3;7(290):290ps13. doi: 10.1126/scitranslmed.aab0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard DE, Moeckel F, Villa MS, Housman LT, McCarty CA, McLeod HL. Strategies for integrating personalized medicine into healthcare practice. Personalized Medicine. 2017;14(2):141–52. doi: 10.2217/pme-2016-0064. [DOI] [PubMed] [Google Scholar]
- 33.Phillips KA, Trosman JR, Kelley RK, Pletcher MJ, Douglas MP, Weldon CB. Genomic sequencing: assessing the health care system, policy, and big-data implications. Health affairs. 2014;33(7):1246–53. doi: 10.1377/hlthaff.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Personalized Medicine Report: 2017 Opportunity, Challenges, and the Future: Personalized Medicine Coalition. 2017 [Available from: http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The_PM_Report.pdf.
- 35.Dzau VJ, Ginsburg GS. Realizing the Full Potential of Precision Medicine in Health and Health Care. JAMA. 2016;316(16):1659–60. doi: 10.1001/jama.2016.14117. [DOI] [PubMed] [Google Scholar]
- 36.Bergin J. DNA Sequencing: Emerging Technologies and Applications. Wellesley, MA: BCC Research; 2016. May, [Google Scholar]
- 37.Phillips KA, Deverka PA, Hooker G, Douglas MP. Genetic Test Availability, Spending, and Market Trends. Where Are We Now? Where Are We Going? Health affairs. 2018 doi: 10.1377/hlthaff.2017.1427. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trosman JR, Weldon CB, Gradishar WJ, Benson ABr, Cristofanilli M, Kurian AW, et al. Modernizing the framework for insurance coverage policy for contemporary precision medicine in the era of next-generation sequencing. Health affairs. 2018 this issue. [Google Scholar]
- 39.Ramsey SD, Sullivan SD. A new model for reimbursing genome-based cancer care. Oncologist. 2014;19(1):1–4. doi: 10.1634/theoncologist.2013-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips KA, Deverka PA, Trosman JR, Douglas MP, Chambers JD, Weldon CB, et al. Payer coverage policies for multigene tests. Nature biotechnology. 2017;35(7):614–7. doi: 10.1038/nbt.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berm EJ, Looff M, Wilffert B, Boersma C, Annemans L, Vegter S, et al. Economic Evaluations of Pharmacogenetic and Pharmacogenomic Screening Tests: A Systematic Review. Second Update of the Literature. PLoS One. 2016;11(1):e0146262. doi: 10.1371/journal.pone.0146262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Andrea E, Marzuillo C, Pelone F, De Vito C, Villari P. Genetic testing and economic evaluations: a systematic review of the literature. Epidemiologia e prevenzione. 2015;39(4 Suppl 1):45–50. [PubMed] [Google Scholar]
- 43.Carlson JJ, Henrikson NB, Veenstra DL, Ramsey SD. Economic analyses of human genetics services: a systematic review. Genetics in medicine: official journal of the American College of Medical Genetics. 2005;7(8):519–23. doi: 10.1097/01.gim.0000182467.79495.e2. [DOI] [PubMed] [Google Scholar]
- 44.Rogowski W. Genetic screening by DNA technology: a systematic review of health economic evidence. Int J Technol Assess Health Care. 2006;22(3):327–37. doi: 10.1017/s0266462306051221. [DOI] [PubMed] [Google Scholar]
- 45.Assasi N, Schwartz L, Tarride JE, Goeree R, Xie F. Economic evaluations conducted for assessment of genetic testing technologies: a systematic review. Genetic testing and molecular biomarkers. 2012;16(11):1322–35. doi: 10.1089/gtmb.2012.0178. [DOI] [PubMed] [Google Scholar]
- 46.Phillips KA, Van Bebber SL. A systematic review of cost-effectiveness analyses of pharmacogenomic interventions. Pharmacogenomics. 2004;5(8):1139–49. doi: 10.1517/14622416.5.8.1139. [DOI] [PubMed] [Google Scholar]
- 47.Beaulieu M, de Denus S, Lachaine J. Systematic review of pharmacoeconomic studies of pharmacogenomic tests. Pharmacogenomics. 2010;11(11):1573–90. doi: 10.2217/pgs.10.145. [DOI] [PubMed] [Google Scholar]
- 48.Oosterhoff M, van der Maas ME, Steuten LM. A Systematic Review of Health Economic Evaluations of Diagnostic Biomarkers. Applied health economics and health policy. 2016;14(1):51–65. doi: 10.1007/s40258-015-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plumpton CO, Roberts D, Pirmohamed M, Hughes DA. A Systematic Review of Economic Evaluations of Pharmacogenetic Testing for Prevention of Adverse Drug Reactions. Pharmacoeconomics. 2016;34(8):771–93. doi: 10.1007/s40273-016-0397-9. [DOI] [PubMed] [Google Scholar]
- 50.Djalalov S, Musa Z, Mendelson M, Siminovitch K, Hoch J. A review of economic evaluations of genetic testing services and interventions (2004–2009) Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13(2):89–94. doi: 10.1097/GIM.0b013e3182003294. [DOI] [PubMed] [Google Scholar]
- 51.Hatz MH, Schremser K, Rogowski WH. Is individualized medicine more cost-effective? A systematic review. Pharmacoeconomics. 2014;32(5):443–55. doi: 10.1007/s40273-014-0143-0. [DOI] [PubMed] [Google Scholar]
- 52.Frank M, Prenzler A, Eils R, Graf von der Schulenburg JM. Genome sequencing: a systematic review of health economic evidence. Health economics review. 2013;3(1):29. doi: 10.1186/2191-1991-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips KA, Ann Sakowski J, Trosman J, Douglas MP, Liang SY, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genetics in medicine: official journal of the American College of Medical Genetics. 2014;16(3):251–7. doi: 10.1038/gim.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khoury MJ, Galea S. Will Precision Medicine Improve Population Health? JAMA. 2016;316(13):1357–8. doi: 10.1001/jama.2016.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016;50(3):398–401. doi: 10.1016/j.amepre.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]