Abstract
The first systematic multi-center study of the clinical use of EPR oximetry has begun, with funding as a PPG from the NCI. Using particulate oxygen sensitive EPR, materials in three complementary forms (India Ink, “OxyChips”, and implantable resonators) the clinical value of the technique will be evaluated. The aims include using repeated measurement of tumor pO2 to monitor the effects of treatments on tumor pO2, to use the measurements to select suitable subjects for the type of treatment including the use of hyperoxic techniques, and to provide data that will enable existing clinical techniques which provide data relevant to tumor pO2 but which cannot directly measure it to be enhanced by determining circumstances where they can give dependable information about tumor pO2.
Keywords: Tumor pO2, Oximetry, EPR, Hypoxia
1 Introduction
It is widely recognized that hypoxia has a profound effect on the radiosensitivity of tissue and that hypoxic cells can be as much as three times less sensitive to radiation than well oxygenated cells [1-5]. A practical consequence of this fact is that tumors with significant hypoxia that are treated with radiation therapy have decreased probabilities for complete tumor control and reduced patient survival rates [1]. Despite the fundamental importance of tumor oxygenation during radiation therapy, measurements of the partial pressure of oxygen (pO2) are not routinely performed in the clinical setting because of a lack of available quantitative measurement techniques that are noninvasive.
Electron Paramagnetic Resonance (EPR) oximetry based on particulates is a technique that is capable of providing repeated, direct, and noninvasive measurements of pO2, following one-time implantation of a paramagnetic oxygen reporter [6]. These capabilities contrast with other techniques currently clinically available to measure oxygenation, which provide indirect measurements and/or are invasive at the time of measurement [7]. Based on the unique capabilities of EPR oximetry, the National Cancer Institute of US National Institutes of Health has funded a 5-year program project grant at Dartmouth (Dartmouth PPG) whose primary aim is to demonstrate the clinical capabilities of EPR oximetry for cancer. An additional goal is to relate EPR measurements to existing clinically available techniques, in order to understand under what circumstances those less direct techniques can give clinically useful information about tumor pO2.
The clinical utility of repeated measurements of tumor oxygen is based on well-established observations of the effects of hypoxia on outcomes, the variability of oxygen levels in apparently similar tumors in different patients, the variation of oxygen levels as a function of growth of the tumor and in response to therapy, and the potential to modify oxygen levels by treatments such as breathing oxygen-rich mixtures [8-10 ]. Therefore, repeated measurements of tumor oxygen would enable the selection of subjects for various therapeutic approaches, evaluation of the effectiveness of methods to modify a patient’s oxygen level, and guidance of the timing of treatments to ensure more optimal oxygen levels at the time of irradiation.
The PPG, while centered at Dartmouth, collaborates with two other academic radiation oncology centers (Emory University in Atlanta, Georgia and the Université catholique de Louvain in Brussels) to evaluate EPR oximetry using three different types of paramagnetic oxygen sensitive materials with complementary properties.
2 Methods
All measurements utilize clinical EPR in vivo oximetry, which has previously been described in some detail in reference [6]. The organizing principle for the PPG is the use of three different methods, which provide complementary capabilities to make the measurements in patients with tumors, These are described in some detail in the following.
The first method uses India ink whose oxygen-dependent broadening of the EPR linewidth reports the pO2 of the tissue. India ink has already been used successfully in a wide array of animal studies [11-13] and in human subjects [6, 14-16]. India ink, because of its extensive prior use in human subjects including its current use for marking boundaries in surgery and radiation therapy, does not require approval by the US Food and Drug Administration (FDA) as a new drug. Consequently, early results for EPR oximetry focused on this sensor and were a major factor in the decision to fund the Dartmouth PPG (discussed in results below, Table 13.1). However, because India ink has a large linewidth, the sensor needs to be placed within 10 mm of the surface to get adequate signal to noise and therefore, while very useful for superficial tumors, it is not suitable for deeper tumors. It is placed in one or more sites in the tumor at depths from 1 to 5 mm, by a simple injection through a small needle (about 23 gauge).
Table 13.1.
EPR oximetry using injected India ink to assess pO2 measurements in tumors measured in vivo while patient breathes room air and enriched oxygena
| Tumor | Tumor pO2 as mmHg | |||
|---|---|---|---|---|
| Type | Site | Breathing room air | Breathing enriched oxygen | Effect of breathing enriched oxygen |
| Lymphoma | Post. thigh | <5 | >100 | Increased |
| Lymphoma | Post. thigh | <3 | >10 | Increased |
| Lymphoma | Post. thigh | ~1 | >5 | Increased |
| Melanoma | Knee | <10 | >50 | Increased |
| Melanoma | Foot | Anoxic | Anoxic | No change |
| Melanoma | Scalp | Anoxic | Anoxic | No change |
| Melanoma | Scalp and neck | ~6 | ~12 | Increased |
| Melanoma | Scapula | <1 | <5 | Increased |
| Melanoma | Chest | ~10 | ~10 | No change |
| Melanoma | Calf | Anoxic | ~3 | Increased |
| Sarcoma | Nose | ~10 | ~10 | No change |
Adapted from a table in Swartz et al. [6]; reproduced with permission.
The second method uses a paramagnetic material that is a derivative of lithium pthalocyanine, lithium octa-n-butoxynaphthalocyanine (LiNc-BuO) (see Fig. 13.1a, b [A]). Because of its very narrow linewidth and high spin density, it can be detected at deeper depths than India ink (discussed further in results below, Fig. 13.3), making it potentially a more effective oximetric material for deeper tumors. Like India ink, it also is very unreactive and very stable, both of which are very desirable characteristics for substances placed into tumors. However, because, unlike India ink, lithium phthalocyanine derivatives have not been commonly used in humans, they would require the very time-consuming and very expensive regulatory processes to get approval from the FDA for direct implantation, even though they are inert. To reduce the regulatory requirements for direct implantation of the crystals, we have developed a method to package them in a widely accepted biocompatible material that is permeable to oxygen, polydimethylsiloxane (PDMS) (Fig. 13.1b [B]). This packaging has enabled us to get an approved Investigation Device Exemption (IDE) from the FDA to conduct an initial Phase 1 safety study in human subjects. The packaged sensor currently in use is 5 mm in length, 0.6 mm in diameter and consists of 40 % of the sensor to 60 % of PDMS; this packaged sensor, called an OxyChip, has been extensively studied in preclinical studies (Fig. 13.1b [C]) [17-19].
Fig. 13.1.
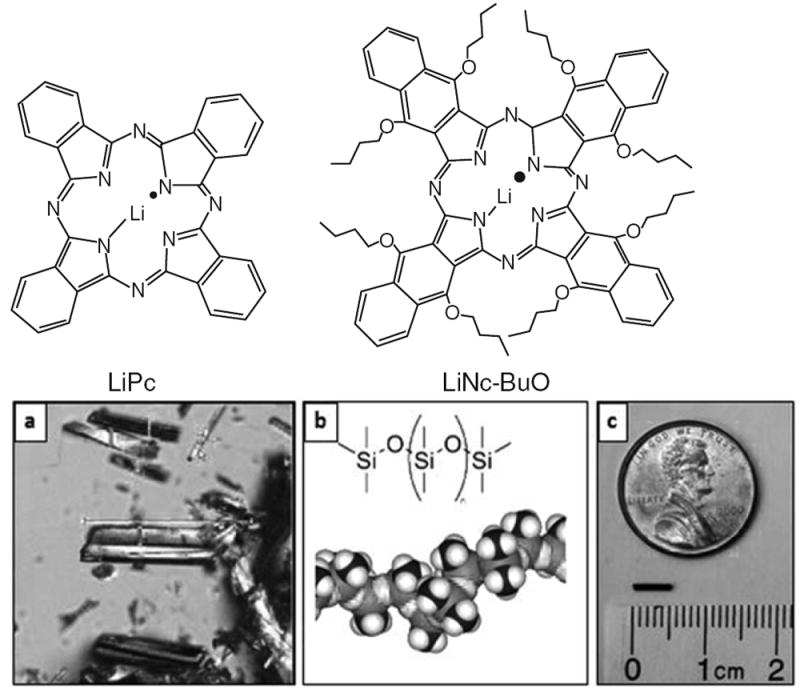
(a) Molecular structure of lithium phthalocyanine (LiPc) and lithium octa-n-butoxynaphthalocyanine (LiNc-BuO) oximetry probes. The probes are prepared in the form of fine crystals which are paramagnetic and biologically inert. The crystals exhibit high spin density (~1020 spins/g), single sharp EPR peak (peak-to-peak width <50 mG under anoxic conditions), and are sensitive to oxygen partial pressure of oxygen (5–9 mG/mmHg pO2). (b) LiNc-BuO crystals, PDMS coating, and the OxyChip. (A) Microcrystals of LiNc-BuO are encapsulated into (B) polydimethylsiloxane (PDMS), a biocompatible and oxygen-permeable polymer, to obtain (C) small pieces of probes (OxyChip) for implantation in tissues. The OxyChip is 0.6 mm in diameter and 5 mm in length and can be conveniently loaded into the tip of an 18-G angio-catheter to be implanted in tumors. (b [B] is reproduced from Courtney 2015 [23])
Fig. 13.3.
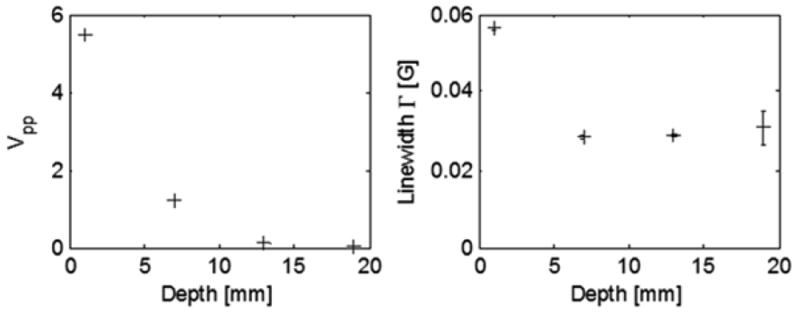
OxyChips were measured at depths of 1, 7, 13, and 19 mm in lossy material. The peak-to-peak amplitude [Vpp] (left) and estimated linewidth (right) are shown. Consistent linewidths, regardless of depth, establish the capability to measure pO2 at 2 cm depth in as little as 5 s per measurement. (Error bars are estimated uncertainty in fit parameter, where the oxygen response is ~7 mG/mmHg)
When this early Phase 1 study using OxyChips is successfully completed, we anticipate not only expanding our clinical studies in humans using the OxyChip but also using this demonstration of the safety of the coated material to receive an IDE to conduct clinical studies using the third method in the PPG, an implantable resonator.
The implantable resonator contains multiple tips, each of which contains an oxygen sensitive material (based on the OxyChip) which is then connected to a loop under the skin by means of a very thin wire. We then can magnetically couple a surface component to the implanted part of the resonant circuit (Fig. 13.2). The FDA has already reviewed preliminary documentation for an IDE for the implantable resonator and, based on these discussions, we anticipate receiving approval once the OxyChip passes the criteria for Phase 1 safety (expected in 2016). The resonator is put in place either through a large biopsy needle or directly at the time of an open biopsy or other procedure that exposures the tumor.
Fig. 13.2.
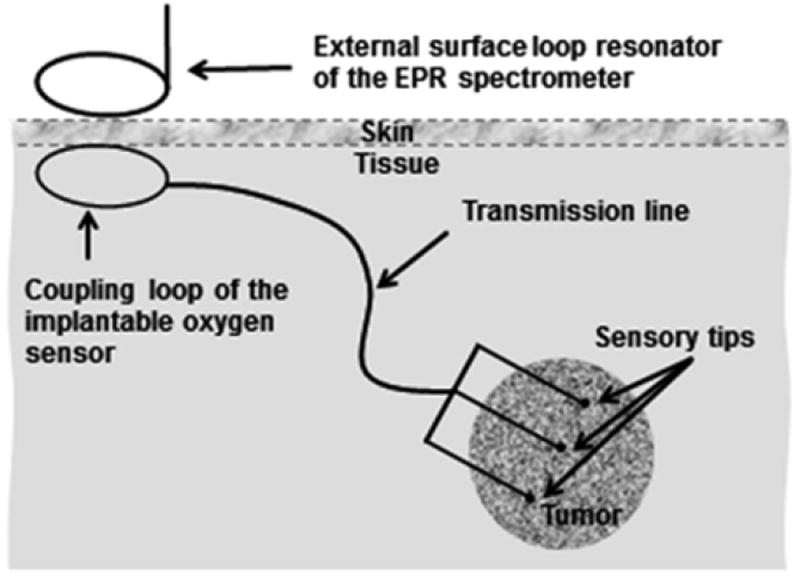
Implantable resonators for deep-tissue EPR oximetry. The implantable sensors can be made with different length of the transmission line (i.e., depth) that can vary from 1 to >10 cm. Multiple implantable deep-tissue oxygen sensors can be implanted with sensory tips inserted into the tissue of interest and coupling loops positioned under the skin. The external surface loop resonator of the EPR spectrometer is used for inductive coupling to acquire EPR spectra
3 Results
Table 13.1 shows clinical measurements of pO2 made in 11 patients with three types of tumors located in several different anatomical sites, all measured using India ink. All patients were measured initially while breathing room air and then again while breathing 100 % oxygen through a face mask. These results show that EPR oximetry can be used to make measurements in tumors under clinically applicable
OxyChip to be reliably equilibrated with the test gas (100 % N2). Single 5 s scans of the spectrum were carried out with constant non-saturating RF power and low modulation amplitude that did not distort the spectrum.
Figure 13.3 shows the peak-to-peak amplitude and estimated linewidth, as the depth was increased from conditions.
Perhaps most importantly, the results also show that not all tumors respond to breathing enriched oxygen. Only seven of the eleven patients had an increase in oxygen after breathing gas mixtures enriched with oxygen. Of the four patients who showed no change after breathing enriched oxygen, two had tumors that were initially assessed as anoxic, and the other two tumors had the highest observed initial levels of oxygen (~10 mmHg).
The finding of tumor variability in response to breathing oxygen has very important implications. The failure of a substantial number of tumors to respond to the increased oxygen in the breathing gas provides an explanation for why previous attempts to improve outcomes by the use of hyperoxic approaches have had at best marginal success [3, 8, 20]. Without monitoring tumor hypoxia directly and repeatedly in response to breathing enriched oxygen (or in some cases without controlling the timing of hypoxic therapy relative to treatment), they could not differentiate between tumors that did and did not respond to the hyperoxic therapy. Therefore, before separating the data into the appropriate subpopulations, they concluded that the procedure did not significantly improved outcomes.
Our results, showing the ability to determine which tumors are responsive to hyperoxic therapy, i.e., which will have increased pO2 after breathing enriched oxygen, support the conclusion that it is possible to assess which tumors respond to hyperoxic therapy so that these patients could be selected to receive concurrent hyperoxic therapy with their standard treatments. Alternatively, some have suggested that treatment could be delivered more aggressively knowing that the tumor is hypoxic and unresponsive to hyperoxic therapy [21] and so oximetry would provide clinically useful information in such cases as well.
In vitro measurements were performed to establish depth sensitivity profiles for oximetry using the second type of sensor, the OxyChip (Fig. 13.3). The non-resonant losses of microwaves which occurs in tissue was modeled using slices of bologna sausage, which allowed the OxyChips to be placed at well-defined depths while maintaining a regular geometry and constant non-resonant losses such as that which occurs in tissues in vivo. Within the block of bologna at four different depths, a single OxyChip was placed within Teflon tubing inside a microcapillary tube that allowed the 1 mm to ~2 cm. Of importance for clinical oximetry, consistent, valid data continued to be obtained throughout the full range of depth. Additional averaging of multiple 5 s scans can be used to increase precision of the measurement and/ or provide the capability to get measurements deeper than 20 mm.
These data support the conclusion that the OxyChip can be used to make measurements directly from the surface up to 20 mm, which would significantly enhance the applications using surface resonators. (The implantable resonator would allow assessment at any depth.)
4 Discussion and Conclusions
The results to date in preclinical and clinical studies indicate that clinical applications of EPR oximetry based on particulates may provide valuable additional capabilities to the current state for measuring hypoxia in tumors and, using that knowledge, to improve clinical care. Furthering this evidence for EPR oximetry and our understanding of the mechanisms involved is the primary aim of the Dartmouth PPG.
There are several existing options available to clinicians to measure hypoxia. While providing much useful information, other current methods cannot provide the quantitative data on tumor pO2 that can be obtained with EPR oximetry with particulates. Therefore, a second but very important goal of the Dartmouth PPG is to systematically gather data using existing methods in current use to characterize hypoxia, in conjunction with EPR oximetry. For a brief listing of the clinically available techniques to measure oxygen in tumors, see the companion paper in this volume by Flood et al. which discusses methods in the context of comparative effectiveness [22]. Here we briefly discuss how the clinical uses of these methods relate to and could be augmented by measurements of EPR oximetry.
The oxygen electrode (which is no longer made for clinical use) while providing very important data, is too invasive for repeated use and is limited to relatively superficial tumors. Other limitations are due to local perturbations when used. Nevertheless, the data from EPR oximetry could delineate where the data obtained with its one time use might provide a better basis for its use for patient selection.
There are several MRI techniques that are widely available clinically. They provide information that, while not directly measuring pO2, is related indirectly to tumor hypoxia, e.g., by providing evidence of poor rates of diffusion of oxygen to the tumor. Techniques that are especially clinically promising include blood oxygen level dependent (BOLD), diffusion-weighted imaging, perfusion weighted imaging, and magnetic resonance spectroscopy. The parameters that they measure can be significant factors in hypoxia. Changes they measure may already be clinically significant, but their effective use could be significantly enhanced if EPR oximetry was used either as an important second source of data or to delineate the circumstances where these techniques would most likely reflect clinically important information on hypoxia in a patient.
The use of optical methods such as near infrared to measure the saturation and volume of hemoglobin are also becoming more widely available. While this information can give pO2 in the vascular system, it cannot then be directly used to estimate pO2 in the tumor. However, coupled with data from EPR, its clinical value could be enhanced if used under conditions when EPR oximetry has shown that the optical method is a reasonable indicator of tumor oxygenation.
The positron emission tomography (PET) tracers of hypoxia localizing drugs are increasing available, providing qualitative indication of the presence of hypoxic areas at the time that the drugs were given. Again, while the information is limited to qualitative indications and describes one point in time, EPR oximetry could help to delineate those circumstances in which the information from hypoxic localizing drugs could be useful in the clinical management of therapy to overcome the effects of tumor hypoxia.
In summary, the translation from preclinical to clinical use of EPR oximetry based on particulates is proceeding effectively with measurements initiated in human subjects. In the near future it should be feasible to test the clinical impact of these measurements. That impact is anticipated to include: improved selection of patients for hyperoxic therapy, enhanced effectiveness of radiation therapy (including optimizing combinations with chemotherapy) by delineating the windows when the radiation will be most effective, and improved clinical utility of existing but indirect methods to characterize tumor hypoxia. If the goals of the PPG are fully achieved the result would be a very significant enhancement of the ability to effectively treat many solid cancers.
Acknowledgments
This work was supported by grants from the National Institutes of Health [P01 CA190193 (the Dartmouth PPG) and R01 EB004031] and pilot project funding from the Prouty Fund of the Norris Cotton Cancer Center at Geisel Medical School.
Disclaimers: HMS and ABF are owners of Clin-EPR, LLC, which manufacturers clinical EPR instruments for investigational use only. PK holds patents related to the OxyChip.
Contributor Information
Harold M. Swartz, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA Department of Medicine, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA.
Benjamin B. Williams, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA Department of Medicine, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA.
Huagang Hou, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA.
Nadeem Khan, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA.
Lesley A. Jarvis, Department of Medicine, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA
Eunice Y. Chen, Department of Surgery, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA
Philip E. Schaner, Department of Medicine, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA
Arif Ali, Department of Radiation Oncology, Emory Medical School, Atlanta, GA, USA.
Bernard Gallez, Louvain Drug Research Institute, Université Catholique de Louvain, Brussels, Belgium.
Periannan Kuppusamy, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA; Department of Medicine, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA.
Ann B. Flood, Department of Radiology, Geisel School of Medicine at Dartmouth, One Medical Center Drive Lebanon, Lebanon, NH, USA
References
- 1.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Mayer A. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2015;10:878–879. doi: 10.1089/ars.2014.6155. [DOI] [PubMed] [Google Scholar]
- 3.Walsh JC, Lebedev A, Aten E, et al. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21:1516–1554. doi: 10.1089/ars.2013.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon C, Fraker DL. Tumor oxygenation status as a prognostic marker. Cancer Lett. 2005;221:225–235. doi: 10.1016/j.canlet.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Dhani N, Fyles A, Hedley D, et al. The clinical significance of hypoxia in human cancers. Semin Nucl Med. 2015;45:110–121. doi: 10.1053/j.semnuclmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Swartz HM, Williams BB, Zaki BI, et al. Clinical EPR: unique opportunities and some challenges. Acad Radiol. 2014;21:197–206. doi: 10.1016/j.acra.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springett R, Swartz HM. Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid Redox Signal. 2007;9:1295–1301. doi: 10.1089/ars.2007.1620. [DOI] [PubMed] [Google Scholar]
- 8.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 9.Vaupel P. Prognostic potential of the pre-therapeutic tumor oxygenation status. Adv Exp Med Biol. 2009;645:241–246. doi: 10.1007/978-0-387-85998-9_36. [DOI] [PubMed] [Google Scholar]
- 10.Hoogsteen IJ, Marresy HAM, van der Kogel AJ, et al. The hypoxic tumour microenvironment, patient selection and hypoxia-modifying treatments. Clin Oncol (R Coll Radiol) 2007;19:385–396. doi: 10.1016/j.clon.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Goda F, O’Hara JA, Rhodes ES, et al. Changes of oxygen tension in experimental tumors after a single dose of X-ray irradiation. Cancer Res. 1995;55:2249–2252. [PubMed] [Google Scholar]
- 12.O’Hara JA, Goda F, Demidenko E, et al. Effect on regrowth delay in a murine tumor of scheduling split-dose irradiation based on direct pO2 measurements by electron paramagnetic resonance oximetry. Radiat Res. 1998;150:549–556. doi: 10.2307/3579872. [DOI] [PubMed] [Google Scholar]
- 13.O’Hara JA, Goda F, Liu KJ, et al. The pO2 in a murine tumor after irradiation: an in vivo electron paramagnetic resonance oximetry study. RadiatRes. 1995;144:222–229. doi: 10.2307/3579262. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Hou H, Hein P, et al. Black magic and EPR oximetry: from lab to initial clinical trials. In: Okunieff P, Williams J, Chen Y, editors. Advances in experimental medicine and biology: Oxygen transport to tissues XXVI; 31st annual meeting of the International Society on Oxygen Transport to Tissue; Rochester, NY. 16–20 August 2003; 2005. [DOI] [PubMed] [Google Scholar]
- 15.Khan N, Williams BB, Swartz HM. Clinical applications of in vivo EPR: rationale and initial results. Appl Magn Reson. 2006;30:185–199. doi: 10.1007/BF03166718. [DOI] [Google Scholar]
- 16.Swartz HM, Khan N, Buckey J, et al. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17:335–351. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 17.Meenakshisundaram G, Eteshola E, Pandian RP, et al. Oxygen sensitivity and biocompatibility of an implantable paramagnetic probe for repeated measurements of tissue oxygenation. Biomed Microdevices. 2009;11:817–826. doi: 10.1007/s10544-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinguizli M, Jeumont S, Beghein N, et al. Development and evaluation of biocompatible films of polytetrafl uoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21:1015–1022. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hou H, Dong R, Li H, et al. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson. 2012;214:22–28. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30:1777–1783. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- 21.Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed. 2004;17:240–262. doi: 10.1002/nbm.900. [DOI] [PubMed] [Google Scholar]
- 22.Flood AB, Satinsky VA, Swartz HM. 2015 Comparing the effectiveness of methods to measure oxygen in tissues for prognosis and treatment of cancer. Advances in experimental medicine and biology: oxygen transport to tissues XXXVIII; 43rd annual meeting of the International Society on Oxygen Transport to Tissue; Wu-han, China. 11–16 July 2015; submitted to same volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courtney A. The synthesis of bouncing putty: a cross-linked silicone polymer. [20 August 2015];2015 [image in Fig. 1b [B] from https://www.wou.edu/las/physci/ch462/BouncingPutty.htm.


