Abstract
Background
Skeletal and respiratory muscle dysfunction constitutes an important pathophysiological feature of heart failure (HF). We assessed the relationships between respiratory muscle function, skeletal muscle mass, and physical fitness in men with HF with reduced left ventricular ejection fraction (HFrEF), and investigated the hypothesis of whether iron deficiency (ID) contributes to respiratory muscle dysfunction in these patients.
Methods
We examined 53 male outpatients with stable HFrEF without asthma or chronic obstructive pulmonary disease (age: 64 ± 10 years; New York Heart Association [NYHA] class I/II/III: 36/51/13%; ischaemic aetiology: 83%; all with left ventricular ejection fraction ≤40%) and 10 middle‐aged healthy men (control group). We analysed respiratory muscle function (maximal inspiratory and expiratory pressure at the mouth [MIP and MEP, respectively]), appendicular lean mass/body mass index (ALM/BMI; ALM was measured using dual‐energy X‐ray absorptiometry), physical fitness (components of Functional Fitness Test for Older Adults), and iron status.
Results
MIP, MEP, and ALM/BMI (but not MIP adjusted for ALM/BMI) were lower in men with HFrEF vs. healthy men. MIP, MEP, and MIP adjusted for ALM/BMI (but not ALM/BMI) were lower in men with HFrEF with vs. without ID. In a multivariable linear regression model lower serum ferritin (but not transferrin saturation) was associated with lower MIP independently of ALM/BMI, left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and haemoglobin concentration. In multivariable linear regression models, lower MIP was associated with worse results in Functional Fitness Test when adjusted for ALM/BMI or relevant clinical variables (NYHA class, estimated glomerular filtration rate, NT‐proBNP, and haemoglobin concentration).
Conclusions
In men with HFrEF, low ferritin reflecting depleted iron stores is associated with inspiratory muscle weakness independently of skeletal muscle mass. Inspiratory muscle dysfunction correlates with worse physical fitness independently of either skeletal muscle mass or disease severity.
Keywords: Heart failure, Iron deficiency, Respiratory muscles, Physical fitness
Introduction
Skeletal and respiratory muscle dysfunction constitutes an important pathophysiological feature of heart failure (HF), contributes to debilitating symptomatology of this disease syndrome (diminished exercise capacity, increased perception of dyspnoea—also during submaximal exercises), and predicts worse outcomes in these patients.1, 2, 3, 4, 5, 6, 7 Pathogenetic processes underlying decreased respiratory muscle strength and endurance in patients with HF remain not fully understood, and potentially involved pathomechanisms include, among others, respiratory muscle underperfusion and generalized muscle atrophy (skeletal muscle mass loss).3, 4, 5, 6
Iron deficiency (ID) constitutes a frequent comorbidity in HF,8, 9 and is associated with reduced exercise capacity in these patients.10 Being the crucial micronutrient for several intracellular processes responsible for energy generation, iron plays the important role for the effective functioning of skeletal muscle tissue.11 In experimental studies (including animal models), ID is associated with complex alterations within skeletal muscle tissue and decreased work capacity,12, 13, 14, 15 and there are some indirect data from sports medicine suggesting the relationship between ID and skeletal muscle dysfunction in humans.16, 17, 18, 19
In the current study, we aimed to (i) assess the relationships between respiratory muscle function, skeletal muscle mass, and physical fitness in men with HF with reduced left ventricular ejection fraction (HFrEF) and (ii) to investigate our recent hypothesis11 of whether ID contributes to respiratory muscle dysfunction in these patients independently of disease severity and indices of skeletal muscle mass.
Methods
Patients
We examined 53 male outpatients with stable HFrEF participating in the large study investigating the associations between skeletal muscle dysfunction and iron status in men with HFrEF. The inclusion criteria in the study were (i) male sex and age >18 years, (ii) a documented history of chronic HF (established diagnosis of chronic HF according to the criteria of the European Society of Cardiology20) of at least 6‐month duration, (iii) left ventricular ejection fraction (LVEF) ≤40% as assessed in the latest echocardiography examination, (iv) New York Heart Association (NYHA) class I–III, and (v) clinical stability along with unchanged HFrEF pharmacotherapy for at least 1 month preceding the enrolment. Exclusion criteria included (i) acute coronary syndrome or acute decompensation of HF (an episode of acute HF) within 3 months preceding the study, (ii) the therapy for anaemia (in particular the use of erythropoiesis‐stimulating agents) or/and ID (in particular intravenous iron therapy) within 12 months preceding the study, (iii) chronic infectious disease or symptoms of infection at enrolment, (iv) a history of autoimmune, haematological, or malignant disease (cancer), (v) muscular or neuro‐muscular disorders, (vi) dementia or cognitive dysfunction, or (vii) simultaneous participation in an interventional clinical trial. Additionally, for the purposes of the current study, patients diagnosed with either asthma or chronic obstructive pulmonary disease (COPD) were not included in the following analyses.
Respiratory muscle function was also assessed in 10 healthy men without the history of cardiovascular disease (exception: well‐controlled arterial hypertension), who were considered the control group. Healthy subjects were recruited among the volunteers, relatives, and the colleagues of the staff or patients.
The study protocol was approved by the local ethics committee (Bioethics Committee, Wroclaw Medical University), and all subjects gave written informed consent at inclusion. The study was conducted in accordance with the Helsinki Declaration.
Respiratory muscle function
Respiratory muscle strength was assessed non‐invasively using the MicroRPM respiratory pressure meter (Becton, Dickinson and Company), which is a portable, hand‐held device with a flanged mouthpiece. We measured maximal inspiratory mouth pressure (MIP) and maximal expiratory mouth pressure (MEP), which reflect inspiratory and expiratory muscle strength, respectively.2, 4, 21 Briefly, MIP reflects the strength of the diaphragm, whereas MEP reflects the strength of abdominal and intercostal muscles.22, 23 These tests belong to the group of volitional tests of respiratory muscle strength, and their advantages are simplicity and high tolerability by the patients.24 MIP and MEP tests were performed according to the manufacturer's guide. Prior to performing the MIP test, the patient is seated comfortably in an air‐conditioned examination room, and informed about the course of the procedure. Subsequently, the patient is asked to exhale ‘to residual volume’, and afterwards, to perform a forced inhalation against the device with as much effort as possible for as long as possible (minimum 2 s). The result of the test is the maximum average inspiratory pressure sustained over a 1 s period of the test, in centimetres of water (cm H2O). The measurement of MEP is analogous, but during the procedure, the patient firstly inhales ‘to total lung capacity’, and then performs a forced exhalation manoeuvre. Similarly to MIP, the result of MEP test is presented in cm H2O. We measured both MIP and MEP five times, and the average values of five tests were used for the following analyses.
Spirometry test
Standard spirometry tests were performed using the cardiopulmonary exercise system Ergostik combined with Blue Cherry diagnostic software (Geratherm Respiratory). The system meets the American Thoracic Society (ATS)/European Respiratory Society (ERS) standards for the assessment of cardiorespiratory function. Spirometry tests were conducted in an air‐conditioned examination room, and patients were thoroughly informed about the course of the procedure before the first test. Spirometry tests were performed according to ATS/ERS guidelines,25 and before each test, the Ergostik system was calibrated according to manufacturer's instructions. In this study, we analysed the following spirometry parameters: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and peak expiratory flow (PEF). All spirometry parameters were presented and analysed as a percentage of predicted value. For each patient, we aimed to obtain two to three comparable tests with sufficient compliance and satisfactory quality of data (parameters meeting ATS/ERS criteria), and for each of aforementioned parameters, the average value (percentage of predicted value) from these two to three tests was used for the following analyses.
Physical fitness
Physical fitness was evaluated using selected physical tasks from the Functional Fitness Test (FFT) for Older Adults.26, 27, 28 For the purpose of this study, we used the following elements of FFT (the remaining two components of FFT assess the patient's lower and upper body flexibility and were not analysed):
‘Up and go’ (assessment of agility and dynamic balance)—patient is asked to stand up from a chair as fast as possible, walk around a pole placed within 2.44 m, and return to the baseline position. The time of the test is measured with an accuracy of 0.1 s.
‘Chair stand’ (assessment of lower body strength)—patient repeats full stands from a chair within 30 s (arms are folded across the chest). The result of the test is the number of completed full stands.
‘Arm curl’ (assessment of upper body strength)—patient repeats 3.5 kg weight lifts with his forearm within 30 s. At baseline, examined person is sitting on a chair with a weight in the dominant forearm in an intermediate position. The patient is asked to lift the weight by flexion and pronation of the forearm and then return to the baseline position. The number of completed full lifts is the result of the test.
Two‐minute step test (2MST; assessment of aerobic endurance) ‐ the patient marches lifting his knees to the appropriate height, defined as the midway between the patella and iliac crest. The patient is asked to perform as many steps as possible (reaching the predefined height) within 2 min. During the test, examined subjects are allowed to hold on to a stable table to improve their balance during the test. The result of the test is the number of correctly completed steps.
Skeletal muscle mass
Skeletal muscle mass in examined men with HFrEF was evaluated using total‐body dual‐energy X‐ray absorptiometry (DXA) measurement (Discovery DXA system, Hologic). DXA is a widely applied, non‐invasive method for the assessment of body composition. It allows to precisely measure both lean and fat mass in different body regions, and lean body mass in DXA scans reflects skeletal muscle mass. Total‐body DXA has been widely applied in the research regarding muscle wasting (sarcopenia) in HF patients.29, 30 For the purpose of the study, we analysed appendicular lean mass/body mass index (ALM/BMI) (m2) (appendicular lean mass = lean mass of lower and upper limbs).30
Haematological parameters, iron status, and other laboratory measurements in peripheral blood
In all participants, venous blood samples were taken in the morning following an overnight fast. Haematological measurements were made in fresh venous blood with EDTA. Haemoglobin concentration (Hb), red cell indices, and reticulocytes were measured using the ADVIA 2120 system (Siemens). Anaemia was defined according to World Health Organization as haemoglobin concentration <12 g/dL in women and <13 g/dL in men.31
The following blood biomarkers/parameters reflecting iron metabolism were measured directly (from fresh venous blood): serum ferritin (μg/L), iron (mg/dL), and total iron binding capacity (TIBC, mg/dL). Transferrin saturation (TSAT) was calculated as the ratio of serum iron (mg/dL) and TIBC (mg/dL) multiplied by 100 and expressed as a percentage. Serum ferritin was measured using an immunoassay based on electrochemiluminescence with the Elecsys 2010 system (Roche). Serum iron and TIBC were assessed using a substrate method with the Konelab Prime 60i system (Thermo Scientific). ID was defined as serum ferritin level <100 μg/L or serum ferritin 100–299 μg/L in combination with a TSAT <20%.8 Serum soluble transferrin receptor (sTfR; mg/L) was measured from frozen serum (after centrifuging, serum was frozen at −70°C until further measurements) using immunonephelometry with BN II System (Siemens). Additionally, the following parameters obtained from automated blood count (ADVIA 2120 automated system) were considered indices of iron status: reticulocyte haemoglobin content (CHR, pg) and hypochromic red cells (PHRC, %).8
Plasma level of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP; pg/mL) was measured using an immunoassay based on chemiluminescence with Dimension RxL system (Siemens).
Serum level of high‐sensitivity C‐reactive protein (hs‐CRP; mg/L) was assessed using immunonephelometry with BN II System (Siemens).
Estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the Modification of Diet in Renal Disease equation.32
Statistical analyses
Most continuous variables had a normal distribution, and were expressed as a mean ± standard deviation of the mean. NT‐proBNP, hs‐CRP, ferritin, and PHRC had a skewed distribution, and were log‐transformed (a natural logarithm, ln) before further inclusion in linear regression analyses. These variables were expressed as a median with an interquartile range. Categorized variables were expressed as a number and percentage. The intergroup differences were tested using the Mann–Whitney U‐test for unpaired samples.
In the first part of statistical analyses we performed univariable linear regression analyses to establish the relationships between respiratory muscle function (MIP, MEP) and spirometry parameters (FVC, FEV1, PEF), and (i) clinical parameters (demographics, ALM/BMI, aetiology and severity of HFrEF), (ii) laboratory and haematological parameters, (iii) indices of iron status, (iv) comorbidities, and (v) administered pharmacotherapy. Further, we constructed a set of multivariable linear regression models to establish the associations between inspiratory muscle strength (MIP as dependent variable in the model) and haematological parameters, and indices of iron status (the latter continuous and categorized) after adjustment for significant (P < 0.05) correlates of MIP from univariable analyses.
Further, we performed univariable linear regression analyses to establish the relationships between the indices of physical fitness (quantitative results of FFT) and (i) clinical parameters (demographics, ALM/BMI, aetiology and severity of HFrEF), (ii) laboratory and haematological parameters, (iii) indices of iron status, (iv) comorbidities, (v) pharmacotherapy, and (vi) respiratory muscle function (MIP, MEP, and spirometry parameters). Subsequently, we constructed a set of multivariable linear regression models to establish the associations between the indices of physical fitness (quantitative results of FFT as dependent variable in the model) and inspiratory muscle strength after adjustment for significant (P < 0.05) correlates of the results of FFT from univariable analyses.
P‐value of <0.05 was considered statistically significant. Statistical analyses were performed using the STATISTICA 13.1 data analysis software system (Statistica).
Results
Respiratory muscle strength, spirometry parameters, and appendicular lean mass in men with HFrEF vs. controls and in HFrEF patients with vs. without ID
Baseline characteristics of 53 examined men with HFrEF are presented in Table 1.
Table 1.
Baseline characteristics of examined men with HFrEF
| Parameter | N | Baseline characteristics |
|---|---|---|
| Age (years) | 53 | 64 ± 10 |
| Body mass index (kg/m2) | 53 | 29.3 ± 4.5 |
| Appendicular lean mass/body mass index (m2) | 41 | 0.81 ± 0.10 |
| New York Heart Association class (I/II/III) | 53 | 19 (36%)/27 (51%)/7 (13%) |
| Heart failure aetiology, ischaemic (n, %) | 53 | 44 (83%) |
| Left ventricular ejection fraction (%) | 53 | 31 ± 7 |
| Left ventricular end‐diastolic diameter (mm) | 53 | 65 ± 8 |
| Moderate or severe mitral regurgitation (n, %) | 53 | 12 (23%) |
| High‐sensitive CRP (mg/L) | 53 | 1.60 (0.64–4.93) |
| Plasma NT‐proBNP (pg/mL) | 53 | 625 (257–1129) |
| eGFR (mL/min/1.73m2) | 53 | 84 ± 23 |
| Haematological parameters and indices of iron status | ||
| Haemoglobin concentration (g/dL) | 53 | 14.4 ± 1.2 |
| Mean corpuscular haemoglobin (pg) | 53 | 30 ± 2 |
| Iron deficiency (n, %) | 53 | 30 (57%) |
| Serum iron (μg/dL) | 53 | 84 ± 27 |
| Serum ferritin (μg/L) | 53 | 114 (80–160) |
| Soluble transferrin receptor (mg/L) | 49 | 1.37 ± 0.51 |
| Transferrin saturation (%) | 53 | 24 ± 7 |
| Reticulocyte haemoglobin content (pg) | 53 | 32 ± 2 |
| Hypochromic red cells (%) | 52 | 0.6 (0.3–1.6) |
| Comorbidities | ||
| Coronary artery disease, yes (n, %) | 53 | 46 (87%) |
| Arterial hypertension, yes (n, %) | 53 | 30 (57%) |
| Peripheral artery disease, yes (n, %) | 53 | 8 (15%) |
| Atrial fibrillation, yes (n, %) | 53 | 5 (9%) |
| Diabetes mellitus, yes (n, %) | 53 | 17 (32%) |
| Previous stroke or transient ischaemic attack | 53 | 4 (8%) |
| Treatment and devices | ||
| ICD/CRT (n, %) | 53 | 27 (51%)/6 (11%) |
| ACE‐I or ARB, yes (n, %) | 53 | 51 (96%) |
| β‐blocker, yes (n, %) | 53 | 52 (98%) |
| Aldosterone antagonist, yes (n, %) | 53 | 47 (89%) |
| Ivabradine, yes (n, %) | 53 | 7 (13%) |
| Oral diuretic, yes (n, %) | 53 | 29 (55%) |
| Antiplatelet drug (n, %) | 53 | 44 (83%) |
| Statin (n, %) | 53 | 50 (94%) |
| Physical fitness | ||
| ‘Up and go’ test (s) | 50 | 6 ± 1 |
| ‘Chair stand’ test — 30 s (repetitions) | 50 | 16 ± 4 |
| ‘Arm curl’ test (repetitions) | 50 | 17 ± 4 |
| 2‐min step test (steps) | 51 | 83 ± 16 |
| Respiratory muscle function | ||
| MIP (cm H2O) | 53 | 74 ± 23 |
| MEP (cm H2O) | 53 | 122 ± 23 |
| FVC (% of predicted value) | 51 | 101 ± 26 |
| FEV1 (% of predicted value) | 51 | 89 ± 28 |
| PEF (% of predicted value) | 51 | 66 ± 27 |
Data are presented as a mean ± standard deviation of the mean, a median (with an interquartile range) or number (with percentage), where appropriate. N, number of patients for whom the parameter was available. Abbreviations: HFrEF, heart failure with reduced left ventricular ejection fraction; CRP, C‐reactive protein; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; CRT, cardiac resynchronization therapy; ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; FVC, forced vital capacity, FEV‐1, forced expiratory volume in 1 s; PEF, peak expiratory flow. For details, see the ‘Methods’ section.
A control group composed of 10 middle‐aged (55 ± 5 years) healthy men (without cardiovascular disease) with BMI 27.8 ± 3.1 kg/m2. DXA scans were available for 41 patients and 8 healthy subjects only, and statistical analyses/models including ALM/BMI are limited to this number of enrolees. Due to low compliance during the procedure, and therefore unreliable data, we excluded spirometry of two patients from the following analyses.
MIP, MEP, and ALM/BMI, but not MIP/(ALM/BMI) were lower in men with HFrEF vs. healthy men (Figure 1). MIP, MEP, and MIP/(ALM/BMI) (but not ALM/BMI) were lower in men with HFrEF with vs. without ID (Figure 1).
Figure 1.
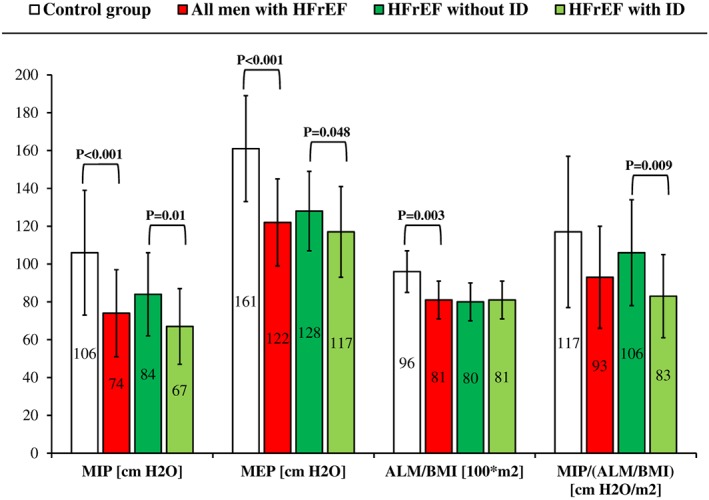
Respiratory muscle strength and skeletal muscle mass in men with heart failure with reduced left ventricular ejection fraction (HFrEF) vs. healthy men, and in men with HFrEF without vs. with iron deficiency (ID). P‐value is presented for significant inter‐group differences only. MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; ALM/BMI, appendicular lean mass/body mass index. For details, see the ‘Methods’ section.
There were no differences regarding spirometry parameters in either men with HFrEF vs. controls or patients with vs. without ID (all P > 0.05).
Clinical and laboratory predictors of respiratory muscle strength and spirometry parameters
In univariable linear regression analyses, MIP correlated with MEP (r = 0.49, P < 0.001), FEV1 (r = 0.39, P = 0.005), and PEF (r = 0.31, P = 0.03), but not with FVC (P = 0.1). MEP correlated with PEF (r = 0.31, P = 0.03), but neither FVC nor FEV1 (all P > 0.3). Spirometry parameters correlated with each other (all r ≥ 0.44, all P ≤ 0.001).
In univariable linear regression analyses, MIP was related to (all P < 0.05): LVEF (r = 0.31), ALM/BMI (r = 0.35), NT‐proBNP (r = −0.30), Hb (r = 0.36), mean corpuscular haemoglobin (MCH, r = 0.36), the presence of ID (r = −0.38), serum ferritin (r = 0.42), sTfR (r = −0.31), CHR (r = 0.45), and PHRC (r = −0.30). MIP was not related to: age, BMI, NYHA class, hs‐CRP, eGFR, comorbidities, and medications (all P > 0.05). MEP correlated neither with clinical nor laboratory parameters (all P > 0.05). In univariable linear regression analyses, FVC was related to (all P < 0.05): BMI (r = −0.42), MCH (r = 0.28), CHR (r = 0.38), PHRC (r = −0.32), sTfR (r = −0.37), and concomitant diabetes mellitus (r = −0.28); FEV1 ‐ MCH (r = 0.28), CHR (r = 0.37), PHRC (r = −0.39), sTfR (r = −0.34), and atrial fibrillation (r = 0.31); PEF ‐ Hb (r = 0.28), MCH (r = 0.29), CHR (r = 0.31), and the use of aldosterone antagonist (r = 0.36).
In multivariable linear regression models, lower CHR was related to lower MIP, when adjusted for either ALM/BMI or relevant clinical predictors of MIP from univariable analyses (LVEF, NT‐proBNP, and Hb) (Table 2). Eventually, in multivariable linear regression model, lower serum ferritin was associated with lower MIP independently of ALM/BMI, LVEF, NT‐proBNP, and Hb (Table 2).
Table 2.
Relationships between inspiratory muscle strength and haematological parameters and indices of iron status in men with HFrEF
| Applied adjustment | Haemoglobin concentration (g/dL) | Mean corpuscular haemoglobin (pg) | Iron deficiency (yes vs. no) | Serum iron (μg/dL) | Serum ferritin (ln μg/L) | Serum ferritin ≥100 vs. <100 μg/L | Transferrin saturation (%) | Transferrin saturation ≥20 vs. <20% | Serum soluble transferrin receptor (mg/L) | Reticulocyte haemoglobin content (pg) | Hypochromic red cells (ln %) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIP (cm H2O) as dependent variable | None (unadjusted) | 0.36** | 0.36** | −0.38** | — | 0.42** | 0.42** | — | — | −0.31* | 0.45** | −0.30* |
| ALM/BMI | 0.37* | 0.32* | −0.39** | — | 0.41** | 0.45** | — | — | — | 0.40** | — | |
| LVEF | 0.33* | 0.30* | −0.33* | — | 0.38** | 0.37** | — | — | — | 0.41** | — | |
| NT‐proBNP (ln) | 0.29* | 0.30* | −0.31* | — | 0.35* | 0.36* | — | — | — | 0.39** | — | |
| Haemoglobin concentration | — | — | 0.34* | 0.34* | — | — | — | 0.37** | — | |||
| LVEF, NT‐proBNP (ln), haemoglobin concentration | — | — | 0.33* | 0.30* | — | — | — | 0.34* | — | |||
| ALM/BMI, LVEF, NT‐proBNP (ln), haemoglobin concentration | — | — | 0.37* | 0.37* | — | — | — | — | — |
Data are presented as standardized regression coefficients β (both in univariable and multivariable models). Abbreviations: HFrEF, heart failure with reduced left ventricular ejection fraction; MIP, maximal inspiratory pressure; ln, natural logarithm; ALM/BMI, appendicular lean mass/body mass index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide. For details, see the ‘Methods’ section.
P < 0.05,
P < 0.01,
P < 0.001.
Respiratory muscle function, spirometry parameters, and physical fitness in men with HFrEF
The associations between clinical variables (including parameters of iron status), respiratory muscle function, and physical fitness (results of FFT) are presented in Table 3. In univariable linear regression analyses, lower ferritin and CHR, and higher sTfR and PHRC were associated with worse results of ‘Up and go’, ‘Chair stand’, and 2MST (Table 3). TSAT and serum iron were not associated with indices of physical fitness (Table 3). Lower MIP (but not MEP and spirometry parameters) correlated with worse results in FFT (results of ‘Up and go’, ‘Chair stand’, ‘Arm curl’, and 2MST) (Table 3). In multivariable linear regression models, lower MIP was associated with worse results in ‘Up and go’, ‘Arm curl’, and 2MST, when adjusted for either ALM/BMI or other determinants of physical fitness from univariable analyses (Table 4).
Table 3.
Relationships between clinical variables, respiratory muscle function, and physical fitness in men with HFrEF.
| Variables | ‘Up and go’ test (s) | ‘Chair stand’ test ‐ 30 s (repetitions) | ‘Arm curl’ test (repetitions) | 2‐min step test (steps) |
|---|---|---|---|---|
| Age (years) | 0.43** | — | −0.45** | — |
| Body mass index (kg/m2) | — | — | — | — |
| Appendicular lean mass/body mass index (m2) | −0.53*** | 0.47** | 0.39* | 0.51** |
| New York Heart Association class (1 class) | 0.32* | −0.39** | −0.48*** | −0.32* |
| Heart failure aetiology, ischaemic (yes vs. no) | — | — | — | — |
| Left ventricular ejection fraction (%) | — | — | 0.28* | — |
| Left ventricular end‐diastolic diameter (mm) | — | — | — | — |
| Moderate or severe mitral regurgitation (yes vs. no) | — | −0.30* | — | −0.26* |
| High‐sensitive CRP (ln mg/L) | — | — | — | — |
| Plasma NT‐proBNP (ln pg/mL) | 0.43** | −0.46*** | −0.51*** | −0.28* |
| eGFR (mL/min/1.73m2) | −0.46*** | 0.44** | 0.52*** | 0.29* |
| Haematological parameters and indices of iron status | ||||
| Haemoglobin concentration (g/dL) | −0.31* | 0.37** | 0.32* | 0.40** |
| Mean corpuscular haemoglobin (pg) | −0.41** | 0.29* | — | — |
| Iron deficiency (yes vs. no) | — | — | −0.32* | — |
| Serum iron (μg/dL) | — | — | — | — |
| Serum ferritin (ln μg/L) | −0.37** | 0.41* | 0.50*** | 0.42** |
| Transferrin saturation (%) | — | — | — | — |
| Serum soluble transferrin receptor (mg/L) | 0.48** | −0.37* | — | −0.38** |
| Reticulocyte haemoglobin content (pg) | −0.44** | 0.38** | — | 0.37** |
| Hypochromic red cells (ln %) | 0.42** | −0.53*** | — | −0.38** |
| Comorbidities | ||||
| Coronary artery disease (yes vs. no) | — | — | — | — |
| Arterial hypertension (yes vs. no) | — | — | — | — |
| Peripheral artery disease (yes vs. no) | — | −0.27* | −0.28* | — |
| Atrial fibrillation (yes vs. no) | — | — | — | 0.27* |
| Diabetes mellitus (yes vs. no) | — | — | −0.27* | — |
| Previous stroke or transient ischaemic attack (yes vs. no) | — | — | — | — |
| Pharmacotherapy | ||||
| ACE‐I or ARB (yes vs. no) | — | — | — | — |
| β‐blocker (yes vs. no) | — | — | — | — |
| Aldosterone antagonist (yes vs. no) | — | 0.32* | 0.28* | 0.37** |
| Ivabradine (yes vs. no) | — | — | — | — |
| Oral diuretic (yes vs. no) | 0.37** | — | −0.45** | −0.38** |
| Antiplatelet drug (yes vs. no) | 0.28* | −0.33 | — | −0.45*** |
| Statin (yes vs. no) | — | — | — | — |
| Respiratory muscle function | ||||
| MIP (cm H2O) | −0.51*** | 0.38** | 0.50*** | 0.56*** |
| MEP (cm H2O) | — | — | — | — |
| FVC (% of predicted value) | — | — | — | — |
| FEV1 (% of predicted value) | — | — | — | — |
| PEF (% of predicted value) | — | — | — | — |
Data are presented as standardized regression coefficients β (univariable analyses). Abbreviations: HFrEF, heart failure with reduced left ventricular ejection fraction; CRP, C‐reactive protein; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; CRT, cardiac resynchronization therapy; ACE‐I angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow. For details, see the ‘Methods’ section.
P < 0.05,
P < 0.01,
P < 0.001.
Table 4.
Relationships between inspiratory muscle strength and physical fitness in men with HFrEF
| Dependent variable reflecting physical fitness | Relationship with MIP (cm H2O) | |||||
|---|---|---|---|---|---|---|
| Adjustment: ALM/BMI | Adjustment: ALM/BMI, NYHA class | Adjustment: ALM/BMI, eGFR | Adjustment: ALM/BMI, haemoglobin concentration | Adjustment: ALM/BMI, NT‐proBNP (ln) | Adjustment: NYHA class, eGFR, haemoglobin concentration and NT‐proBNP (ln) | |
| ‘Up and go’ test (s) | −0.37** | −0.35* | −0.27* | — | −0.33* | −0.38** |
| ‘Chair stand’ test ‐ 30 s (repetitions) | — | — | — | — | — | — |
| ‘Arm curl’ test (repetitions) | 0.41** | 0.36* | 0.36* | 0.33* | 0.33* | 0.32** |
| 2‐min step test (steps) | 0.43** | 0.42** | 0.41** | 0.31* | 0.40** | 0.42** |
Data are presented as standardized regression coefficients β (multivariable analyses). Abbreviations: HFrEF, heart failure with reduced left ventricular ejection fraction; MIP, maximal inspiratory pressure; ALM/BMI, appendicular lean mass/body mass index; NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; ln, natural logarithm. For details, see the ‘Methods’ section.
P < 0.05,
P < 0.01,
P < 0.001.
Discussion
There are two major findings arising from our study: (i) in men with HFrEF, low serum ferritin reflecting depleted iron stores is associated with inspiratory muscle weakness (as reflected by lower MIP) independently of skeletal muscle mass and clinical variables, (ii) inspiratory muscle weakness adjusted for skeletal muscle mass correlates with decreased physical fitness in these patients.
Since the introduction of ‘the muscle hypothesis’,1 skeletal and respiratory myopathy has been considered an important pathophysiological feature of chronic symptomatic HF. Pathological process affecting muscle tissue is not only responsible for increased exertional dyspnoea and fatigue in these patients, but also constitutes the key element of the vicious cycle of HF. Importantly, striated muscle myopathy (as reflected by both morphological and functional alterations) is visible on different levels of tissue organization (from overall muscle mass to sub‐cellular organella such as mitochondria).11, 33 Although in patients with HF both respiratory muscle strength (as reflected by MIP) and endurance (assessed by maximal sustainable ventilatory capacity) are significantly reduced, precise pathomechanisms underlying specific respiratory myopathy in the course of this disease syndrome are largely unknown.2, 4, 6, 11 More than two decades ago Mancini et al.3 have shown using non‐invasive near‐infrared spectroscopy that in patients with HF (non‐smokers without known lung disease) accessory respiratory muscle (serratus anterior) deoxygenation occurs during maximal bicycle exercise, which is suggestive of the underperfusion process. In another study21 regarding patients with chronic HFrEF, it has been shown that beyond increased accessory respiratory muscle deoxygenation, there is also an increased work of the diaphragm at rest and at peak exercise on bicycle ergometer. Furthermore, the authors have demonstrated that different parameters reflecting the function of respiratory muscles (MIP, MEP, diaphragmatic work, FEV1) were closely associated with the perception of dyspnoea during exercise as assessed using the Borg scale.21
From the clinical perspective, in patients with HF, concomitant ID has been traditionally perceived as an aetiological factor of anaemia.34 In cross‐sectional analyses, there has also been demonstrated the relationship between the presence of ID and the severity of HF (more severe symptoms, higher circulating natriuretic peptides), and between ID and lower body mass index.35 The key finding of the current study is that in men with HFrEF, lower serum ferritin reflecting depleted iron stores is associated with inspiratory muscle weakness (lower MIP) independently of Hb concentration, severity of left ventricular systolic dysfunction (LVEF), magnitude of neurohormonal activation (NT‐proBNP level), and skeletal muscle mass (ALM/BMI). Apart from serum ferritin level, which is a reliable surrogate of total iron stores, similar associations were observed for CHR (even after adjustment for Hb), which is another indicator of iron restriction.8 Being the key component of oxidative enzymes and respiratory chain proteins within the mitochondria, iron plays the prominent role in oxidative energy metabolism and several intracellular processes.36 Recently, we established the hypothesis that skeletal and respiratory muscle dysfunction due to ID constitutes a potential pathophysiological link between impaired iron status and decreased exercise capacity in patients with chronic diseases such as HF or COPD.11 It needs to be acknowledged that ID predicts diminished exercise capacity in HF patients independently of decreased haemoglobin concentration,10 and beneficial effects of intravenous iron therapy (ferric carboxymaltose) in HFrEF (improved exercise capacity) have been reported for both anaemic and non‐anaemic enrolees.37, 38 Importantly, we have demonstrated that lower ferritin is related to lower MIP independently of appendicular skeletal muscle mass (ALM/BMI). Inspiratory muscle weakness associated with depleted iron stores should therefore be considered independent of the skeletal muscle mass loss.
In this study, we have shown that impaired iron status (low ferritin and CHR, high sTfR and PHRC) is associated with worse physical fitness as assessed using different components of FFT. These results are consistent with a recent cross‐sectional study investigating the relationships between iron status and submaximal exercise capacity in more than 500 patients with stable HF with either reduced or preserved LVEF.39 In cited study, the authors have demonstrated, that the presence of ID, higher ferritin index (sTfR/log10 ferritin) and sTfR, and lower TSAT were associated with a shorter distance covered in a 6‐min walking test. Importantly, in the current study, we have also demonstrated that decreased inspiratory muscle strength (lower MIP) is an independent predictor of decreased physical fitness in men with HFrEF. It needs to be emphasized that selected elements of FFT (‘Up and go’, ‘Arm curl’, 2MST) imitate patient's daily physical activities better than maximal exercise tests (e.g. cardiopulmonary exercise test on a treadmill). Indeed, patients with symptomatic HF experience reduced functional capacity even during daily standard activities, which in fact require only submaximal physical effort. Therefore, for these patients, the ability to perform repeated submaximal exercises (important in everyday functioning) is much more important than peak (maximal) exercise capacity.40 Our study contributes to the knowledge on the associations between respiratory muscle dysfunction (decreased inspiratory muscle strength) and the symptomatology of an overt HF (decreased functional capacity). These associations were independent of skeletal muscle mass (ALM/BMI), overall HF symptoms (NYHA class), neurohormonal activation (plasma NT‐proBNP), renal dysfunction (eGFR), or anaemia (Hb concentration). Our results suggest that the relationship between inspiratory muscle weakness and worse physical fitness is not simply parallel with either the process of skeletal muscle mass loss or the advancement of primary cardiac disease.
It needs to be emphasized that neither expiratory muscle strength (as reflected by average MEP value) nor spirometry parameters (the measurement of FEV1 and PEF is also based on the expiration manoeuvre) correlated with physical fitness in these patients. As mentioned earlier, from the physiological perspective, MIP reflects the strength of the diaphragm,22 which is the key muscle involved in the process of breathing in humans and larger mammals.4, 23 Indeed, in patients with HF, neuro‐muscular abnormalities regarding diaphragm (related to cardiac disease) are considered primary pathophysiological mechanism responsible for inspiratory dysfunction.23 It is worth noting that these abnormalities frequently overlap with those related to the process of aging or comorbidities, for example, COPD.23 In the current study, inspiratory muscle weakness was associated with lower serum ferritin (reflecting depleted iron stores), but not with TSAT (index of iron availability for metabolizing tissues). The hypothesis of whether diaphragmatic dysfunction and subsequent inspiratory muscle weakness are associated exclusively with an absolute depletion of iron stores, but are not affected by functional ID (restricted delivery of iron to dedicated tissues), needs further studies.
Conclusions
In men with HFrEF, depleted iron stores (as reflected by low serum ferritin) are associated with inspiratory muscle weakness independently of skeletal muscle mass. Importantly, the dysfunction of inspiratory muscles in these patients is associated with worse physical fitness independently of either skeletal muscle mass or disease severity. The results of our study support the hypothesis that inspiratory muscle dysfunction due to decreased iron stores can partially explain decreased functional capacity in iron‐deficient men with HFrEF.
Study limitations
We examined relatively small number of subjects with HF, and the control group was younger than patients. Furthermore, we examined men with HFrEF only and there is no data regarding either women or remaining strata of LVEF (mid‐range and preserved). Moreover, DXA scans were available for 41 patients and 8 healthy men (control group) only, and therefore, statistical analyses with ALM/BMI do not include all examined patients. It is worth mentioning that pathophysiological associations between inspiratory muscle weakness and lower ferritin require confirmation in an interventional study with the assessment of MIP before and after a period of intravenous iron therapy.
Disclosures
Wroclaw Medical University received an unrestricted grant from Vifor Pharma. M.D. reports personal fees from Vifor Pharma and Fresenius. W.B. reports personal fees from Vifor Pharma. P.P. reports grants and personal fees from Vifor Pharma and Fresenius, and personal fees from AMGEN. E.A.J. reports grants and personal fees from Vifor Pharma and Fresenius. Other authors have nothing to disclose.
Acknowledgements
This research was financially supported by the National Science Centre (Kraków, Poland) grant allocated on the basis of the decision number DEC‐2012/05/E/NZ5/00590. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.41
Tkaczyszyn, M. , Drozd, M. , Węgrzynowska‐Teodorczyk, K. , Flinta, I. , Kobak, K. , Banasiak, W. , Ponikowski, P. , and Jankowska, E. A. (2018) Depleted iron stores are associated with inspiratory muscle weakness independently of skeletal muscle mass in men with systolic chronic heart failure. Journal of Cachexia, Sarcopenia and Muscle, 9: 547–556. doi: 10.1002/jcsm.12282.
References
- 1. Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol 1996;28:2255–2262. [DOI] [PubMed] [Google Scholar]
- 2. Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kübler W, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 2001;103:2153–2158. [DOI] [PubMed] [Google Scholar]
- 3. Mancini DM, Ferraro N, Nazzaro D, Chance B, Wilson JR. Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near‐infrared spectroscopy. J Am Coll Cardiol 1991;18:492–498. [DOI] [PubMed] [Google Scholar]
- 4. Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest 1990;98:1091–1094. [DOI] [PubMed] [Google Scholar]
- 5. Lindsay DC, Lovegrove CA, Dunn MJ, Bennett JG, Pepper JR, Yacoub MH, et al. Histological abnormalities of muscle from limb, thorax and diaphragm in chronic heart failure. Eur Heart J 1996;17:1239–1250. [DOI] [PubMed] [Google Scholar]
- 6. Mancini DM, Henson D, LaManca J, Levine S. Evidence of reduced respiratory muscle endurance in patients with heart failure. J Am Coll Cardiol 1994;24:972–981. [DOI] [PubMed] [Google Scholar]
- 7. Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation 1995;91:320–329. [DOI] [PubMed] [Google Scholar]
- 8. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582.e3. [DOI] [PubMed] [Google Scholar]
- 10. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 11. Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail 2016;18:762–773. [DOI] [PubMed] [Google Scholar]
- 12. Ackrell BA, Maguire JJ, Dallman PR, Kearney EB. Effect of iron deficiency on succinate‐ and NADH‐ubiquinone oxidoreductases in skeletal muscle mitochondria. J Biol Chem 1984;259:10053–10059. [PubMed] [Google Scholar]
- 13. Cartier LJ, Ohira Y, Chen M, Cuddihee RW, Holloszy JO. Perturbation of mitochondrial composition in muscle by iron deficiency. Implications regarding regulation of mitochondrial assembly. J Biol Chem 1986;261:13827–13832. [PubMed] [Google Scholar]
- 14. Mackler B, Grace R, Finch CA. Iron deficiency in the rat: effects on oxidative metabolism in distinct types of skeletal muscle. Pediatr Res 1984;18:499–500. [DOI] [PubMed] [Google Scholar]
- 15. Finch CA, Miller LR, Inamdar AR, Person R, Seiler K, Mackler B. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest 1976;58:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron‐depleted, nonanemic women. J Appl Physiol (1985) 2000;88:1103–1111. [DOI] [PubMed] [Google Scholar]
- 17. Hinton PS, Sinclair LM. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron‐deficient nonanemic athletes. Eur J Clin Nutr 2007;61:30–39. [DOI] [PubMed] [Google Scholar]
- 18. Brutsaert TD, Hernandez‐Cordero S, Rivera J, Viola T, Hughes G, Haas JD. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron‐depleted, nonanemic women. Am J Clin Nutr 2003;77:441–448. [DOI] [PubMed] [Google Scholar]
- 19. Brownlie T 4th, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 2004;79:437–443. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21. Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation 1992;86:909–918. [DOI] [PubMed] [Google Scholar]
- 22. Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med 1994;149:430–438. [DOI] [PubMed] [Google Scholar]
- 23. Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev 2017;22:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ATS/ERS Statement on respiratory muscle testing . American Thoracic Society/European Respiratory Society. Am J Respir Crit Care Med 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- 25. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 26. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community‐residing older adults. J Aging Phys Act 1999;7:129–161. [Google Scholar]
- 27. Wegrzynowska‐Teodorczyk KL, Rudzińska E, Jankowska E, Grzesło A, Nowakowska K, Lazorczyk M, et al. Determinants of physical fitness in males with systolic heart failure. Kardiol Pol 2010;68:146–154. [PubMed] [Google Scholar]
- 28. Węgrzynowska‐Teodorczyk K, Mozdzanowska D, Josiak K, Siennicka A, Nowakowska K, Banasiak W, et al. Could the two‐minute step test be an alternative to the six‐minute walk test for patients with systolic heart failure? Eur J Prev Cardiol 2016;23:1307–1313. [DOI] [PubMed] [Google Scholar]
- 29. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 30. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016;222:41–46. [DOI] [PubMed] [Google Scholar]
- 31. Blanc B, Finch CA, Hallberg L, Lawkowicz W, Layrisse M, Mollin DL. Nutritional anaemias. Report of a WHO scientific group. WHO Tech Rep Ser 1968;405:1–40. [PubMed] [Google Scholar]
- 32. Levey ASL, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 33. Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev 2013;18:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol 2011;8:485–493. [DOI] [PubMed] [Google Scholar]
- 35. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872–1880. [DOI] [PubMed] [Google Scholar]
- 36. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131:568S–579S. [DOI] [PubMed] [Google Scholar]
- 37. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 38. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Enjuanes C, Bruguera J, Grau M, Cladellas M, Gonzalez G, Meroño O, et al. Iron status in chronic heart failure: impact on symptoms, functional class and submaximal exercise capacity. Rev Esp Cardiol (Engl Ed) 2016;69:247–255. [DOI] [PubMed] [Google Scholar]
- 40. Clark AL, Poole‐Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 1996;28:1092–1102. [DOI] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


