Abstract
Various types of vaccines have been proposed as approaches for prevention or delay of the onset of cancer by boosting the endogenous immune system. We previously developed a senescent‐cell‐based vaccine, induced by radiation and veliparib, as a preventive and therapeutic tool against triple‐negative breast cancer. However, the programmed death receptor‐1/programmed death ligand‐1 (PD‐1/PD‐L1) pathway was found to play an important role in vaccine failure. Hence, we further developed soluble programmed death receptor‐1 (sPD1)‐expressing senescent cells to overcome PD‐L1/PD‐1‐mediated immune suppression while vaccinating to promote dendritic cell (DC) maturity, thereby amplifying T‐cell activation. In the present study, sPD1‐expressing senescent cells showed a particularly active status characterized by growth arrest and modified immunostimulatory cytokine secretion in vitro. As expected, sPD1‐expressing senescent tumor cell vaccine (STCV/sPD‐1) treatment attracted more mature DC and fewer exhausted‐PD1+ T cells in vivo. During the course of the vaccine studies, we observed greater safety and efficacy for STCV/sPD‐1 than for control treatments. STCV/sPD‐1 pre‐injections provided complete protection from 4T1 tumor challenge in mice. Additionally, the in vivo therapeutic study of mice with s.c. 4T1 tumor showed that STCV/sPD‐1 vaccination delayed tumorigenesis and suppressed tumor progression at early stages. These results showed that STCV/sPD‐1 effectively induced a strong antitumor immune response against cancer and suggested that it might be a potential strategy for TNBC prevention.
Keywords: immunotherapy, senescent cell, soluble PD‐1, triple‐negative breast cancer, vaccine
Abbreviations
- DC
dendritic cell
- ER
estrogen receptor
- IFN‐γ
interferon‐γ
- IL‐10
interleukin‐10
- IL‐12
interleukin‐12
- IL‐17
interleukin‐17
- PD‐1
programmed death receptor‐1
- PD‐L1
programmed death ligand‐1
- PR
progesterone receptor
- sPD‐1
soluble programmed death receptor‐1
- STCV
senescent tumor cell vaccine
- TGF‐β
transforming growth factor‐β
- TME
tumor microenvironment
- TNBC
triple‐negative breast carcinoma
- TNF‐α
tumor necrosis factor‐α
1. INTRODUCTION
Breast cancer is the most common cancer in women globally, with an annual incidence of approximately 170 million, according to the International Agency for Research on Cancer (IACR).1 Breast cancer therapy is guided by clinicopathological information that mainly reflects molecular features of cells.2 In recent years, combination approaches including chemotherapy, surgery, radiotherapy, targeted therapy and hormonal therapy have proven more successful than conventional single treatment methods.3 However, patients with TNBC, characterized by lack of expression of human epithelial growth factor receptor‐2, estrogen, progesterone receptor (ER−, PR−, Her2/neu−) have poor prognosis.4, 5 This is because therapy for TNBC patients remains limited.
More recently, immunotherapy has been reported to be associated with favorable outcomes for patients with carcinoma.6, 7, 8 Vaccination is a therapeutic modality that stimulates endogenous immune responses against carcinoma.9, 10 After vaccination, cytokines are required to improve tumor antigen presentation for priming and enhancing T‐cell response against cancer cells. As a result of high metabolic rates or low expression of cytokines, tumor vaccines, including peptides, tumor‐associated antigens and inactivated tumor cells, usually result in low efficacy.10, 11
Cell senescence is a special active state characterized by irreversible growth arrest and modified immunostimulatory cytokine secretion.12, 13, 14, 15 Cellular senescence is caused not only by telomere shortening after multiple replications, but also when cells experience environmental insults (stress‐induced senescence).2 Senescent cells typically extensively alter their cytokine secretion profiles, resulting in development of the senescence‐associated secretory phenotype (SASP).2 In our study, senescent 4T1 cells, a TNBC cell line, were acquired after exposure to radiation combined with the poly ADP ribose polymerase (PARP) inhibitor veliparib. SASP of senescent 4T1 cells showed a protective role against tumor progression, suggesting potential for senescent cells to function as tumor vaccines.
The PD‐1/PD‐L1 pathway is a major immunosuppressive mechanism for vaccination during antigen uptake by DC to prime T‐cell immune response.16, 17 PD‐1 is primarily expressed on activated T cells, and PD‐L1 is expressed by tumor cells and antigen‐presenting cells.18 Interaction of PD‐1 and its ligands PD‐L1 or PD‐L2 induced apoptosis and exhaustion of activated immune cells.19 In our study, expression of PD‐L1 was increased on 4T1 triple‐negative mammary cancer cells after receiving radiotherapy or with the presence of IFN‐γ in vitro. This meant that PD‐1/PD‐L1 signaling inhibited the efficiency of STCV against 4T1 tumor. Therefore, STCV require further improvement to induce adequate efficacy. One potential strategy to overcome the PD‐1/PD‐L1 mechanism in vaccine‐based immunotherapy is optimization of blockade. The clinical effect of blockade has been shown in a number of advanced malignancies.20, 21 Another possible approach for reversing vaccination failure is to combine sPD‐1, which is a soluble form of PD‐1 detected in the blood of patients with autoimmune disease (AID), associated with intense inflammation through blockade of the PD‐1 suppressive pathway by interaction with PD‐L1.22, 23
In the present study, we found that senescent whole‐tumor‐cell vaccination significantly shifted DC towards maturity followed by T‐cell activation in vivo. Moreover, stress‐induced senescent cells re‐engineered to secrete sPD‐1 were safer and much more effective than control when vaccinated into mice.
2. MATERIALS AND METHODS
2.1. Cell line
BALB/c‐derived 4T1 triple‐negative breast cancer cells were obtained from the China Center for Type Culture Collection (CCTCC, Wuhan, China). Cells were grown in RPMI‐1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Gibco) and 1% penicillin‐streptomycin at 37°C and 5% CO2.
2.2. Mice
Six‐ to eight‐week‐old female BALB/c mice were purchased from the Laboratory Animal Center of Southern Medical University (Guangzhou, China). All procedures were conducted in accordance with relevant laws and institutional guidelines.
2.3. Flow cytometry analysis
To determine the effect of 10 Gy radiation and various concentrations of IFN‐γ (5, 10, 20, 30 ng/mL) on PD‐L1 expression of 4T1, cells were harvested from each experimental group on days 3 and 5 after radiation or 24 hours after IFN‐γ stimulation. Then, anti‐PDL1 antibody (BD Biosciences, Franklin Lakes, NJ, USA) was used for flow cytometry analysis.
For PD‐1 detection, 4T1 cells pretreated with IFN‐γ (30 ng/mL) were collected and incubated with PBS or supernatant collected from 4T1/sPD‐1 cells media for 30 minutes. Anti‐PD1 antibody (BD Biosciences) was used for flow cytometry analysis.
On day 5 after vaccine or PBS injections, splenocytes and blood were isolated from mice of each group to count mature DC and PD‐1+ T‐cell subsets by staining cells with anti‐CD3, anti‐CD45, anti‐CD11c, anti‐CD83, anti‐CD86, anti‐CD8, anti‐CD4, and anti‐PD‐1 antibodies (BD Biosciences). Data acquired were processed with FlowJo version software (TreeStar, Ashland, OR, USA).
2.4. Lentivirus‐mediated sPD‐1 gene transfer
Lentiviral particles containing the genetic material for sPD‐1 and negative control mock sequence were purchased from GeneChem (Shanghai, China). 4T1 cells were infected with lentiviral particles according to the manufacture's protocol. Stabilized cells were selected by puromycin (GeneChem) and infected cells were denoted as 4T1/sPD‐1 and 4T1/NC. Enhanced green fluorescent protein (EGFP) expression was examined 72 hours post‐infection under fluorescence microscopy. Expression of sPD‐1 was monitored 72 hours later by qRT‐PCR and western blotting.
2.5. Cytotoxicity study
To investigate the impact of lentivirus infection on cells, 4T1, 4T1/NC and 4T1/sPD‐1 cell characteristics were tested. 5‐Ethynyl‐2′‐deoxyuridine (EdU), Transwell and wound healing assays were used to measure proliferation, invasion and migration ability. For the EdU assay, proliferating cells were examined using an EdU Kit (RiboBio, Guangzhou, China) according to the manufacturer's protocol. Briefly, after incubation with 10 mmol L−1 EdU for 2 hours, cells were fixed with 4% paraformaldehyde, permeabilized in 0.3% Triton X‐100 and stained with Apollo fluorescent dyes (RiboBio, Guangzhou, China). Hoechst stain (Hoechst, Höchst, Germany) was used to stain cell nuclei for 10 minutes. Number of EdU‐positive cells was counted under a microscope.
For the Transwell invasion assay, Matrigel‐precoated Transwell chambers (8 μm; Corning, Corning, NY, USA) were used. Briefly, 1 × 105 cells were plated in the upper chamber in serum free RPMI‐1640 medium, while medium with 10% FBS was added to the lower chamber as a chemoattractant. Following incubation for 12 hours, cells in the upper chamber were removed, and cells that had migrated to the lower surface of the filter were stained with crystal violet and analyzed by bright‐field microscopy.
For the wound healing assay, 1 × 105 cells were seeded in 6‐well plates and incubated in complete medium overnight. Wounds were made with a 200‐μL tip and the wells were washed several times to remove non‐adherent cells. Wound repair was documented at 12 hours and at 24 hours using a microscope.
2.6. Quantitative real‐time PCR (qRT‐PCR)
Total RNA was extracted from 4T1, 4T1/NC and 4T1/sPD‐1 cell subsets using Trizol (Takara, Japan). Cycling conditions were 95°C for 10 minutes to activate DNA polymerase followed by 45 cycles of 95°C for 15 seconds, 60°C for 15 seconds and 72°C for 10 seconds. Specificity of amplification products was confirmed by melting curve analysis. Independent experiments were done in triplicate. Specific sense primers for sPD‐1 were: forward, 5′‐TGACTTCCACATGAACATCCT‐3′; reverse, 5′‐CTTGTTGAGGTCTCCAGGATT‐3′. The comparative CT method was used to calculate the relative expression of the gene under analysis.
2.7. Western blotting
4T1,4T1/NC and 4T1/sPD‐1 cells were lysed and separated by 12% SDS‐PAGE, then transferred onto PVDF membranes. After blocking, membranes were incubated with antibodies for sPD‐1 (GeneChem), or P21 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with secondary antibody (Abcam, Cambridge, UK) and washes, proteins were detected with an electrochemiluminescence detection kit according to the manufacturer's instructions.
2.8. Senescence‐associated‐β‐galactosidase staining assay
4T1, 4T1/NC and 4T1/sPD‐1 cells were seeded in 6‐well plates and received 10 Gy radiation. Then, cells were cultured in complete medium containing 10 μmol/L veliparib for 5 days. Senescence‐associated β‐galactosidase staining assay was carried out using an SA‐β‐Gal Staining Kit (Beyotime, Shanghai, China). Distorted cells with a bright blue color observed through microscopy were considered positive.
2.9. ELISA assay
4T1, 4T1/NC and 4T1/sPD‐1 cells in 6‐well plates were treated with 10 Gy radiation and were cultured in medium adding veliparib for 5 days. Non‐treated and treated cell supernatants were both collected for IL‐17, IL‐10, sPD‐1, IFN‐γ, TNF‐α, TGF‐β, IL‐12P40, IL‐12P70 detection by using mouse ELISA kits (Cusabio, Wuhan, China).
2.10. Syngeneic tumor models
4T1, 4T1/NC and 4T1/sPD‐1 cells were resuspended to 1 × 107 cells per milliliter in PBS. Mice were anesthetized and s.c. injected with 1 × 106 tumor cells on the right hind flanks. Tumor size was measured every other day using calipers and represented as tumor volume (V = ab 2/2 mm3; a: length of tumor, b: width of tumor).
2.11. Vaccinations
4T1, 4T1/NC and 4T1/sPD‐1 cells were induced into senescence by a single dose of 10 Gy radiation combined with veliparib stimulation for 5 days. Cells were treated with trypsin and washed 3 times with PBS. Before injection, they were adjusted to appropriate concentrations of 1 × 107 cells per milliliter PBS. Mice were s.c. injected with approximately 100 μL of senescent tumor cells on the left or right hind flanks. When assessing vaccine prevention efficacy, 100 μL of senescent tumor cells were s.c. injected into left hind flanks on day 3 and day 5 before 4T1 tumor cell inoculation. For therapeutic vaccination, mice were s.c. injected with 100 μL of senescent tumor cells on the right hind flanks on day 3 and day 5 after 4T1 tumor establishment.
2.12. Statistical analysis
We used GraphPad Prism v5.0 and SPSS v20.0 for statistical analyses. Data are shown as the mean ± standard error. For comparisons between groups, 1‐way analysis of variance or Student's t test was carried out. Survival was analyzed using the Kaplan‐Meier method. All statistical tests were 2‐sided, with statistical significance denoted as *P < .05, **P < .01 and ***P < .001.
3. RESULTS
3.1. Interferon‐γ and radiation stimulation increased PD‐L1 expression by 4T1 TNBC cells
Previous studies showed therapeutic resistance occurring in breast cancer cells as a result of overexpression of PD‐L1. One of the most important explanations for the phenomenon was the rapidly changing TME surrounding the cancer cells. During the schedule of STCV vaccination, senescent cells would be pre‐radiated and incubated in the TME when vaccinating mice. In the present study, we determined the role of radiation and IFN‐γ (a crucial component of TME) in regulating PD‐L1 expression of 4T1 cells. In vitro, 4T1 cells were cultured in the presence of IFN‐γ for 24 hours with doses of 0, 5, 10, 20 and 30 ng/mL, or received 10 Gy irradiation and were maintained for 3 and 5 days before harvest and measurement by flow cytometry. Both treatments significantly increased expression of PD‐L1. With stimulation of IFN‐γ, the PD‐L1+ variant of 4T1 cells increased in a dose‐dependent way from 2.61 ± 0.23% to 19.3 ± 1.59%, 48.51 ± 1.14%, 63.79 ± 0.92% and 86.69 ± 1.04%, respectively (P < .001, Figure 1A,B). After irradiation, the proportions of PD‐L1+ cells in the groups were 3.93 ± 0.19%, 5.14 ± 0.19% and 9.25 ± 0.34%, respectively (P < .001, Figure 1C,D).
Figure 1.
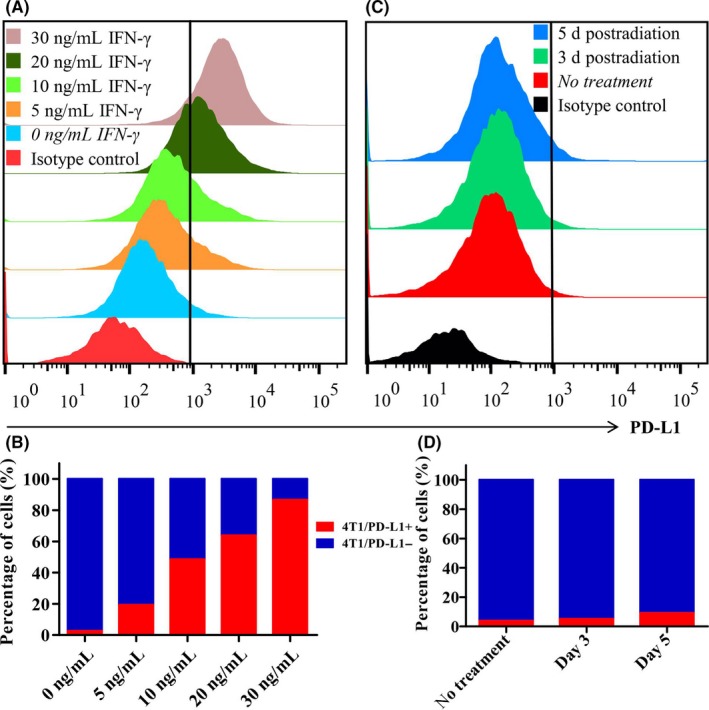
Analysis of programmed death ligand‐1 (PD‐L1) expression on 4T1 cells after receiving radiation or interferon (IFN‐γ) treatment. A,B, Percentage of PD‐L1+ 4T1 cells was assayed by flow cytometry with the presence of different doses of IFN‐γ of 0, 5, 10, 20 and 30 ng/mL. C,D, Percentage of PD‐L1+ 4T1 cell was assessed on days 3 and 5 after receiving 10 Gy radiation
3.2. 4T1 cells were re‐engineered to secrete biological sPD‐1 through lentivirus‐mediated gene delivery
The results above suggest that PD‐1/PD‐L1 signaling would weaken STCV effects through tumor antigen presentation and T‐cell response interference. Thus, we took advantage of sPD‐1, a soluble form of PD‐1, for blockade to enhance the immune response. 4T1 cells were infected with lentiviral particles carrying sPD‐1 vector or negative control mock sequence (Figure 2A). Three days post‐infection, both 4T1/NC and 4T1/sPD‐1 cell subsets presented strong fluorescence intensity on microscopy (Figure 2B). Consistent expression of sPD‐1 at the RNA and protein levels were confirmed in 4T1/sPD‐1 cells but not in cells of control groups by qRT‐PCR and western blotting (Figure 2C,D). We also assessed the binding ability of sPD‐1 to ligand by flow cytometry. As seen in Figure 2E, adding supernatant from 4T1/sPD‐1 cell culture medium significantly increased the population of PD‐1+ 4T1 cells that had been pretreated with IFN‐γ. This increase was not seen in other groups. These results showed successful genetic modification via lentivirus. Functional‐sPD‐1‐expressing 4T1 cell subsets were developed.
Figure 2.
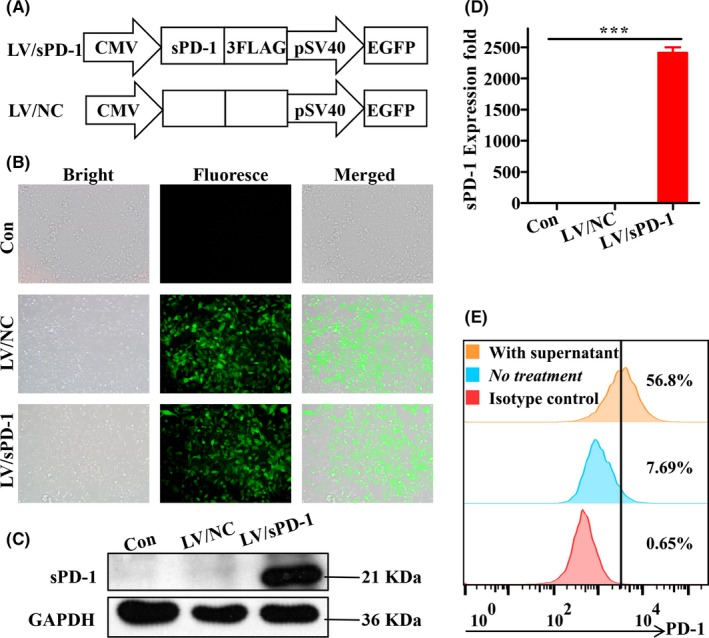
Confirmation of soluble programmed death receptor‐1 (sPD‐1) expression post‐lentivirus infection. A, Schematics of sPD‐1 overexpressing lentivirus (LV/sPD‐1) and negative control lentivirus (LV/NC). EGFP, enhanced green fluorescent protein. B, Verification of infected efficiency of lentivirus based on fluorescence microscopy of EGFP expression on 4T1 cells (magnification ×200). C, Western blotting analysis of sPD‐1 protein expression on 4T1 cells infected by LV/NC and LV/sPD‐1, with 4T1 cells as a control. D, qRT‐PCR analysis of sPD‐1 RNA expression on 4T1 cells infected by LV/NC and LV/sPD‐1, with 4T1 cells as a control. ***P < .001. E, 4T1 cells were pretreated with IFN‐γ to increase programmed death ligand‐1 expression. Culture medium from 4T1/sPD‐1 cells was added to 4T1 cells as mentioned above and incubated for approximately 30 min before flow cytometry assay for detecting ratio of PD‐1+ 4T1 cells. PD‐1, programmed death receptor‐1
3.3. Lentivirus‐mediated sPD‐1 gene transfer had no impact on cell variant characteristics in vitro
We conducted several experiments to ascertain whether lentivirus infection, including positive and negative control lentivirus, would make a difference on cell proliferation, invasion and migration. On EdU assay, typical pictures suggested similar vitality among 4T1, 4T1/NC and 4T1/sPD‐1 cells. Percentages of EdU‐positive cells were 50.33 ± 2.52%, 51.33 ± 3.21% and 50.67 ± 2.52%, respectively (P > .05, Figure 3A,B). In the Transwell assay, 4T1 cells showed similar performance in terms of invasion as did 4T1/NC and 4T1/sPD‐1 cells. Migration index for 4T1 was 905.30 ± 26.27 vs 903.70 ± 23.03 for 4T1/NC and vs 897.70 ± 13.05 for 4T1/sPD‐1 cells (P > .05, Figure 3C,D). For wound repair, 4T1 showed a mean percentage of repair of 12.33 ± 0.58% vs 12.33 ± 1.15% for 4T1/NC vs 12.67 ± 1.53 for 4T1/sPD‐1 cells after 12 hours and 49.67 ± 2.08% vs 50.67 ± 1.53% vs 51.70 ± 1.53%, respectively, after 24 hours (P > .05, Figure 3E,F). These data suggested that lentivirus‐mediated sPD‐1 gene transfer had no impact on cell aggressiveness.
Figure 3.
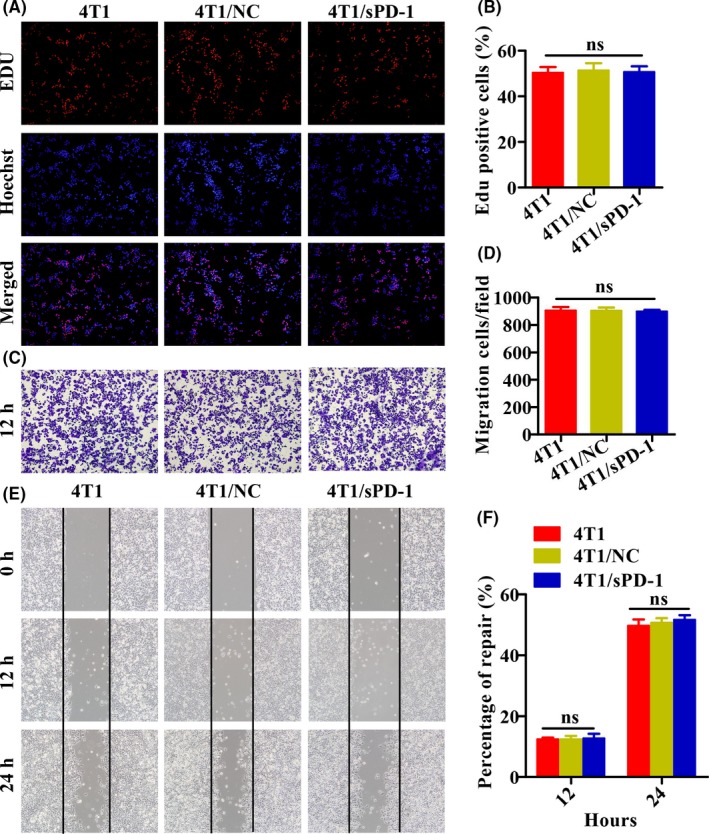
Aggressive characteristics of 4T1, 4T1/NC and 4T1/sPD‐1 cells. A,B, 5‐Ethynyl‐2′‐deoxyuridine (EdU) assay was carried out to assess proliferation ability among 4T1, 4T1/NC and 4T1/sPD‐1 cells. NC, negative control; sPD‐1, soluble programmed death receptor‐1. C,D, Transwell assay was conducted to compare invasion ability among 4T1, 4T1/NC and 4T1/sPD‐1 cells at 12 h. E,F, Wound healing assay was used to assess migration ability among 4T1, 4T1/NC and 4T1/sPD‐1 cells at 12 and 24 h (magnification ×200) (ns, P > .05)
3.4. Senescent cells induced by radiation and veliparib showed vaccine potential
We previously hypothesized that senescent cells could be explored as novel vaccines because of their special characteristics. In order to test our assumption, tumor cell subsets were exposed to radiation and were incubated in medium containing veliparib for 5 days. Cells were harvested for senescence testing by SA‐β‐gal staining and senescence marker p21 expression examination by western blotting. Supernatants were also collected for measurement of sPD‐1, IL‐10, IL‐12P40, IL‐12P70, IL‐17, IFN‐γ, TNF‐α and TGF‐β. Flattened cellular morphology, enhanced SA‐β‐gal staining (Figure 4A) and increased p21 expression (Figure 4B) of 4T1, 4T1/NC, 4T1/sPD‐1 cells indicated that tumor cells shifted toward senescence. As expected, senescent tumor cells expressed higher levels of immunostimulatory cytokines including IFN‐γ (P < .01), TNF‐α (P < .001), and IL‐12P70 (P < .05). In addition, IL‐17 secretion increased but did not reach statistical significance (P > .05, Figure 4C‐F). Secretion of immunosuppressive cytokines including TGF‐β (P > .05) and IL‐12P40 (P > .05) were not significantly altered, except for that of IL‐10 (P < .01, Figure 4G‐I). We also measured expression of sPD‐1 in cell variants and found that 4T1/sPD‐1 cells, either non‐senescent or senescent, generated more sPD‐1 molecules than did other cells (Figure 4J). This finding suggested that senescent tumor cells most likely worked as vaccines against cancer.
Figure 4.
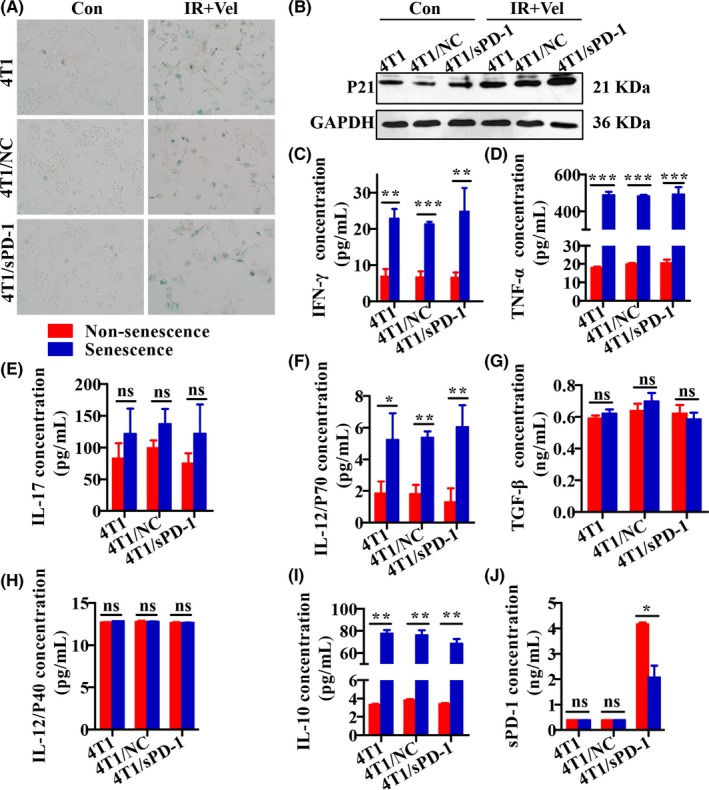
Senescence‐associated secretory phenotype of 4T1, 4T1/NC, and 4T1/sPD‐1 cells treated with radiation and veliparib. NC, negative control; sPD‐1, soluble programmed death receptor‐1. A, SA‐β‐gal staining was used to detect senescence. Bright blue cells were regarded as senescent (magnification ×200). B, Western blotting analysis of senescence marker p21 protein expression. C‐J, Secretion of interferon (IFN)‐γ, tumor necrosis factor‐α (TNF)‐α, interleukin (IL)‐17, IL‐12P70, transforming growth factor‐β (TGF‐β), IL‐12P40, IL‐10 and sPD‐1 was compared between non‐senescent and senescent cells (ns, non‐significant, *P < .05,**P < .01,***P < .001)
3.5. sPD‐1 expression suppressed the growth of 4T1 tumor in vivo
To determine whether a soluble form of PD‐1 effectively enhanced the safety of STCV, we compared tumor incidence after senescent or non‐senescent cell challenge among 4T1, 4T1/NC and 4T1/sPD‐1 cells. We observed that mice inoculated with non‐senescent 4T1/sPD‐1 cells had smaller tumor volumes and longer survival times than did PBS, 4T1 and 4T1/NC inoculation groups (Figure 5). When challenged with senescent cells, mice were divided into different circumstances: injected senescent cells alone, injected senescent cells before 4T1 cell implantation and injected senescent cells after 4T1 cell implantation. In senescent 4T1 or 4T1/NC cell‐injected mice, tumors occurred but complete remission was found a couple of days later in mice receiving senescent cells alone (Figure S1A,C). Senescent 4T1/sPD‐1 cells showed no tumorigenesis in all circumstances (Figures S1B, S2B, S3B). In the condition of senescent 4T1 or 4T1/NC cell injection before or after 4T1 cell implantation, few mice showed tumorigenesis and those that did had slower growth (Figures S2A,C, S3A,C). This suggested that sPD‐1 overexpression contributed to inhibition of tumor occurrence and growth in vivo. Based on these results, we confirmed that STCV/sPD‐1 acted as a safer vaccine.
Figure 5.
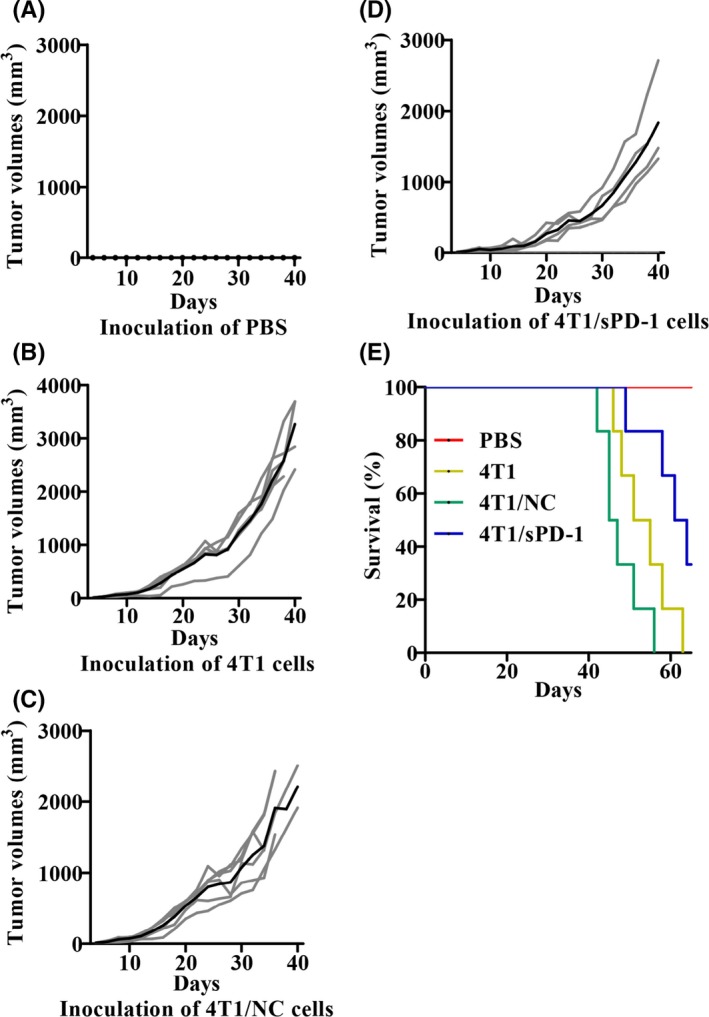
Tumor burden and prognosis of mice with s.c. tumor of 4T1, 4T1/NC and 4T1/sPD‐1 cells. NC, negative control; sPD‐1, soluble programmed death receptor‐1. Mice were inoculated with 4T1, 4T1/NC or 4T1/sPD‐1 cells as indicated and PBS injection was used as a control. A‐D, Individual tumor growth curves (gray lines) and mean tumor growth (black line) of mice in different groups are shown. E, Survival curves of mice in different groups
3.6. STCV/sPD‐1 recruited more CD11c+CD83+/CD86+ DC and fewer PD‐1+ T cells
To further confirm the role of senescent tumor cells in inducing the immune response, we carried out in vivo experiments in a murine model. Mice were divided randomly to various groups, receiving vaccine or PBS injections twice. Five days after treatment, splenocytes and blood were collected for flow cytometry analysis. More mature DC coexpressing CD11c and CD83 or CD86 were detected in splenocytes of mice treated with 4T1/sPD‐1 STCV (Figure 6A,B). Increased frequency of PD‐1+CD4+ T and PD‐1+CD8+ T cells was found in the blood of mice treated with STCV/4T1 and STCV/NC, but not in STCVsPD‐1 (Figure 6C,D). STCV/sPD‐1 promoted DC maturity and rescued exhausted PD‐1+ T cells.
Figure 6.
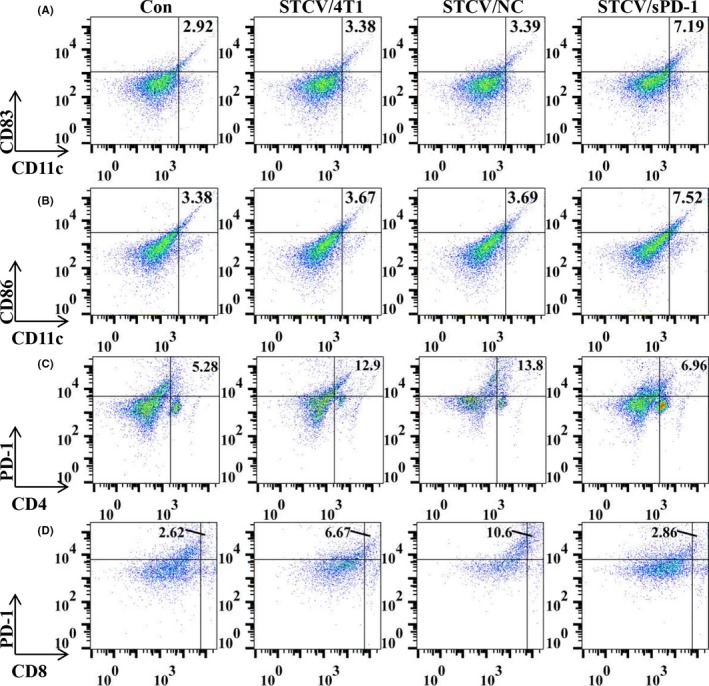
Analysis of dendritic cells (DC) and T‐cell subsets after vaccinations. To evaluate the effect of STCV/4T1, STCV/NC and STCV/sPD‐1, splenocytes and blood were collected from each experimental group on day 5 after vaccination. DC of splenocytes and T‐cell subsets of blood were assayed by flow cytometry. A,B, Proportions of mature DC (CD11c+ CD83+, CD11c+ CD86+) in splenocytes were analyzed by flow cytometry. Typical data from a representative experiment in mice of each group. C,D, Proportions of T‐cell subsets (PD‐1+ CD4+, PD‐1+ CD8+) in blood were analyzed by flow cytometry. Typical data from a representative experiment in mice of each group. NC, negative control; sPD‐1, soluble programmed death receptor‐1; STCV, senescent tumor cell vaccine
3.7. sPD‐1 expression enhanced the efficacy of STCV
Vaccine efficacy is crucial to novel vaccine development. We carried out an in vivo study to evaluate the efficacy of STCV as a preventive and therapeutic approach against 4T1 tumors. STCV or PBS was s.c. injected into mice hind flanks twice on days 3 and 5 before or after 4T1 tumor cell inoculation. Surprisingly, tumor volumes and tumor incidence were significantly lower and survival was longer in STCV‐treated mice, particularly in STCV/sPD‐1, compared with PBS treatment (Figure 7). As a therapeutic vaccine, results suggested a successful delay of tumorigenesis, and decreased tumor burden in STCV/sPD‐1‐treated mice in early stages (Figure 8A‐E). However, a similar survival rate was found among mice of all the groups (Figure 8F). Taken together, STCV/sPD‐1 exerted a stronger growth‐suppressive effect on tumors.
Figure 7.
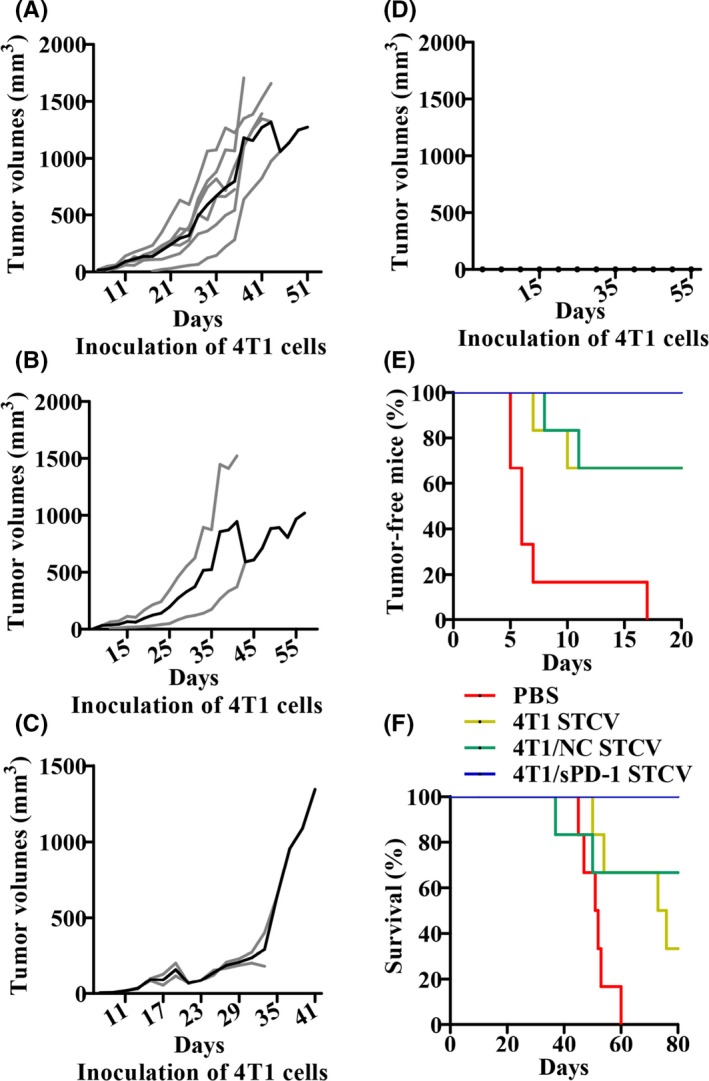
Efficacy of STCV/4T1, STCV/NC and STCV/sPD‐1 on 4T1 tumor prevention. Mice were injected with STCV/4T1, STCV/NC, or STCV/sPD‐1 on days 3 and 5 twice before 4T1 tumor cells were challenged as indicated. A‐D, Individual tumor growth (gray lines) and mean tumor growth (black line) in groups of PBS, STCV/4T1, STCV/NC, and STCV/sPD‐1 treatment are shown. E, Tumor‐free curves of mice in each group. F, Survival curves of mice in each group. NC, negative control; sPD‐1, soluble programmed death receptor‐1; STCV, senescent tumor cell vaccine
Figure 8.
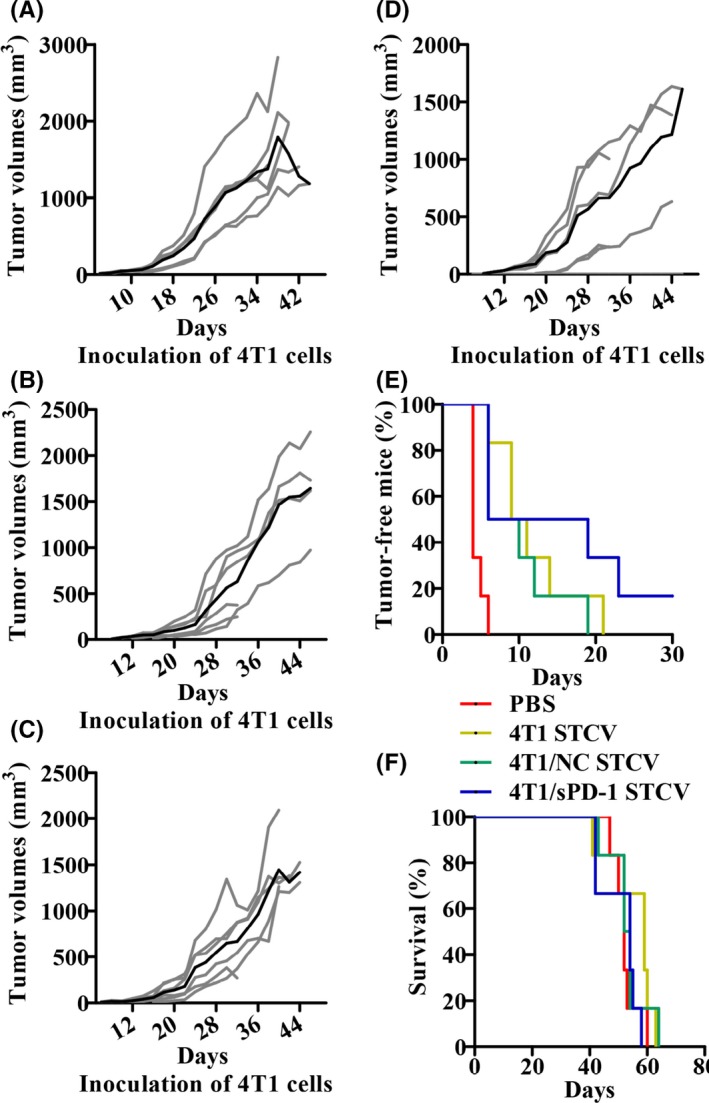
Efficacy of STCV/4T1, STCV/NC and STCV/sPD‐1 on 4T1 tumor treatment. Mice were injected with STCV/4T1, STCV/NC, or STCV/sPD‐1 on days 3 and 5 for twice after 4T1 tumor cells were challenged as indicated. A‐D, Individual tumor growth (gray lines) and mean tumor growth (black line) in groups of PBS, STCV/4T1, STCV/NC, and STCV/sPD‐1 treatment are shown. E, Tumor‐free curves of mice in each group. F, Survival curves of mice in each group. NC, negative control; sPD‐1, soluble programmed death receptor‐1; STCV, senescent tumor cell vaccine
4. DISCUSSION
Vaccine therapeutic methods have been extensively regarded as crucial approaches to generate adaptive tumor‐specific T cells.24 However, antitumor immunity induced by traditional vaccines has been far from desirable.25 One possible explanation for vaccination failure was lack of cytokines acting as key factors for improving antigen presentation following T‐cell response to tumors. In contrast, vaccine therapy increased PD‐L1 expression on cancer cells, leading to immune resistance.10, 26
At present, cellular senescence is an area of intense investigation because of unique cell conditions. Unlike death or apoptotic cells, despite the fact that a senescent cell would no longer proliferate, it remains viable and metabolically active.27 Our study showed that an accumulation of senescent cells with various SASP would cause pro‐inflammatory microenvironments. This suggested the possibility to exploit a novel vaccine different from conventional vaccines. In this study, we exploited a cell‐based vaccine that consisted primarily of stress‐induced senescent tumor cells. The vaccine not only maintained a large number of tumor antigens, but also maintained high local cytokine concentrations.27
In the present study, we induced 4T1 cells into senescent status and found higher concentrations of immunostimulatory cytokines, including IFN‐γ, TNF‐α, IL‐17 and IL‐12P70. Meanwhile, we assessed secretion of TGF‐β, IL‐12P40 and IL‐10. These immunosuppressive cytokines were not extensively altered, except for IL‐10. Researchers have already shown that senescent cells significantly enabled or weakened immunity by SASP.28, 29, 30 Our data suggested the SASP of senescent 4T1 cells accelerated immunity. Therefore, further studies were conducted to test the potential of senescent 4T1 cell vaccines against tumor. During vaccine preparation, we discovered an increase of PD‐L1 expression on 4T1 cells when exposed to irradiation or IFN‐γ. This suggested a possibility for tumor relapse or failure of antigen presentation for T‐cell activation in the senescent tumor cell vaccination context. However, it also suggested the use of blockade when targeting PD‐L1 expressed on tumor cells, and that DC would reverse this phenomenon.
PD‐L1 inhibitor has been approved for use as monotherapy.31 Generally, preclinical trials have managed to combine cancer vaccines with commercially available antibodies against PD‐1/PD‐L1 or other inhibitory receptors.32 However, a recent study showed that not only commercial antibodies interrupted PD‐1/PD‐L1 interaction, but a similar effect was also obtained by the soluble form of PD‐1, detected in blood from patients with autoimmune diseases.33, 34, 35 Therefore, we sought to evaluate whether the safety and efficacy of STCV could be improved when senescent cells expressed sPD‐1 by themselves. We conducted lentivirus infection to generate stable sPD1‐expressing 4T1 cell subsets. Expression of biological sPD‐1 was confirmed consistently in RNA and protein levels after lentivirus infection. To determine the influence of sPD‐1 overexpression on STCV safety, mice were randomly assigned to several groups with different immunization schedules. As a result, STCV/sPD1‐treated mice showed no tumor occurrence, but mice injected with STCV/4T1 or STCV/NC alone were seen to bear tumor and were eventually free from tumor. Moreover, similar tumorigenesis rates caused by senescent cells were obtained in mice with STCV/4T1 or STCV/NC treatment before/after 4T1 implantation. This suggested that STCV/sPD‐1 were safer than other STCV, and that STCV safety was influenced by mouse conditions. Simultaneously, we inoculated non‐senescent 4T1, 4T1/NC and 4T1/sPD‐1 cells s.c. into mice and observed substantially smaller tumor sizes and longer survival times in 4T1/sPD‐1‐injected mice. Previously, we discovered a similarity in proliferation, invasion and migration among 4T1, 4T1/NC and 4T1/sPD‐1 cells in vitro. Therefore, the discrepancy in tumor arising above indicated that sPD‐1 inhibited non‐senescent or senescent tumor cell growth in vivo. In the meantime, we observed and recorded alterations in mental state, weight, activity, bowel function, skin and hair loss. The data showed that there were few side‐effects caused by soluble PD‐1 overexpression in vivo. Briefly, sPD‐1 markedly suppressed tumorigenicity and boosted the safety of STCV. We then explored the mechanisms of STCV in altering organism immunity. Splenocytes and blood cells were obtained from mice after receiving vaccinations or PBS injection. STCV/sPD‐1 stimulated DC to maturity and recruited fewer dysfunctional PD‐1+ T cells. We further verified the efficacy of STCV against cancer in vivo. In a prevention tumor model, we discovered that STCV induced intense antitumor immunity, resulting in a higher tumor‐free rate, longer survival time and lower tumor volumes. The percentage of tumor‐free mice and survival rates rose to 100% in the STCV/sPD‐1 group. Similarly, the phenomenon was seen in the therapeutic setting. Tumor onset and growth were suppressed for a period of time in STCV‐treated tumor‐bearing mice, especially in the STCV/sPD‐1 group.
We developed a sPD1‐expressing senescent‐cell‐based vaccine that shifted DC toward maturity followed by T‐cell activation. STCV/sPD‐1 showed stronger protection against solid subcutaneous 4T1 tumors than did control. Clearly, we offered a potential avenue to deal with TBNC. More extensive studies in experimental animal models and clinical trials are needed to further confirm our finding.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
This study was supported by research grants from National Nature Science Foundation of China (grant number 81372449) and the Science & Technology Planning Project of Guangdong (grant numbers 2014A020212537, 2016A020215103).
Chen Z, Hu K, Feng L, et al. Senescent cells re‐engineered to express soluble programmed death receptor‐1 for inhibiting programmed death receptor‐1/programmed death ligand‐1 as a vaccination approach against breast cancer. Cancer Sci. 2018;109:1753–1763. https://doi.org/10.1111/cas.13618
Zehong Chen and Kang Hu contributed equally to this work.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Rakha EA, Ellis IO. Modern classification of breast cancer: should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18:255‐267. [DOI] [PubMed] [Google Scholar]
- 3. Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:324‐354. [DOI] [PubMed] [Google Scholar]
- 4. Liao HY, Zhang WW, Sun JY, Li FY, He ZY, Wu SG. The clinicopathological features and survival outcomes of different histological subtypes in triple‐negative breast cancer. J Cancer. 2018;9:296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quintero M, Adamoski D, Reis L, et al. Guanylate‐binding protein‐1 is a potential new therapeutic target for triple‐negative breast cancer. BMC Cancer. 2017;17:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McNutt M. Cancer immunotherapy. Science. 2013;342:1417. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335‐3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sau S, Alsaab HO, Bhise K, Alzhrani R, Nabil G, Iyer AK. Multifunctional nanoparticles for cancer immunotherapy: a groundbreaking approach for reprogramming malfunctioned tumor environment. J Control Release. 2018;274:24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jahan ST, Sadat SM, Haddadi A. Design and immunological evaluation of anti‐CD205‐tailored PLGA‐based nanoparticulate cancer vaccine. Int J Nanomedicine. 2018;13:367‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi X, Zhang X, Li J, et al. PD‐1/PD‐L1 blockade enhances the efficacy of SA‐GM‐CSF surface‐modified tumor vaccine in prostate cancer. Cancer Lett. 2017;406:27‐35. [DOI] [PubMed] [Google Scholar]
- 11. Grenier JM, Yeung ST, Qiu Z, Jellison ER, Khanna KM. Combining adoptive cell therapy with cytomegalovirus‐based vaccine is protective against solid skin tumors. Front Immunol. 2017;8:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sikora E, Mosieniak G, Sliwinska MA. Morphological and functional characteristic of senescent cancer cells. Curr Drug Targets. 2016;17:377‐387. [DOI] [PubMed] [Google Scholar]
- 13. Vilgelm AE, Johnson CA, Prasad N, et al. Connecting the dots: therapy‐induced senescence and a tumor‐suppressive immune microenvironment. J Natl Cancer Inst. 2016;108:v406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toso A, Revandkar A, Di Mitri D, et al. Enhancing chemotherapy efficacy in Pten‐deficient prostate tumors by activating the senescence‐associated antitumor immunity. Cell Rep. 2014;9:75‐89. [DOI] [PubMed] [Google Scholar]
- 15. Chua CE, Chan SN, Tang BL. Non‐cell autonomous or secretory tumor suppression. J Cell Physiol. 2014;229:1346‐1352. [DOI] [PubMed] [Google Scholar]
- 16. Soares KC, Rucki AA, Wu AA, et al. PD‐1/PD‐L1 blockade together with vaccine therapy facilitates effector T‐cell infiltration into pancreatic tumors. J Immunother. 2015;38:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death‐1 blockade enhances the antitumor effects of peptide vaccine‐induced peptide‐specific cytotoxic T lymphocytes. Int J Oncol. 2015;46:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar‐Sela G. The role of tumor‐infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti‐PD1 therapy in patients with metastatic non‐small cell lung cancer or metastatic melanoma. Med Oncol. 2018;35:25. [DOI] [PubMed] [Google Scholar]
- 19. Roemer M, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018;36:942‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD‐1/PD‐L1 pathway in solid tumors. Immunotherapy. 2016;8:583‐600. [DOI] [PubMed] [Google Scholar]
- 21. Jensen KH, Persson G, Bondgaard AL, Pohl M. Development of pulmonary tuberculosis following treatment with anti‐PD‐1 for non‐small cell lung cancer. Acta Oncol. 2018;1‐2. https://doi.org/10.1080/0284186X.2018.1433877. [DOI] [PubMed] [Google Scholar]
- 22. Kruger S, Legenstein ML, Rosgen V, et al. Serum levels of soluble programmed death protein 1 (sPD‐1) and soluble programmed death ligand 1 (sPD‐L1) in advanced pancreatic cancer. Oncoimmunology. 2017;6:e1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukasawa T, Yoshizaki A, Ebata S, et al. Contribution of soluble forms of programmed death 1 and programmed death ligand 2 to disease severity and progression in systemic sclerosis. Arthritis Rheumatol. 2017;69:1879‐1890. [DOI] [PubMed] [Google Scholar]
- 24. Matsumoto M, Takeda Y, Tatematsu M, Seya T. Toll‐like receptor 3 signal in dendritic cells benefits cancer immunotherapy. Front Immunol. 2017;8:1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNeel DG. Therapeutic cancer vaccines: how much closer are we? BioDrugs. 2017;32:1‐7. https://doi.org/10.1007/s40259-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163‐3174. [DOI] [PubMed] [Google Scholar]
- 27. Ong SM, Hadadi E, Dang TM, et al. The pro‐inflammatory phenotype of the human non‐classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng Y, Efimova EV, Hamzeh KW, et al. Radiation‐inducible immunotherapy for cancer: senescent tumor cells as a cancer vaccine. Mol Ther. 2012;20:1046‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simova J, Sapega O, Imrichova T, et al. Tumor growth accelerated by chemotherapy‐induced senescent cells is suppressed by treatment with IL‐12 producing cellular vaccines. Oncotarget. 2016;7:54952‐54964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Byun HO, Lee YK, Kim JM, Yoon G. From cell senescence to age‐related diseases: differential mechanisms of action of senescence‐associated secretory phenotypes. BMB Rep. 2015;48:549‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCall NS, Dicker AP, Lu B. Beyond concurrent chemoradiation: the emerging role of PD‐1/PD‐L1 inhibitors in stage III lung cancer. Clin Cancer Res. 2018;24:1271‐1276. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasan P, Wu X, Basu M, Rossi C, Sandler AD. PD‐L1 checkpoint inhibition and anti‐CTLA‐4 whole tumor cell vaccination counter adaptive immune resistance: a mouse neuroblastoma model that mimics human disease. PLoS Med. 2018;15:e1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pen JJ, Keersmaecker BD, Heirman C, et al. Interference with PD‐L1/PD‐1 co‐stimulation during antigen presentation enhances the multifunctionality of antigen‐specific T cells. Gene Ther. 2014;21:262‐271. [DOI] [PubMed] [Google Scholar]
- 34. Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine‐induced primary and memory CD8(+) T‐cell responses by soluble PD‐1. J Immunother. 2011;34:297‐306. [DOI] [PubMed] [Google Scholar]
- 35. Zhou J, Cheung AK, Tan Z, et al. PD1‐based DNA vaccine amplifies HIV‐1 GAG‐specific CD8 + T cells in mice. J Clin Invest. 2013;123:2629‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


