Abstract
Background
Little is known about the brain mechanisms underlying cancer‐associated weight loss (C‐WL) in humans despite this condition negatively affecting their quality of life and survival. We tested the hypothesis that patients with C‐WL have abnormal connectivity in homeostatic and hedonic brain pathways together with altered brain activity during food reward.
Methods
In 12 patients with cancer and 12 healthy controls, resting‐state functional connectivity (RSFC, resting brain activity observed through changes in https://en.wikipedia.org/wiki/Cerebral_blood_flow in the brain which creates a https://en.wikipedia.org/wiki/Blood-oxygen-level_dependent signal that can be measured using https://en.wikipedia.org/wiki/Functional_magnetic_resonance_imaging) was used to compare three brain regions hypothesized to play a role in C‐WL: the hypothalamus (homeostatic), the nucleus accumbens (hedonic), and the habenula (an important regulator of reward). In addition, the brain reward response to juice was studied.
Participants included 12 patients with histological diagnosis of incurable cancer (solid tumours), a European Cooperative Oncology Group performance status of 0–2, and a ≥5% involuntary body weight loss from pre‐illness over the previous 6 months and 12 non‐cancer controls matched for age, sex, and race. RSFC between the hypothalamus, nucleus accumbens, and habenula and brain striatum activity as measured by functional MRI during juice reward delivery events were the main outcome measures.
Results
After adjusting for BMI and compared with matched controls, patients with C‐WL were found to have reduced RSFC between the habenula and hypothalamus (P = 0.04) and between the habenula and nucleus accumbens (P = 0.014). Patients with C‐WL also had reduced juice reward responses in the striatum compared with controls.
Conclusions
In patients with C‐WL, reduced connectivity between both homeostatic and hedonic brain regions and the habenula and reduced juice reward were observed. Further research is needed to establish the relevance of the habenula and striatum in C‐WL.
Keywords: Cancer cachexia, Cancer anorexia, Resting‐state functional connectivity, Habenula, Hypothalamus, Nucleus accumbens
Introduction
Involuntary weight loss is commonly seen in patients with chronic illnesses such as cancer, chronic obstructive pulmonary disease, liver disease, renal failure, congestive heart failure, and chronic infection.1 Depending on tumour type, between 50 and 90% of patients with advanced‐stage cancer experience weight loss.2 This is highly predictive of increased mortality and contributes to the decreased quality of life that accompanies end‐stage disease.3, 4, 5
Cancer‐associated weight loss (C‐WL) is a significant burden to patients with cancer and their families. Unfortunately, the mechanisms underlying this condition are not fully understood in humans, hampering the development of effective treatments. A common finding in these patients is a paradoxical decrease in appetite despite significant weight loss, known as anorexia. Tumours have anorexigenic properties, and decreased food intake is commonly reported in treatment‐naïve patients.6 The emetogenic properties of chemotherapy and radiation treatment often worsen anorexia and C‐WL.7 Factors such as depression, pain, gastrointestinal obstruction, constipation, and altered taste may also play a role in the development of C‐WL.8
Central nervous system regulation of appetite involves a homeostatic metabolic need (hunger) and a hedonic, reward‐based consumption component (pleasure associated with eating). Homeostatic control is largely regulated by the hypothalamus, based in part on caloric requirements.9, 10, 11, 12 The hedonic signalling present when eating highly palatable foods is likely mediated by the dopaminergic limbic system, which reinforces rewarding behaviours.13 In particular, activation of hedonic pathways including the ventral tegmental area, nucleus accumbens, and striatum has been observed following food reward.14
Prior research in pre‐clinical non‐cancer models has identified a potential link between the homeostatic and hedonic reward systems: the habenula.15 This small, midbrain structure receives information from several areas including the hypothalamus and relays those signals to other systems (dopaminergic, serotonergic, noradrenergic) that influence emotion and motivation.16, 17, 18 The habenula is activated by negative events or in the absence of expected positive rewards. Activation of the habenula decreases downstream dopaminergic activity and thus may inhibit the hedonic system if the expected positive reward from food is forgone.19, 20, 21
Further support for a connection between habenula signalling and hunger comes from rat studies, wherein efferent projections from the lateral hypothalamus to the lateral habenula have been reported.22, 23, 24 The lateral hypothalamus regulates hunger, suggesting interplay between the habenula and brain regions regulating homeostatic processes. However, comparable connections between habenula and hypothalamus in humans have not been conclusively demonstrated. Indeed, the hypothalamus and reward pathways, of which the habenula is a component, have been implicated in eating disorders such as anorexia nervosa. To date, the habenula has not been specifically linked to hedonic and homeostatic pathways in cachectic patients.25
We postulate that C‐WL is likely the result of alterations in these central nervous and endocrine systems that regulate food intake and mediate reward. While past studies of C‐WL in humans have focused on peripheral cytokine and hormonal signalling, little is known about the neurocognitive processes underlying C‐WL, with most hypotheses driven by findings from mouse models that suggest a key role of inflammation particularly in the hypothalamus.26, 27 Functional magnetic resonance imaging (fMRI), a non‐invasive method of measuring blood oxygen level‐dependent signal as a proxy for brain activity,28 may provide insight into how the brains of patients with C‐WL differ from non‐cancer controls.
The present study set out to identify a functional connection between the habenula and homeostatic and hedonic pathways by comparing resting‐state functional connectivity (RSFC) in patients with C‐WL and healthy, non‐cancer controls. We hypothesized reduced RSFC between the habenula and homeostatic (hypothalamus) and hedonic (nucleus accumbens) brain regions in patients with C‐WL. In addition, we studied juice reward‐associated activity in the striatum. We hypothesized that deficient habenular connectivity may be linked to deficient processing of rewarding signals in the brain.
Methods
Protocol and study participants
All protocols were approved by the Baylor College of Medicine Institutional Review Board and the Research and Development Committee of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, TX. The study was conducted between October 2011 and February 2015. All clinical investigations described in the paper were conducted according to Declaration of Helsinki guidelines.
All study participants were older than 18 years of age and provided signed, informed consent. Two groups of participants were recruited: (1) 12 individuals with histological diagnosis of incurable cancer (solid tumour), a European Cooperative Oncology Group performance status of 0–2, and presence of C‐WL as defined as involuntary weight loss of at least 5% of the pre‐illness body weight over the previous 6 months,29 and (2) 12 non‐cancer controls matched by age, gender, and race without prior/current history of cancer. Resting‐state functional connectivity has been shown to be age‐dependent and sex‐dependent in healthy adults.30 Patients with C‐WL presented with a range of cancers, including small cell lung cancer, non‐small cell lung cancer, prostate cancer, cholangiocarcinoma, hepatocellular carcinoma, esophageal cancer, sigmoid adenocarcinoma, neuroendocrine carcinoma, gastrointestinal stromal tumour, multiple myeloma, squamous cell carcinoma, malignant myxoid tumour, and maxillary cancer. The cancer stage ranged from II to IV. In all C‐WL cases, the investigators deemed the weight loss to be due to cancer. Subjects with stage II disease were deemed not candidates for curative treatment by the team providing care for these patients.
Exclusion criteria for both groups included obesity (body weight > 140 kg); recent active excessive alcohol or illicit drug use; severe depression; other causes of weight loss (e.g. renal failure, untreated thyroid disease, class III–IV congestive heart failure, acquired immune deficiency syndrome, severe chronic obstructive pulmonary disease, liver disease); inability to increase food intake (e.g. esophageal obstruction, intractable vomiting); any condition that would prevent participant from performing research procedures; use of growth hormone, megestrol, marinol, or any other anabolic agents, appetite stimulants, tube feedings, or parenteral nutrition 1 month prior to entering the study; recent administration of highly emetogenic chemotherapy (Hesketh scale class 4–5); pregnant, breastfeeding, or of childbearing potential; and contraindication to magnetic resonance imaging such as severe claustrophobia, pacemaker, or metallic foreign body. For the C‐WL group, appetite was assessed by a previously validated visual analogue scale (1–100).31
Magnetic resonance imaging data acquisition
All MRI procedures were completed at Baylor College of Medicine in the Core for Advanced MRI.
Structural imaging
A T1*‐weighted, three‐dimensional acquisition (MPRAGE, echo time = 2.66 ms, repetition time = 1200 ms, flip angle = 12°, 256 × 256 matrix) was used to obtain 160 contiguous, 1‐mm‐thick axial slices of the brain with 1 × 1 × 1 mm voxels. Structural images were used to rule out possible gross anatomical abnormalities such as tumours or stroke and to co‐register the functional data.
Resting‐state imaging
Participants were scanned for 5 min while resting with eyes either open or closed. An ‘X’ was displayed on the screen, and patients were instructed to relax but not sleep. Whole brain images were collected as 3.4 × 3.4 × 4 mm voxels, echo time = 40 ms, repetition time = 2 s, flip angle = 90.
Pre‐processing
The functional time series for each participant were realigned to the first image and unwarped. Images were co‐registered to the mean image and normalized to the standard Montreal Neurological Institute atlas. Artefact detection and scrubbing (Artefact Detection Tools, Whitfield‐Gabrieli, MGH, Boston, MA) was performed to obtain outliers. An 8 mm full width at half‐maximum Gaussian smoothing kernel was used to smooth the data, and physiological low‐frequency effects were removed using a temporal bandpass filter (0.01–0.08 Hz). The CONN Matlab Toolbox (Matlab, Torrance, CA) and SPM8 were used for image pre‐processing and statistical analysis.32, 33
Regions of interest
ROIs were created in AFNI34 using the Montreal Neuroscience Institute (MNI) atlas for nucleus accumbens and hypothalamus. One voxel (3.4 × 3.4 × 4 mm) right and left habenula ROIs were manually created in AFNI using each participant's structural T1 MRI as a guide (Figure 1).
Figure 1.
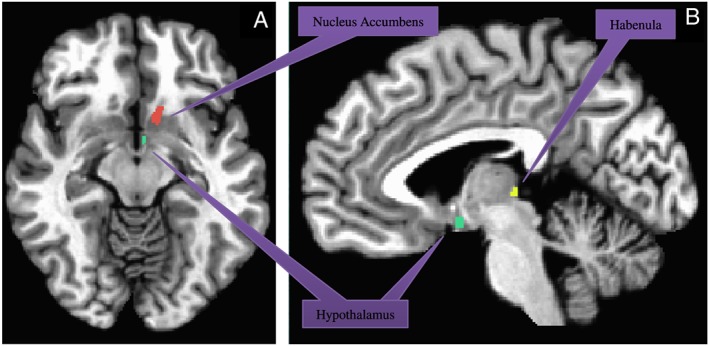
Significant regions of interest used in analysis: nucleus accumbens (peach), hypothalamus (green), and habenula (yellow). (A) axial view and (B) sagittal view.
Resting‐state functional connectivity analysis
The CONN Matlab toolbox35 was used to segment grey matter and correct for outlier data points and signal spikes identified using the artefact detection and scrubbing toolbox or during pre‐processing, respectively.
The cerebrospinal fluid and white matter signals from segmentation were used as regressors of no interest. After processing the data through CONN, correlation coefficients between the homeostatic ROI, hedonic ROI, and the habenula were analysed by ANOVA in SPSS. Mean and standard error were adjusted for body mass index (BMI) because this was the only baseline characteristic found to be significantly different between the two groups and because body weight is known to influence the fMRI outcomes measured.36 The statistical test used to control for this variable was ANCOVA performed in CONN (Matlab, Torrance, CA).
Juice reward
Using the same scanner parameters as in resting state, the juice reward was designed to deliver a 1 mL bolus of sweet juice (a choice of flavour was offered) after a visual cue (a large yellow circle that lasted 1 s). The cue presentation/juice delivery happened 22 times. This paradigm has been used to study striatal reward‐related signals, and this number was sufficient to induce a strong striatal signal in our previous juice reward experiments.21, 37
Analysis of juice reward
Raw DICOM images were converted to NIFTI format, followed by anatomical and functional image analysis using a standard AFNI processing stream (Cox, 1996). Briefly, voxels above a 15% outlier threshold were rejected by 3dToutcount. Remaining functional data were slice‐time corrected using 3dTshift and then aligned to the first image and measured for motion using 3dvolreg. fMRI data were then registered to the high‐resolution MPRAGE and transformed into MNI space using a single spatial transform (@auto_tlrc, 3dAllineate). A 4.5 mm smoothing kernel was applied in 3dmerge and submitted to a general linear model regression in 3dDeconvolve. The general linear model design matrix included regressors for linear, quadratic, and cubic linear trends, three translational and three rotational motion parameters, and two stimulus conditions of interest: visual cue and juice reward. Group analysis of healthy controls and age‐matched, gender‐matched, and race‐matched controls was performed using 3dMVM. Given our previous experience with juice reward,21 we analysed only a putamen mask to avoid multiple comparison issues.
Results
The C‐WL and control groups were matched for age, sex, and race. Although not part of our exclusionary medication list, no subjects had recently received corticosteroids. Compared with control participants, BMI and weight were significantly lower in participants with C‐WL at time of fMRI scan. This difference at time of scan was expected, given that a ≥5% drop in pre‐illness BMI was required for inclusion in the C‐WL group. When the pre‐illness weight and BMI of patients with C‐WL were compared with controls, no differences were observed. Patients with C‐WL had experienced a significant degree of weight loss at the time of recruitment and had a wide range of appetite scores (Table 1). There was no correlation between the degree of weight loss and appetite scores (R 2 0.03, P value NS).
Table 1.
Baseline characteristics in cancer‐associated weight loss and control groups
| C‐WL (n = 12) | Controls (n = 12) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Stand. deviat. | Range | Mean | Stand. deviat. | Range | P value | |
| Age (years) | 66.5 | 7.3 | 58–78 | 62.8 | 9.2 | 52–79 | 0.293 |
| Sex (M/F) | 11/1 | — | — | 7/5 | — | — | 0.067 |
| Race (White/AfA) | 10/2 | 11/1 | 0.558 | ||||
| Weight (kg) | 65.45 | 13.1 | 52–90 | 77.23 | 11.0 | 59–95 | 0.026* |
| Pre‐illness weight (kg) | 75.12 | 16.2 | 58–109 | 0.713a | |||
| BMI (kg/m2) | 22.60 | 4.3 | 16–31 | 25.8 | 2.7 | 21–33 | 0.012* |
| Pre‐illness BMI (kg/m2) | 25.96 | 5.41 | 18–37 | NA | 0.648a | ||
| Weight loss (%) | 12.6 | 4.3 | 6.4–16.7 | NA | |||
| Appetite VAS (1–100) | 44.75 | 26.18 | 3–90 | NA | |||
When pre‐illness weight and BMI in C‐WL group were compared with weight and BMI at time of fMRI in controls.
P < 0.05, significant difference between the two groups.
Resting‐state functional connectivity
When RSFC between the hedonic nucleus accumbens and homeostatic hypothalamus was measured, no significant difference was observed in patients with C‐WL and controls (P = 0.171). However, when RSFC between the habenula and either nucleus accumbens or hypothalamus were measured, a significant reduction in patients with C‐WL was found compared with controls (P = 0.014, P = 0.040, respectively, refer to Figure 2). For this comparison, the habenula was used as a ‘seed’ region and BMI was controlled for statistically (refer to “Methods” section). No significant correlation between appetite and RSFC between the habenula and the hypothalamus or between the habenula and the nucleus accumbens was observed (not shown).
Figure 2.
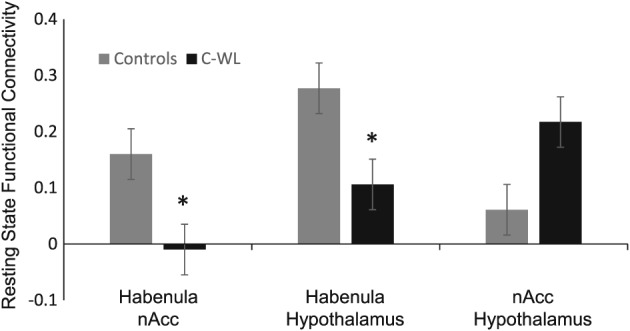
Mean resting‐state function connectivity of control participants compared with paticipants with cancer‐associated weight loss controlling for BMI. The resting‐state function connectivity between habenula and nucleus accumbens was significantly lower in participants with cancer‐associated weight loss as compared with control participants (P = 0.014). The resting‐state function connectivity between habenula and hypothalamus is also significantly lower in participants with cancer‐associated weight loss (P = 0.04). This pattern of lower resting‐state function connectivity does not hold for nucleus accumbens and hypothalamus directly. All analysis performed controlling for BMI, the only variable significantly different between groups.
Reward‐related activity
Reward‐related activity was found in striatal regions in controls as expected. When patients with C‐WL and controls were compared, a strong signal was observed in the striatum of controls (Figure 3A and 3B), but not in patients with C‐WL (Figure 3C and 3D). No significant correlation between appetite and reward‐induced striatal activity was observed (not shown).
Figure 3.
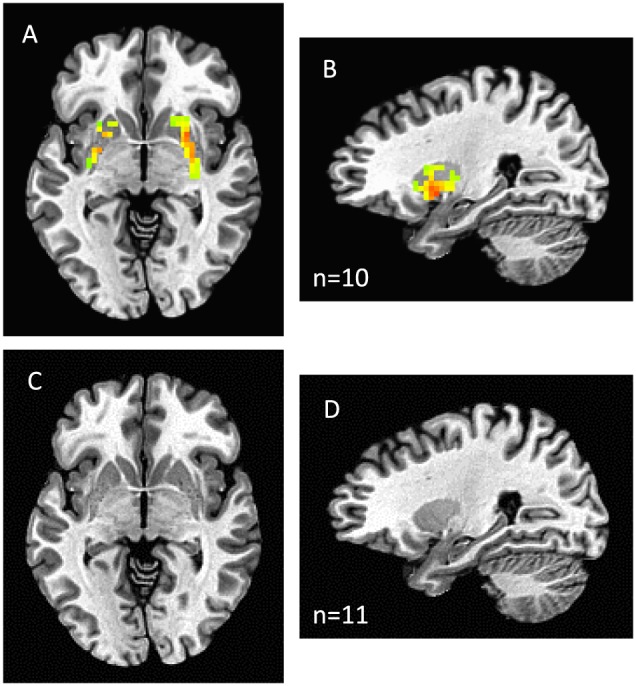
Juice reward activity in healthy controls (A axial; B sagittal) and patients with cancer‐associated weight loss (C axial; D sagittal). A dorsal striatum mask was used. Activity within the dorsal striatum mask was observed in controls (P < 0.05, family‐wise error rate corrected) but not in cancer‐associated weight loss. Group activity is shown over a standard Montreal Neuroscience Institute template image of the brain (http://www.bic.mni.mcgill.ca).
Discussion
This fMRI study explores resting‐state brain connectivity and reward‐related brain activity differences between patients with cancer with significant recent weight loss and in healthy controls. We hypothesized that functional connectivity between the homeostatic pathway mediating hunger (in which the hypothalamus is a key component) and hedonic or pleasure pathway (which includes the nucleus accumbens) would be impaired in patients with cancer with weight loss.
The discovery of two orexigenic neuropeptides—melanin concentrating hormone and orexins—in the hypothalamus gave further credence to this area of the brain as a regulator of hunger.38, 39 As a non‐invasive measure of brain function, fMRI provides insights into the regulation of hunger previously only available through animal studies. For example, the role of inflammation in modulating food intake in the setting of weight loss and the role of tumour‐secreted factors in inducing anorexia via the hypothalamus have been derived mostly from studies in rodents.26, 27
Past fMRI research on obesity has shown that obesity‐prone participants do not exhibit attenuated brain activation of hypothalamus and visual cortical areas observed in obesity‐resistant participants presented with hedonically positive food images following food intake.40 In fMRI studies of patients with anorexia nervosa, perceptions of satiety and hunger were influenced by body image stimuli, and when compared with healthy controls, they had reduced activation in the cerebellar vermis bilaterally and increased activation in the right visual cortex. Activity in the hypothalamus, nucleus accumbens, or the habenula was not reported.25 Recent reports suggest that in the setting of cancer‐related anorexia, activity in the hypothalamic and frontal brain regions in the premotor and the prefrontal cortices is decreased.41, 42
Although no direct changes in RSFC between the hypothalamus and nucleus accumbens were observed in our study, we identified decreased RSFC between the habenula and both the hedonic nucleus accumbens and the hypothalamus in patients with C‐WL compared with healthy controls. This suggests that the habenula links reward and hunger signalling in the brain and that this mechanism is impaired in this setting (Figure 4).
Figure 4.
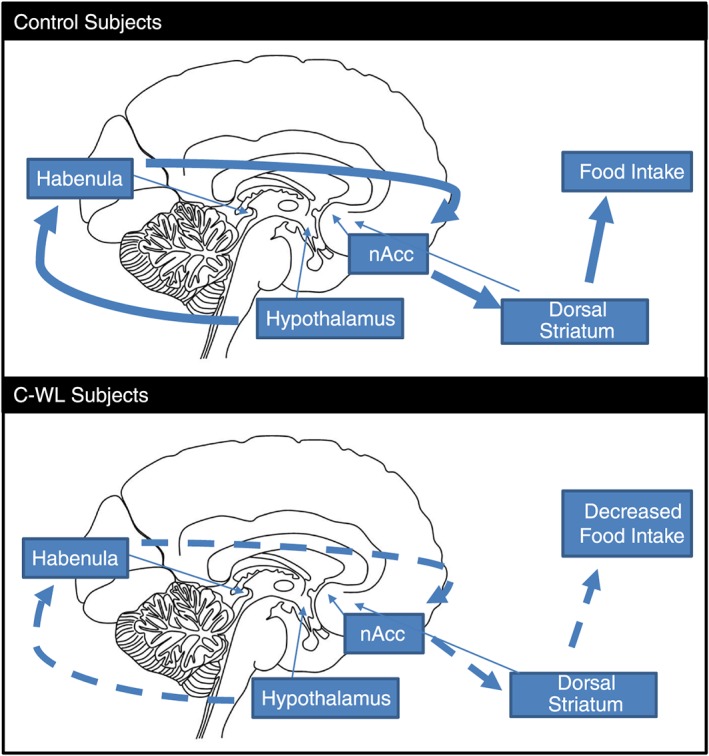
Integration of homeostatic and hedonic inputs. In the control group, homeostatic hunger signals from the hypothalamus modulate the perception of food reward through the habenula. The habenula projects to the nucleus accumbens thus heightening the negative reward of forgoing food within the hedonic pathway. Participants with cancer‐associated weight loss had lower levels of connectivity between the hypothalamus and the habenula and between the habenula and the nucleus accumbens thus decreasing the negative reward of forgoing food and decreasing motivation for food and food intake, which translates in less reward‐related dorsal striatal activity. Large solid arrows indicate pathways active in non‐cancer, healthy controls; dotted arrows indicate same pathways that are downregulated in subjects with cancer‐associated weight loss, and small arrows indicate anatomical location of these brain structures.
We showed that when patients with C‐WL are given juice reward, their striatal activity is not increased as it is in healthy controls. This is consistent with previous reports that patients with cancer with poor appetite have decreased brain activity in response to food or food images, although the study by Molfino et al. only included two control subjects and the study by Sanchez‐Lara et al. detected activity in controls only while watching unpleasant stimuli. In this particular study, fMRI based on https://en.wikipedia.org/wiki/Blood-oxygen-level_dependent signals were analysed while the patients perceived pleasant and unpleasant food pictures based on a food preference questionnaire provided to patients.41, 42
Generalized anhedonia has been reported in patients with cancer,43 and our group and others have reported altered appetite and hedonic perception of food in this setting, particularly in those patients receiving chemotherapy.31, 44 Furthermore, these symptoms are associated with weight loss and malnutrition.45 The mechanisms proposed behind these alterations are not well characterized in humans. Although the possible roles of other brain regions were not studied to avoid possible multiple comparison problems due to small sample size, this study provides mechanistic insight into areas of the brain known to regulate appetite and hedonic signals and supports the idea that the activity and connectivity of these regions are affected in this setting. We also expand previous findings and characterize a novel circuitry that is impaired in this setting: the habenula and reward processing.
Anatomical studies of the hypothalamus have identified efferent projections to several brain areas, including the medial hypothalamus nuclei, amygdala, and ventral tegmental area. One of the strongest lateral hypothalamus projections is to the lateral habenula.22, 23, 24 In fact, it has been shown that hypothalamic neurons and their projections to the habenula regulate feeding and reward.46 As noted in the preceding texts, the habenula is the brain's primary regulator of negative reward, including that due to anxiety, stress, pain, or the absence of an expected reward, including food rewards.20, 47, 48, 49 While the brain normally rewards positive signals, such as eating palatable food, with the release of dopamine, negative rewards activate the habenula, which then suppresses dopaminergic neurons in the ventral tegmental area and inhibits positive reward in the hedonic nucleus accumbens.20, 50
In the present study, reduced RSFC between hypothalamus and habenula in patients with C‐WL suggests a disruption of hunger signalling. Similarly, reduced RSFC between the nucleus accumbens and habenula in patients with C‐WL suggests a disruption of reward signalling. In addition, juice delivery did not elicit the presumably dopaminergic signals in C‐WL as it did in controls. Combined, these data suggest that food may have a less rewarding effect while forgone food is also not accompanied by a negative hunger signal, ultimately resulting in less motivation for food intake in this setting, and less striatal activity when food is delivered. Interestingly, we found decreased connectivity between the habenula and the nAcc, not the striatum. However, there was diminished juice‐stimulated activity in the striatum (mainly dorsal striatum) and not in the nAcc. We hypothesize that diminished functional connectivity in the hypothalamus/habenula/nAcc circuit results in the nAcc influencing activity in the dorsal striatum, and in reward having a smaller effect on those areas. This could have an important role in C‐WL, as the dorsal striatum uses reward information from the nAcc to affect decision making and action initiation.51
We did not find a correlation between appetite scores and any of the other variables measured including weight loss, RSFC, or reward‐induced striatal activity. Given the wide range of appetite scores we found, it is possible that a larger sample size is needed to establish an association between appetite and other variables. Also, it is possible that appetite is a more complex construct that involves other peripheral component in the gastrointestinal tract (such as taste, gastric emptying, and constipation) that may not be captured by brain fMRI of the habenula and striatum. Lastly, appetite scores were not collected for controls, and this will be needed in the future to better understand the relationship between appetite and RSFC or reward‐induced striatal activity.
Given the role the habenula may play in mediating food intake, this brain structure needs to be explored further in future studies of appetite regulation in patients with C‐WL. Activity in the habenula may predict weight loss susceptibility, and this region could serve as an early biomarker for the effectiveness of orexigenic agents. Future research should explore differences in the reward brain system including the habenula and the striatum between C‐WL and control groups in response to food stimuli—both visual and gustatory—and in response to orexigenic and dopaminergic agents. In addition, direct comparisons between patients with cancer with and without weight loss could be conducted to rule out changes in RSFC and brain activity due to cancer or cancer therapies, rather than to weight loss as a separate condition. Subjects with inability to increase food intake (e.g. esophageal obstruction, intractable vomiting) or those receiving highly emetogenic chemotherapy (Hesketh scale class 4–5) were excluded from our study. However, prior chemotherapy (Hesketh scale class 1–3) or anti‐emetics were not exclusionary. Controls were deemed healthy, but a weight history was not obtained. These factors should also be taken into account in the design of future studies.
Finally, future research should address the primary limitation of this study, namely a relatively small sample size and resulting heterogeneity of tumour types to improve the generalizability of the current findings and confirm these results. The development of therapies for cancer‐associated anorexia and weight loss is desperately needed. fMRI may be a useful tool in the development of these therapies.
Acknowledgements
This study was funded by a MEDVAMC Seed grant (JG, RS), VHA MERIT grants (I01BX002807 to JG, CX000174 to JG, and CX000994 to RS), the NCI (3P30CA125123‐08S2 to RS) and the NIA (T32AG000183 and AG040583 to JG). Funding was also provided by the American Federation of Aging Research via the Medical Student Training in Aging Research program to MM and the McNair Medical Institute to RS. Both Jose M. Garcia and Ramiro Salas had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Authors report no conflicts of interest. The authors acknowledge the help of Eduardo Aramayo, Kenia Velasquez, and Gina Cardwell in data collection and the Baylor College of Medicine Core for Advanced MRI. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.52
Maldonado, M. , Molfese, D. L. , Viswanath, H. , Curtis, K. , Jones, A. , Hayes, T. G. , Marcelli, M. , Mediwala, S. , Baldwin, P. , Garcia, J. M. , and Salas, R. (2018) The habenula as a novel link between the homeostatic and hedonic pathways in cancer‐associated weight loss: a pilot study. Journal of Cachexia, Sarcopenia and Muscle, 9: 497–504. doi: 10.1002/jcsm.12286.
Trial Registration: http://clinicaltrials.gov Identifier: NCT01614990.
Contributor Information
Jose M. Garcia, Email: jg77@uw.edu
Ramiro Salas, Email: rsalas@bcm.edu.
References
- 1. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 2. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 3. Adamopoulos S, Parissis JT, Georgiadis M, Karatzas D, Paraskevaidis J, Kroupis C, et al. Growth hormone administration reduces circulating proinflammatory cytokines and soluble Fas/soluble Fas ligand system in patients with chronic heart failure secondary to idiopathic dilated cardiomyopathy. Am Heart J 2002;144:359–364. [DOI] [PubMed] [Google Scholar]
- 4. Lasheen W, Walsh D. The cancer anorexia‐cachexia syndrome: myth or reality? Support Care Cancer 2010;18:265–272. [DOI] [PubMed] [Google Scholar]
- 5. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez C, Rosell R, Abad‐Esteve A, Monras P, Moreno I, Serichol M, et al. Quality of life during chemotherapy in non‐small cell lung cancer patients. Acta Oncol 1989;28:29–33. [DOI] [PubMed] [Google Scholar]
- 7. Staal‐van den Brekel AJ, Schols AM, ten Velde GP, Buurman WA, Wouters EF. Analysis of the energy balance in lung cancer patients. Cancer Res 1994;54:6430–6433. [PubMed] [Google Scholar]
- 8. Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 2005;20:369–376. [DOI] [PubMed] [Google Scholar]
- 9. Plata‐Salaman CR. Regulation of hunger and satiety in man. Dig Dis 1991;9:253–268. [DOI] [PubMed] [Google Scholar]
- 10. Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage 2006;32:1273–1280. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 12. Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 1998;280:1378–1383. [DOI] [PubMed] [Google Scholar]
- 13. Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 2005;8:1445–1449. [DOI] [PubMed] [Google Scholar]
- 14. Delgado MR. Reward‐related responses in the human striatum. Ann N Y Acad Sci 2007;1104:70–88. [DOI] [PubMed] [Google Scholar]
- 15. Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple‐function networks. Med Sci Monit 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- 16. Blander A, Wise RA. Anatomical mapping of brain stimulation reward sites in the anterior hypothalamic area: special attention to the stria medullaris. Brain Res 1989;483:12–16. [DOI] [PubMed] [Google Scholar]
- 17. Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber‐of‐passage problem. J Comp Neurol 1977;173:123–146. [DOI] [PubMed] [Google Scholar]
- 18. Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev 1982;6:1–13. [DOI] [PubMed] [Google Scholar]
- 19. Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci 2004;19:2551–2560. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007;447:1111–1115. [DOI] [PubMed] [Google Scholar]
- 21. Salas R, Baldwin P, de Biasi M, Montague PR. BOLD responses to negative reward prediction errors in human habenula. Front Hum Neurosci 2010;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull 1982;8:511–526. [DOI] [PubMed] [Google Scholar]
- 23. Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol 2008;507:1465–1478. [DOI] [PubMed] [Google Scholar]
- 24. Swanson LW. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol 1976;167:227–256. [DOI] [PubMed] [Google Scholar]
- 25. Brooks SJ, O'Daly O, Uher R, Friederich HC, Giampietro V, Brammer M, et al. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One 2012;7:e34000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav 2010;100:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, et al. Tumor‐induced anorexia and weight loss are mediated by the TGF‐beta superfamily cytokine MIC‐1. Nat Med 2007;13:1333–1340. [DOI] [PubMed] [Google Scholar]
- 28. Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level‐dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 1993;64:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 30. Zhang C, Cahill ND, Arbabshirani MR, White T, Baum SA, Michael AM. Sex and age effects of functional connectivity in early adulthood. Brain Connect 2016;6:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia JM, Garcia‐Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer‐induced cachexia. J Clin Endocrinol Metab 2005;90:2920–2926. [DOI] [PubMed] [Google Scholar]
- 32. Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp 1999;7:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012;59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 35. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 36. Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS One 2015;10:e0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron 2003;38:339–346. [DOI] [PubMed] [Google Scholar]
- 38. Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin‐concentrating hormone system of the rat brain: an immuno‐ and hybridization histochemical characterization. J Comp Neurol 1992;319:218–245. [DOI] [PubMed] [Google Scholar]
- 39. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behavior. Cell 1998;92:573–585. [DOI] [PubMed] [Google Scholar]
- 40. Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr 2007;86:965–971. [DOI] [PubMed] [Google Scholar]
- 41. Molfino A, Iannace A, Colaiacomo MC, Farcomeni A, Emiliani A, Gualdi G, et al. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite‐regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle 2017;8:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanchez‐Lara K, Arrieta O, Pasaye E, Laviano A, Mercadillo RE, Sosa‐Sanchez R, et al. Brain activity correlated with food preferences: a functional study comparing advanced non‐small cell lung cancer patients with and without anorexia. Nutrition 2013;29:1013–1019. [DOI] [PubMed] [Google Scholar]
- 43. Brintzenhofe‐Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics 2009;50:383–391. [DOI] [PubMed] [Google Scholar]
- 44. Comeau TB, Epstein JB, Migas C. Taste and smell dysfunction in patients receiving chemotherapy: a review of current knowledge. Support Care Cancer 2001;9:575–580. [DOI] [PubMed] [Google Scholar]
- 45. Mattes RD, Curran WJ Jr, Alavi J, Powlis W, Whittington R. Clinical implications of learned food aversions in patients with cancer treated with chemotherapy or radiation therapy. Cancer 1992;70:192–200. [DOI] [PubMed] [Google Scholar]
- 46. Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci 2016;36:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor‐mediated mechanism. J neurosci 2007;27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lecourtier L, Deschaux O, Arnaud C, Chessel A, Kelly PH, Garcia R. Habenula lesions alter synaptic plasticity within the fimbria‐accumbens pathway in the rat. Neuroscience 2006;141:1025–1032. [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 2009;12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine‐containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci 1986;6:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision‐making. J Neurosci 2007;27:8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


