Abstract
In 1993, megestrol acetate (MA) was approved by the US Food and Drug Administration for the treatment of anorexia, cachexia, or unexplained weight loss in patients with acquired immunodeficiency syndrome. The mechanism by which MA increases appetite is unknown, and its effectiveness for anorexia and cachexia in neoplastic, elderly, and acquired immunodeficiency syndrome patients is under investigation. This is an updated version of a Cochrane systematic review first published in 2005 and later updated in 2013 entitled ‘Megestrol acetate for the treatment of anorexia–cachexia syndrome’. MA vs. placebo: in studies where MA was compared with placebo, the overall results showed that MA patients gained weight (mean difference, MD 2.25 kg, 95% CI [1.19, 3.3]) but did not gain quality of life (QOL) (standarized mean difference, SMD 0.5, 95% CI [−0.13, 1.13]), with more adverse events (relative risk, RR 1.46, 95% CI [1.05, 2.04]), but no difference in deaths (RR 1.26, 95% CI [0.70, 2.27]). MA vs. no treatment: MA patients gained weight (MD 1.45 kg, 95% CI [0.15, 2.75]) but did not gain QOL (standardized mean difference 3.89 95% CI [−14, 6.28]). There was no increase in adverse events (RR 0.90, 95% CI [0.39, 2.08]) or deaths (RR 1.01, 95% CI [0.42, 2.45]). MA vs. active drugs: MA patients gained weight (MD 2.5 kg, 95% CI [0.37, 4.64]) but did not gain QOL (MD 0.20 95% CI [−0.02, 0.43]) and did not report an increase in adverse events (RR 1.05 95% CI [0.95, 1.16]) or in deaths (RR 1.53, 95% CI [1.02, 2.29]) Different doses of MA: in studies where lower doses of MA were compared with higher doses of MA, we did not find differences either in weight gain (MD −0.94 kg, 95% CI [−3.33, 1.45]), QOL (MD 0.31 95% CI [−0.19, 0.81]), or adverse events (RR 1.34, 95% CI [0.65, 2.76]). Thus, we cannot reach a conclusion for an optimal dose of MA.
Keywords: Anorexia, Appetite Stimulants, Cachexia, Systematic Review, Megestrol Acetate, Randomized Controlled Trials
Background
This review is the second update of a review first published in The Cochrane Library (2005, Issue 2) and previously updated in 2013, on megestrol acetate (MA) for anorexia–cachexia syndrome.1, 2, 3 Anorexia–cachexia syndrome is a common clinical problem that has a substantial impact on the quality of life and survival of affected patients. It is characterized by loss of appetite, weight loss, and tissue wasting, accompanied by a decrease in muscle mass and adipose tissue, worsening quality of life, and often preceding the patient's death.4, 5
More than two‐thirds of patients dying from advanced cancer suffer from anorexia–cachexia syndrome.6 Apart from cancer, anorexia–cachexia syndrome is also described in other pathologies such as in acquired immune deficiency syndrome (AIDS), anorexia nervosa, degenerative illnesses of the central nervous system, and other terminal illnesses.7 Its incidence is variable and difficult to determine, but in general, the syndrome may occur in 15% to 40% of patients with cancer and in more than 80% of patients with advanced illness.8 In the pathophysiology of anorexia–cachexia syndrome, several factors were involved, and many substances were released from the tumour: pro‐inflammatory cytokines, lactate, parathormone‐related peptide, dysphagia, zinc deficiency, hypoxia, increase in peripheral tryptophan leading to increasing central serotonin, or alterations in the release of peripheral hormones related to feeding (e.g. peptide tyrosine and ghrelin).9
Methods
For this updated systematic review, we included only randomized controlled trials, regardless of blinding, both in inpatient and outpatient study settings. In the 2013 update, we decided not to include cross‐over trials because the time between the two phases was too short to be certain whether any adverse event or outcome was due to MA or the placebo.
Study design and settings
Participants
We included participants with a clinical diagnosis of anorexia–cachexia related to cancer, AIDS, or another underlying pathology (independent of sex, age, or ethnicity). In addition, we included participants with previous weight loss.
Types of interventions
The review focused on the following treatment comparisons:
Megestrol acetate at any dose vs. placebo.
Megestrol acetate at any dose vs. no treatment.
Megestrol acetate at any dose vs. other active drugs (appetite stimulants such as dronabinol, cytokine inhibitors such as cyproheptadine, eicosapentaenoic acid, and anabolic agents such as nandrolone decanoate or corticosteroids).
Megestrol acetate at different doses.
Types of outcome measures
Weight gain assessed as a continuous variable in kilograms (i.e. weight difference between baseline and end of treatment).
Quality of life gain measured as a continuous variable assessed as described in trials (such as means of the Therapy impact Questionnaire, Padilla Index, Measure of health status for chronic airflow limitation, The St. George's Respiratory Questionnaire, etc.).
Adverse events, measured by the number of participants who suffered any adverse event as reported by the authors of the trial.
Deaths.
Searches
We searched the following electronic databases to identify relevant studies:
Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 11);
MEDLINE (OVID) (May 2012 to November 2016);
Embase (OVID) (May 2012 to November 2016).
We designed and executed a specific search strategy for each database. The search strategies are available in Data S1.
Other resources
We checked reference lists from systematic reviews of MA and from the included studies to identify further trials. We did not exclude studies on the basis of language or publication status (published, unpublished, in press, and in progress). We sought additional data from published trials by contacting authors. We consulted the information made available by the main researchers/sponsors. Pharmaceutical companies were contacted to retrieve additional information. We also reviewed information on the clinical trial metaregister databases: http://www.who.int/ictrp/es and https://clinicaltrials.gov with the following keywords: megestrol acetate, anorexia, and cachexia (May 2012 to November 2016).
Results
A total of 132 references were retrieved from electronic databases; after de‐duplication, 112 remained.
A total of 26 references were identified from clinical trial registries.
We present a flowchart of included studies, according to the PRISMA recommendations, shown in Figure 1, and a risk of summary bias in Figure 2 and risk of bias of each trial in Figure 3.
Figure 1.
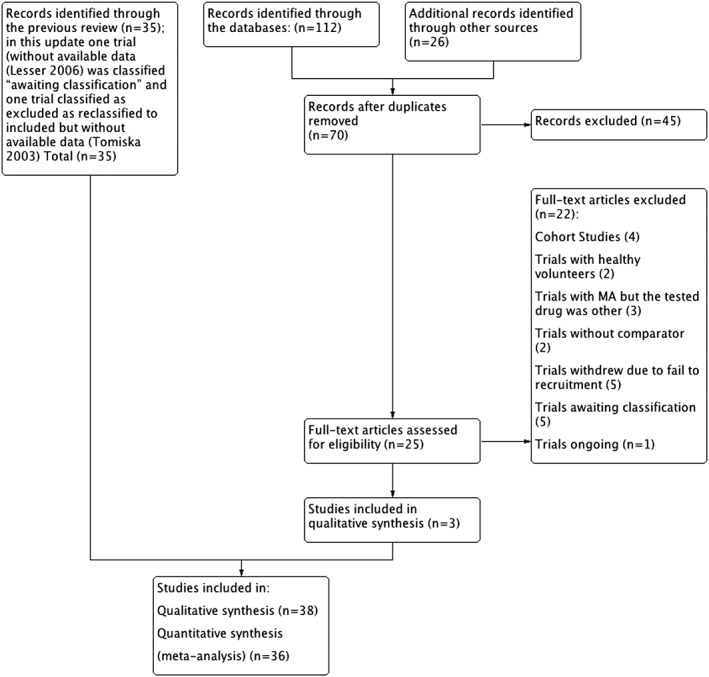
Flow chart. MA, megestrol acetate.
Figure 2.
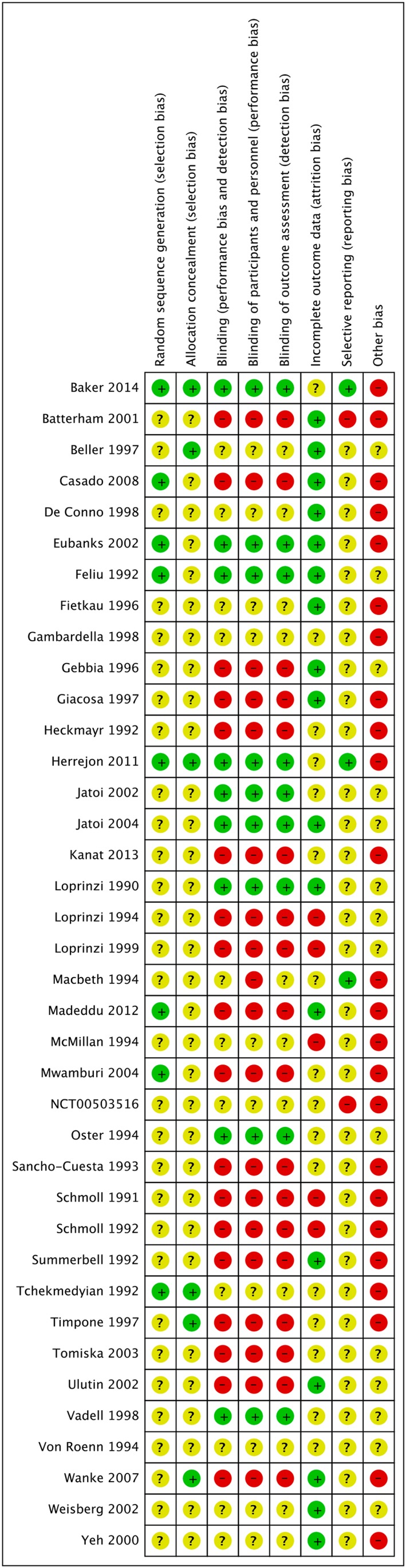
Risk of bias: summary.
Figure 3.
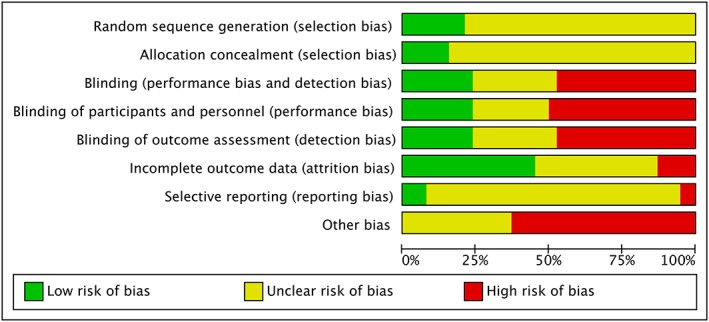
Risk of bias graph.
We analysed data from 38 studies,10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 with 4304 participants.
Additionally, in accordance with the MECIR standard, we reclassified the trial of Tomíska44 as included. We did not have available data on this trial because it compared two doses of MA, but the results were shown as only one arm.
In this update, we excluded 70 studies and included three new studies.45, 46, 47 We identified one ongoing study48 and reclassified a previously included study as awaiting classification due to lack of available data.49
Three studies were awaiting classification.50, 51, 52
In the end, this review update includes 38 trials involving 4304 participants.
Megestrol acetate at any dose versus placebo
Weight gain
The overall results showed weight gain (in kilograms) in participants treated with MA (mean difference, MD 2.25 kg, 95% confidence interval, CI [1.19, 3.30]; 9 studies, 575 participants) (Figure 4).
Figure 4.
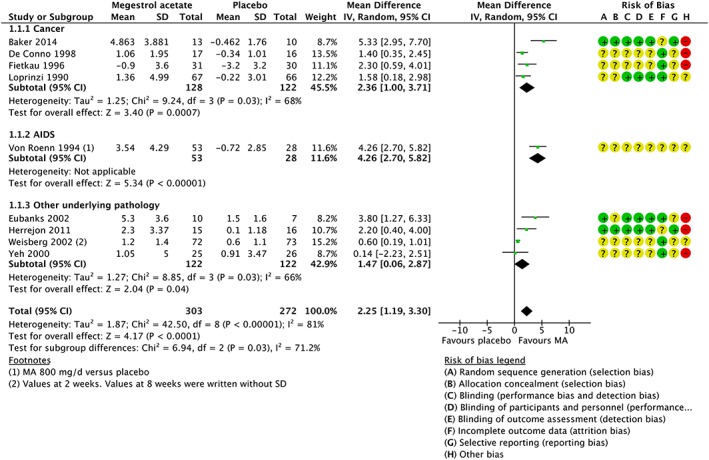
Meta‐analysis of weight. CI, confidence interval; SD, standard deviation.
We judged the quality of evidence for weight gain to be moderate. We downgraded the quality of evidence by one level because of unclear risk of bias, because of unclear generation of the randomization sequence and unclear allocation concealment.
Quality of life gain
Megestrol acetate and placebo participants did not report changes in quality of life (standardized mean difference 0.50, 95% CI [−0.13, 1.13]; 2 studies, 70 participants) (Figure 5).
Figure 5.
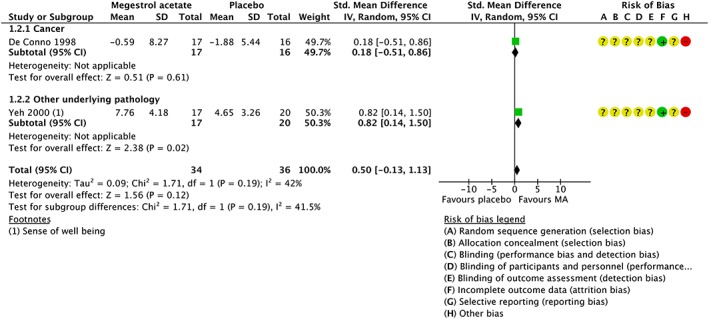
Meta‐analysis of the quality of life. CI, confidence interval; SD, standard deviation.
We judged the quality of evidence for the quality of life gain to be very low. We downgraded the quality of evidence by two levels because of the risk of bias due to unclear generation of the randomization sequence, unclear allocation concealment, and high risk of bias in blinding and one additional level for imprecision due to the fact that the total number of events was less than 400, and the pooled result included both benefits and negative health effects.
Adverse events
Overall, participants treated with MA at any dose reported a higher number of adverse events (relative risk, RR 1.46, 95% CI [1.05, 2.04]; 8 studies, 638 participants).
We judged the quality of evidence for adverse events to be moderate. We downgraded the quality of evidence by one level, because of an unclear risk of bias due to an unclear generation of the randomization sequence and unclear allocation concealment.
Deaths
The overall results showed no differences for deaths for participants treated with MA (RR 1.26, 95% CI [0.70, 2.27]; 6 studies, 877 participants).
We judged the quality of evidence for death to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment; and one additional level because of imprecision due to the fact that the 95% CI included both negative health effects and lack of negative effects.
Megestrol acetate at any dose versus no treatment
Weight gain
Regarding weight gain, we found two trials with 101 cancer participants reporting weight gain, and the results showed small differences (MD 1.45 kg, 95% CI [0.15, 2.75]).
We judged the quality of evidence for weight gain to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment and one additional level because of imprecision due to the fact that the total population size was less than 400.
Quality of life gain
We found two studies for the quality of life gain analysis, both in cancer participants. 28 , 46 The results did not show differences (standardized mean difference −3.89, 95% CI [−14.07, 6.28]; 2 studies, 99 participants).
We judged the quality of evidence for the quality of life gain to be very low. We downgraded the quality of evidence by two levels because of the risk of bias due to unclear generation of the randomization sequence, unclear allocation concealment, and lack of blinding; one additional level of inconsistency due to forest plot with CI trials which no overlap; and one additional level for imprecision due to the fact that the total size population was less than 400.
Adverse events
We found two trials with 101 cancer participants.28 , 46 The results showed no differences in adverse events (RR 0.90, 95% CI [0.39, 2.08]). We judged the quality of evidence for adverse events to be very low. We downgraded the quality of evidence by two levels, because of the risk of bias due to unclear generation of the randomization sequence, unclear allocation concealment, and lack of blinding; and one additional level because of imprecision due to the fact that the total number of events was less than 400, and the 95% CI included both appreciable negative health effects and lack of negative effects.
Deaths
We identified two trials measuring the death outcome among 90 participants.19 , 28 The results showed no differences in deaths (RR 1.01, 95% CI [0.42, 2.45]). We judged the quality of evidence for the deaths to be very low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment and two additional levels because of imprecision due to the fact that the total population size was less than 400, and the pooled value included both appreciable negative health effects and lack of negative effects.
Megestrol acetate at any dose versus other active drugs treatments
Weight gain
Regarding weight gain, the overall results for the outcome of weight gain showed improvement (MD 2.50 kg, 95% CI [0.37, 4.64]; 4 studies, 541 participants).
We judged the quality of evidence for weight gain to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment; and one additional level because of inconsistency due to high heterogeneity in trials of patients with cancer and AIDS with CIs of trials that did not overlap.
Quality of life gain
There was only one trial22 with cancer participants for the quality of life gain that showed no difference between the two groups (MD 0.20, 95% CI [−0.02, 0.43]; 469 participants).
We judged the quality of evidence for the quality of life gain to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment; and one additional level due to imprecision given that the 95% CI (or alternative estimate of precision) around the pooled or best estimate of effect included no effect and appreciable benefit or appreciable negative effect.
Adverse events
The overall results showed no increase in adverse events for participants treated with MA (RR 1.05, 95% CI [0.95, 1.16] 7 studies, 1175 participants). When we analysed the drugs used as comparators: eicosapentaenoic acid, anabolic steroids (Nandrolone or Oxandronolone), corticosteroids, dronabinol, and cyproheptadine, we did not find differences (RR 0.98, 95% CI [0.89, 1.09]), (RR 1.79, 95% CI [0.58, 5.48]), (RR 1.11 95% CI [0.90, 1.37]) (RR 1.07, 95% CI [0.94, 1.21]), and (RR 4 95% CI [0.58, 27.41]), respectively.
We judged the quality of evidence for adverse events to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment; and one additional level due to inconsistency given that the total number of events is less than 300, and the 95% CI (or alternative estimate of precision) around the pooled or best estimate of effect includes no effect and appreciable benefit or appreciable negative effect.
Megestrol acetate at different doses
We analysed low doses vs. high doses of MA. We used the definitions of low dose and high dose as delineated in each trial. Accordingly, in some trials, such as Beller and Tattersall's,11 low doses of MA were described as 160 mg and high doses as 480 mg, while in the trial of Wanke et al.,41 low doses were defined as 575 mg and high doses as 800 mg. This variability could potentially affect the strength of the treatment effect, and results should be interpreted with caution.
Weight gain
We found two trials in the subcategory of AIDS participants for the outcome weight gain, and the meta‐analysis showed no statistical differences (MD −0.94, 95% CI [−3.33, 1.45]; 283 participants). Thus, we could not determine the optimal doses with MA for the overall participants.
We judged the quality of evidence for weight gain to be very low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment; another level because of inconsistency due to no CI overlap of trials; and one additional level because of imprecision given that the 95% CI included both effect and lack of effect.
Quality of life gain
Only one trial (Wanke et al.)41 assessed the quality of life gain and did not find any differences either (MD 0.31, 95% CI [−0.19, 0.81]; 1 study, 63 participants).
We judged the quality of evidence for the quality of life gain to be very low. We downgraded the quality of evidence by two levels because of the risk of bias due to unclear generation of the randomization sequence and unclear blindness and one level for imprecision because the 95% CI of trials included both effect and lack of effect.
Adverse events
The overall results showed no differences for participants treated with MA at different doses (RR 1.34, 95% CI [0.65, 2.76]; 3 studies, 356 participants).
We judged the quality of evidence for adverse events to be low. We downgraded the quality of evidence by one level because of the risk of bias due to unclear generation of the randomization sequence and unclear allocation concealment and one additional level due to imprecision given that the total number of events was less than 400, and the 95% CI (or alternative estimate of precision) around the pooled or best estimate of effect included both no effect and appreciable benefit or appreciable negative effect.
Deaths
No data available.
Discussion
Our systematic review confirms the effect of MA in weight gain, as seen in recent animal models.53, 54 However, the aim of the present update of the review was to assess the efficacy and adverse events of MA for the management of anorexia–cachexia syndrome in humans, a common clinical problem that substantially impacts upon the quality of life and survival of affected participants.
The current update does not present any relevant changes for MA effectiveness. However, the inclusion of three relevant studies allows us to be more confident of the results obtained because the results are in line with the previous ones. Participants treated with MA showed a slight increase in weight compared with the placebo group participants. However, the weight gain was not clinically relevant (i.e. not leading to full weight loss recovery). Moreover, participants treated with MA did not gain quality of life.
On the contrary, the disaggregated analysis performed for the adverse events outcomes changed the conclusions with respect to our previous review. We only detected an increase in adverse events in the comparison with the placebo. No increase in deaths was found in any of the comparisons. These overall results were obtained from small trials lasting from 14 to 180 days. Most of the trials had a follow‐up of about 56 to 84 days. We cannot reach a conclusion for an optimal dose of MA regarding weight gain, quality of life gain, or adverse events. Overall, the risk of bias due to unclear generation of the randomization sequence, unclear allocation concealment, and imprecision were the main factors for downgrading the quality of evidence.
We have conducted a robust search, study selection, data collection, and bias risk assessment. In addition, we contacted the authors and retrieved more information from the sponsors. We evaluated the studies in pairs. We consider the potential bias in this review to be low.
Authors' conclusions
Participants treated with MA gained small amounts of weight. The disaggregated analysis performed for adverse event outcomes changed the conclusions with respect to our previous review. We only detected an increase in adverse events in the comparison with the placebo. None of the comparisons found any benefit in quality of life or increase in deaths or withdrawals in patients treated with MA.
Conflict of interest
All the authors declare that they have no conflicts of interest and certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.55
Supporting information
Data S1: Supporting information
Ruiz‐García, V. , López‐Briz, E. , Carbonell‐Sanchis, R. , Bort‐Martí, S. , and Gonzálvez‐Perales, J. L. (2018) Megestrol acetate for cachexia–anorexia syndrome. A systematic review. Journal of Cachexia, Sarcopenia and Muscle, 9: 444–452. doi: 10.1002/jcsm.12292.
CASPe and the ‘Red de investigación en servicios de Salud y Enfermedades Crónicas’
References
- 1. Berenstein EG, Ortiz Z. Megestrol acetate for the treatment of anorexia‐cachexia syndrome. Cochrane Database Syst Rev 2003. [DOI] [PubMed] [Google Scholar]
- 2. Berenstein EG, Ortiz Z. Megestrol acetate for the treatment of anorexia‐cachexia syndrome. Cochrane Database Syst Rev 2005. [DOI] [PubMed] [Google Scholar]
- 3. Ruiz Garcia V, Lopez‐Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort‐Marti S. Megestrol acetate for treatment of anorexia‐cachexia syndrome. Cochrane Database Syst Rev 2013;3:CD004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson KA, Walsh D, Sheehan FA. The cancer anorexia‐cachexia syndrome. J Clin Oncol 1994;12:213–225. [DOI] [PubMed] [Google Scholar]
- 5. Splinter TA. Cachexia and cancer: a clinician's view. Ann Oncol Off J Eur Soc Med Oncol ESMO 1992;3:25–27. [DOI] [PubMed] [Google Scholar]
- 6. Argilés JM, Meijsing SH, Pallares‐Trujillo J. Cancer cachexia. A therapeutic approach. Med Res Rev US 2001;21:83–101. [DOI] [PubMed] [Google Scholar]
- 7. Von Roenn JH, Knopf K. Anorexia/cachexia in patients with HIV: lessons for the oncologist. Oncology 1996;10:1049–1056. [PubMed] [Google Scholar]
- 8. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers‐update 2014. J Cachexia Sarcopenia Muscle 2014;5:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle 2015;6:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Batterham MJ, Garsia R. A comparison of megestrol acetate, nandrolone decanoate and dietary counselling for HIV associated weight loss. Int J Androl 2001;24:232–240. [DOI] [PubMed] [Google Scholar]
- 11. Beller E, Tattersall M. Improved quality of life with megestrol acetate in patients with endocrine‐insensitive advanced cancer: a randomised placebo‐controlled trial. Ann Oncol 1997;8:277–283. [DOI] [PubMed] [Google Scholar]
- 12. Casado Herráez A, Díaz‐Rubio GE. Estudio de Fase II Randomizado de Dos Niveles Posológicos de Acetato de Megestrol versus PLacebo para el Tratamiento de la Anorexia y la Caquexia en Pacientes con Cáncer Locoregional Avanzado o Metastásico. Madrid: Universidad Complutense de Madrid, Servicio de Publicaciones; 2008. [Google Scholar]
- 13. De Conno F, Martini C. Megestrol acetate for anorexia in patients with far‐advanced cancer: a double‐blind controlled clinical trial. Eur J Cancer 1998;34:1705–1709. [DOI] [PubMed] [Google Scholar]
- 14. Eubanks V, Koppersmith N, Wooldridge N, Clancy JP, Lyrene R, Arani RB, et al. Effects of megestrol acetate on weight gain, body composition, and pulmonary function in patients with cystic fibrosis. J Pediatr 2002;140:439–444. [DOI] [PubMed] [Google Scholar]
- 15. Feliu J, Gonzalez‐Baron M, Berrocal A, Artal A, Ordonez A, Garrido P, et al. Usefulness of megestrol acetate in cancer cachexia and anorexia. A placebo‐controlled study. Am J Clin Oncol 1992;15:436–440. [DOI] [PubMed] [Google Scholar]
- 16. Fietkau R, Riepl M, Kettner H, Hinke A, Sauer R. Supportive treatment with megestrol acetate during radio‐(chemo‐)therapy. A randomized trial. Strahlenther Onkol 1996;172:162–168. [PubMed] [Google Scholar]
- 17. Gambardella A, Pesce L, Bolognino P, Lombardi G, Barbieri M, Rinaldi C. Megestrol acetate prevents cachexia in elderly cancer patient. Ann Oncol 1998;9:72–72. [Google Scholar]
- 18. Gebbia V, Testa A, Gebbia N. Prospective randomised trial of two dose levels of megestrol acetate in the management of anorexia‐cachexia syndrome in patients with metastatic cancer. Br J Cancer 1996;73:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giacosa A, Frascio F, Sukkar SG, Costantini M, Baracco G, Capelli M. Changes of nutritional and psychological status after megestrol acetate treatment of cancer cachexia. Riv Ital Nutr Parenter Ed Enterale 1997;15:20–23. [Google Scholar]
- 20. Heckmayr M, Gatzemeier U. Treatment of cancer weight loss in patients with advanced lung cancer. Oncology 1992;49:32–34. [DOI] [PubMed] [Google Scholar]
- 21. Herrejon A, Palop J, Inchaurraga I, Lopez A, Banuls C, Hernandez A, et al. Low doses of megestrol acetate increase weight and improve nutrition status in patients with severe chronic obstructive pulmonary disease and weight loss. Med Clin Barc 2011;137:193–198. [DOI] [PubMed] [Google Scholar]
- 22. Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer‐associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 23. Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer‐associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469–2476. [DOI] [PubMed] [Google Scholar]
- 24. Loprinzi CL, Ellison NM, Schaid DJ, Krook JE, Athmann LM, Dose AM, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127–1132. [DOI] [PubMed] [Google Scholar]
- 25. Loprinzi CL, Bernath AM. Phase III evaluation of 4 doses of megestrol acetate for patients with cancer anorexia and/or cachexia. Oncology 1994;51:2–7. [DOI] [PubMed] [Google Scholar]
- 26. Loprinzi CL, Kugler JW. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 1999;17:3299–3306. [DOI] [PubMed] [Google Scholar]
- 27. Macbeth FR, Grgeor A, Cottier B. A randomised study of megestrol acetate (MA) and prednisolone (P) for anorexia and weight loss in patients with lung cancer. Lung Cancer 1994;11 Suppl 1:88–88. [Google Scholar]
- 28. Madeddu C, Maccio A, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment with megestrol acetate+carnitine+celecoxib+antioxidants vs. megestrol acetate alone for patients with cancer cachexia syndrome. Support Care Cancer 2012;20:S57–S58. [Google Scholar]
- 29. McMillan DC, Simpson JM, Preston T, Watson WS, Fearon KC, Shenkin A, et al. Effect of megestrol acetate on weight loss, body composition and blood screen of gastrointestinal cancer patients. Clin Nutr 1994;13:85–89. [DOI] [PubMed] [Google Scholar]
- 30. Mwamburi DM, Gerrior J, Wilson IB, Chang H, Scully E, Saboori S, et al. Comparing megestrol acetate therapy with oxandrolone therapy for HIV‐related weight loss: similar results in 2 months. Clin Infect Dis 2004;38:895–902. [DOI] [PubMed] [Google Scholar]
- 31. Oster MH, Enders SR, Samuels SJ, Cone LA, Hooton TM, Browder HP, et al. Megestrol acetate in patients with AIDS and cachexia. Ann Intern Med 1994;121:400–408. [DOI] [PubMed] [Google Scholar]
- 32. Sancho‐Cuesta JF. Megestrol acetate and weight loss in advanced cancer [Abstract no: 1141]. Eur J Cancer 1993;29A:S204–S204. [Google Scholar]
- 33. Schmoll E. Risks and benefits of various therapies for cancer anorexia. Oncology 1992;49:43–45. [DOI] [PubMed] [Google Scholar]
- 34. Schmoll E, Wilke H, Thole R, Preusser P, Wildfang I, Schmoll HJ. Megestrol acetate in cancer cachexia. Semin Oncol 1991;18:32–34. [PubMed] [Google Scholar]
- 35. Summerbell CD, Youle M, McDonald V, Catalan J, Gazzard BG. Megestrol acetate vs cyproheptadine in the treatment of weight loss associated with HIV infection. Int J STD AIDS 1992;3:278–280. [DOI] [PubMed] [Google Scholar]
- 36. Tchekmedyian NS, Hickman M, Siau J, Greco FA, Keller J, Browder H, et al. Megestrol acetate in cancer anorexia and weight loss. Cancer 1992;69:1268–1274. [DOI] [PubMed] [Google Scholar]
- 37. Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, et al. The safety and pharmacokinetics of single‐agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. AIDS Res Hum Retroviruses 1997;13:305–315. [DOI] [PubMed] [Google Scholar]
- 38. Ulutin HC, Arpaci F, Pak Y. Megestrol acetate for cachexia and anorexia in advanced non‐small cell lung cancer: a randomized study comparing two different doses. Tumori 2002;88:277–280. [DOI] [PubMed] [Google Scholar]
- 39. Vadell C, Segui MA, Gimenez‐Arnau JM, Morales S, Cirera L, Bestit I, et al. Anticachectic efficacy of megestrol acetate at different doses and versus placebo in patients with neoplastic cachexia. Am J Clin Oncol 1998;21:347–351. [DOI] [PubMed] [Google Scholar]
- 40. Von Roenn JH. Randomized trials of megestrol acetate for AlDS‐associated anorexia and cachexia. Oncology 1994;51:19–24. [DOI] [PubMed] [Google Scholar]
- 41. Wanke C, Gutierrez J, Kristensen A, MacEarchern L. Safety and efficacy of two preparations of megestrol acetate in HIV‐infected individuals with weight loss in Africa, India, and the United States. J Appl Res 2007;7:206–216. [Google Scholar]
- 42. Weisberg J, Wanger J, Olson J, Streit B, Fogarty C, Martin T, et al. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest 2002;121:1070–1078. [DOI] [PubMed] [Google Scholar]
- 43. Yeh SS, Wu SY, Lee TP, Olson JS, Stevens MR, Dixon T, et al. Improvement in quality‐of‐life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double‐blind, placebo‐controlled study. J Am Geriatr Soc 2000;48:485–492. [DOI] [PubMed] [Google Scholar]
- 44. Tomíska M, Tomisková M, Salajka F, Adam Z, Vorlícek J. Palliative treatment of cancer anorexia with oral suspension of megestrol acetate. Neoplasma 2003;50:227–233. [PubMed] [Google Scholar]
- 45. Baker TJ, Peddie EF, Casey LM, Lambert PJ, Distefano DS, Wardle MG, et al. A randomized, double‐blind, placebo‐controlled clinical trial of megestrol acetate as an appetite stimulant in children with weight loss due to cancer and/or cancer therapy. Pediatr Blood Cancer 2014;61:672–679. [DOI] [PubMed] [Google Scholar]
- 46. Kanat O, Cubukcu E, Avci N, Budak F, Ercan I, Canhoroz M, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori 2013;Volume|;229–233, https://doi.org/10.1700/1283.14197. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez P. Study to Evaluate the Effect of Megestrol Acetate in Weight Loss in Dementia Patients n.d. https://clinicaltrials.gov/ct2/show/NCT00503516 (accessed September 10, 2017). [Google Scholar]
- 48. Currow D. Randomised, double blind control trial of megestrol acetate, dexamethasone and placebo in the management of anorexia in people with cancer. Https://Www.http://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000405314 2008.
- 49. Lesser GJ, Case D, Sharp S, Choksi J, Miller A, Atkins J, et al. A Phase III randomized study comparing the effects of oxandrolone (Ox) and megestrol acetate (Meg) on weight (wt), lean body mass (LBM) and quality of life (QOL) in solid tumor patients (pts) receiving chemotherapy (chemo) [abstract]. J Clin Oncol ASCO Annu Meet Proc 2006;24:691–691. [Google Scholar]
- 50. Anonymous . A study of megestrol acetate in HIV‐infected children n.d. https://clinicaltrials.gov/ct2/show/NCT00002182 (accessed September 10, 2017).
- 51. Hayden J. Randomized placebo‐controlled study of aerobic exercise and resistance training plus megestrol acetate for hiv‐wasting ‐ Full text view ‐ http://clinicaltrials.gov n.d. https://www.clinicaltrials.gov/ct2/show/NCT00004664 (accessed September 10, 2017).
- 52. Greven KM. Megestrol to limit weight loss and improve quality of life in treating patients with head and neck cancer n.d. https://clinicaltrials.gov/ct2/show/study/NCT00006799 (accessed September 10, 2017).
- 53. Toledo M, Penna F, Oliva F, Luque M, Betancourt A, Marmonti E, et al. A multifactorial anti‐cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J Cachexia Sarcopenia Muscle 2016;7:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Musolino V, Palus S, Tschirner A, Drescher C, Gliozzi M, Carresi C, et al. Megestrol acetate improves cardiac function in a model of cancer cachexia‐induced cardiomyopathy by autophagic modulation. J Cachexia Sarcopenia Muscle 2016;7:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015: editorial. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information


