Abstract
Purpose
Retinal ischemic injury depends on grade and duration of an ischemic insult. We developed a method to induce ischemic injury in rats permitting: (1) Variable grades of retinal blood flow (F) reduction, (2) controllable duration of F reduction, (3) injury without collateral neural damage, and (4) optical measurements of F and O2-related factors: O2 delivery (DO2), O2 extraction fraction (OEF), and metabolic rate of O2 (MO2).
Methods
In five anesthetized rats the left common carotid artery (CA) was ligated and the right CA was exposed. A variable clamp having a backstop and a rod mounted on a micromanipulator straddled the right CA. Advancing the rod with the micromanipulator produced graded compressions of the CA. F and O2-related factors were measured with established optical techniques.
Results
Four to seven grades of F for at least 10 minutes were achieved per rat. F decreased only with compressions of over 60%. DO2 changed in proportion to F, particularly at low F. As F decreased, OEF initially changed little, but then rose steeply to its maximum of 1 when F was approximately 4 μL/min. MO2 was stable with reduced F until OEF maximized, after which it decreased progressively.
Conclusions
This model in rats permits acute, graded inner retinal ischemia that is reversible after prescribed durations, does not otherwise injure the eye and allows optical measurement of important physiologic factors during ischemia.
Translational Relevance
This model will allow improved understanding of retinal ischemic injury and enable better management of this common, sight-threatening affliction.
Keywords: animal model, retinal ischemia, retinal blood flow, oxygen metabolism
Introduction
Retinal ischemia is a major cause of visual loss in several common eye diseases, including diabetic retinopathy and retinal vessel occlusions. Much information about retinal ischemic injury has been derived from animal models. Two main methods have been used to induce retinal ischemia in animals. First, ischemia can be induced by compressing the wall of a vessel. Ligation has been used to compress and close the common carotid artery (CA),1 pterygopalatine artery,2 ophthalmic vessels,3 and central retinal artery.4 Additionally, retinal vessel walls can be compressed by an intraocular rod.5 Second, the vessel lumen can be obstructed by emboli,6 thrombosis,7 an intraluminal suture,8 diathermy,9 or photocoagulation.10 Elevation of the intraocular pressure (IOP) also has been used in many studies to interrupt the ocular blood flow,11 but there now is good evidence that other factors, including mechanical effects, contribute substantially to the resulting injury; thus, limiting its use to characterize the effects of ischemia.12–14
Despite the multiplicity of methods, two major knowledge gaps remain. The first gap in knowledge is that the ischemic retinal insult depends on the level of reduced blood flow and the duration of that reduction. Nevertheless, only IOP elevation, with the limitations noted above,13,14 has been used to induce levels of reduced inner retinal blood flow (F). In fact, most of the aforementioned models induce total cessation of F, often permanently. When attempts have been made to vary the ischemic insult, the approach usually has been to vary the duration of total F cessation. The assumption underlying these attempts has been that the ischemic insults induced by these durations would be equivalent to the insults induced by varying the grade of F reduction. Nonetheless, this assumption has not been verified, and the relative ischemic effects of these two approaches may well differ. For example, abrupt, total ischemia may preclude certain metabolic responses to ischemia that may have time to develop during partial (graded) ischemia. In any case, reduction as opposed to cessation of F appears to be more similar to the clinical situation, since some retinal blood flow tends to be retained in patients with vascular occlusions.
The second gap in knowledge is that the roles of several key factors in the pathophysiology of ischemic retinal injury have not been determined given the lack of models for graded retinal ischemia in which they could be measured. These factors include F, blood O2 concentration, O2 delivery (DO2, the product of F and arterial O2 concentration, O2A), the inner retinal O2 extraction fraction (OEF), and the inner retinal rate of O2 metabolism (MO2). These O2-related factors (i.e., DO2, OEF, and MO2) are particularly crucial because O2 is the substance with the largest net flux between the blood and retinal cells.15 Accordingly, the first deficiency to occur during an ischemic insult is inadequate DO2. Clearly, the impact of combinations of levels and durations of F and O2-related factors on retinal ischemic injury cannot be determined in models in which there is total cessation of F.
We addressed these two gaps in knowledge by devising a method for graded retinal ischemia in rats. This model is based on previous work revealing that ligation of one CA led to minimal retinal changes, whereas ligation of both CAs caused more severe retinal changes.1,16 We tested the hypothesis that by ligating one CA and variably compressing the other CA we could induce controlled grades of acute retinal ischemia and measure changes in the O2-related factors DO2, OEF, and MO2.
Methods
Animals
The study was performed on five male Long Evans rats, which were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The study was approved by the Animal Care Committee at the University of Illinois at Chicago. Mean age ± standard deviation (SD) was 78 ± 5 days (range, 71–84). The rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg) with additional injections given to maintain anesthesia as necessary. The right pupil was dilated with 2.5% phenylephrine and 1% tropicamide. The femoral artery was cannulated and a catheter was attached. An incision then was made parallel to and to the left of the midline in the neck and, using blunt dissection, the salivary glands, lymph nodes, and ventral neck muscles were retracted to expose the left CA. The branches of the sympathetic nerve close to the CA were carefully dissected from the artery so that the CA was ligated without involving the nerve. A similar incision was made to the right of the midline, and the right CA was isolated.
The rats were placed on an animal holder that had copper tubing perfused with heated water to maintain body temperature. A custom-built clamp, consisting of a rod mounted on a micromanipulator and a backstop (Fig. 1), was positioned to straddle the right CA (Fig. 2). The positions of the rod and backstop were adjusted so that both were in contact with the CA without compressing it. The backstop was fixed in location using a flexible holder that could be locked accurately in position (Flexbar Machine Corporation, Islandia, NY). Graded compressions of the CA were achieved by rotating the knob of the micromanipulator to advance the rod towards the backstop with micrometer resolution. A glass cover slip with 1% hydroxypropyl methylcellulose was applied to the right cornea to minimize its refractive power and prevent dehydration. Retinal vascular O2 tension (PO2) and blood flow imaging were performed in the right eye of each rat, starting 10 minutes after CA compressions that varied from 0% (full flow) to 100% (minimal/no flow). The time needed for making measurements was approximately 10 minutes. For PO2 imaging, an oxygen-sensitive molecular probe, Pd-porphine (Frontier Scientific, Logan, UT), was administered through the femoral arterial catheter (20 mg/kg). For retinal blood flow imaging, 2-μm polystyrene fluorescent microspheres (Invitrogen, Grand Island, NY) were injected through the catheter.
Figure 1.
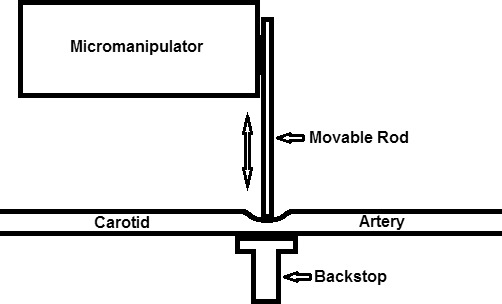
Schematic diagram of apparatus for graded compression of the CA in rats. The micromanipulator allows for precise positioning of the movable rod in the directions indicated by the double-headed arrow. This permits controllable compression of the CA between the movable rod and backstop.
Figure 2.
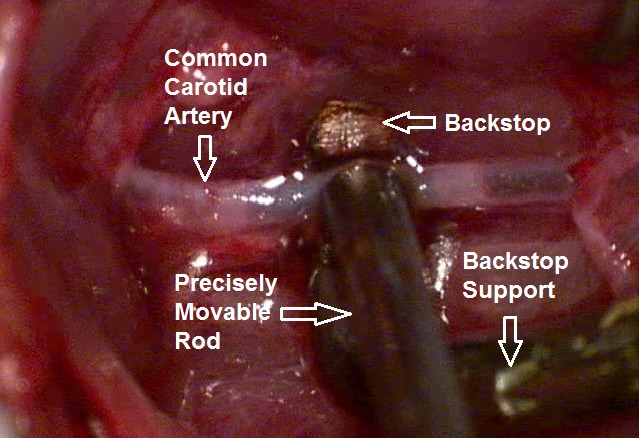
Backstop and precisely movable rod on the exposed CA in a rat.
Imaging
The methods to measure F and O2-related factors were similar to those reported previously.17,18 Retinal vascular PO2 was measured using our optical section phosphorescence lifetime imaging system.17,19 Briefly, a vertical laser line (532 nm) was projected onto the retina, and phosphorescence lifetimes in the retinal vessels were determined by imaging analysis using a frequency-domain approach19,20 and converted to PO2 measurements using the Stern-Volmer equation. Mean arterial (PO2A) and venous (PO2V) values were calculated from the individual artery and vein measurements in each animal, respectively.
Our previously described blood flow imaging system17 was used to measure retinal venous blood velocity and retinal arterial and venous vessel diameters. Briefly, a diode laser beam (488 nm) was projected on the retina after intravenous injection of fluorescent microspheres. Image sequences of the intravascular motion of the microspheres were acquired, and blood velocity in all individual veins (V) was measured by manually tracking displacements of the microspheres over time.17 Correspondingly, diameters of all individual major retinal veins (DV) were measured using dedicated software applied to red-free retinal images.17 Blood flow (F) in each vein was calculated according to F = (V*π*DV2)/4 and summed among all veins to determine total venous blood flow.
Global Inner Retinal Oxygen Delivery, Extraction Fraction, and Metabolism
The O2 of blood in the retinal arteries (O2A) and veins (O2V) was calculated as the sum of oxygen bound to hemoglobin and dissolved in blood: O2 = SO2*C*HGB + PO2*k, where SO2 is the oxygen saturation (%), C is the oxygen-carrying capacity of hemoglobin (1.39 mL O2/g),21 HGB is the hemoglobin concentration (13.8 g/dL), and k is the oxygen solubility in blood (0.003 mL O2/dL·mm Hg).22 SO2 was calculated using the measured PO2 and the hemoglobin oxygen dissociation curve in rats.18,23,24 The arteriovenous oxygen content difference was calculated as: O2A–V = O2A – O2V.
DO2 was defined as the rate that oxygen enters the retinal circulation, and it was calculated as the product of F and O2A. MO2 was defined as the rate that oxygen is metabolized by the inner retinal tissue, and under steady state it equals the rate O2 is extracted from the retinal circulation. Thus, MO2 was calculated as the product of F and O2A–V. In the steady state, OEF equals the ratio of MO2 to DO2 and was calculated as O2A–V/O2A.25 By definition, OEF varies between 0 and 1.
The primary outcome of applying these methods was the induction of varying grades of retinal ischemia. The secondary outcome was measurement of the O2-related factors DO2, OEF, and MO2 at the induced grades of ischemia.
Results
Using the rod attached to the micromanipulator, we were able to make precise compressions of the CA. Between 4 and 7 grades of compression per rat were induced for a total of 27 compressions. Progressive compression was related to progressive reduction of F as displayed in Figure 3. However, the relation was nonlinear in that there were minimal or no changes in F at CA compressions less than approximately 60%, whereas F tended to decrease progressively as the CA compression increased above 60%.
Figure 3.
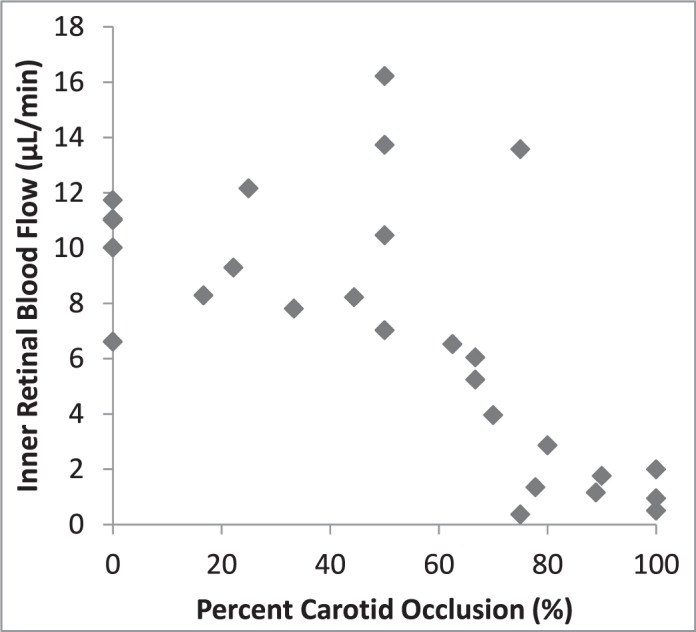
F by percent CA occlusion. Note that no clear changes in F are apparent at occlusions of less than approximately 60%, whereas F tended to decrease progressively as the occlusion increased above 60%.
Since the animal model allowed measurement of F and PO2 during the periods of graded ischemia, we were able to measure the O2-related factors over a range of F values. DO2 declined progressively with reduction of F (Fig. 4). A linear relationship was expected since the variation of O2A was small and DO2 is the product of F and O2A. With reductions of F, OEF initially remained relatively constant and then increased progressively to a maximum approximating 1.0 when F was approximately 4 μL/min (Fig. 5). Since all O2 in the blood is removed when OEF equals 1.0, it did not change with further reduction of F. Figure 6 indicates that MO2 declined minimally as F decreased to approximately 5 μL/min, but it declined steeply as the reduction of F progressed. We noted that MO2 began to decline as OEF approached the maximum.
Figure 4.
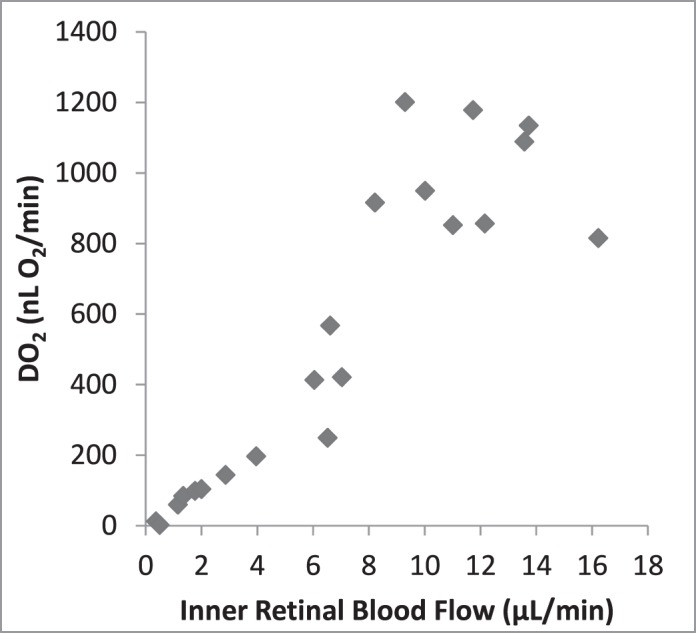
Retinal DO2 by F. DO2 declined progressively with reduction of F in a way that was consistent with a linear relationship.
Figure 5.
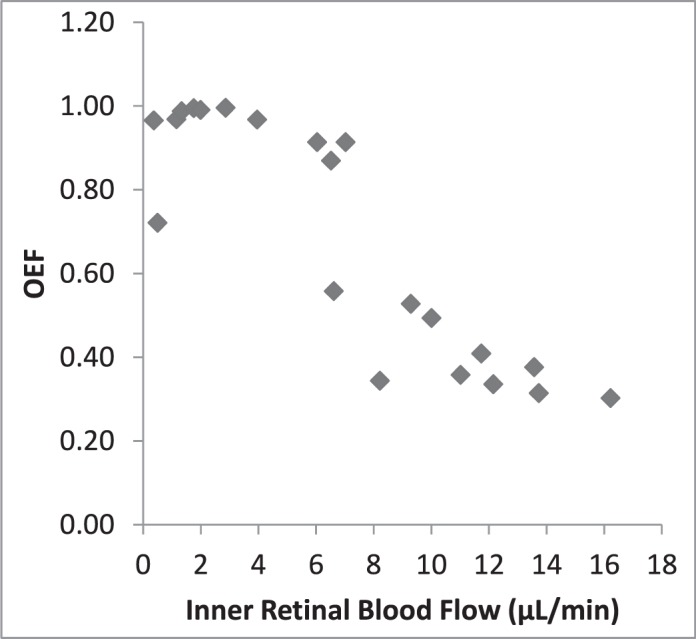
Retinal OEF by F. With reductions of F, OEF initially changed little and then increased progressively to a maximum approximating 1.0 when F was approximately 4 μL/min. OEF remained essentially constant with further reduction of F.
Figure 6.
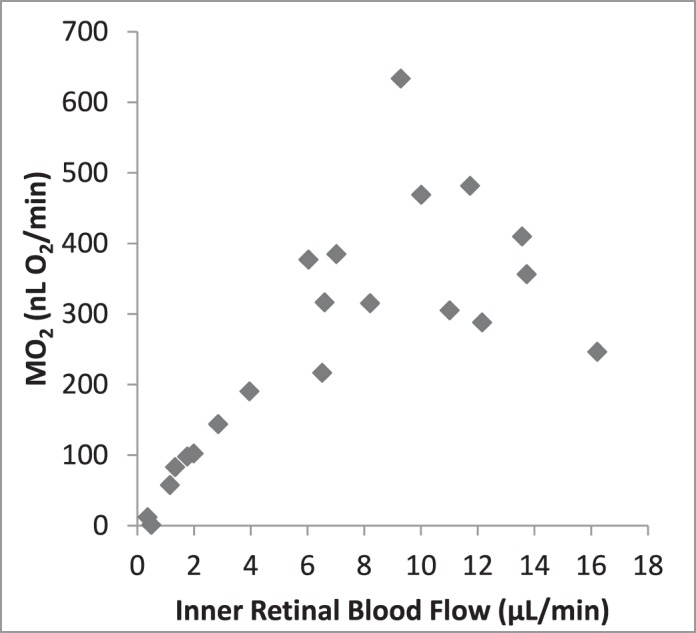
Retinal MO2 by IF. MO2 declined minimally as F decreased to approximately 5 μl/min, but it declined steeply as the reduction of F progressed.
Discussion
We presented a rat model that provides graded, temporally controllable acute, retinal ischemic insults. This could allow correlation of these two key prognostic factors, namely grade and duration, with the resulting ischemic retinal injury. While the many models of retinal ischemia1–11 have various advantages and have permitted discovery of many features of retinal ischemic injury, only elevated IOP can produce graded ischemic insults. He et al.13,14 produced multiple grades of retinal ischemia by progressively increasing IOP in rats. They calculated OEF (called OER) from ocular laser Doppler flow and preretinal PO2 measurements taken between major retinal vessels. However, their F, PO2, and OEF measurements included contributions from the choroidal circulation, whereas the values in our study were derived only from the retinal circulation. Furthermore, they showed that a substantial component of the injurious effects of elevated IOP was not related to ischemia. In contrast, our method does not add injurious effects to those of ischemia, manipulate or distort the eye, or cause corneal clouding, which precludes clear imaging of the ocular posterior segment and measurement of O2-related factors.
Our model permitted preliminary noninvasive measurements of F, DO2, OEF, and MO2 during graded ischemia. To our knowledge, there have been no previous reports in which a comparable combination of these inner retinal O2-related factors were measured in experimental graded ischemia. Of note, we showed for the first time to our knowledge in retinal ischemia that OEF reaches 1 (indicating that all vascular oxygen is being extracted), even while there still is substantial F. The relationship of F to the percent CA occlusion appeared to be nonlinear in which changes in F occurred mainly with compressions greater than 60%. This was the result of retinal vascular compensatory vasodilation, which eventually became maximized, and, probably, a nonlinear relationship between CA compression and the resulting cross-sectional area of the vessel. We interpreted the findings of DO2, OEF, and MO2 in the following manner. At mildly reduced levels of F and DO2, MO2 is maintained by extracting a higher percentage of the O2 in the blood, that is, by increasing OEF. With further reductions in the F and DO2 levels, OEF eventually reaches its maximum value of 1 when all O2 in the blood is extracted. At more severely reduced levels of F and DO2, MO2 decreases proportionately and, when maintained for long enough durations, energy failure and the complex metabolic sequences of ischemic retinal injury must supervene, which terminate in cell death and loss of visual function.26
At 100% occlusion of the CA there may have been a small amount of F, at least in some rats. This is consistent with findings of other studies with long-term total bilateral CA occlusion that showed various electroretinographic, biochemical, and histologic changes.27–32 In fact, with total CA occlusion, the circle of Willis may provide some blood flow to the ophthalmic artery either directly into the orbit33 or by way of retrograde flow through the internal carotid artery and then antegrade flow into the pterygopalatine artery (this artery is the normal blood supply to the eye in rats). Nonetheless, in the present short-term study, MO2 was essentially reduced to zero with maximal compression of one CA and ligature of the other CA.
Our study had limitations. First, this method reduced flow in the retinal and choroidal circulations, which differs from clinical retinal vascular occlusions, which do not involve the choroid. Thus, findings here of DO2, OEF, and MO2, with F may differ from those that occur during retinal vascular occlusions only. However, it does mimic carotid occlusive disease and ophthalmic artery occlusion. Second, we made measurements during progressive grades of occlusion. Accordingly, some effects of the previous levels of occlusion probably were continuing at each grade of compression, as depicted in the presented data variability. However, we were particularly interested in the general pattern of how DO2, OEF, and MO2 related to F over a wide range. Future studies designed to collect data in groups of animals at each F reduction level will introduce less variability and better delineation of the relationships. Third, the CA blood flow was not measured. Thus, there may have been some variability among animals in the actual amount of compression. This also would contribute to measurement variability. Fourth, we did not measure the arterial pH, which can be reduced in ischemia. Lowering the pH shifts the oxygen hemoglobin dissociation curve to the right and causes SO2 to become lower for a given PO2 value. While our SO2 and O2 content values may be somewhat high, it is unlikely that this would have a major impact on the overall relationships among the O2 biomarkers. Fifth, the sample size was small. Nonetheless, it was adequate to demonstrate the feasibility of generating the model and obtaining data.
In conclusion, we described a new model for acute retinal ischemia in rats that can be delivered with controlled grade and duration without otherwise injuring the eye. We also showed that the methods we described previously to measure O2-related factors can be applied, and we presented preliminary combined measurements in this model of retinal ischemia.
Acknowledgments
Supported by the National Eye Institute, Bethesda, MD, EY017918 and EY001792, and Research to Prevent Blindness, New York, NY, Senior Scientific Investigator award (MS), and an unrestricted departmental award.
Disclosure: N.P. Blair, None; A.E. Felder, None; M.R. Tan, None; M. Shahidi, None
References
- 1. Slakter JS, Spertus AD, Weissman SS, Henkind P. . An experimental model of carotid artery occlusive disease. Am J Ophthalmol. 1984; 97: 168– 172. [DOI] [PubMed] [Google Scholar]
- 2. Lelong DC, Bieche I, Perez E,et al. Novel mouse model of monocular amaurosis fugax. Stroke. 2007; 38: 3237– 3244. [DOI] [PubMed] [Google Scholar]
- 3. Lafuente MP, Villegas-Perez MP, Selles-Navarro I, Mayor-Torroglosa S, Miralles de Imperial J, Vidal-Sanz M. . Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002; 109: 157– 168. [DOI] [PubMed] [Google Scholar]
- 4. Hayreh SS, Kolder HE, Weingeist TA. . Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980; 87: 75– 78. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Nun J, Alder VA, Cringle SJ, Constable IJ. . A new method for oxygen supply to acute ischemic retina. Invest Ophthalmol Vis Sci. 1988; 29: 298– 304. [PubMed] [Google Scholar]
- 6. Ashton N, Henkind P. . Experimental occlusion of retinal arterioles: using graded glass ballotini. Br J Ophthalmol. 1965; 49: 225– 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosinger JL, Olney JW. . Photothrombosis-induced ischemic neuronal degeneration in the rat retina. Exp Neurol. 1989; 105: 110– 113. [DOI] [PubMed] [Google Scholar]
- 8. Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. . Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett. 1997; 232: 45– 48. [DOI] [PubMed] [Google Scholar]
- 9. Stefansson E, Novack RL, Hatchell DL. . Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990; 31: 284– 289. [PubMed] [Google Scholar]
- 10. Ernest JT, Archer DB. . Vitreous body oxygen tension following experimental branch retinal vein obstruction. Invest Ophthalmol Vis Sci. 1979; 18: 1025– 1029. [PubMed] [Google Scholar]
- 11. Buchi ER, Suivaizdis I, Fu J. . Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991; 203: 138– 147. [DOI] [PubMed] [Google Scholar]
- 12. Gehlbach PL, Purple RL. . A paired comparison of two models of experimental retinal ischemia. Curr Eye Res. 1994; 13: 597– 602. [DOI] [PubMed] [Google Scholar]
- 13. He Z, Lim JK, Nguyen CT, Vingrys AJ, Bui BV. . Coupling blood flow and neural function in the retina: a model for homeostatic responses to ocular perfusion pressure challenge. Physiol Rep. 2013; 1: e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Z, Nguyen CT, Armitage JA, Vingrys AJ, Bui BV. . Blood pressure modifies retinal susceptibility to intraocular pressure elevation. PLoS One. 2012; 7: e31104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ames A III, Maynard KI, Kaplan S. . Protection against CNS ischemia by temporary interruption of function-related processes of neurons. J Cereb Blood Flow Metab. 1995; 15: 433– 439. [DOI] [PubMed] [Google Scholar]
- 16. Spertus AD, Slakter JS, Weissman SS, Henkind P. . Experimental carotid occlusion: funduscopic and fluorescein angiographic findings. Br J Ophthalmol. 1984; 68: 47– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wanek J, Teng PY, Albers J, Blair NP, Shahidi M. . Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomed Optics Exp. 2011; 2: 2562– 2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wanek J, Teng PY, Blair NP, Shahidi M. . Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in rat. Invest Ophthalmol Vis Sci. 2013; 54: 5012– 5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahidi M, Wanek J, Blair NP, Mori M. . Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Invest Ophthalmol Vis Sci. 2009; 50: 820– 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shahidi M, Shakoor A, Blair NP, Mori M, Shonat RD. . A method for chorioretinal oxygen tension measurement. Curr Eye Res. 2006; 31: 357– 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nathan AT, Singer M. . The oxygen trail: tissue oxygenation. Br Med Bull. 1999; 55: 96– 108. [DOI] [PubMed] [Google Scholar]
- 22. Pittman RN. . Regulation of Tissue Oxygenation. San Rafael, CA: Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 23. Cartheuser CF. . Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comp Biochem Physiol Comp Physiol. 1993; 106: 775– 782. [DOI] [PubMed] [Google Scholar]
- 24. Wanek J, Teng PY, Blair NP, Shahidi M. . Inner retinal oxygen delivery and metabolism in streptozotocin diabetic rats. Invest Ophthalmol Vis Sci. 2014; 55: 1588– 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teng PY, Wanek J, Blair NP, Shahidi M. . Inner retinal oxygen extraction fraction in rat. Invest Ophthalmol Vis Sci. 2013; 54: 647– 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. . Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004; 23: 91– 147. [DOI] [PubMed] [Google Scholar]
- 27. Barnett NL, Osborne NN. . Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res. 1995; 61: 83– 90. [DOI] [PubMed] [Google Scholar]
- 28. Block F, Schwarz M, Sontag KH. . Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci Lett. 1992; 144: 124– 126. [DOI] [PubMed] [Google Scholar]
- 29. Lavinsky D, Arterni NS, Achaval M, Netto CA. . Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe's Arch Clin Exp Ophthalmol. 2006; 244: 199– 204. [DOI] [PubMed] [Google Scholar]
- 30. Osborne NN, Safa R, Nash MS. . Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vision Res. 1999; 39: 3995– 4002. [DOI] [PubMed] [Google Scholar]
- 31. Stevens WD, Fortin T, Pappas BA. . Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke. 2002; 33: 1107– 1112. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Yamamoto H, Parel JM. . Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp Eye Res. 2006; 82: 767– 779. [DOI] [PubMed] [Google Scholar]
- 33. Steele EC Jr, Guo Q, Namura S. . Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008; 39: 2099– 2104. [DOI] [PubMed] [Google Scholar]


