Abstract
Objective
The aim of this study was to perform the first resting‐state functional MRI (RS‐fMRI) analysis in Friedreich's ataxia (FRDA) patients to assess possible brain functional connectivity (FC) differences in these patients, and test their correlations with neuropsychological performances.
Methods
In total, 24 FRDA patients (M/F: 15/9, mean age 31.3 ± 15.0) and 24 healthy controls (HC; M/F: 15/9, mean age 30.7 ± 15.5) were enrolled in this cross‐sectional study. All patients underwent a thorough neuropsychological battery, investigating different cognitive domains. RS‐fMRI data were analyzed using a seed‐based approach, probing the FC of cortical areas potentially referable to specific executive and cognitive functions compromised in FRDA.
Results
Compared to HC, FRDA patients showed overall worse neuropsychological scores in several domains, including global cognitive assessment, spatial memory, visuoperception and visuospatial functions, and executive functions. Analysis of RS‐fMRI data showed a higher FC in FRDA patients compared to HC between paracingulate gyri and the medial frontal gryrus, between the superior frontal gyrus and bilateral angular gyri, and between the middle temporal gyrus and the cingulate gyrus, with a reduced FC between the medial frontal gryrus and the cerebellum.
Interpretation
We found a reduction in FC between frontal areas and the contralateral cerebellar cortex in FRDA, in line with the known alteration in cerebello‐cortical pathway in this condition. On the other hand, a higher FC between different cortical areas was shown, possibly reflecting a compensatory phenomenon. These results, in conjunction with clinical findings, may shed new light on the pattern of supratentorial and infratentorial involvement, and on dynamics of brain plasticity in this disease.
Introduction
Friedreich's ataxia (FRDA) is the most frequent inherited ataxia (1/50,000) caused by homozygous hyperexpansion of GAA triple‐repeat in the first intron of the frataxin gene (FXN).1 Most common symptoms include progressive cerebellar dysfunction with limb and gait ataxia, dysarthria, oculomotor disturbances, and lack of tendon reflex response,2 with nonspecific brain MRI findings that are present only in more advanced stages of the disease.3 Hypertrophic cardiomyopathy, skeletal abnormalities, and increased risk of diabetes mellitus have also been described.4 In addition to progressive neurological disability, a mild impairment of neuropsychological functions is present. In particular, among nonmotor symptoms, impaired emotion recognition and a variable impairment in visuospatial abilities, information processing speed and executive functions (with specific reference to long‐term working memory) have been recently described.5, 6 Most of the available studies have explored specific cognitive domains,7, 8 while a comprehensive neuropsychology battery has been implemented only in few cases.6, 9
Advanced MR techniques such as voxel‐based morphometry (VBM),10, 11, 12 functional MRI (fMRI).13, 14 or MR spectroscopy15 have been used to detect possible biomarkers to elucidate the pathophysiological mechanisms underlying the clinical disability in FRDA patients. Recently, a disconnection was found between associative cortical areas and neocerebellar regions in FRDA patients, areas known to be the anatomical substrate of the cerebellum involvement in cognition.16, 17 Furthermore, only few activation fMRI studies have been performed to clarify cerebello‐cerebral connectivity deficits in FRDA, showing lower activation patterns during both motor and behavioral tasks.13, 14, 18, 19, 20
Resting‐state functional MRI (RS‐fMRI) is a powerful and noninvasive tool that allows the evaluation of functional connectivity (FC) between different areas of the brain when a subject is not performing any specific task. This technique has allowed to demonstrate the presence of supratentorial and infratentorial FC changes in other cerebellar ataxias.21, 22
However, to date, no RS‐fMRI analysis has been performed in FRDA patients. With this knowledge, the aim of this study was to assess for the first time possible brain FC alterations in FRDA patients compared to a group of healthy controls (HC), and their possible correlations with clinical scores, in order to shed new light on the mechanisms of cognitive dysfunctions underlying the disease.
Material and Methods
Subjects
A total of 24 patients with FRDA were enrolled at the University “Federico II” of Naples. Inclusion criteria were a molecular diagnosis of FRDA with a homozygous expansion of the FXN gene, carried out by performing conventional genetic test with short and long triplet repeat primed polymerase chain reaction. Exclusion criteria were the presence of any psychiatric disorder. Patients were recruited at our outpatient clinic for movement disorders. We recruited an equivalent number of age‐, sex‐, and education‐matched HC through patient's relatives and university personnel. All patients were preliminarily tested with a complete neuropsychological battery (Table 1) and underwent MRI brain scan within 1 week from the clinical assessment. Two FRDA patients refused to undergo the MRI examination because of anxiety and claustrophobia, while three additional patients were excluded because of low compliance resulting in massive motion artifacts during the RS‐fMRI acquisition.
Table 1.
Neuropsychological battery
| Type of test | List of tests | Time |
|---|---|---|
| Global assessment | The Montreal Cognitive Assessment | 10 |
| Language | Naming Nouns and Pointing | 10 |
| Intelligence | Raven Colored Progressive Matrices | 15 |
| Executive functions |
Symbol Digit Modalities Test Attentional Matrices Trail Making Test Brief Stroop Test Weigl's Sorting Test Phonetic and Semantic Fluencies |
60 |
| Memory |
Digit span 10/36 Spatial Recall Test Rey Auditory Verbal Learning Test |
20 |
| Visuoperception and visuospatial functions |
Segment length discrimination Mental rotation |
30 |
Time is shown in minutes.
The study was formerly approved by the “Carlo Romano” Ethical Committee of “Federico II” University of Naples (Italy) (approval no. 47/15), and performed in accordance to the Declaration of Helsinki. Written informed consent was obtained from both patients and HC.
Neuropsychological tests and correction formulas
All patients were preliminarily tested with a complete neuropsychological battery investigating different cognitive domains, including a global cognitive assessment, the evaluation of language, intelligence and executive functions, as well as memory visuoperception and visuospatial functions. A complete list of the neuropsychological tests is listed on Table 1.
In order to correct neuropsychological test scores for upper limbs and speech impairment, we applied our recently reported normalization method.23 Briefly, this method uses the patient's PATA Rate Test (PRT) and Nine‐hole Pegboard Test (9HPT) score to modify the fixed time, or the test result itself if time is the output. Time is corrected only if the patient's PRT or 9HPT fall outside the limit of normality. The tests involved in this correction process were the Attentional Matrices, Trail Making Test (TMT) A and B, Phonemic and Semantic Fluencies, and Symbol Digit Modalities Test (SDMT). Fixed time and test time are modified only in their motor/verbal component following specifically developed formulas and calculations.23
MRI data acquisition
All MRI studies have been performed on the same 3 Tesla MR scanner (Trio, Siemens Medical Systems, Erlangen, Germany). MRI protocol included a volumetric T1w acquisition for anatomical coregistration of fMRI data (MPRAGE; axial planes; TR = 1900 msec; TE = 3.4 msec; TI = 900 msec; flip angle = 9°; voxel size = 1 × 1 × 1 mm3; number of slices = 160), and a T2*‐weighted sequence (axial planes; TR = 2500 msec; TE = 40 msec; 64 × 64 acquisition matrix; 30 slices; voxel size = 3 × 3 × 4 mm3; gap 1 mm; 200 time points; acquisition time 8′27″) used for the RS‐fMRI analysis. During the entire acquisition, subjects were laying supine with their head lightly fixed by foam pads and straps (to minimize head movement), and were asked to relax with eyes closed, without falling asleep.
MRI data analysis
RS‐fMRI data were processed using the FC toolbox (CONN, v. 16.a, McGovern Institute for Brain Research, Massachusetts Institute of Technology, http://www.nitrc.org/projects/conn), which contains libraries for fMRI analysis based on SPM8.
Preprocessing steps of RS‐fMRI data included the removal of the first five time points (to allow for instability of the initial MRI signal), leaving a total number of 195 time points, a motion correction procedure, a slice timing correction, and a temporal despiking (by means of an hyperbolic tangent squashing function to limit outlier values), followed by band‐pass filtering (0.008 Hz < f < 0.09 Hz) and spatial smoothing using a 6‐mm Gaussian kernel. The motion correction procedure performed by SPM realigns the volumes of each study to the first one, iteratively finding the translation and rotation parameters that minimize a least‐squares cost function derived from the voxel‐by‐voxel intensity difference from the reference image. This approach proved to be accurate in realigning fMRI volumes for motion correction purposes.24 From this motion correction procedure, for each brain volume, the mean displacement was computed as the root‐mean‐square (RMS) of the translation parameters at each time point, and those studies that showed a mean relative RMS of 0.20 or higher (according to25), or with more than 2.0 mm displacement along or 2.0 degrees rotation around any axis were discarded from the analysis. In addition, a “scrubbing” procedure was applied to time points (along with the preceding and the two following ones) with a framewise differential of signal intensity >9 z‐values, which were deleted and substituted with the mean framewise value to remove their effect.
RS‐fMRI data were then normalized to a standard EPI template (Montreal Neurological Institute [MNI]), and resampled to a voxel size of 2 × 2×2 mm3. All images were visually assessed case‐by‐case by an experienced operator in fMRI analysis, to evaluate the overall accuracy of the processing.
For each subject, BOLD signal time course was calculated over 39 different cortical regions potentially referable to specific executive and cognitive functions compromised in FRDA patients.6 A complete list of the investigated regions, as defined in the Automated Anatomical Labeling Atlas,26 is reported in Table 2. For each seed, the corresponding correlation maps of the BOLD signal across the brain were generated, including in the model the time courses of White Matter (WM) and Cerebrospinal Fluid (CSF) signals, and the six parameters (translations and rotations along the X, Y, and Z axes) of spatial transformation derived from the coregistration step.
Table 2.
Selected seed for the RS‐fMRI analysis
| Frontal lobe | Superior frontal gyrus (right and left) | Temporal lobe | Middle temporal gyrus – anterior division (right and left) |
| Middle frontal gyrus (right and left) | Middle temporal gyrus – posterior division (right and left) | ||
| Inferior frontal gyrus (right and left) | Middle temporal gyrus – temporoccipital part (right and left) | ||
| Supplementary motor cortex | Temporal fusiform cortex – anterior division (right and left) | ||
| Paracingulate cortex (right and left) | Temporal fusiform cortex – posterior division (right and left) | ||
| Anterior cingulate cortex | Temporal occipital fusiform cortex (right and left) | ||
| Frontal orbital cortex (right and left) | Hippocampus (right and left) | ||
| Left parahippocampal gyrus (anterior and posterior) | |||
| Lingual gyrus (right and left) | |||
| Parietal lobe | Superior parietal lobule (right and left) | Occipital lobe – deep gray matter | Intralcalcarine cortex (right and left) |
| Right supramarginal gyrus (anterior and posterior) | Cuneal cortex | ||
| Thalamus (right and left) |
Anatomical labeling is according to.26
The resulting FC maps were entered in a second‐level analysis, to test for the presence of differences between the two groups in the strength of the correlation with each seed, including age and sex as nuisance covariates for each subject, along with the average RMS, to remove potential residual movement effects.
Statistical analysis
Differences in demographic variables (age and education) and in PRT and 9HPT scores between controls and FRDA patients were analyzed by the Mann–Whitney U test and chi‐square test as appropriate. Effect size was calculated using the partial η 2 method , using the Mann–Whitney U test's Z score, and with a N = 48. P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using Statistical Package for Social Sciences, SPSS Inc, Chicago, Ill, USA version 23.0.0.1 running on MacOS 10.11.6.
For the RS‐fMRI analysis, both contrasts were probed for possible differences when comparing the two groups (HC > FRDA and HC < FRDA). Differences were considered significant for P < 0.0012 (0.05/the number of the tested seed), corrected for the family wise error at cluster level (cluster‐defining threshold P = 0.001). Where significant FC differences emerged, the first eigenvariate of each cluster was extracted, corrected for age, sex, and RMS, and tested, in the FRDA patients, for correlation with the above‐mentioned neuropsychological tested variables, as well as the Scale for the Assessment and Rating of Ataxia (SARA) scores, number of GAA triplets and disease duration, by nonparametric correlation analysis using the Spearman's coefficient (P = 0.05, Bonferroni‐corrected).
Results
Neuropsychology
We enrolled 24 FRDA patients and 24 HC that underwent the extensive neuropsychological testing procedures (Table 3).
Table 3.
Demographic variables for all subjects included in the study
| Variable | FRDA | Controls | P |
|---|---|---|---|
| Age | 31.3 ± 15.0 | 30.7 ± 15.5 | 0.789 |
| Education | 12.1 ± 2.9 | 12.5 ± 3.2 | 0.719 |
| Gender (M/F) | 15/9 | 15/9 | 1.000 |
| GAA1 | 677.0 ± 282.8 | – | – |
| GAA2 | 906.3 ± 310.4 | – | – |
| Ventricular hypertrophy | 13/24 | – | – |
| Reduced ejection fraction | 4/24 | – | – |
| Ambulant | 18/24 | – | – |
| SARA | 18.7 ± 7.2 | – | – |
| 1 – Gait | 5.6 ± 2.0 | – | – |
| 2 – Stance | 4.2 ± 1.6 | – | – |
| 3 – Sitting | 1.2 ± 1.1 | – | – |
| 4 – Speech disturbance | 1.8 ± 1.2 | – | – |
| 5 – Finger chase | 1.3 ± 0.8 | – | – |
| 6 – Nose‐finger test | 1.2 ± 0.7 | – | – |
| 7 – Fast alternating hand movements | 1.7 ± 0.8 | – | – |
| 8 – Heel shin slide | 2.2 ± 1.0 | – | – |
Age and education are expressed in years. FRDA, Friedreich's ataxia; SARA, Scale for the Assessment and Rating of Ataxia.
Neuropsychological battery administration resulted in lower scores for FRDA patients compared to HC (Table 4) encompassing several domains. The global cognitive assessment at the Montreal Cognitive Assessment (MOCA) showed a difference of almost four points between FRDA patients and HC, implying the existence of a mild cognitive impairment. Clear‐thinking ability and nonverbal assessment of intelligence, as assessed by Raven Colored Progressive Matrices (RCPM), were not impaired in FRDA patients, although this neared significance. Similarly, language was not affected, with comparable results at the Pointing Names and Naming Nouns Tests between the two groups. On the other hand, spatial memory was impaired, with significant differences between FRDA and HC at both the Immediate and Recall Modified 10/36 Spatial Tests (SPART and SPART‐D). In contrast, verbal memory was not impaired with results approaching significance at the Immediate Recall Rey Auditory Verbal Learning Test (RAVLT). Working memory's number storage capacity tested with the digit‐span task was also preserved.
Table 4.
Neuropsychological tests in FRDA and HC
| Test | FRDA (SD) | Controls (SD) | Effect size | P |
|---|---|---|---|---|
| MOCA | 22.3 (3.6) | 26.2 (2.3) | 0.33 | <0.001 |
| Pointing names | 23.9 (0.3) | 24 (0.2) | 0.02 | 0.301 |
| Naming nouns | 13.9 (1.3) | 14.2 (1.2) | 0.01 | 0.568 |
| RCPM | 29.6 (6.3) | 33 (5.4) | 0.08 | 0.050 |
| Digit Span | 7 (4.4) | 6.5 (1.1) | 0.01 | 0.454 |
| SPART | 18.5 (6.6) | 22.9 (3.8) | 0.12 | 0.017 |
| SPART‐D | 6.5 (2.4) | 8.2 (1.8) | 0.13 | 0.012 |
| RAVLT | 40.8 (12.6) | 47.5 (9.4) | 0.08 | 0.052 |
| RAVLT‐D | 9.3 (3.9) | 10.5 (3.4) | 0.03 | 0.250 |
| SLD | 26.9 (2.2) | 28.5 (1.7) | 0.18 | 0.003 |
| Mental rotation | 93.3 (15.9) | 105.8 (4.7) | 0.15 | 0.008 |
| SDMT | 35.8 (10) | 57.3 (13.6) | 0.50 | <0.001 |
| Attentional matrices | 50.5 (8.7) | 55.5 (3.2) | 0.05 | 0.118 |
| TMT‐A | 67 (68.9) | 29.8 (8.8) | 0.27 | <0.001 |
| TMT‐B | 127.3 (57.7) | 73.2 (24.2) | 0.33 | <0.001 |
| Brief Stroop test | 50.1 (20.3) | 26.9 (7.4) | 0.44 | <0.001 |
| Semantic fluency | 18.5 (5.8) | 23.7 (4.9) | 0.25 | 0.001 |
| Phonemic fluency | 27.5 (11.7) | 41.3 (8.8) | 0.31 | <0.001 |
Significance shows the P value of the Mann–Whitney U test. FRDA, Friedreich ataxia patients; HC, healthy controls; MOCA, Montreal Cognitive Assessment; RCPM, Raven Colored Progressive Matrices; SPART, 10/36 Spatial Recall Test; SPART‐D, SPART delayed; RAVLT, Rey Auditory Verbal Learning Test Immediate Recall; RAVLT‐D, RAVLT Delayed Recall; SLD, Segment Length Discrimination; SDMT, Symbol Digit Modalities Test; TMT, Trail Making Test. Significant differences are reported in bold.
Visuoperception and visuospatial functions were impaired both at the segment length discrimination (SLD) and mental rotation tests, with a significant difference in final scores between the two groups.
When testing for executive functions, including attention and motivation tasks and linguistic skills, FRDA patients showed reduced scores compared to HC, with the most significant differences obtained at the TMT.
MRI
Clusters of significant difference in FC between FRDA patients and HC were observed for the following seed regions: right paracingulate gyrus (r_PaCiG) and left paracingulate gyrus (l_PaCiG), right superior frontal gyrus (r_SFG), right medial frontal gyrus (r_MFG), and left middle temporal gyrus (l_MTG).
In particular, when evaluating the r_PaCiG seed, the HC < FRDA contrast provided a cluster of significant difference encompassing the r_MFG (P = 10−8), similar to those obtained when testing the same contrast of the contralateral seed (l_PaCiG, P = 10−6). When testing the r_SFG seed, the HC < FRDA contrast provided two clusters of significant difference at the level of the right (P < 5 × 10−5) and left (P = 0.0001) angular gyri. When evaluating the r_MFG seed, the HC > FRDA contrast provided a cerebellar cluster of significant difference encompassing the lobule six and the vermis (P = 0.0001). Finally, when testing the temporoccipital l_MTG seed, the HC < FRDA contrast provided a cluster of borderline significant difference located at the level of the cingulate gyrus (P = 0.0011). No clusters of significant difference emerged for any of the other tested seeds. Results of the between‐group RS‐fMRI analysis are shown in Figure 1.
Figure 1.
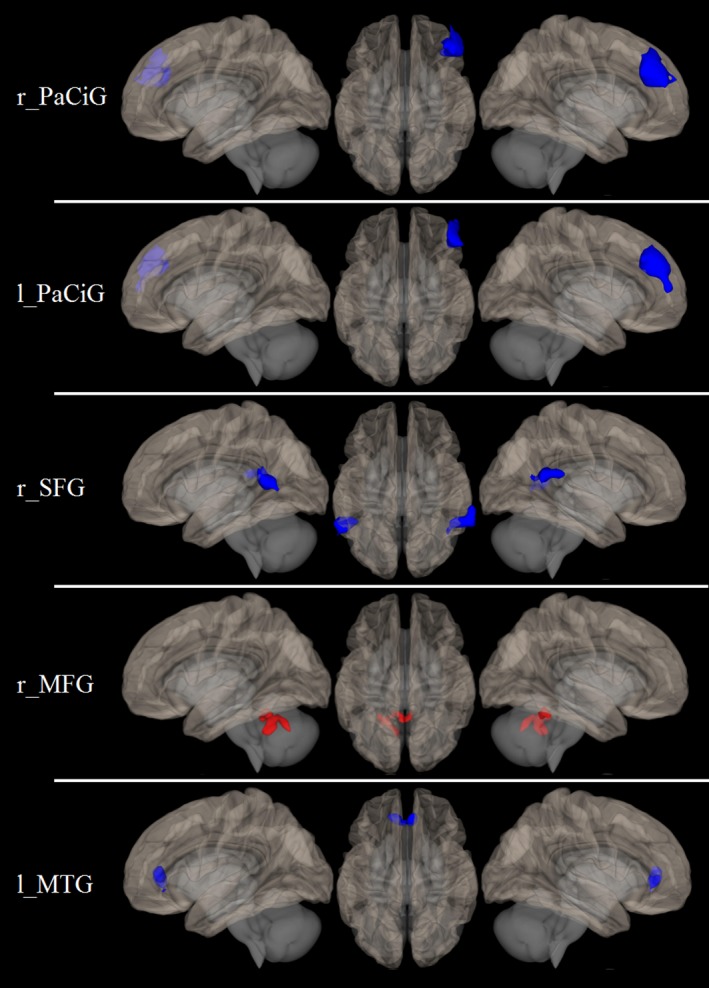
Results of the between‐group RS‐fMRI analysis. Brain regions showing a different functional connectivity between FRDA patients and healthy controls, superimposed on the left lateral (left), right lateral (right), and upper (central column) view of a three‐dimensional rendering of a healthy brain in the MNI space. Areas of increased connectivity in patients compared to controls are shown in blue, while areas of reduced connectivity are shown in red. RS‐fMRI, resting‐state functional MRI; FRDA, Friedreich's Ataxia; MNI, Montreal Neurological Institute; r_PaCiG, right paracingulate gyrus; l_PaCiG, left paracingulate gyrus; r_SFG, right superior frontal gyrus; r_MFG, right medial frontal gyrus; l_MTG, left middle temporal gyrus.
Table 5 shows the results of the correlation between RS‐fMRI data and neuropsychological tests. After Bonferroni correction, no correlation emerged between clusters of altered FC and neuropsychological tests in FRDA patients, as well as with the other tested clinical variables.
Table 5.
Correlations between RS‐fMRI and neuropsychological tests
| r_MFG cluster (r_PaCiG seed) | r_MFG cluster (l_PaCiG seed) | Angular gyri (r_SFG seed) | Cerebellar cluster (r_MFG seed) | Cingulate cluster (l_MTG seed) | |
|---|---|---|---|---|---|
| MOCA | n.s. | n.s. | n.s. | n.s. | n.s. |
| Pointing names | n.s. | n.s. | n.s. | n.s. | n.s. |
| Naming nouns | n.s. | n.s. | n.s. | n.s. | n.s. |
| RCPM | n.s. | n.s. | n.s. | n.s. | n.s. |
| Digit span | P = 0.009a | n.s. | P = 0.047a , b | n.s. | n.s. |
| 1036 immediate | n.s. | n.s. | n.s. | n.s. | n.s. |
| 1036 recall | n.s. | n.s. | n.s. | n.s. | n.s. |
| RAVLT immediate | n.s. | n.s. | n.s. | n.s. | n.s. |
| RAVLT recall | n.s. | n.s. | n.s. | n.s. | n.s. |
| SLD | n.s. | n.s. | n.s. | n.s. | n.s. |
| Mental rotation | P = 0.014a | n.s. | n.s. | n.s. | n.s. |
| SDMT | n.s. | n.s. | n.s. | n.s. | n.s. |
| Attentional matrix | P = 0.037a | n.s. | n.s. | n.s. | n.s. |
| TMT‐A | n.s. | n.s. | P = 0.007c , b | n.s. | n.s. |
| TMT‐B | P = 0.006c | n.s. | P = 0.024c , b | n.s. | n.s. |
| Brief Stroop test | P = 0.027c | P = 0.036c |
P = 0.007c
,
b
P = 0.036c , d |
n.s. | n.s. |
| Phonemic fluency | n.s. | n.s. | n.s. | n.s. | n.s. |
| Semantic fluency | n.s. | n.s. | n.s. | n.s. | n.s. |
Results of the correlation analysis between RS‐fMRI data and neuropsychological tests. All results did not survive a multiple comparisons correction. RS‐fMRI, resting‐state functional MRI; r_PaCiG, right paracingulate gyrus; l_PaCiG, left paracingulate gyrus; r_SFG, right superior frontal gyrus; r_MFG, right medial frontal gyrus; l_MTG, left middle temporal gyrus. n.s. = not significant (P > 0.05).
Direct correlation.
Left angular gyrus.
Inverse correlation.
Right angular gyrus.
Discussion
In this paper, we examined cognitive functioning in FRDA patients using a comprehensive battery of neuropsychological tests and a seed‐based RS‐fMRI approach to assess brain functional modifications compared to a matched group of HC. We also identified possible correlations of MRI data with neuropsychological scores, to better elucidate the mechanisms of cognitive functioning underlying this disease.
At neuropsychological tests, we found a global cognitive impairment with preserved digit span, language, verbal long‐term, and categorization abilities. Particularly surprising was the poor performance on MOCA, with most patients under the cutoff of normative values. These results are in contrast with previously reported data that suggested no difference with controls when administrating global screening scales.6, 19
We can exclude language disorders as our patients showed no difficulties in both nouns naming (production) and pointing (receptive language) subtests.
Executive deficits have been previously described in FRDA.6, 19, 27 We observed severe impairment of phonemic and semantic verbal fluency tests, with the latter being less impaired. This cannot be explained by articulatory deficits, as correction formulas can exclude any role of dysarthria in the final score.23
Compared to phonemic fluency, lower scores of semantic fluency are not surprising, as they require different retrieval strategies.28 The “theory of prototype” states that semantic fluency works by using prototypes relative to each category, and then retrieves several examples making the process more automatic.29 On the contrary, phonemic verbal fluency requires a more controlled retrieval as it uses phonological and not prototypical cues, resulting in a major involvement of frontal regions.
With the exception of verbal working memory (digit span) and categorization (Weigl's Sorting Test), patients performed worse than controls in all tests assessing executive and attentive abilities. We found a slowed information processing speed in SDMT and Stroop Test, confirming previous studies.9, 27, 30, 31 Similarly, patients performed worse than controls on remaining selective (TMT‐A) and divided attention tests (TMT‐B), with latter being more compromised. For several tests that use visual abilities (TMT‐A/‐B, SDMT), the impairment in executive functions may not be the only cause of poor performance. Indeed, tests exploring visuospatial abilities (SLD, mental rotation task, visual memory on both learning and delayed recall) were impaired in our population, supporting this hypothesis.
FRDA can be interpreted as a condition in which many executive and cognitive functions are impaired in different ways, by extensively affecting cerebello‐cerebral connections. Nonmotor symptoms in FRDA have already been proposed to be linked to deficits in connectivity between regions associated with executive and cognitive functions,8 with specific reference to cerebellar projections to prefrontal and temporo‐parietal cortex.17
Cerebellar atrophy can be detected in FRDA patients via VBM analysis, and consistent Gray Matter (GM) alterations and volume reduction in vermis, dentate, and posterior lobes have been described.10, 11, 12, 32, 33 At the same time, VBM and Tract‐Based Spatial Statistics (TBSS) studies demonstrated cerebellar WM volume loss in cerebellar peduncles, peridentate white matter,34 and midbrain,35 with only a mild supratentorial WM volume reduction limited to posterior cyngulate and middle frontal gyri and paracentral lobule.15, 19, 36 The superior cerebellar peduncle atrophy seems to be significantly related to disease severity.37
Together with studies that investigated cerebellar GM and WM in FRDA, only few fMRI studies have been performed to investigate possible functional modifications in FRDA patients. These studies showed the presence of an abnormal activation pattern during motor and behavioral tasks in FRDA patients. Indeed, a higher activity in left premotor, left somatosensory, and left inferior parietal cortices, coupled to a decreased activation in the primary motor cortex and cerebellum, were proven in FRDA patients compared to HC during motor tasks.13, 14 On the other hand, during behavioral stimuli and verbal fluency performance FRDA patients showed a reduced prefrontal and parietal activation compared to HC, with a mixed activity pattern involving the Broca Area, prefrontal, cingulate, and premotor cortices during behavioral stimuli and verbal fluency performance.18, 19, 20 Taken together, these findings are in line with our results showing a lower cerebello‐cerebral and a higher cerebro‐cerebral FC in FRDA patients, confirming the occurrence of these alteration in this condition.19, 20
It has been clearly demonstrated that the frontal cortex, with particular reference to the medial frontal gyrus and cingulate‐paracingulate gyri, is implicated in motor planning as well as in nonmotor tasks involving decision making, discrimination, computation, and reasoning.38 Therefore, it is presumable that in FRDA, because of the disruption of cerebello‐cerebral circuits involved in cognition, different mechanisms of neuroplasticity could lead to a gradual adaptation of supratentorial cortex. In fact, beside the reduced FC between the left cerebellum and the contralateral medial frontal gyrus, the increased connectivity between superior and medial frontal gyri with other associative cortical areas described above, could be interpreted as a possible compensatory mechanism to the structural basis of this condition.
In this study, we performed a seed‐based analysis of RS‐fMRI data because we were interested in investigating possible FC changes of those cortical areas referable to specific functions known to be clinically compromised in FRDA. Such approach was chosen because the use of a priori clinical information regarding specific altered functions prevailed over the possibility to investigate different aspects of possible functional changes in FRDA (i.e., possible network alterations using an independent componenet analysis) without a priori hypotheses, especially considering the relatively small number of subjects.
When testing the relationships between these clusters of altered FC in FRDA patients and neuropsychological scores, no significant correlations emerged. A possible explanation of this lack of results has to be researched both in the relatively small number of patients enrolled, as well as in the restrictive statistical thresholding. However, it should be noted that a trend of significance for positive correlation was observed for all the digit span, mental rotation, and attentional matrixes. In contrast, a trend for inverse correlation was present for TMT‐A/‐B and for the Stroop test, consistent with the fact that a better performance in the former tests is associated with higher scores, whereas in the latter tests it is associated with a lower score.
Accordingly, the present results suggest that a correlation between neuropsychological performance and FC in the studied areas may exist, so that future studies with the inclusion of a larger number of patients are warranted, in order to confirm the present findings.
In this study, the SARA score was used to determine the severity of ataxia symptoms. Such score was chosen over the Friedreich′s Ataxia Rating Scale because the latter, while time consuming, does not appear to offer significant advantages over the SARA with regard to ataxia assessment. Furthermore, SARA has been used in observational studies, clinical trials, scale comparative studies, and it is the clinical scale used in the European collaborative network EFACTS.39, 40, 41, 42
Conclusion
In FRDA patients, widespread alterations of FC are present compared to HC. In particular, the observed reduction in FC between the medial frontal gyrus and the contralateral cerebellar cortex reflects the presence of alterations in cerebello‐cortical pathway, in line with the clinical degeneration of the cognitive circuits of the disease. On the other hand, the higher FC between different cortical areas, such as paracingulate gyri or superior and medial frontal gyri could speculatively reflect a compensatory activation of associative areas in response to the deterioration of a portion of the physiological cerebello‐cortical routes of connection. These results, in conjunction with clinical findings and neurofunctional tests, may shed new light on the pattern of supratentorial and infratentorial involvement and on the dynamics of brain plasticity in FRDA.
Author Contributions
S.C. conceptualized, organized, and executed the research and writing of the manuscript. T.C. organized and executed the research, writing, and review of the manuscript. E.T. organized and executed the research and writing of the manuscript. F.A. conceptualized and executed the research and review of the manuscript. C.R. conceptualized the research, writing, and review of the manuscript. A.L. organized and executed the research and review of the manuscript. W.D.V. organized and executed the research and review of the manuscript. F.P. conceptualized the research and review of the manuscript, and critically reviewed the manuscript. M.Q. critically reviewed the statistical analysis, and the manuscript. A.F. conceptualized the research and review of the manuscript, and critically reviewed the manuscript. A.B. wrote and critically reviewed the manuscript. F.S. designed, executed, wrote, and critically reviewed the manuscript
Conflict of Interest
All authors declare no conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
References
- 1. Campuzano V, Montermini L, Molto MD, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996;271:1423–1427. [DOI] [PubMed] [Google Scholar]
- 2. Pandolfo M, Manto M. Cerebellar and afferent ataxias. Continuum (Minneap Minn) 2013;19(5 Movement Disorders):1312–1343. [DOI] [PubMed] [Google Scholar]
- 3. Mascalchi M. The cerebellum looks normal in Friedreich ataxia. AJNR Am J Neuroradiol 2013;34:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol 2009;256(Suppl 1):3–8. [DOI] [PubMed] [Google Scholar]
- 5. Costabile T, Capretti V, Abate F, et al. Emotion recognition and psychological comorbidity in Friedreich's ataxia. Cerebellum 2018; 1–10. https://doi.org/10.1007/s12311-018-0918-5 [DOI] [PubMed] [Google Scholar]
- 6. Nieto A, Correia R, de Nobrega E, et al. Cognition in Friedreich ataxia. Cerebellum 2012;11:834–844. [DOI] [PubMed] [Google Scholar]
- 7. de Nobrega E, Nieto A, Barroso J, Monton F. Differential impairment in semantic, phonemic, and action fluency performance in Friedreich's ataxia: possible evidence of prefrontal dysfunction. J Int Neuropsychol Soc 2007;13:944–952. [DOI] [PubMed] [Google Scholar]
- 8. Klopper F, Delatycki MB, Corben LA, et al. The test of everyday attention reveals significant sustained volitional attention and working memory deficits in Friedreich ataxia. J Int Neuropsychol Soc 2011;17:196–200. [DOI] [PubMed] [Google Scholar]
- 9. Mantovan MC, Martinuzzi A, Squarzanti F, et al. Exploring mental status in Friedreich's ataxia: a combined neuropsychological, behavioral and neuroimaging study. Eur J Neurol 2006;13:827–835. [DOI] [PubMed] [Google Scholar]
- 10. Della Nave R, Ginestroni A, Giannelli M, et al. Brain structural damage in Friedreich's ataxia. J Neurol Neurosurg Psychiatry 2008;79:82–85. [DOI] [PubMed] [Google Scholar]
- 11. Selvadurai LP, Harding IH, Corben LA, et al. Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE‐FRDA study. J Neurol 2016;263:2215–2223. [DOI] [PubMed] [Google Scholar]
- 12. Silva CB, Yasuda CL, D'Abreu A, et al. Neuroanatomical correlates of depression in Friedreich's ataxia: a voxel‐based morphometry study. Cerebellum 2013;12:429–436. [DOI] [PubMed] [Google Scholar]
- 13. Akhlaghi H, Corben L, Georgiou‐Karistianis N, et al. A functional MRI study of motor dysfunction in Friedreich's ataxia. Brain Res 2012;1471:138–154. [DOI] [PubMed] [Google Scholar]
- 14. Ginestroni A, Diciotti S, Cecchi P, et al. Neurodegeneration in Friedreich's ataxia is associated with a mixed activation pattern of the brain. A fMRI study. Hum Brain Mapp 2012;33:1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franca MC Jr, D'Abreu A, Yasuda CL, et al. A combined voxel‐based morphometry and 1H‐MRS study in patients with Friedreich's ataxia. J Neurol 2009;256:1114–1120. [DOI] [PubMed] [Google Scholar]
- 16. Palesi F, Tournier JD, Calamante F, et al. Contralateral cerebello‐thalamo‐cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct Funct 2015;220:3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zalesky A, Akhlaghi H, Corben LA, et al. Cerebello‐cerebral connectivity deficits in Friedreich ataxia. Brain Struct Funct 2014;219:969–981. [DOI] [PubMed] [Google Scholar]
- 18. Georgiou‐Karistianis N, Akhlaghi H, Corben LA, et al. Decreased functional brain activation in Friedreich ataxia using the Simon effect task. Brain Cogn 2012;79:200–208. [DOI] [PubMed] [Google Scholar]
- 19. Dogan I, Tinnemann E, Romanzetti S, et al. Cognition in Friedreich's ataxia: a behavioral and multimodal imaging study. Ann Clin Transl Neurol 2016;3:572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harding IH, Corben LA, Storey E, et al. Fronto‐cerebellar dysfunction and dysconnectivity underlying cognition in Friedreich ataxia: the IMAGE‐FRDA study. Hum Brain Mapp 2016;37:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cocozza S, Sacca F, Cervo A, et al. Modifications of resting state networks in spinocerebellar ataxia type 2. Mov Disord 2015;30:1382–1390. [DOI] [PubMed] [Google Scholar]
- 22. Hernandez‐Castillo CR, Galvez V, Mercadillo RE, et al. Functional connectivity changes related to cognitive and motor performance in spinocerebellar ataxia type 2. Mov Disord 2015;30:1391–1399. [DOI] [PubMed] [Google Scholar]
- 23. Saccà F, Costabile T, Abate F, Liguori A, Paciello F, Pane C, De Rosa A, Manganelli F, De Michele G, Filla A. Normalization of timed neuropsychological tests with the PATA rate and nine‐hole pegboard tests. J Neuropsychol 2017. https://doi.org/10.1111/jnp.12125 [DOI] [PubMed] [Google Scholar]
- 24. Ardekani BA, Bachman AH, Helpern JA. A quantitative comparison of motion detection algorithms in fMRI. Magn Reson Imaging 2001;19:959–963. [DOI] [PubMed] [Google Scholar]
- 25. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 27. White M, Lalonde R, Botez‐Marquard T. Neuropsychologic and neuropsychiatric characteristics of patients with Friedreich's ataxia. Acta Neurol Scand 2000;102:222–226. [DOI] [PubMed] [Google Scholar]
- 28. Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry 2000;69:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vita MG, Marra C, Spinelli P, et al. Typicality of words produced on a semantic fluency task in amnesic mild cognitive impairment: linguistic analysis and risk of conversion to dementia. J Alzheimers Dis 2014;42:1171–1178. [DOI] [PubMed] [Google Scholar]
- 30. Ciancarelli I, Cofini V, Carolei A. Evaluation of neuropsychological functions in patients with Friedreich ataxia before and after cognitive therapy. Funct Neurol 2010;25:81–85. [PubMed] [Google Scholar]
- 31. Wollmann T, Barroso J, Monton F, Nieto A. Neuropsychological test performance of patients with Friedreich's ataxia. J Clin Exp Neuropsychol 2002;24:677–686. [DOI] [PubMed] [Google Scholar]
- 32. Pagani E, Ginestroni A, Della Nave R, et al. Assessment of brain white matter fiber bundle atrophy in patients with Friedreich ataxia. Radiology 2010;255:882–889. [DOI] [PubMed] [Google Scholar]
- 33. Solbach K, Kraff O, Minnerop M, et al. Cerebellar pathology in Friedreich's ataxia: atrophied dentate nuclei with normal iron content. Neuroimage Clin 2014;6:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Della Nave R, Ginestroni A, Tessa C, et al. Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract‐based spatial statistics and voxel‐based morphometry. Neuroimage 2008;40:19–25. [DOI] [PubMed] [Google Scholar]
- 35. Santos TA, Maistro CE, Silva CB, et al. MRI texture analysis reveals bulbar abnormalities in Friedreich ataxia. AJNR Am J Neuroradiol 2015;36:2214–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rezende TJ, Silva CB, Yassuda CL, et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich's ataxia. Mov Disord 2016;31:70–78. [DOI] [PubMed] [Google Scholar]
- 37. Akhlaghi H, Corben L, Georgiou‐Karistianis N, et al. Superior cerebellar peduncle atrophy in Friedreich's ataxia correlates with disease symptoms. Cerebellum 2011;10:81–87. [DOI] [PubMed] [Google Scholar]
- 38. Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no‐go decisions based on “what”, “when”, and “where” related information: an fMRI study. J Cogn Neurosci 2005;17:981–993. [DOI] [PubMed] [Google Scholar]
- 39. Burk K, Malzig U, Wolf S, et al. Comparison of three clinical rating scales in Friedreich ataxia (FRDA). Mov Disord 2009;24:1779–1784. [DOI] [PubMed] [Google Scholar]
- 40. Reetz K, Dogan I, Hilgers RD, et al. Progression characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS): a 2 year cohort study. Lancet Neurol 2016;15:1346–1354. [DOI] [PubMed] [Google Scholar]
- 41. Romano S, Coarelli G, Marcotulli C, et al. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol 2015;14:985–991. [DOI] [PubMed] [Google Scholar]
- 42. Tanguy Melac A, Mariotti C, Filipovic Pierucci A, et al. Friedreich and dominant ataxias: quantitative differences in cerebellar dysfunction measurements. J Neurol Neurosurg Psychiatry 2017. https://doi.org/10.1136/jnnp-2017-316964 [DOI] [PubMed] [Google Scholar]


