Abstract
Background
Hormone replacement therapy (HRT) is often seen as the treatment of choice for preventing fractures in women. We undertook a recent meta-analysis of randomised trials which suggested that HRT reduced non-vertebral fractures by 30%. In this analysis we extend that analysis to vertebral fractures.
Methods
We searched the main electronic databases until the end of August 2001. We sought all randomised controlled trials (RCTs) of HRT where women had been randomised to at least 12 months of HRT or to no HRT.
Results
We found 13 RCTs. Overall there was a 33% reduction in vertebral factures (95% confidence interval (CI) 45% to 98%).
Conclusions
This review and meta-analysis showed a significant reduction in vertebral fractures associated with HRT use.
Background
Hormone replacement therapy (HRT) is often considered to reduce vertebral fractures by about 60% [1]. This view is based upon the results of one trial, which counted the number of fractures rather than the number of women with fractures. If an analysis is undertaken looking at the number of women with an incident vertebral fracture the reduction is less and is not statistically significant [2]. We have recently reported in a systematic review of 22 randomised-controlled trials that hormone replacement therapy (HRT) reduces non-vertebral fractures by about 30% [3]. To see if there were a similar effect on vertebral fractures we have extended our review to include such fractures.
Methods
Our search strategy has been previously reported; [3] however, in brief, we searched all the main electronic databases for any RCT of HRT and contacted investigators for unpublished data. There were no language restrictions. To be included in the review trials had to be longer than 12 months and include a comparator group who were either taking an inactive placebo, calcium with or without vitamin D, or using no treatment. Up until the end of August 2001, after excluding duplicate reports, we identified 72 potentially relevant trials.
Results
We identified 13 eligible studies. Nine of which came from our original review of 22 trials [4-12]. The four additional trials, not previously included, were identified as follows. Two trials were excluded from our previous review as they only reported vertebral fractures [1,13] and are now included and two further studies were identified in a recent update of our search [14,15]. We combined the trials in a meta-analysis using a random effects model.
We assessed 12 studies for quality, the remaining study being available in abstract form only [15]. Trial quality was generally good. All studies were reported as randomised controlled trials with seven reporting the method of randomisation used. In addition nine trials were double blind by design and almost all trials reported on drop-outs or withdrawals and document the reasons for these events.
The table shows the characteristics of the included trials. Eight [1,7,10-15] of the 13 studies assessed fracture incidence using radiographs whilst the remaining five appeared to report only symptomatic fractures.
Table 1.
Description of HRT Trials.
| Study | Length months | Type of Oestrogen | Progestin+ Addition of calcium* | Study Population | Outcome measure | Age (SD/range) |
| Alexandersen 1999 [12] | 22 | 50-ug transdermal estradiol | +* | Healthy postmenopausal women with low BMD | BMD | 65 (2.2) |
| Delmas 2000 [5] | 24 | Oral 1 mg estradiol | +* | Healthy >1 year postmenopausal women with normal BMD. | BMD | 58 (5) |
| Gallagher 2001 [14] | 36 | Oral 0.625 mg conjugated estrogens | +* | Elderly women with normal bone density | BMD | 72(± 4) |
| Cauley 2001 [4] | 49 | Oral 0.625 conjugated estrogen | + | Women with established coronary disease >5 years post menopause | MI or CHD | 67 |
| Herrington 2000 [6] | 38 | Oral 0.625 conjugated estrogen | + | Women with coronary arterial disease (CAD) | CAD Progression | 66 (7.0) |
| Ishida 2001 [15] | 12 | Oral 0.625 conjugated estrogen. | + | Women with established osteoporosis. | BMD | 70 (7.6) |
| Lindsay 1990 [13] | 24 | Oral 0.625 mg conjugated estrogen | +* | Postmenopausal women with 1+ vertebral fracture & low BMD | BMD | 48 (1.0) |
| Lufkin 1992 [1] | 12 | Transdermal 0.1 mg 17β-estradiol | + | Postmenopausal white women with documented osteoporosis | BMD | 64.8 (54.9 to 71.3) |
| Mosekilde 2000 [7] | 60 | Oral 1 mg or 2 mg estradiol | + | Healthy women 3–24 months post menopause | Fractures | 50 (2.8) |
| PEPI 1996 [8] | 36 | Oral 0.625 mg conjugated estrogen | + | Healthy women 1–10 years post menopause normal BMD | BMD | 56 (0.3) |
| Ravn 1999 [9] | 48 | Oral 0.625 conjugated estrogen or 2 mg estradiol | + | Healthy 6+ months postmenopausal women under 60 years | BMD | 55 |
| Recker 1999 [10] | 42 | 0.3 mg conjugated estrogen | +* | Healthy women average BMD t-score-3.5 at femur | BMD | 73 (5.0) |
| Wimalawansa 1998 [11] | 48 | Oral 0.625 conjugated estrogen | +* | Women with established osteoporosis (1+vertebral fracture) | BMD | 65 (0.9) |
Figure 1 shows the number of women in each treatment group and their relative risk of fracture. As the figure shows there was an approximate 33% reduction in vertebral fractures among women randomised to HRT (p = 0.04). Three of the studies were undertaken among women who had established osteoporosis [1,11,15]. The relative risk of fracture among these women was 0.47 (95% CI 0.25 to 0.89, p = 0.02), whilst the relative risk of the 10 trials among women without osteoporosis was 0.81 (95% CI 0.50 to 1.33, p = 0.40). Five trials were undertaken among women with a mean age of less than 60 years [5,7-9,13]: the pooled relative risk of fracture for these women was 0.61 (95% CI 0.16 to 2.36), whilst for women older than 60 years it was 0.63 (95%CI 0.41 to 0.96).
Figure 1.
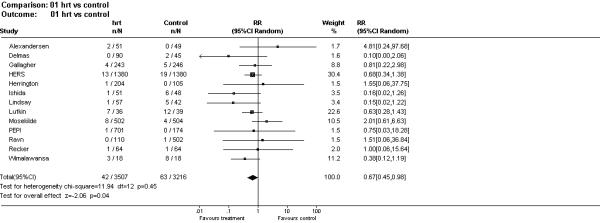
Forest plot of randomised trials of HRT and vertebral fracture incidence.
Discussion
This review of the effects of HRT on vertebral fractures showed a similar reduction in events as did our previous analysis on non-vertebral fractures. As in our previous review the quality of the trials was generally good [3]. Our previous review noted a decreasing effect of HRT on non vertebral fractures for women starting therapy when older than 60 years [3]. In this study we did not observe a similar relationship. Although the relative risk of fracture reduction for younger women was not statistically significant it was virtually identical to that for older women (i.e RR = 0.61 and 0.63 for younger and older women respectively). There were fewer events and fewer participants in trials among women with a mean age of less than 60 years and this may explain the lack of statistical significance.
Studies of other anti-fracture drugs, such as the bisphosphonates, have suggested an enhanced effect among patients with established osteoporosis [16]. This review may support an interaction between HRT and the presence of osteoporosis on vertebral fracture incidence, although the number of trials in that sub-group are small, so definitive conclusions cannot be drawn on this issue. This may be a chance finding, however, because our previous review, where nearly all included women were not osteoporotic, showed an HRT effect on non-vertebral fractures [3]. With respect to the effects, or otherwise, of HRT among women who have low bone density without a prior vertebral fracture there were no studies that allowed us to explore HRT's effects on this sub-group.
Interestingly, taking the results of this review along with our previous analysis shows a similar effect on fractures as the large RCT of the Selective Estrogen Receptor Modulator (SERM) raloxifene [17]. In that trial, among women with a mean age of 67 years, a 50% reduction in new vertebral fractures was observed among osteoporotic women whilst a small, non-significant reduction in non-vertebral fractures was observed [17]. The issue as to whether HRT does significantly reduce vertebral and other fractures needs to be tested in large randomised trials with fracture as an endpoint. Fortunately, ongoing trials of HRT are large enough to answer this important question.
Conclusion
In summary, our review has shown that HRT use is associated with reduction in vertebral fractures, particularly among osteoporotic women.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This review was partly funded from an unrestricted educational grant from Wyeth Pharmaceuticals. We thank Cynthia Iglesias and Andrea Manca for screening Spanish and Italian language papers for us.
Contributor Information
David J Torgerson, Email: djt6@york.ac.uk.
Sally EM Bell-Syer, Email: sembs1@york.ac.uk.
References
- Lufkin EG, Wahner HW, O'Fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117:1–9. doi: 10.7326/0003-4819-117-1-1. [DOI] [PubMed] [Google Scholar]
- Windeler J, Lange S. Events per person year – a dubious concept. BMJ. 1995;310:454–456. doi: 10.1136/bmj.310.6977.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson DJ, Bell-Syer SEM. Hormone Replacement Therapy and Prevention of Nonvertebral Fractures: A Meta-analysis of Randomized Trials. JAMA. 2001;285:2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Black DM, Barrett-Connor E, Harris F, Shields K, Applegate W, Cummings SR. Effects of Hormone Replacement Therapy on Clinical Fractures and Height Loss: The Heart and Estrogen/Progestin Replacement Study (HERS). Am J Med. 2001;110:442–450. doi: 10.1016/S0002-9343(01)00647-7. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Confavreux E, Garnero P, Fardellone P, Vernejoul MC, Cormier C, Arce JC JC. A combination of low doses of 17b-Estradiol and Norethisterone Acetate Prevents Bone Loss and Normalizes bone turnover in postmenopausal women. Osteoporosis International. 2000;11:177–187. doi: 10.1007/pl00004180. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Broshnihan KB, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of Estrogen Replacement on the Progression of Coronary-Artery Atherosclerosis. N Engl J Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- Mosekilde L, Beck-Nielsen H, Sorensen OH, Nielsen SP, Charles P, et al. Hormonal Replacement Therapy Reduces Forearm Fracture Incidence in Recent Postmenopausal Women: Results of the Danish Osteoporosis Prevention Study. Maturitas. 2000;36:181–193. doi: 10.1016/S0378-5122(00)00158-4. [DOI] [PubMed] [Google Scholar]
- The Writing Group for the PEPI Trial Effect of Hormone Therapy on Bone Mineral Density. JAMA. 1996;276:1389–1396. [PubMed] [Google Scholar]
- Ravn P, Bidstrup M, Wasnich RD, Davis JW, McClung MR, Balske A, et al. Alendronate and Estrogen-Progestin in the Long Term Prevention of Bone Loss: Four-Year Results from the early Postmenopausal Intervention Cohort Study. Ann Intern Med. 1999;131:935–942. doi: 10.7326/0003-4819-131-12-199912210-00005. [DOI] [PubMed] [Google Scholar]
- Recker RR, Davies KM, Heaney RP. The Effect of Low-Dose Continuous Estrogen and Progesterone Therapy with Calcium and Vitamin D on Bone in Elderly Women: A Randomized Controlled Trial. Ann Intern Med. 1999;130:897–904. doi: 10.7326/0003-4819-130-11-199906010-00005. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. A Four-Year Randomized Controlled Trial of Hormone Replacement Therapy and Bisphosphonate, Alone or in Combination, in Women with Postmenopausal Osteoporosis. Am J Med. 1998;104:219–226. doi: 10.1016/S0002-9343(98)00029-1. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Riis BJ, Christiansen C. Monoflurophosphate Combined with Hormone Replacement Therapy Induces a Synergistic Effect on Bone Mass by Dissociating Bone Formation and Resoprtion in Postmenopausal Women: A Randomized Study. J Clin Endocrinol Metab. 1999;84:3013–3020. doi: 10.1210/jcem.84.9.5988. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Hart DM, Forrest C, Baird C. Prevention of Spinal Osteoporosis in Oophorectomised Women. Lancet. 1980;ii:1151–1154. doi: 10.1016/S0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination Treatment with Estrogen and Calcitriol in the Prevention of Age-Related Bone Loss. J Clin Endocrinol Metab. 2001;86:3618–3628. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Soh H, Tsuchida M, Kawahara S, Murata H. Comparison of the Effectiveness of Hormone Replacement Therapy, Bisphosphonate, Calcitonin, Vitamin D and Vitamin K in Postmenopausal Osteoporosis: A One Year Prospective Randomized Controlled Trial. Bone. 2001;28 Supp 1:S224. [Google Scholar]
- McClung MR, Geusens P, Miller PD, Zipple H, Bensen WG, Roux C, et al. Effect of Risedronate on the Risk of Hip Fracture in Elderly Women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Mitlak BH, Nickelson T, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from 3-year randomised clinical trial. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]


