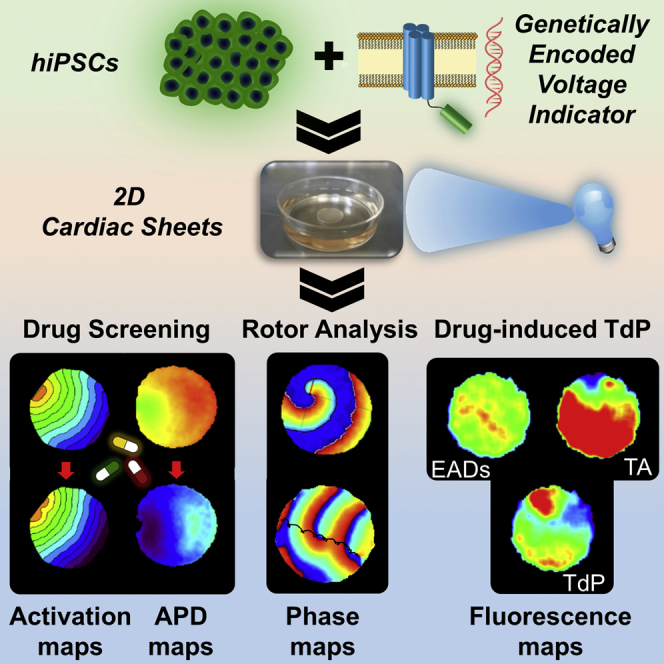
Summary
Fulfilling the potential of human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes for studying conduction and arrhythmogenesis requires development of multicellular models and methods for long-term repeated tissue phenotyping. We generated confluent hiPSC-derived cardiac cell sheets (hiPSC-CCSs), expressing the genetically encoded voltage indicator ArcLight. ArcLight-based optical mapping allowed generation of activation and action-potential duration (APD) maps, which were validated by mapping the same hiPSC-CCSs with the voltage-sensitive dye, Di-4-ANBDQBS. ArcLight mapping allowed long-term assessment of electrical remodeling in the hiPSC-CCSs and evaluation of drug-induced conduction slowing (carbenoxolone, lidocaine, and quinidine) and APD prolongation (quinidine and dofetilide). The latter studies also enabled step-by-step depiction of drug-induced arrhythmogenesis ("torsades de pointes in the culture dish") and its prevention by MgSO4 and rapid pacing. Phase-mapping analysis allowed biophysical characterization of spiral waves induced in the hiPSC-CCSs and their termination by electrical cardioversion and overdrive pacing. In conclusion, ArcLight mapping of hiPSC-CCSs provides a powerful tool for drug testing and arrhythmia investigation.
Keywords: cardiomyocytes, induced pluripotent stem cells, optical mapping, reentry, arrhythmias, genetically encoded voltage indicators, drug screening
Graphical Abstract
Highlights
-
•
Optical mapping of hiPSC-derived cardiac cell sheets expressing ArcLight
-
•
Evaluating effects of drugs and time (weeks) on conduction and APD
-
•
Mapping drug-induced TdP and electrically induced spiral waves (rotors)
-
•
Evaluating interventions aiming to prevent or terminate arrhythmias in the model
Shaheen and Shiti et al. combine hiPSC-CMs, two-dimensional cardiac tissue models, and optical mapping of a genetically encoded voltage indicator (ArcLight) to enable both short-term and repeated tissue electrophysiological phenotyping (over weeks). This allowed tracking of electrophysiological tissue remodeling over time; drug effects on conduction, repolarization, and pro-arrhythmia; and investigation of arrhythmia mechanisms and treatments.
Introduction
The introduction of the human induced pluripotent stem cell (hiPSC) technology (Takahashi et al., 2007) coupled with improved methods for cardiomyocyte differentiation (Burridge et al., 2014, Mummery et al., 2012) brought a unique value to the fields of cardiac disease modeling (Bellin and Mummery, 2016, Itzhaki et al., 2011, Matsa et al., 2016, Moretti et al., 2010, Shaheen et al., 2017, Sun et al., 2012) and drug testing (Liang et al., 2013, Shinnawi et al., 2015, Stillitano et al., 2017, Zwi et al., 2009). In the field of cardiac electrophysiology, most studies examined the electrical properties of the hiPSC-derived cardiomyocytes (hiPSC-CMs) at the single-cell level, focusing primarily on action potential (AP) and ionic current characteristics. Only a few studies have utilized multicellular hiPSC-based cardiac tissue models to evaluate conduction and arrhythmogenesis (Herron, 2016, Kadota et al., 2013, Laksman et al., 2017, Lee et al., 2012).
Traditional methodologies used for studying conduction in cardiomyocyte cultures include multielectrode extracellular recordings (Yankelson et al., 2008), which may be limited in terms of spatial resolution and degree of complexity of the information gained, or optical mapping (Herron et al., 2012). The latter approach utilizes voltage-sensitive dyes (VSDs) to follow changes in membrane potential (Lopez-Izquierdo et al., 2014, Matiukas et al., 2007). These indicators, although of great utility, can cause phototoxicity and hamper the ability to obtain long-term and repeated recordings.
To overcome the aforementioned challenges, we propose to combine the hiPSC technology, two-dimensional (2D) cardiac tissue models, and genetically encoded voltage indicators (GEVIs), such as ArcLight (Leyton-Mange et al., 2014, Shinnawi et al., 2015), VSFP-CR (Chen et al., 2017), CaViar (Dempsey et al., 2016), and VSFP2.3 (Liao et al., 2015), for probing membrane potentials. Specifically, we chose to focus on ArcLight and hypothesized that the generated ArcLight-expressing hiPSC-derived cardiac cell sheets (hiPSC-CCSs) will allow gaining high-resolution information regarding tissue conduction and repolarization in both acute and long-term studies. Following establishment of this unique multicellular model, we aimed to evaluate its potential for drug testing using agents known to alter conduction and/or repolarization with specific emphasis on studying the mechanisms of drug-induced arrhythmias at the tissue level. Finally, we aimed to evaluate the biophysical properties of reentrant arrhythmias (spiral waves) induced in this model through either a drug-related pro-arrhythmia mechanism or following programmed electrical stimulation and to test the efficacy of clinically relevant therapeutic interventions.
Results
Generation of ArcLight-Expressing hiPSC-CCSs
ArcLight-expressing hiPSCs from a stable transgenic line established in our laboratory (Shinnawi et al., 2015) were differentiated into the cardiac lineage using a modified directed small-molecule monolayer-based differentiation system (Burridge et al., 2014). Following differentiation, beating monolayers, containing ≥85% cardiomyocytes (Figure S1A), were enzymatically dissociated and seeded as circular homogeneous hiPSC-CCSs (Figure S1B).
A series of experimental calibration and optimization steps were then performed to determine the optimal seeding density required to obtain hiPSC-CCSs, which will be highly confluent but without evidence for cell death due to improper diffusion. The optimal approach, according to these studies, consisted of using only highly enriched early-stage differentiated cardiomyocytes (>85% cardiac troponin T positive, 8–14 days post differentiation). Tissues were generated from single-cell suspensions, derived following enzymatic dissociation and the use of a 100 μm cell strainer, at a seeding density of 38,000–56,000 cells/mm2. We chose to generate hiPSC-CCSs with a ∼0.5 cm diameter, as this size was sufficient for monitoring electrical propagation, measuring local conduction velocities (CVs), and inducing arrhythmias.
We next performed cell content analysis by enzymatically digesting hiPSC-CCSs at different developmental stages. This time-series analysis (Figure S1C) revealed a major drop in cell content between day 0 (d0) and d1, and a relatively stable cell content after d6–10. The final cell count was ∼400,000 cells/hiPSC-CCS, which was sufficient to achieve homogeneous tissues without any structural discontinuities in 80%–90% of independent sessions.
Immunostaining of sarcomeric α-actinin and connexin 43 (Cx43) confirmed the presence of high-density electrically coupled cardiomyocytes arranged in an isotropic pattern within the hiPSC-CCSs (Figure S1). Structural characterization using three-dimensional reconstruction (Figures S1D–S1F) and orthogonal views (Figure S2G) revealed that the final tissues consisted of approximately four cell layers (range, 3–7; n = 13 from four independent experiments) with a thickness of 19.1 ± 8.4 μm.
Comparison of ArcLight and Di-4-ANBDQBS-Based Optical Mapping
We hypothesized that GEVIs can be utilized for long-term and repeated electrophysiological phenotyping without affecting cell viability. The ability of ArcLight to evaluate human cardiomyocyte AP morphology and duration was already demonstrated at the cellular level (Leyton-Mange et al., 2014, Shinnawi et al., 2015) but has not been utilized at the tissue level to evaluate conduction. One potential limitation of GEVIs in general and ArcLight specifically is their relatively slow kinetics (Jin et al., 2012), a property that may hinder their potential use for activation mapping. To evaluate whether, despite this limitation, ArcLight imaging can still be used to reliably evaluate tissue conduction, we compared the results of ArcLight-based activation mapping with similar maps obtained using conventional VSD-based mapping over a wide array of conditions (Figures 1 and S2–S4).
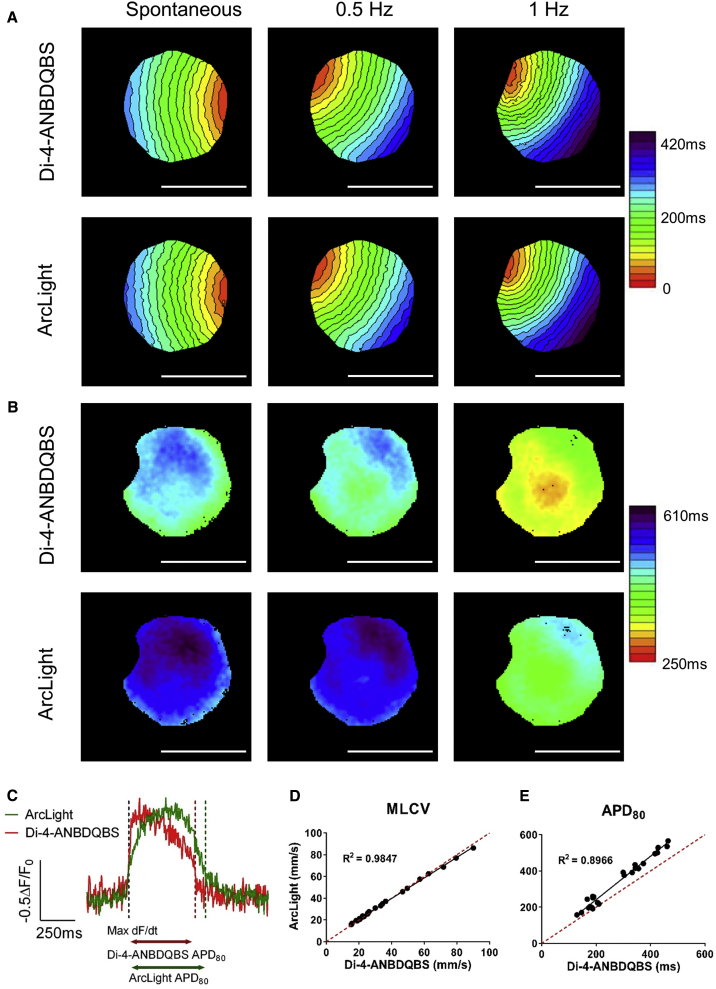
Figure 1.
ArcLight versus Di-4-ANBDQBS: Mapping Comparison
(A and B) Activation (A) and APD80 (B) maps of the same ArcLight-hiPSC-CCS generated from analysis of Di-4-ANBDQBS (top) and ArcLight (bottom) signals during spontaneous activity, 0.5 Hz and 1 Hz pacing frequencies. Single isochrone: 20 ms. Scale bars: 5 mm.
(C) Representative traces of Di-4-ANBDQBS (red) and ArcLight (green) optical APs recorded from the same region of interest (ROI) (3 × 3 pixels).
(D) Correlation between MLCVs values, as derived from ArcLight and Di-4-ANBDQBS activation maps in the same cultures. Notice the high correlation (R2 = 0.9847, n = 25 from four independent experiments) in MLCVs measurements made by the two methods.
(E) Similar comparison of the mean APD80 as measured from ArcLight and Di-4-ANBDQBS recordings (R2 = 0.8966, n = 25 from four independent experiments).
See also Figures S1–S4 and Video S1.
ArcLight-hiPSC-CCSs (6–35 days post plating) were loaded with the VSD, Di-4-ANBDQBS, paced at variable cycle lengths (CLs, 300–2,000 ms) and alternatingly imaged by manual interchanging between two filter sets, one for obtaining ArcLight optical signals and the other for Di-4-ANBDQBS signals (Figure S2A). These studies verified the development of a functional syncytium, enabled monitoring of AP wave propagation (Video S1), and allowed construction of detailed activation and APD maps using both ArcLight and Di-4-ANBDQBS signals (Figures 1, S2, and S3).
We found that the slower kinetics of ArcLight affected mostly the final portion of phase 0 of the AP (Figures 1C and S2E). Consequentially, by defining the local activation time (LAT) as the time point of the maximum first derivative of the recorded signal at each pixel (Figures 1C and S2E), we were able to construct ArcLight-based activation maps that did not differ from those generated using Di-4-ANBDQBS mapping (Figures 1A, S2, and S3). This was also confirmed quantitatively, as a high correlation (R2 = 0.985, Figure 1D, n = 25) was found between the mean local conduction velocities (MLCV) measured from the corresponding ArcLight and Di-4-ANBDQBS maps across a wide range of MLCV values. The agreement between ArcLight and Di-4-ANBDQBS mapping was also validated when using different signal-acquisition rates (260 and 520 frames/s, Figures S3A–S3C). Similarly, the average APD80 (APD at 80% repolarization) values measured by ArcLight correlated (R2 = 0.897, Figure 1E, n = 25) with the measurements derived from Di-4-ANBDQBS recordings over a wide range of values. Notice that, despite this high correlation, ArcLight APD80 values tended to overestimate APD as compared with Di-4-ANBDQBS recordings (Figures 1B and 1E).
ArcLight Displays Decreased Phototoxicity
We next attempted to compare ArcLight- with VSD-based mapping in regards to signal-to-noise ratio (SNR) and phototoxicity. In a series of preliminary studies, we found that the optimal loading conditions of Di-4-ANBDQBS were 60 μg/mL for 20 min. Using these conditions, we noted that the SNR values of Di-4-ANBDQBS were comparable with that of ArcLight (p > 0.05, n = 9, Figure S4A). This finding was verified using additional four different formulas previously utilized to calculate SNR (data not shown).
In order to compare the potential cytotoxic effects of both indicators, we illuminated a focused area within the ArcLight-hiPSC-CCSs for 10 min (central portion in Figure S4B). These studies were conducted in ArcLight-hiPSC-CCSs that were also loaded with Di-4-ANBDQBS and compared with hiPSC-CCSs expressing ArcLight alone or Di-4-ANBDQBS alone (Figure S4C). ArcLight-hiPSC-CCSs were also exposed to red illumination to rule out the contribution of this factor. Our results show a significant decline in the optical signals in the two groups containing Di-4-ANBDQBS ("ArcLight + Di-4-ANBDQBS″ and "Di-4-ANBDQBS alone"; Figure S4C). Importantly, the decline in optical signal in the ArcLight + Di-4-ANBDQBS group was not restricted to Di-4-ANBDQBS signal but was also present in ArcLight recordings (Figure S4C). In contrast, the decline in the optical signal was minimal in the "ArcLight alone" hiPSC-CCSs groups exposed to either blue or red illumination (Figure S4C).
The aforementioned results suggest a potential phototoxic effect induced by Di-4-ANBDQBS. To test this hypothesis, we illuminated only the central portion of hiPSC-CCSs (Figure S4B) for 20 min and then labeled the tissues with DAPI. We then quantified the DAPI signal (as a marker of membrane damage or cell death) in the central illuminated area and normalized it by the DAPI signal in the non-illuminated peripheral area (center/periphery DAPI ratio). As shown in Figures S4C and S4D, an increased toxicity at the illuminated center was only observed in the two groups loaded with Di-4-ANBDQBS (ArcLight + Di-4-ANBDQBS and Di-4-ANBDQBS alone) and was lacking in the blue or red-illuminated ArcLight alone group and in the control hiPSC-CCS group (lacking both reporters) (Figures S4C and S4D). These results suggest that ArcLight-based imaging may be less cytotoxic and therefore more suitable for long-term and repeated tissue phenotyping.
Repeated Long-Term Analysis of the ArcLight-hiPSC-CCSs
To test the hypothesis that ArcLight-based optical mapping can allow repeated and long-term analysis, we followed the electrical remodeling process of the ArcLight-hiPSC-CCSs for more than a month. Figures 2A and 2B depict changes in mean APD80 and MLCV values respectively, at each time point, for the seven cultures studied while paced at 1 Hz. These long-term studies revealed gradual shortening of mean APD values (Figure 2A, p < 0.0001, n = 7) and a gradual increase in CV values (Figure 2B), with the fastest CVs peaking at d16–20 (p < 0.0001, n = 7) and then slightly declining (p > 0.05) at d26–35.
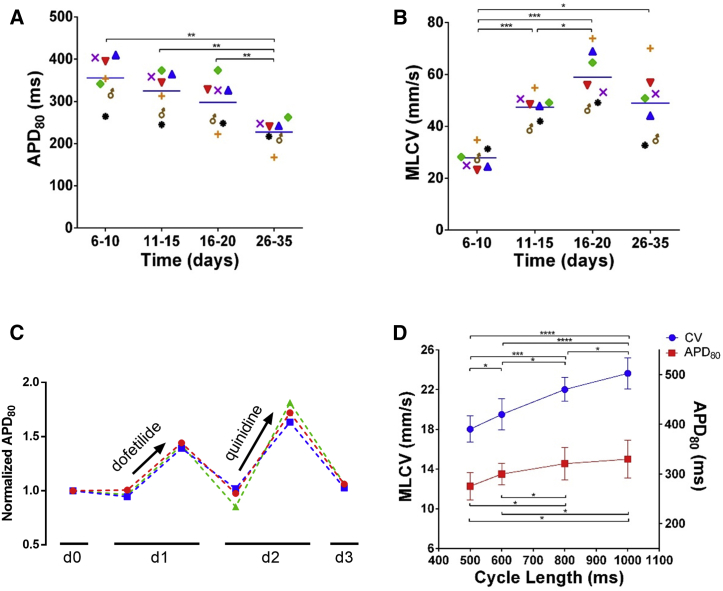
Figure 2.
Effects of Culture Time, Pacing Frequency, and Repeated Drug Applications on APD80 and CV Measurements
(A and B) Summary of mean APD80 (A) and MLCV (B) values as a function of culture time in ArcLight-hiPSC-CCSs (repeated-measurements one-way ANOVA followed by Tukey post-hoc analysis, n = 7 from three independent experiments).
(C) Summary of changes in the normalized APD80 values over time in three hiPSC-CCSs during repeated drug experiments (dofetilide and quinidine).
(D) Restitution curves summarizing the changes in MLCV and mean APD80 values in hiPSC-CCS (6–10 days post plating) as function of pacing cycle length (repeated-measurements one-way ANOVA followed by Tukey post-hoc analysis, n = 7 and 6 respectively, from three independent experiments).
Values are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. See also Figures S3 and S5.
To address the mechanisms underlying the CV changes over time, we focused on Cx43 expression (as a surrogate for intercellular coupling) and on Na+ current recordings (as a surrogate for membrane excitability). As shown in the western blot analysis in Figures S5A and S5B, we did not observe any consistent changes in Cx43 expression between hiPSC-CCSs at 6–10 days versus 16–20 days. Next, we conducted voltage-clamp studies to measure INa+ from single dispersed hiPSC-CMs, derived from hiPSC-CCSs at 6 days or 20 days post plating. As depicted in the current-voltage curves in Figure S5C, cardiomyocytes derived from hiPSC-CCSs at d20 displayed significantly higher peak-current densities (106.5 ± 13.2 versus 70.1 ± 9.9 pA/pF, p < 0.01, n = 15 and 13).
Finally, we aimed to test the suitability of ArcLight-hiPSC-CCSs for repeated pharmacological studies by conducting multiple recordings from the same culture over several days and applying different repolarization-altering drugs at each day (10 nmol/L dofetilide and 5 μmol/L quinidine). The results demonstrate APD80 prolongation in response to each compound followed by recovery to baseline values following drug washout (Figure 2C).
Pharmacological and Pacing Studies
We next aimed to monitor the effects of different interventions on the electrophysiological properties of the hiPSC-CCSs. We first tested the effects of altering pacing frequency (1, 1.25, 1.67, 2 Hz) on the tissues' AP (APD) and conduction (MLCV) properties. Notice in the resulting restitution plots (Figure 2D) the expected APD shortening (p < 0.01, n = 6) and CV slowing (p < 0.0001, n = 7) with higher pacing frequencies.
We next tested the effects of different drugs on the electrical properties of the hiPSC-CCSs. The drugs tested included agents known to slow conduction via two different mechanisms: (1) carbenoxolone (50 μmol/L), a gap-junction blocker; and (2) lidocaine (1–300 μmol/L), a class Ib antiarrhythmic agent that blocks the fast voltage-gated Na+ channels. Both agents (n = 5) significantly slowed conduction in all cultures, as depicted in the activation maps presented prior to and after drug application (Figure 3A, left panels). Note also the typical dose-response effect of lidocaine on MLCV (Figure 3A, middle-right panels).
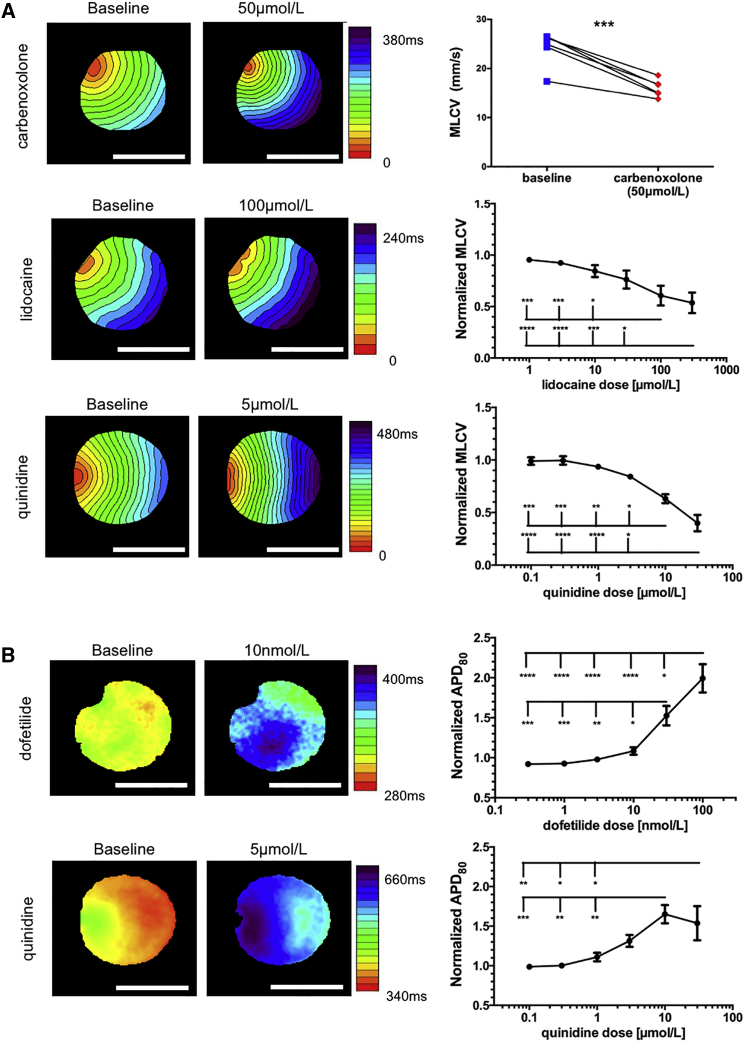
Figure 3.
Pharmacological Effects on Repolarization and Conduction
(A) Representative activation maps (left and middle) and dose-response curves (right) of CV slowing drugs: carbenoxolone (50 μmol/L, n = 5 from three independent experiments, paired t test), lidocaine (1, 3, 10, 30, 100, and 300 μmol/L), and quinidine (0.1, 0.3, 1, 3, 10, and 30 μmol/L). Multiple comparisons were made using one-way ANOVA followed by Tukey post-hoc analysis, both n = 5 from four and three independent experiments, respectively.
(B) Representative APD80 maps (left and middle) and dose-response curves (right) of APD prolonging drugs: dofetilide (0.3, 1, 3, 10, 30, and 100 nmol/L) and quinidine (0.1, 0.3, 1, 3, 10, and 30 μmol/L). Multiple comparisons made using one-way ANOVA followed by Tukey post-hoc analysis, both n = 5 from three independent experiments).
Scale bars: 5 mm. Values are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001. See also Figure S3.
We next tested the ability to detect repolarization changes by applying dofetilide (0.3–100 nmol/L), a class III antiarrhythmic agent that blocks IKr. As expected, dofetilide led to significant APD prolongation, as depicted in the APD80 map example (Figure 3B, top-left panels) and in the plot summarizing the dose-response changes in mean APD80 for all cultures (Figure 3B, top-right panel; n = 5).
Finally, we tested the effects of quinidine (0.1–30 μmol/L), a Na+ channel blocker with additional non-specific potassium channels blocking activity. The resulting activation (Figure 3A, bottom-left panels) and APD80 (Figure 3B, bottom-left panels) maps and the corresponding dose-response curves (bottom-right panels, n = 5) revealed the dual effect of quinidine, both slowing conduction and prolonging APD in the hiPSC-CCSs.
Modeling Reentrant Arrhythmia (Spiral Waves)
We next aimed to evaluate whether ArcLight imaging could go beyond assessment of conduction in the hiPSC-CCSs and also be used to detect, monitor, and study reentrant arrhythmias. To this end, we aimed to induce arrhythmias in the hiPSC-CCSs through a systematic electrical stimulation approach: starting by an increase in stimulation frequency followed by burst pacing and finally direct current injection (Video S2). Using this induction protocol, we were able to induce reentrant arrhythmias in 30% of the hiPSC-CCSs and to monitor the resulting rotors' activities using ArcLight optical signals. Once initiated, the rotors (spiral waves) were highly stable and were associated with a significant increase (p < 0.05) in the frequency of activation (CL = 476 ± 237 ms) as compared with baseline spontaneous activity (CL = 2,853 ± 1,114 ms, n = 5).
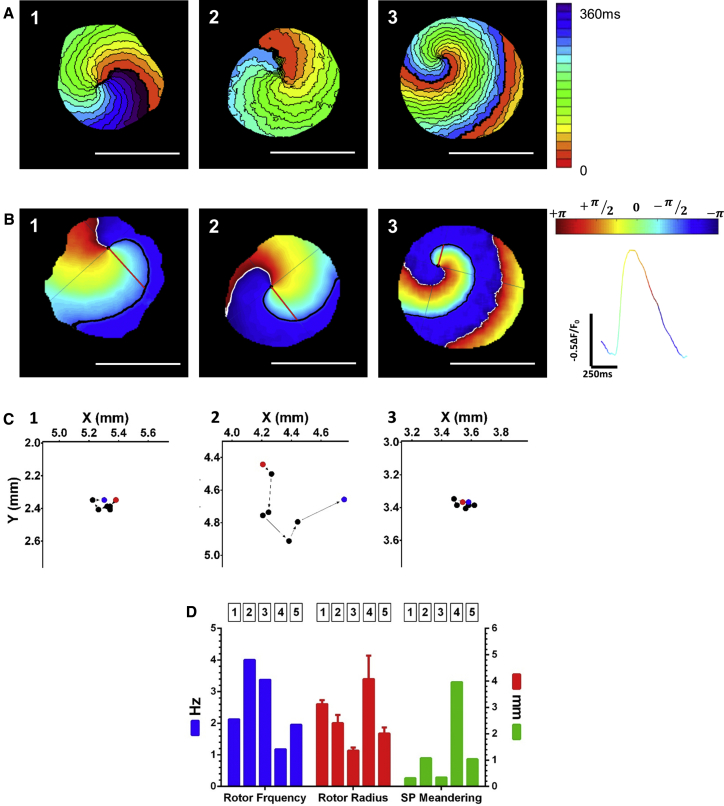
The propagation of the spiral waves could be followed by ArcLight optical mapping, either as dynamic displays depicting the changes in ArcLight fluorescence (Video S3, upper panels) or by constructing detailed activation maps (Figure 4A). An alternative strategy to characterize complex reentrant activity is phase mapping (Gray et al., 1998, Hou et al., 2010, Iyer and Gray, 2001, Pandit and Jalife, 2013). The constructed phase maps, generated by mathematical processing of the optical signals, could be used to characterize the spiral waves either as snapshots (Figure 4B) or as dynamic displays (Video S3, lower-panels). This approach makes it possible to follow the propagation of the rotor's wave front, its singularity point (SP) (the point where all phases conjoin, depicted as black dots in Figure 4B and Video S3), and its meandering path (Figure 4C). Such analysis also allows quantification of various spiral wave biophysical properties, including the rotor's frequency (2.53 ± 1.14 Hz, n = 5, Figure 4D), radius (inversely proportional to curvature, 2.59 ± 1.48 mm; Figure 4D), and the spatial quantification of SP meandering (1.34 ± 1.50 mm; Figures 4C and 4D).
Figure 4.
Analysis of Spiral Waves (Rotors)
(A) Activation maps of the induced rotors in three ArcLight-hiPSC-CCSs. Single isochrones: 20 ms.
(B) Snapshots of phase maps of the same rotors. Colors represent phase, as in the color bar and the colored signal (right). Singularity points (SPs) are indicated as black dots. Black and white lines indicate the wave fronts and tails respectively. Red lines extending from the SP to the wave front, indicate radiuses of the curvatures.
(C) Trajectory of SP meandering for each of the three rotors; total pathway of six SP steps over one rotation cycle.
(D) Summary of rotating frequency (2.526 ± 1.141 Hz), rotor radius (2.594 ± 1.48 mm; error bars represent SEM), and SP meandering pathway (1.342 ± 1.505 mm) of five rotors (from three independent experiments).
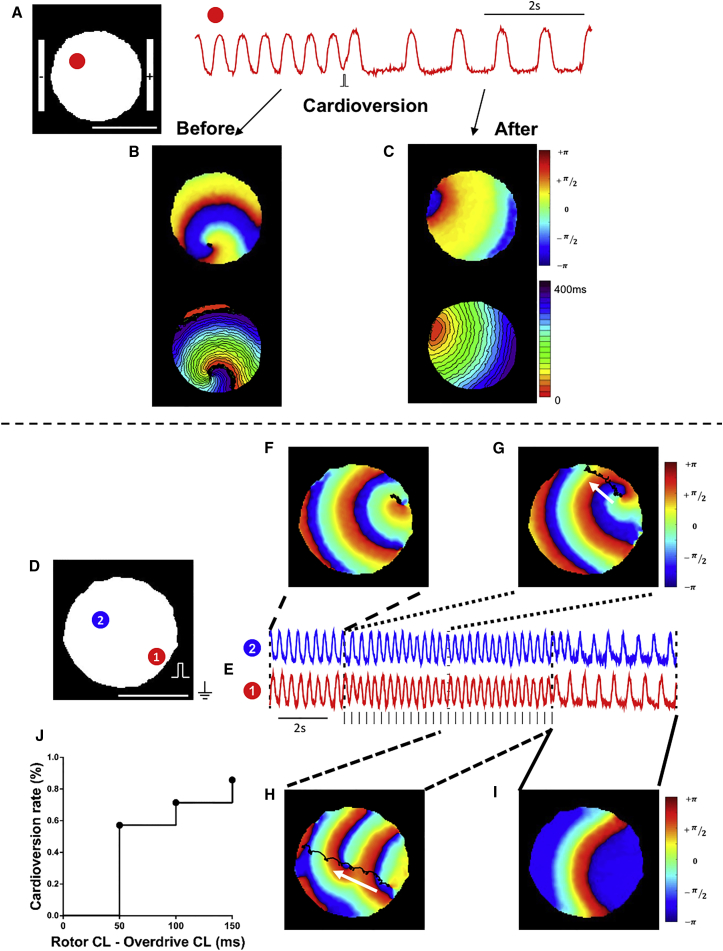
We next evaluated whether clinically relevant maneuverers such as electrical cardioversion and overdrive pacing can terminate such reentrant arrhythmias. To allow field electrical cardioversion, we placed bipolar electrodes at opposing borders of the hiPSC-CCSs (Figure S2D) and applied a 50 ms biphasic pulse over the entire tissue. This resulted in arrhythmia termination in seven out of seven cultures studied, as shown in Figures 5A–5C and Video S4.
Figure 5.
Spiral Waves Termination by Electrical Cardioversion and Overdrive Pacing
(A) Fluorescent trace from one area (red) in an ArcLight-hiPSC-CCS exhibiting stable spiral wave.
(B and C) Phase (upper panel) and activation (lower panel) maps before (B) and after (C) electric cardioversion.
(D and E) Fluorescence traces from two points, 1 (red) and 2 (blue), in an ArcLight-hiPSC-CCS exhibiting stable spiral wave. Vertical marks denote timing of pacing pulses.
(F–I) Phase maps during the arrhythmia (F), during overdrive pacing (G and H), and after recovery to spontaneous normal activity (I). Black trajectories depict the meandering of the SP during each time period.
(J) Cumulative arrhythmia conversion rates in relation to rotor CL minus pacing CL (n = 7 from four independent experiments).
As for overdrive pacing, we performed point stimulation of the hiPSC-CCSs at rates faster than arrhythmia's CL, as shown in Figures 5D–5I and Video S5. Figures 5D and 5E show the fluorescent signals recorded from two sites in a hiPSC-CCS with stable rotor activity (phase map in Figure 5F) with a CL of 350 ms. Next, the electrical wave initiated by the high-frequency pacing (CL = 300 ms) was able to drive the reentrant activity to the top edge of the hiPSC-CCS (Figure 5G, note the black trace and white arrow showing the migration path of the rotor's SP). Occasionally, as shown in this example, new rotors emerged during overdrive pacing, which were also driven to the edge of the culture (Figure 5H), leading eventually to arrhythmia termination and restoration of normal activity (Figure 5I). Figure 5J summarizes the cumulative cardioversion rate in all hiPSC-CCSs studied following sequential increases in pacing frequency (defined as "rotor CL-pacing CL″), leading to termination of reentrant activity in six out of seven cultures studied.
Drug-Induced Pro-arrhythmia (Torsades de Pointes in the Dish)
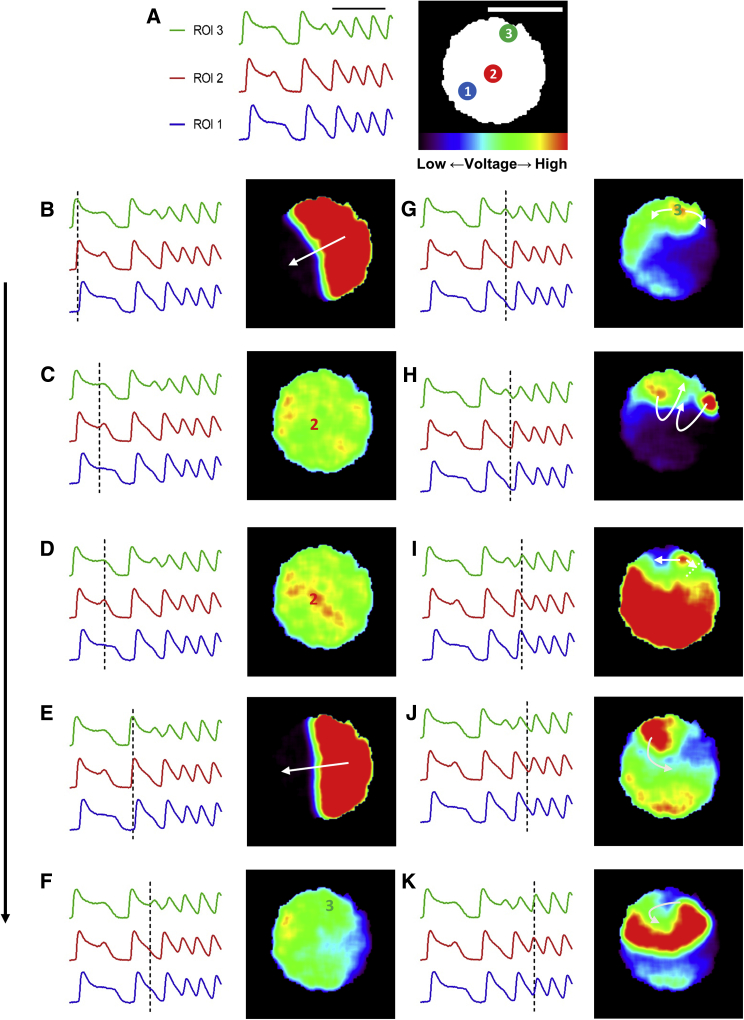
Recent studies demonstrated the potential of hiPSC-CMs in the field of safety pharmacology by detecting drug-induced APD prolongation at the cellular level (Leyton-Mange et al., 2014, Liang et al., 2013, Shinnawi et al., 2015, Stillitano et al., 2017, Zwi et al., 2009). Nevertheless, APD prolongation and even development of early after-depolarizations (EADs) and triggered activity (TA) are not sufficient for development of the relevant clinical arrhythmia (torsades de pointes [TdP]), which requires also the presence of an abnormal substrate at the tissue level (Antzelevitch, 2005). To study the process of drug-induced arrhythmias also at the tissue level we treated the hiPSC-CCSs with a high dose of dofetilide (50 nmol/L, Figure 6). This led to significant and diffuse APD prolongation throughout the culture, eventually causing sustained reentrant arrhythmias in five out of 12 examined hiPSC-CCSs.
Figure 6.
Fluorescence Time Lapse of Dofetilide-Induced Arrhythmogenesis
(A) Three ROIs are highlighted: (1) region with no detected triggered activity, (2) region with EAD/TA that did not develop to subsequent arrhythmia, and (3) region with TA that deteriorated into stable arrhythmia. Black scale bar: 2 s. White scale bar: 5 mm. Color scale bar represents fluorescence intensity (corresponding to voltage changes).
(B–K) Fluorescence time-lapse snapshots describing dofetilide-induced arrhythmogenesis. Note that following propagation of the first activation wave-front (B–C) and EAD/TA developed that failed to propagate and did not induce a stable arrhythmia (D). In contrast, following the second activation wave (E–F), a TA developed (G) that caused "figure-of-eight" reentry (H), unidirectional conduction block and eventually the development of a stablespiral wave (I–K).
See also Video S6. Dofetilide-Induced Arrhythmogenesis #1, Related to Figures 6 and 7 and Videos S7 and S8, Video S7. Dofetilide-Induced Non-propagating EADs, Related to Figures 6 and 7 and Videos S6, S8, and S9, Video S8. Dofetilide-Induced Propagating TA, Related to Figures 6 and 7 and Videos S6, S7, and S9, Video S9. Dofetilide-Induced Arrhythmogenesis #2, Related to Figures 6 and 7 and Videos S6–S8.
By performing long-term mapping of ArcLight-hiPSC-CCSs, we were able to detect the cascade of events that led to the establishment of stable dofetilide-induced arrhythmia (Video S6 and Figure 6). Notice in both the video and the resulting snapshots of the fluorescent ("potential") maps, the development of EADs/TA in localized spots (high voltage, red signal) within the hiPSC-CCS. Note that during the first activation, while APD was prolonged throughout the culture, an EAD could be detected only at one area at the center of the culture (localized increase in membrane potential [red area] at recording site 2 in Figure 6D). This EAD could not be propagated since all areas surrounding it were still relatively depolarized (homogeneous green/yellow). In the next beat, an EAD/TA developed at a different site (site 3 in Figure 6G). Due to the localized heterogeneity in the tissue, with neighboring cells already being repolarized (adjacent blue-purple areas in Figure 6G), this new ectopic activity could be propagated, generating an initial figure-of-eight reentry (Figures 6G–6I) before deteriorating into stable rotor-like activity (Figures 6I–6K).
Prevention of Dofetilide-Induced Pro-arrhythmia
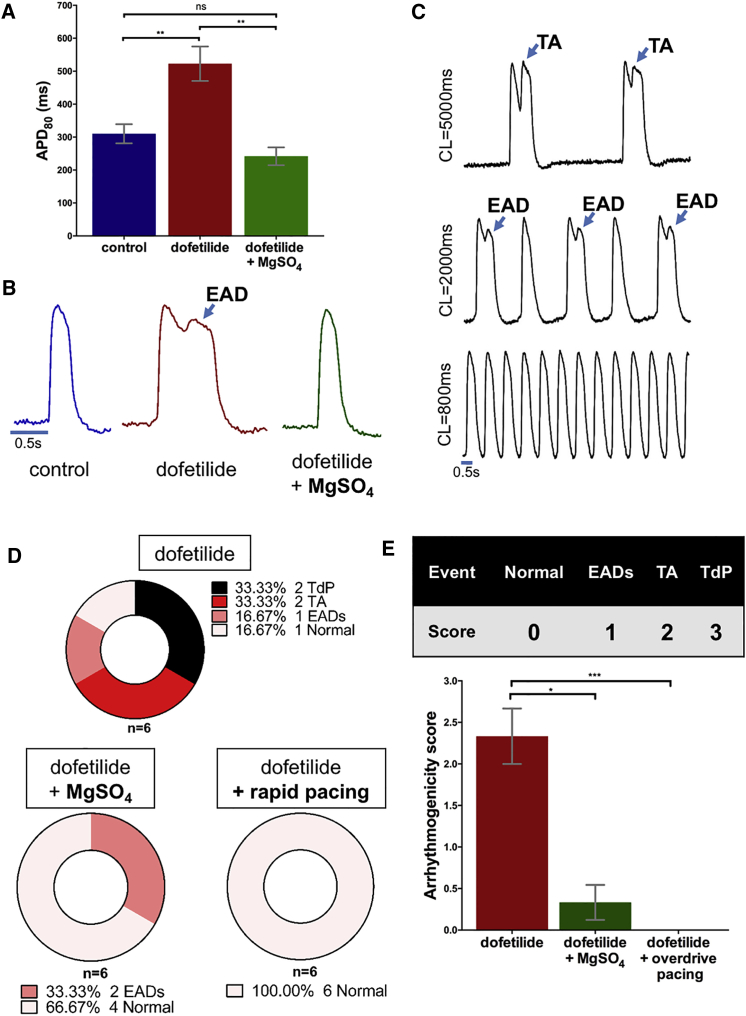
To evaluate the potential role of clinically relevant treatments for TdP in our model, we tested the effects of rapid pacing and magnesium administration in preventing dofetilide-induced arrhythmias. To this end, we took advantage of the ability to study the same ArcLight-hiPSC-CCS over time and tested the incidence of arrhythmic activity following application of dofetilide alone as compared with dofetilide together with magnesium administration or rapid pacing. The electrical activity was closely monitored and resulting pro-arrhythmia was classified and scored as either non-propagating EADs/TA (Video S7, arrhythmogenic score 1), isolated propagated TA (Video S8, score 2), or stable reentrant activity (TdP, Videos S6 and S9, score 3).
Application of dofetilide resulted in significant prolongation of APD (Figures 7A and 7B) and in the generation of non-propagated EADs/TA alone in one of six cultures studied, isolated TA in two specimens, and stable reentrant activity in an additional two cultures (Figure 7D, top panel). Pacing of the dofetilide-treated hiPSC-CCSs at relatively fast rates (CL: 800–1,000 ms) shortened APD and prevented all arrhythmic events (EAD, TA, or TdP), significantly lowering the arrhythmic risk score to 0 (p < 0.01, n = 6, Figures 7D and 7E).
Figure 7.
Prevention of Dofetilide-Induced Arrhythmogenicity by Rapid Pacing and Magnesium Sulfate
(A) Summary of dofetilide and dofetilide + MgSO4 supplementation effects on APD80 (one-way ANOVA followed by Tukey post-hoc analysis, n = 6 from three independent experiments for each group).
(B) Representative optical APs from ArcLight-hiPSC-CCS after treatment with dofetilide or dofetilide + MgSO4.
(C) Optical APs from ArcLight-hiPSC-CCS showing arrhythmogenic activity (TA and EAD) at slow pacing frequencies (CL, 5,000 and 2,000 ms) and their suppression at higher pacing frequency (CL, 800 ms).
(D) Summary of dofetilide-induced arrhythmogenicity in ArcLight-hiPSC-CCSs and its suppression by MgSO4 supplementation and rapid pacing.
(E) Arrhythmogenicity scoring system and summary of MgSO4 supplementation and rapid pacing on ArcLight-hiPSC-CCSs' arrhythmogenicity score (Kruskal-Wallis test followed by Dunn's multiple comparison, n = 6 from three independent experiments for each group).
Values are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001. See also Video S6. Dofetilide-Induced Arrhythmogenesis #1, Related to Figures 6 and 7 and Videos S7 and S8, Video S7. Dofetilide-Induced Non-propagating EADs, Related to Figures 6 and 7 and Videos S6, S8, and S9, Video S8. Dofetilide-Induced Propagating TA, Related to Figures 6 and 7 and Videos S6, S7, and S9, Video S9. Dofetilide-Induced Arrhythmogenesis #2, Related to Figures 6 and 7 and Videos S6–S8.
Co-administration of 2.1 mmol/L of MgSO4 with dofetilide also prevented APD prolongation in the hiPSC-CCSs (Figures 7A and 7B, p < 0.01, n = 6). Magnesium also reduced arrhythmogenicity in the cultures with only two specimens developing dofetilide-induced EADs and none developing any propagated TA or TdP, significantly reducing the arrhythmogenicity score (Figures 7D and 7E).
Discussion
The ability to derive hiPSC-CMs offers unique opportunities for cardiac regenerative medicine, disease modeling, and drug testing. In the cardiac electrophysiology field, most efforts have focused on studying the cellular properties of hiPSC-CMs, namely their AP properties and ionic current profile, with only limited studies evaluating the tissue electrical properties. However, fulfilling the unique potential of the hiPSC technology for studying more complex electrophysiological phenomena (conduction and reentry) and for evaluating long-term tissue remodeling processes and drug effects requires the development of multicellular models as well as methods for long-term, repeated, tissue phenotyping. To address these challenges, we combined hiPSC-CMs, GEVIs, and optical mapping technologies to establish a robust 2D cardiac tissue model for cardiac mapping. The established ArcLight-hiPSC-CCSs model enabled long-term and repeated phenotyping of the same cultures; detailed pharmacological studies evaluating drug effects on conduction, repolarization, and pro-arrhythmia mechanisms; and complex arrhythmia modeling focusing on the biophysical properties of rotors.
Two-dimensional rather than cellular models are important since they resemble more the native organization of cardiomyocytes within the heart and in parallel allow the induction and high-resolution mapping of tissue-related arrhythmias (Herron, 2016, Tung and Zhang, 2006). Moreover, the human origin of our cardiac cell sheets may be advantageous over the conventional animal-derived in vitro models such as neonatal rat ventricular myocyte cultures because of the significant interspecies differences in the expression of relevant cardiac ion channels (Wymore et al., 1997). For example, it was reported that IKr blockers could terminate spiral waves in cardiomyocyte monolayers derived from hPSCs but not from neonatal rat cardiomyocytes (Kadota et al., 2013). This difference stems from the important role of IKr in repolarization in the human, but not in the rodent, heart (Wymore et al., 1997).
The circular hiPSC-CCSs model established here follows similar efforts in the field (Herron et al., 2016, Kadota et al., 2013, Laksman et al., 2017, Lee et al., 2012) and was made possible by advancements in hPSC cardiomyocyte differentiation protocols (Burridge et al., 2014, Mummery et al., 2012), allowing the derivation of large quantities of relatively pure populations of cardiomyocytes. In this study, we generated confluent, high-density hiPSC-CCSs with high cardiomyocyte content. These characteristics are important since previous studies showed that arrhythmia inducibility was inversely correlated with cell seeding density (Kadota et al., 2013) and cardiomyocyte content (Zlochiver et al., 2008), probably because myocyte scarce areas may act as anatomical substrates for complex and fractionated conduction and reentry.
Previous reports have examined similar cell sheets by mapping of calcium transients or APs using calcium or VSDs respectively (Herron, 2016, Kadota et al., 2013, Laksman et al., 2017, Lee et al., 2012). A major limitation of using such dyes for optical mapping of cardiac cultures is their phototoxicity, which can affect cell viability and potentially alter the studied electrophysiological properties (Lopez-Izquierdo et al., 2014, Matiukas et al., 2007, Shinnawi et al., 2015), thereby significantly hampering long-term or repeated mapping. Importantly, we demonstrated that hiPSC-CCSs expressing ArcLight alone did not suffer from phototoxic effects following continuous excitation. This is opposed to sheets loaded with Di-4-ANBDQBS alone or together with ArcLight, which displayed augmented cellular damage in the illuminated centers.
One limitation of ArcLight is its relatively slow kinetics at high frequencies, leading to alterations in the fluorescent representation of phase 0 of the AP (Jin et al., 2012, Leyton-Mange et al., 2014). Since activation mapping is based on measuring LAT from the timing of phase 0 of the optical APs at each sampled pixel, this limitation could lead to inaccurate maps. When comparing the ArcLight-based optical signals with Di-4-ANBDQBS, which does not interfere with ArcLight spectrum (Matiukas et al., 2007), we could detect the typical two-stage exponential kinetics of ArcLight, characterized by an initial rapid phase that closely matches the VSD signal and a second phase where the ArcLight signal is significantly lagging behind the VSD. By targeting the initial rapid stage of phase 0 (timing of the maximal first derivative of the signal) to determine LATs, we could derive values that were almost identical between the ArcLight and VSD signals, including when using different signal-acquisition sampling rates. This allowed derivation of ArcLight-based activation and CV maps that matched those obtained by Di-4-ANBDQBS.
Based on these findings we reasoned that ArcLight-based mapping could enable reliable, long-term, and repeated phenotyping of the same specimens, allowing the study of long-term electrical remodeling processes and chronic effects of drugs or other interventions. Consequentially, by subjecting the same ArcLight-hiPSC-CCSs to repeated imaging over weeks, we identified the continuous shortening of APD over time and gradual increases in CV. To address the mechanisms underlying such CV changes, we focused on quantifying Cx43 expression (as a surrogate for changes in cellular coupling) and on Na+ current recordings (as a surrogate for membrane excitability). Our results did not show any consistent changes in the expression of Cx43 but did reveal a significant increase in Na+ current density over time in the hiPSC-CCSs. It is worth mentioning, however, that this increased CV is still one magnitude slower than in the native myocardium.
To demonstrate the utility of the ArcLight-hiPSC-CCS model for drug testing, we evaluated four different pharmacological agents and observed the expected dose-response effects on conduction slowing induced by suppression of gap-junction coupling (carbenoxolone) or membrane excitability (lidocaine and quinidine) and APD prolongation (dofetilide and quinidine). Moreover, the ability to use the suggested model for repeated long-term phenotyping studies can maximize the drug-screening potential, as the same specimens can be used for multiple “drug/wash” cycles to evaluate different drugs.
Importantly, while assays at the cellular level can detect APD prolongation (Caspi et al., 2009, Leyton-Mange et al., 2014, Lopez-Izquierdo et al., 2014), they lack the ability to address the degree of its heterogeneity and spatial distribution in the tissue and cannot predict drug effects on conduction. Since both CV and APD (as a surrogate for refractory period) play a role in determining the tissue wavelength (a key parameter inversely proportional to arrhythmogenic susceptibility), APD alone is not sufficient to determine the tissue's arrhythmogenic propensity.
A potential application of the hiPSC technology is in the field of safety pharmacology and specifically for “QT screening”, since drug-induced APD prolongation causing life-threatening ventricular arrhythmias (TdP) is the leading cause for withdrawal of approved drugs from the market (Roden, 2004). Recent studies focused on developing screens to identify drug-induced APD prolongation using cardiomyocytes derived from human embryonic stem cells (Caspi et al., 2009, Leyton-Mange et al., 2014) or hiPSC (Liang et al., 2013, Shinnawi et al., 2015, Stillitano et al., 2017, Zwi et al., 2009). These studies, utilizing optical recordings (Lopez-Izquierdo et al., 2014) or intracellular (Liang et al., 2013) and extracellular (Caspi et al., 2009, Stillitano et al., 2017) electrophysiological measurements, primarily focused on the use of single-cell or small cell clusters and were able to detect APD prolongation and even EADs and TA. While APD prolongation, EADs, and TA are prerequisites for development of pro-arrhythmia, they are not sufficient, since development of clinical TdP requires also the presence of the necessary substrate at the tissue level (Antzelevitch, 2005).
Using continuous imaging of dofetilide-treated ArcLight-hiPSC-CCSs, we were able to map step by step the process of arrhythmia initiation and sustainment as a result of drug-induced APD prolongation (“TdP in the dish”). Our results highlight the importance of heterogeneity of repolarization in the mechanism of drug-induced arrhythmias. The development of dofetilide-induced APD prolongation and EAD/TA formation was necessary but not sufficient by itself to induce arrhythmia. The latter required, in addition, spatiotemporal repolarization heterogeneities, resulting in the presence of an already repolarized (and therefore excitable) tissue in close proximity to the site of EAD/TA formation, allowing the initiation and maintenance of complex reentrant activity.
We next demonstrated that clinically relevant interventions could prevent drug-induced arrhythmias in our model. Rapid pacing and magnesium sulfate are commonly used in the clinical management of TdP. Importantly, both treatments were also demonstrated to prevent dofetilide-induced EADs and TA and to eliminate the risk for TdP in our hiPSC-CCSs model.
In addition to drug-related arrhythmias, we were also able to induce stable spiral waves by incremental electrical stimulation protocols. These arrhythmias were then mapped and analyzed either as dynamic displays, activation maps, or by the use of phase mapping. The latter approach facilitates detailed analysis of rotor stability parameters (such as curvature and SP meandering). Since rotors may play an important role in cardiac fibrillation (Pandit and Jalife, 2013), the development of our human model may provide insights into the mechanisms of their initiation and perpetuation as well as for monitoring the effects of different therapeutic modalities. Consequentially, we were able to use our model to demonstrate the ability to interrupt and terminate rotor activity by clinically relevant interventions such as overdrive pacing and electrical cardioversion.
Among the limitations of the hiPSC-CCS model is the relatively immature state of hiPSC-CMs (Yang et al., 2014), which is an important shortcoming of the field in general. An obvious consequence is the slow CV observed within our tissues. Our measured CV values (2–7 cm/s) are in the range of some of the studies using hPSC-CM-derived tissues (Kadota et al., 2013, ∼2–12 cm/s; Laksman et al., 2017, ∼5.4 cm/s) but slower than values reported in others (Zhang et al., 2012, ∼13–18 cm/s). This variability in CV values may stem from differences in the hPSCs lines used, in the cardiomyocyte differentiation protocols, in the culture density and cardiomyocyte content, in the culture-media used, and in the maturation stage of the hPSC-CMs. One advantage of the observed slow CV is that it leads to a shortened wavelength, which in turn can facilitate arrhythmia induction in smaller tissues, allowing down-scaling the size of the hiPSC-CCSs.
Although shown to be suitable for mapping in the current experimental conditions, the slow kinetics of ArcLight (Jin et al., 2012) may hinder perfect recapitulation of the AP morphology. This shortcoming may be solved in the future by using GEVIs with faster kinetics, such as ASAP1 (St-Pierre et al., 2014) and Arch(D95N) (Kralj et al., 2012). Lastly, our model utilizes an already established transgenic hiPSC line that expresses ArcLight constitutively (Shinnawi et al., 2015). Generating multiple stable hiPSC lines to model different disease- or patient-specific abnormalities may not be practical. This limitation may be solved by using transient lenti- or adeno-viral-based transductions of the differentiating cardiomyocytes.
In conclusion, we have established a platform for generation and mapping of 2D hiPSC-CCSs expressing a GEVI. This robust and promising platform can be widely utilized for safety pharmacology, studying arrhythmia mechanisms, and for testing various antiarrhythmic approaches.
Experimental Procedures
Supplemental Experimental Procedures section is available online.
Cardiomyocyte Differentiation of the ArcLight-hiPSCs
We utilized a transgenic hiPSC line that stably expresses ArcLight (Shinnawi et al., 2015). Cardiomyocyte differentiation was induced using the monolayer differentiation system with a chemically defined medium supplemented with human albumin and ascorbic acid (Burridge et al., 2014).
Generation of the hiPSC-CCSs
hiPSC-CMs at d8–14 were enzymatically dissociated and seeded as circular cell sheets (∼0.5 cm diameter) on Matrigel-coated culture plates (Corning) at a seeding density between 38,000 and 56,000 cells/mm2 (0.75–1.1 million cells in 50 μL drop, Figure S1B). Tissues were cultured in RPMI/B27 containing 1% penicillin/streptomycin and blebbistatin (5 μmol/L).
Optical Mapping
The optical mapping setup consisted of a high-speed EM-CCD (electron multiplying charge coupled device) camera (Evolve 512Delta, Photometrics, 512 × 512 pixels) mounted on a fluorescent macroscope (MVX10, Olympus). Both ArcLight and Di-4-ANBDQBS containing specimens were excited using light-emitting diodes (X-Cite TURBO, Excelitas Technologies) with peak wavelengths at 475 and 630 nm, respectively. For ArcLight recordings, emission was passed through 495 nm long-pass dichroic mirror and filtered using 525/50 nm band-pass filter. A 660 nm long-pass dichroic mirror and 665 nm long-pass filter were used for Di-4-ANBDQBS recordings (all from Chroma). Fluorescence was acquired at 4 × 4 binning and a sampling interval of ∼3.847 ms.
Acquisition and Analysis of Electrophysiological Parameters
OMProCCD (Huang et al., 2016), a custom-designed software, was kindly provided by Prof. Bum-Rak Choi (Brown University) and was utilized for acquisition and analysis. For generation of activation maps, LAT at each pixel was determined from the optical signals as the time point of the maximal first derivative (dF/dt)max. APD80 was defined as the time interval between the LAT and the time point of 80% repolarization. Activation and APD80 maps were displayed as color-coded maps.
Comparison of ArcLight versus Di-4-ANBDQBS Mapping
ArcLight-hiPSC-CCSs were loaded for 7 min with RPMI/B27 medium containing 15 μg/mL (26.3 μmol/L) Di-4-ANBDQBS (purchased from Prof. Leslie Loew). Both reporters were imaged by alternating filter cubes (Figure S2A). To compare APD80 and MLCV values derived using the two reporters, we evaluated cultures at different developmental stages (6– 35 days) and at various pacing frequencies (CLs, 300–2,000 ms) (Figure S2A).
Drug Studies
Carbenoxolone (50 μmol/L), quinidine (0.1, 0.3, 1, 3, 10, and 30 μmol/L), lidocaine (1, 3, 10, 30, 100, and 300 μmol/L) stock solutions were dissolved in H2O, while dofetilide (0.3, 1, 3, 10, 30, and 100 nmol/L [dose-response], 50 nmol/L [drug-induced pro-arrhythmia]) stock solution was dissolved in DMSO (all from Sigma-Aldrich). All recordings were performed 10 min after each dose application, except for carbenoxolone (30 min).
Electrical Stimulation and Arrhythmia Induction
A stimulus isolation unit (SIU-102, Warner Instruments) was utilized to deliver 5 ms pulses through a platinum iridium electrode (Alpha-Omega) or custom-made platinum electrodes positioned close to the tissue edge (Figures S2B and S2C). Arrhythmia inducibility was evaluated using a three-step pacing protocol: (1) incremental pacing frequency until loss of capture or induction of arrhythmia; (2) burst pacing for 3 s; and (3) direct current injection for up to 3 s.
Phase Mapping
Phase maps and related rotor biophysical parameters were computed similarly to that described previously (Gray et al., 1998, Hou et al., 2010, Iyer and Gray, 2001, Pandit and Jalife, 2013) using a semi-automated custom-written MATLAB script that generated color-coded phase maps. The SP was identified as the point where all phases of the AP conjoin (Iyer and Gray, 2001), then the meandering pathway of the SP (six steps of one rotor rotation) was calculated. Rotor radius, which is inversely proportional to the rotor's curvature, was defined as previously described (Hou et al., 2010).
Experiment Solutions
Experiments were performed at 34°C using Tyrode's solution containing (in mmol/L): NaCl, 140; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.0; HEPES, 10; and glucose, 10 (pH 7.4 with NaOH). Prolonged experiments were performed in RPMI/B27 culture medium incubated with 95% air and 5% CO2.
Statistical Analysis
Data are presented as mean ± SEM. Paired t test was used to compare ArcLight- and Di-4-ANBDQBS-derived SNR values and changes in mean MLCV values following carbenoxolone (50 μmol/L) administration. Repeated-measurements one-way ANOVA followed by Tukey post-hoc multiple-comparison analysis was carried out for comparison of mean MLCV and/or APD80 values in (1) repeated phenotyping experiments over time, (2) pharmacological dose-response experiments, and (3) the dofetilide ± MgSO4 APD studies. Repeated-measurements two-way ANOVA followed by Sidak post-hoc analysis was carried out for Na+ activation currents analysis. Kruskal-Wallis test followed by Dunn's multiple-comparison test was carried out for arrhythmogenicity score comparisons.
Author Contributions
N. Shaheen and L.G. designed the experiments. N. Shaheen and A.S. performed the experiments, analyzed the data, and prepared the figures. I.H. and R.S. established the ArcLight hiPSC line. G.A. and A. Gepstein conducted the hiPSC cardiomyocyte differentiation. N. Setter and A. Gruber performed the western blot experiments. I.G. performed flow cytometry experiments. S.C. performed the immunostaining experiments. L.G. supervised the study. N. Shaheen, A.S., and L.G. wrote the paper.
Acknowledgments
This work was supported in part by the European Research Council (ERC-2017-COG-773181-iPS-ChOp-AF), by the Technion-UHN research collaboration fund, and by the BIRAX initiative (04BX14CDLG).
Published: May 10, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and nine videos and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.04.006.
Supplemental Information
References
- Antzelevitch C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm. 2005;2:9–15. doi: 10.1016/j.hrthm.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin M., Mummery C.L. Inherited heart disease - what can we expect from the second decade of human iPS cell research? FEBS Lett. 2016;590:2482–2493. doi: 10.1002/1873-3468.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi O., Itzhaki I., Kehat I., Gepstein A., Arbel G., Huber I., Satin J., Gepstein L. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2009;18:161–172. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xian W., Bellin M., Dorn T., Tian Q., Goedel A., Dreizehnter L., Schneider C.M., Ward-van Oostwaard D., Ng J.K. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2017;38:292–301. doi: 10.1093/eurheartj/ehw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey G.T., Chaudhary K.W., Atwater N., Nguyen C., Brown B.S., McNeish J.D., Cohen A.E., Kralj J.M. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Gray R.A., Pertsov A.M., Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- Herron T.J. Calcium and voltage mapping in hiPSC-CM monolayers. Cell Calcium. 2016;59:84–90. doi: 10.1016/j.ceca.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Herron T.J., Lee P., Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 2012;110:609–623. doi: 10.1161/CIRCRESAHA.111.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron T.J., Rocha A.M., Campbell K.F., Ponce-Balbuena D., Willis B.C., Guerrero-Serna G., Liu Q., Klos M., Musa H., Zarzoso M. Extracellular matrix-mediated maturation of human pluripotent stem cell-derived cardiac monolayer structure and electrophysiological function. Circ. Arrhythm. Electrophysiol. 2016;9:e003638. doi: 10.1161/CIRCEP.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Deo M., Furspan P., Pandit S.V., Mironov S., Auerbach D.S., Gong Q., Zhou Z., Berenfeld O., Jalife J. A major role for HERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ. Res. 2010;107:1503–1511. doi: 10.1161/CIRCRESAHA.110.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Kim T.Y., Koren G., Choi B.R., Qu Z. Spontaneous initiation of premature ventricular complexes and arrhythmias in type 2 long QT syndrome. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1470–H1484. doi: 10.1152/ajpheart.00500.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I., Maizels L., Huber I., Zwi-Dantsis L., Caspi O., Winterstern A., Feldman O., Gepstein A., Arbel G., Hammerman H. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Iyer A.N., Gray R.A. An experimentalist's approach to accurate localization of phase singularities during reentry. Ann. Biomed. Eng. 2001;29:47–59. doi: 10.1114/1.1335538. [DOI] [PubMed] [Google Scholar]
- Jin L., Han Z., Platisa J., Wooltorton J.R.A., Cohen L.B., Pieribone V.A. Single action potentials and subthreshold electrical events imaged in neurons with a novel fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota S., Minami I., Morone N., Heuser J.E., Agladze K., Nakatsuji N. Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur. Heart J. 2013;34:1147–1156. doi: 10.1093/eurheartj/ehs418. [DOI] [PubMed] [Google Scholar]
- Kralj J.M., Douglass A.D., Hochbaum D.R., Maclaurin D., Cohen A.E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods. 2012;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksman Z., Wauchop M., Lin E., Protze S., Lee J., Yang W., Izaddoustdar F., Shafaattalab S., Gepstein L., Tibbits G.F. Modeling atrial fibrillation using human embryonic stem cell-derived atrial tissue. Sci. Rep. 2017;7:5268. doi: 10.1038/s41598-017-05652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Klos M., Bollensdorff C., Hou L., Ewart P., Kamp T.J., Zhang J., Bizy A., Guerrero-Serna G., Kohl P. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ. Res. 2012;110:1556–1563. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton-Mange Jordan S., Mills Robert W., Macri Vincenzo S., Jang Min Y., Butte Faraz N., Ellinor Patrick T., Milan David J. Rapid cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Reports. 2014;2:163–170. doi: 10.1016/j.stemcr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Lan F., Lee A.S., Gong T., Sanchez-Freire V., Wang Y., Diecke S., Sallam K., Knowles J.W., Wang P.J. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M.L.C., De Boer T.P., Mutoh H., Raad N., Richter C., Wagner E., Downie B.R., Unsöld B., Arooj I., Streckfuss-Bömeke K. Sensing cardiac electrical activity with a cardiac myocyte-targeted optogenetic voltage indicator. Circ. Res. 2015;117:401–412. doi: 10.1161/CIRCRESAHA.117.306143. [DOI] [PubMed] [Google Scholar]
- Lopez-Izquierdo A., Warren M., Riedel M., Cho S., Lai S., Lux R.L., Spitzer K.W., Benjamin I.J., Tristani-Firouzi M., Jou C.J. A near-infrared fluorescent voltage-sensitive dye allows for moderate-throughput electrophysiological analyses of human induced pluripotent stem cell-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1370–H1377. doi: 10.1152/ajpheart.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiukas A., Mitrea B.G., Qin M., Pertsov A.M., Shvedko A.G., Warren M.D., Zaitsev A.V., Wuskell J.P., Wei M.-d., Watras J. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 2007;4:1441–1451. doi: 10.1016/j.hrthm.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa E., Ahrens J.H., Wu J.C. Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol. Rev. 2016;96:1093–1126. doi: 10.1152/physrev.00036.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A., Bellin M., Welling A., Jung C.B., Lam J.T., Bott-Flugel L., Dorn T., Goedel A., Hohnke C., Hofmann F. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- Mummery C.L., Zhang J., Ng E.S., Elliott D.A., Elefanty A.G., Kamp T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S.V., Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ. Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- Shaheen N., Shiti A., Gepstein L. Pluripotent stem cell-based platforms in cardiac disease modeling and drug testing. Clin. Pharmacol. Ther. 2017;102:203–208. doi: 10.1002/cpt.722. [DOI] [PubMed] [Google Scholar]
- Shinnawi R., Huber I., Maizels L., Shaheen N., Gepstein A., Arbel G., Tijsen A.J., Gepstein L. Monitoring human induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem Cell Reports. 2015;5:582–596. doi: 10.1016/j.stemcr.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre F., Marshall J.D., Yang Y., Gong Y., Schnitzer M.J., Lin M.Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 2014;17:884–889. doi: 10.1038/nn.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillitano F., Hansen J., Kong C.W., Karakikes I., Funck-Brentano C., Geng L., Scott S., Reynier S., Wu M., Valogne Y. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. eLife. 2017;6:e19406. doi: 10.7554/eLife.19406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Yazawa M., Liu J., Han L., Sanchez-Freire V., Abilez O.J., Navarrete E.G., Hu S., Wang L., Lee A. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tung L., Zhang Y. Optical imaging of arrhythmias in tissue culture. J. Electrocardiol. 2006;39:S2–S6. doi: 10.1016/j.jelectrocard.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wymore R.S., Gintant G.A., Wymore R.T., Dixon J.E., McKinnon D., Cohen I.S. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ. Res. 1997;80:261–268. doi: 10.1161/01.res.80.2.261. [DOI] [PubMed] [Google Scholar]
- Yang X., Pabon L., Murry C.E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelson L., Feld Y., Bressler-Stramer T., Itzhaki I., Huber I., Gepstein A., Aronson D., Marom S., Gepstein L. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–731. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- Zhang J., Klos M., Wilson G.F., Herman A.M., Lian X., Raval K.K., Barron M.R., Hou L., Soerens A.G., Yu J. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ. Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlochiver S., Muñoz V., Vikstrom K.L., Taffet S.M., Berenfeld O., Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys. J. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi L., Caspi O., Arbel G., Huber I., Gepstein A., Park I.H., Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.