Abstract
We present a technique to implant autogenous meniscal fragments using a fibrin clot to repair a large degenerative meniscal defect. A total of 25 mL of the patient's blood is agitated for 10 minutes using a stainless steel swizzle stick in a sterile glass syringe. The elastic fibrin clot subsequently adheres to the stick in a tubular manner. Using arthroscopic debridement, native meniscal tissue is resected. The meniscal fragments are packaged into the tubular-shaped fibrin clot, and the tube is tied at both ends using 4-0 absorbable sutures. A repair suture is prepared using the inside-out meniscal repair device and woven into the margins of the tubular-shaped fibrin clot. The packaged graft with the tubular-shaped fibrin clot is placed with a horizontal suture across both edges of the meniscal defect and secured with a supplemental vertical suture using an all-inside meniscal repair device. Follow-up arthroscopy performed 6 months postoperatively reveals regeneration of meniscus-like tissue. It is ideal to treat large degenerative defects with meniscal preservation, and the present procedure has the advantage of tissue regeneration with native meniscal tissue and growth factors obtained from the fibrin clot using a simple technique. This method could prove helpful in patients with degenerative meniscal defects.
A complex degenerative meniscal tear with a tissue defect is the most common indication for a partial or subtotal meniscectomy. These tears require extensive resection of the meniscal tissue.
In 2010, Kobayashi et al.1 described a meniscal repair technique in which autogenous meniscal fragments wrapped in a fascia sheath were implanted into large meniscal defects in a rabbit model. We recently described a technique for repairing degenerative meniscal tears using fibrin clots.2, 3 Tubular-shaped fibrin clots are particularly versatile and can be used to wrap or package autogenous meniscal fragments. On the basis of these prior findings, we hypothesized that implanting meniscal fragments using this technique to manage large meniscal defects would be possible without having to harvest the fascia sheath to wrap meniscal fragments for implantation or to cover the meniscal region.1, 4 Growth factors derived from the fibrin clot would promote synthesis and healing between meniscal defects and implanted meniscal fragments, and the fibrin clot would act as a scaffold of the graft-defect interface.
Here, we describe a technique whereby autogenous meniscal fragments are packaged using a tubular-shaped fibrin clot and implanted into the defective site.
Surgical Technique
Indications
Large meniscal defects, resulting from complex tears, degenerative tears, and chronic tears of the meniscus, that cannot be repaired at both torn ends with conventional meniscal repair sutures are the indications for our technique. Coronal views of magnetic resonance imaging (MRI) provide useful information of the diagnosis of meniscal defects and reveal the absence of a clear low signal of the meniscus (Fig 1). A lack of significant osteoarthritis and stable knee without ligament instability are necessary for this technique. No limitation is placed on patients' age, although active patients who could be compliant to postoperative rehabilitation are necessary. Most patients with chronic meniscal defects are at the early stage of osteoarthritis, with some of these being controversial indications. However, cases complicated with significant varus or valgus deformity are contraindications. Meniscal defects that involve the meniscal root as well as total and subtotal meniscal defects could not be repaired using this technique.
Fig 1.
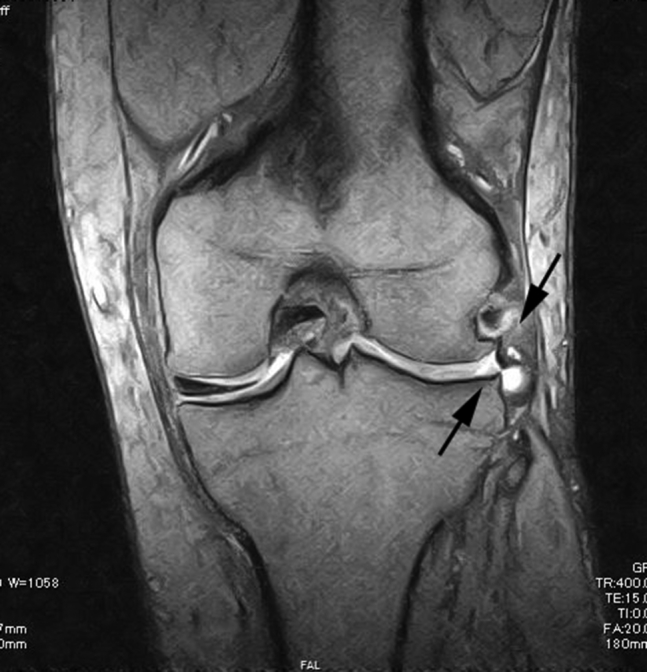
Preoperative coronal view of the magnetic resonance image of the lateral meniscal defect in the left knee. It reveals an absence of a clear low signal of the meniscus (arrows).
Patient Positioning
The patient is placed in a supine position with knee flexion in the figure-4 position on the operating table. The flexed knee is protruded off the side of the operating table in case of a lateral meniscal defect because this makes it easy to retrieve the sutures from the lateral skin incision. Alternatively, the foot of the operating table may be dropped, with the patient's operative leg hanging over the end of the table using a leg holder and/or lateral post, particularly when this technique is performed for medial meniscus cases. This position allows the control of the knee flexion and extension with varus or valgus force. It also aids the management of the arthroscopic view of the meniscal repair site and retrieval of sutures from the medial skin incision. Although a tourniquet is applied on the upper thigh of the operative leg, it is inflated only in cases of bleeding complications during surgery.
Diagnostic Arthroscopy
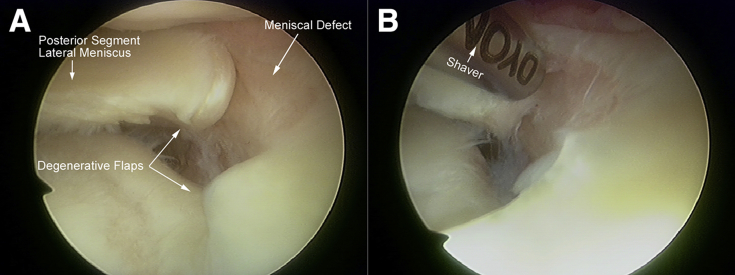
Diagnostic arthroscopy is performed before repair. The standard medial and lateral infrapatellar portals are made for the arthroscopic examination and in preparation of the repair. In the present case, a complete radial defect that extended from the white-white zone to the red-red zone of the middle and posterior segments of the lateral meniscus is observed in the left knee, collapsing its original anatomical shape. The defect comprised 2 stray parts of the meniscus tissue, and both ends had blunted edges with evidence of degeneration (Fig 2A). These degenerative meniscal tissues adjacent to the defect could be the donor of the implantation.
Fig 2.
Intraoperative arthroscopic view of the lateral meniscal defect from the lateral infrapatellar portal in the left knee in the figure-4 position. (A) A large radial defect is observed extending from the middle to posterior segment of the lateral meniscus with degenerative flaps. (B) The blunt edges of the meniscal flaps are resected in a piecemeal manner and debrided.
The meniscus is resected in a piecemeal manner using a basket punch at both ends of the degenerative flaps. The resected meniscal fragments are placed in a sterile bowl for later implantation.
The remaining meniscal tissues surrounding the defect site are abraded using a shaver to expose the clear margins, allowing the size and shape of the defect to be accurately assessed in preparation for the graft (Fig 2B).
Preparation of the Tubular-Shaped Fibrin Clot
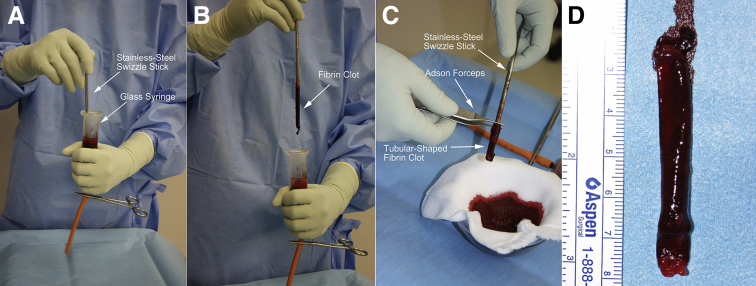
At this time, the fibrin clot is prepared. Approximately 25 mL of blood is collected from the patient's arm into a sterile glass syringe. The blood is stirred in the glass syringe for 10 minutes using a stainless steel swizzle stick measuring approximately 4 mm in diameter (Fig 3 A and B). The elastic fibrin clot adheres to the swizzle stick, resulting in a tubular-shaped fibrin clot that measured approximately 60 mm in length. The tubular-shaped fibrin clot is gently removed using Adson forceps (Fig 3 C and D) and cut to an appropriate length, which is slightly longer than that required for implantation. There are individual differences in the wall thickness of the tubular-shaped fibrin clot (Table 1). If the wall is thin, the clot will be too fragile for packaging the meniscal fragments. Therefore, a tube with 2 layers should be made.
Fig 3.
Preparation of the tubular-shaped fibrin clot. (A) Approximately 25 mL of blood is stirred in the glass syringe for 10 minutes using a stainless steel swizzle stick measuring approximately 4 mm in diameter. (B) The elastic fibrin clot adheres to the swizzle stick (arrow). (C) The fibrin clot adheres to the swizzle stick in a tubular shape and is gently peeled away using Adson forceps. (D) The tubular-shaped fibrin clot measures approximately 60 mm in length.
Table 1.
Tips and Pitfalls for Preparing the Tubular-Shaped Fibrin Clot
|
|
|
Graft Preparation
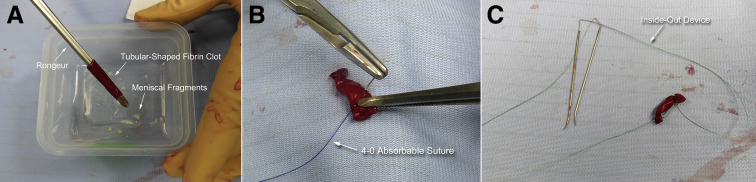
The meniscal fragments are inserted into the tubular-shaped fibrin clot using a rongeur (Fig 4A), and both ends of the tube are tied with 4-0 absorbable sutures (Fig 4B). This creates a construct that resembles a piece of candy. A repair suture is prepared using the inside-out device, including straight needle-suture combination, such as Henning meniscal suture kit (Stryker, Kalamazoo, MI), and woven through the open ends of the packaged graft (Figs 4C and 5).
Fig 4.
Intraoperative preparation of the graft. (A) Using a rongeur, the meniscal fragments are inserted into the tubular-shaped fibrin clot. (B) Both ends of the tubular-shaped fibrin clot are tied, resembling a piece of candy. (C) Graft preparation is completed using an inside-out suture device including straight needle-suture combination.
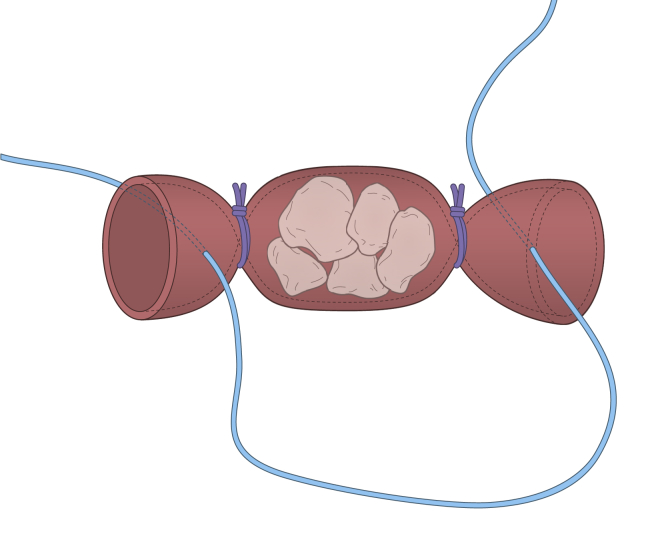
Fig 5.
Schematic of graft preparation.
Implantation of the Graft to the Meniscal Defect
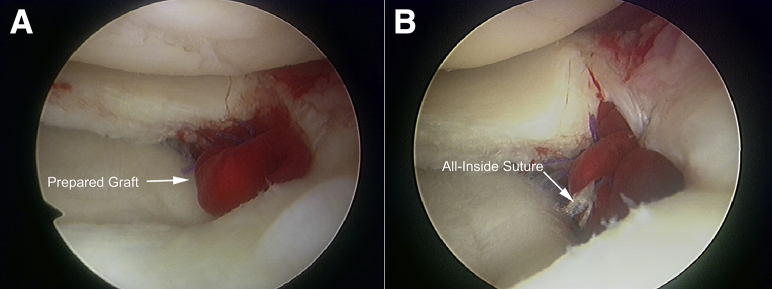
A lateral skin incision is made over the repair site, and the superficial fascia and iliotibial band are divided to allow the sutures to be retrieved later. The arthroscope is then inserted into the joint space from the infrapatellar lateral portal, and a larger cylindrical guide is inserted from the medial portal in the standard approach for an inside-out lateral meniscal repair technique. The prepared graft is positioned using the guide, and horizontal sutures are placed across both edges of the meniscal defect (Fig 6A). The repair suture is tied to the lateral capsule through the lateral incision. The graft implant is secured with a supplemental vertical suture and applied with an all-inside meniscal repair device (FAST-FIX; Smith & Nephew Endoscopy, Andover, MA) (Figs 6B and 7). The graft position is confirmed, and the iliotibial band, fascia, and soft tissues are then closed.
Fig 6.
Implantation of the graft to the meniscal defect. (A) Using a larger cylindrical guide, the graft is obtained with retrieval of the suture from the lateral incision and placed at the defective site. (B) A supplement vertical suture is applied with an all-inside meniscal repair device to complete the repair.
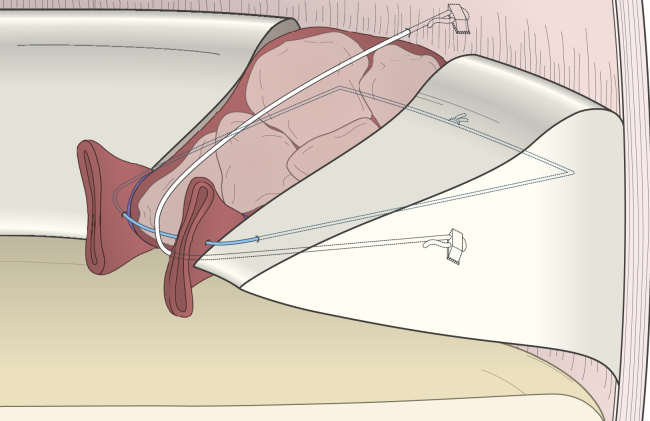
Fig 7.
Schematic of the technique showing implantation of the graft into the meniscus defect.
Postoperative Recovery
A cylinder cast is applied, and the patient is not permitted to bear weight for 4 weeks after surgery. Weight-bearing is then slowly introduced. Moderate impact physical activity such as jogging is permitted 4 months postoperatively, and full squatting and sport participation is permitted after the postoperative MRI and the follow-up arthroscopy performed 6 months postoperatively.
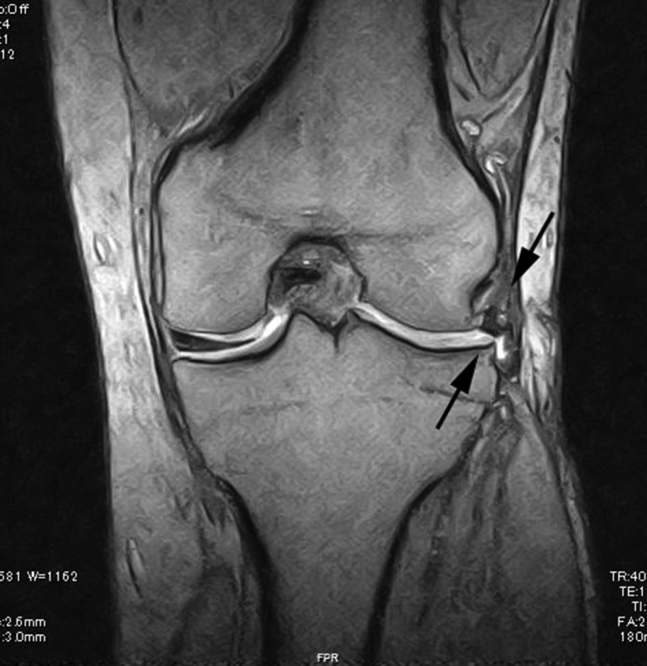
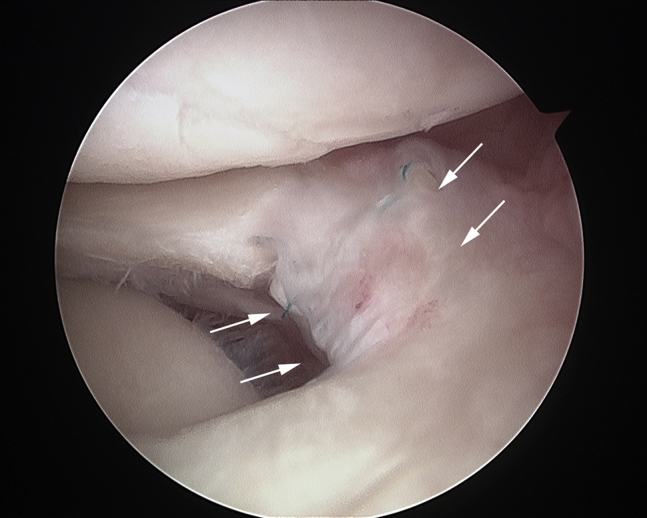
On postoperative MRI, the edge of the lateral meniscus appeared as a clear, low-signal-intensity structure, which is originally absent and slightly extruded on preoperative MRI findings (Fig 8). On follow-up arthroscopy, the defect site is filled with meniscus-like tissue, and the meniscus is now crescent shaped. There is no evidence of displacement of the meniscal fragments from the packaged graft to the other regions of the joint (Fig 9; Video 1).
Fig 8.
Postoperative coronal view of magnetic resonance image of the present case at 6 months postoperatively. A clear low signal appeared at the grafted site (arrows).
Fig 9.
Follow-up arthroscopy of the present case at 6 months postoperatively. A meniscus-like tissue is observed incorporated into the meniscal defect (arrows).
Discussion
The most important finding of this Technical Note is that the described technique permitted tissue regeneration with native meniscal tissue fragments contained in a tubular-shaped fibrin clot. Furthermore, this technique can implant meniscal fragments into the meniscal defect without the need of harvesting other tissues, such as fascia sheath, to package the meniscal fragments and/or cover the recipient region.
Large meniscal defects often result from complex tears, degenerative tears, chronic tears of the meniscus, or after the operative treatment of these injuries. In most cases, an extensive meniscectomy approximating a subtotal meniscectomy is reluctantly performed as a last ditch effort to reduce the symptoms caused by the defect because these menisci have a lower healing potential than those in acute cases. Meniscectomies of complex and degenerative meniscal tears are more extensive than meniscectomies for simple vertical tears in the avascular zone of the meniscus. However, questions have been raised as to whether a meniscectomy is appropriate for symptom relief in these cases, as postoperative osteoarthritis is an inevitable and well-known sequela after the removal of a significant part of the meniscus.5
Based on these previous findings, clinical efforts have been directed toward improving meniscal repair. However, meniscal repair is limited in certain situations, such as tears located in the avascular zone, tears with accompanying meniscal degeneration, complex and extensive tear patterns extending from the avascular to the vascular zone, and large defects resulting from chronic degenerative changes or after an extensive meniscectomy.
There are several studies that have investigated ways to restore the meniscus of patients with large meniscal defects or meniscal tears that involve the avascular zone. Treatment options for meniscal defects have previously been described experimentally and clinically.6, 7, 8, 9 The modern trend toward “meniscus preservation” has further accelerated technological development.
Autogenous grafting was first investigated for use in meniscal reconstruction by using a tendon graft to substitute for the meniscal defect. However, the initial results of this technique were unsatisfactory.6 Allograft transplantation has been used to treat total defects of the meniscus, and substitutes such as collagen meniscus implants and polyurethane meniscus implants for postoperative defects have been employed after partial meniscectomies. Platelet-rich plasma and new tissue engineering techniques7 have also been used to aid in meniscal regeneration, as have scaffolds comprising myoblasts8 and synovial stem cells.9 These innovative implants and tissue engineering techniques are ideal for clinical use, but have been until recently difficult to employ in every situation.
Previous studies have attempted to repair and restore the meniscus using a fibrin clot and patients' native meniscal tissue. In a 1991 clinical study, Henning et al.4 described a new repair technique for complex tears that was developed in response to the high failure rate of conventional meniscal repair. In their technique, a rectangular section of fascia was harvested from the patient's thigh and used to cover the complex tear along with a fibrin clot.
In a 2010 experimental study, Kobayashi et al.1 implanted minced meniscal fragments wrapped in a fascia sheath into large meniscal defects in rabbit models. The animals that received grafted meniscal fragments wrapped with a fascia sheath had enhanced fibrocartilage regeneration in vivo 12 weeks after implantation, and a maximum load stiffness that was significantly higher than in other 2 experimental groups.
For the application of this implantation technique using native meniscal fragments in actual clinical cases, there are 2 concerns. One is the choice of donor site, and the other concern is the difficulty in minimally invasive harvesting of a fascia sheath in current arthroscopic meniscal surgery.
The use of a fibrin clot for meniscal repair in the avascular zone is a common augmentation technique because it is an easy way to provide growth factors directly to the meniscal repair site.10 In our evaluation of the management of degenerative meniscal tears using fibrin clots, we prepared a fibrin clot in a variety of constructs. The tubular-shaped fibrin clot is amenable to a variety of applications and can be used to wrap or package autogenous meniscal fragments. On the basis of the results in our previous studies that used fibrin clots to repair degenerative horizontal meniscus tears,2, 3 we surmised that fibrin clots could provide scaffolding during meniscal repair but may be too small to fill large meniscal defects. Thus, we combined the advantages of the technique described by Kobayashi et al.1 and our recent approach into the present fibrin clot preparation method; the resulting implantation technique is simple and provides the benefits of the fibrin clot, which promotes cellular infiltration and healing.10 In our previous study using fibrin clot, we observed meniscal healing even in surfaces showing degeneration.2, 3 Therefore, we consider the degenerative meniscal tissue adjacent to the defect to be valuable as a donor site.
The advantages of our technique are that implantation is easier to perform than the fascia sheath technique, additional tissue harvesting is not necessary, and growth factors are provided from the fibrin clot. However, it is difficult to obtain the tubular-shaped fibrin clot without using a stainless steel swizzle stick and glass syringe and with individual differences in wall thickness (Table 1). Enough volume of meniscal fragments is not necessarily corrected from the meniscal tissue adjacent to a meniscal defect. In addition, the graft needs to be placed between both ends of the residual meniscal tissues. The graft serves as a continuity of the crescent shape of the meniscus to ensure meniscal regeneration and contribute to the formation of the anatomical crescent shape. Therefore, this technique cannot be applied for defects with one-sided residual tissue, such as the meniscal root tear. In the postoperative rehabilitation of patients with meniscal repair, the rehabilitation protocol restricts patient range of motion and weight-bearing, which is slowly introduced. The risk of complication is the same as that with common arthroscopic procedures (Table 2). A summarized step-by-step description of this procedure is listed in Table 3.
Table 2.
Advantages/Benefits and Disadvantages/Risks of the Procedure
|
|
Table 3.
Step-by-Step Description of the Procedure
|
Although future studies to establish the indications for our technique are required, we believe that using a fibrin clot to deliver meniscal fragments can serve as an option for the repair of large defects and complex tears, irreparable meniscal defects, and chronic degenerative tears after a meniscectomy.
Acknowledgment
The authors thank Yasukazu Kobayashi, M.D., Ph.D., of the Department of Orthopaedic Surgery, Zenshukai Hospital, and Professor Kazunori Yasuda, M.D., Ph.D., of the Department of Sports Medicine and Joint Reconstruction Surgery, Hokkaido University Graduate School of Medicine, Japan. Dr. Kobayashi died peacefully after publishing his investigation conducted at the Hokkaido University. His basic experimental study1 was fundamental to this Technical Note and in our efforts for clinically treating meniscal defects.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Complex degenerative tear with tissue defect of the lateral meniscus in the left knee. A large complete radial defect is observed at the middle to posterior segment of the lateral meniscus, collapsing its original anatomical shape. Using a basket punch, native meniscal tissue adjacent to a meniscal defect is resected. The meniscal fragments are packaged into the tubular-shaped fibrin clot. The packaged graft is placed with a horizontal suture across both edges of the meniscal defect and secured with a supplemental vertical suture using an all-inside meniscal repair device. On follow-up arthroscopy at 6 months after initial surgery, the defect site is filled with meniscus-like tissue, and the meniscus assumes a crescent shape.
References
- 1.Kobayashi Y., Yasuda K., Kondo E. Implantation of autogenous meniscal fragments wrapped with a fascia sheath enhances fibrocartilage regeneration in vivo in a large harvest site defect. Am J Sports Med. 2010;38:740–748. doi: 10.1177/0363546509350749. [DOI] [PubMed] [Google Scholar]
- 2.Kamimura T., Kimura M. Repair of horizontal meniscal cleavage tears with exogenous fibrin clots. Knee Surg Sports Traumatol Arthrosc. 2011;19:1154–1157. doi: 10.1007/s00167-011-1404-5. [DOI] [PubMed] [Google Scholar]
- 3.Kamimura T., Kimura M. Meniscal repair of degenerative horizontal cleavage tears using fibrin clots: Clinical and arthroscopic outcomes in 10 cases. Orthop J Sports Med. 2014;2 doi: 10.1177/2325967114555678. 2325967114555678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henning C.E., Yearout K.M., Vequist S.W., Stallbaumer R.J., Decker K.A. Use of the fascia sheath coverage and exogenous fibrin clot in the treatment of complex meniscal tears. Am J Sports Med. 1991;19:626–631. doi: 10.1177/036354659101900613. [DOI] [PubMed] [Google Scholar]
- 5.Baratz M.E., Fu F.H., Mengato R. Meniscal tears: The effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 6.Johnson L.L., Feagin J.A., Jr. Autogenous tendon graft substitution for absent knee joint meniscus: A pilot study. Arthroscopy. 2000;16:191–196. doi: 10.1016/s0749-8063(00)90035-5. [DOI] [PubMed] [Google Scholar]
- 7.Pereira H., Frias A.M., Oliveira J.M., Espregueira-Mendes J., Reis R.L. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 2011;27:1706–1719. doi: 10.1016/j.arthro.2011.08.283. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y., Zhu W., Hao Y., Lu L., Chen Y., Wang Y. Repair of meniscal defect using an induced myoblast-loaded polyglycolic acid mesh in a canine model. Exp Ther Med. 2012;3:293–298. doi: 10.3892/etm.2011.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horie M., Driscoll M.D., Sampson H.W. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am. 2012;94:701–712. doi: 10.2106/JBJS.K.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnoczky S.P., Warren R.F., Spivak J.M. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–1217. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complex degenerative tear with tissue defect of the lateral meniscus in the left knee. A large complete radial defect is observed at the middle to posterior segment of the lateral meniscus, collapsing its original anatomical shape. Using a basket punch, native meniscal tissue adjacent to a meniscal defect is resected. The meniscal fragments are packaged into the tubular-shaped fibrin clot. The packaged graft is placed with a horizontal suture across both edges of the meniscal defect and secured with a supplemental vertical suture using an all-inside meniscal repair device. On follow-up arthroscopy at 6 months after initial surgery, the defect site is filled with meniscus-like tissue, and the meniscus assumes a crescent shape.