Abstract
Sarcopenia, the age‐related loss of muscle mass and strength, is linked to a range of adverse outcomes, such as impaired physical performance, cognitive function, and mortality. Preventing sarcopenia may reduce the burden of functional decline with aging and its impact on physiological and economic well‐being in older adults. Mitochondria in muscle cells lose their intrinsic efficiency and capacity to produce energy during aging, and it has been hypothesized that such a decline is the main driver of sarcopenia. Oxidative phosphorylation becomes impaired with aging, affecting muscle performance, and contributing to an age‐associated decline in mobility. However, it is unclear whether this deterioration is due to a reduced mitochondria population, decreased mitochondrial energetic efficiency, or a reduced capacity to dynamically transport oxygen and nutrients into the mitochondria, and addressing these questions is an active area of research. Further research in humans will require use of new “omics” technologies, progress in neuroimaging techniques that permit energy production assessment, and visualization of molecules critical for energetic metabolism, as well as proxy biomarkers of muscle perfusion.
Keywords: Mitochondria, Aging, Skeletal muscle, Sarcopenia, Branched chain amino acids, Muscle quality
Ageing, body composition, and muscle quality
Ageing research has substantially advanced by characterizing longitudinal trajectories of ageing phenotypes, although the mechanisms that underlie these changes and the basis for the observed heterogeneity across individuals remain largely unexplored. Longitudinal studies have shown that the ageing process is associated with loss of muscle strength, muscle mass, and aerobic capacity.1, 2 The loss of lean body mass begins around age 30, which then progressively accelerates in older men and women. However, the decline in muscle strength observed in epidemiological studies is greater than what is expected given the magnitude of changes in lean body mass, suggesting that ageing is associated with impaired muscle biomechanical quality (strength/mass).1, 2 This notion is further supported by recent studies, which have observed that low muscle strength is an accurate, independent predictor of gait performance, response to exercise interventions, and mobility disability, while muscle mass is only marginally associated with these outcomes.3, 4, 5 Interestingly, the Baltimore Longitudinal Study of Aging revealed that visceral obesity, nerve conduction velocity, and performance in cognitive tests relative to executive function were the strongest predictors of muscle quality in older persons.2 A subsequent study further demonstrated that the difference between men and women in the rate of muscle quality decline is fully accounted for by differences in percent body fat.6 Fat accumulation is a phenotypic manifestation of metabolic impairment, especially when fat accumulates in visceral compartments, such as in the abdomen, liver, or muscle.7
Amino acid uptake, mitochondrial function, and muscle quality
In order to better understand the mechanisms by which muscle quality declines with ageing, a matched (by sex, age, and height) nested case–control study was performed within the Baltimore Longitudinal Study of Aging, characterizing the plasma metabolic profile associated with low vs. high muscle quality in participants that were 60+ years old.8 In this study, muscle quality was defined as the ratio of muscle torque (assessed by isokinetic dynamometry) to muscle mass, which was estimated from the muscle cross‐sectional area of the mid‐thigh acquired by computed tomography. Using a targeted metabolomics approach, participants with low muscle quality presented significantly higher plasma concentrations of isoleucine and leucine, suggesting that low muscle quality is characterized by impaired transport of amino acids, especially branched chain amino acids (BCAAs), across the muscle cell membrane (Figure 1A). Further validating this hypothesis, BCAA concentrations in biopsy specimens collected from the vastus lateralis were lower in participants with low muscle quality compared with those with high muscle quality (unpublished results, Figure 1B). Reduced muscle BCAA uptake has serious consequences in regulating protein synthesis, as BCAAs activate mTOR, which is the master regulator for nutrient sensing. When BCAA levels are sufficient, mTOR is activated and causes an increase in p70S6K phosphorylation, subsequently activating the S6 ribosomal protein, thereby inducing protein synthesis.9, 10 In contrast, at low BCAA concentrations, mTOR recognizes the limited substrate availability for protein synthesis/recycling and inhibits p70S6K phosphorylation, hindering normal protein recycling, and thus allowing ‘wear and tear’ protein damage to accumulate. Additionally, BCAA supplementation can increase the expression of sirtuin 1 (SIRT1) and other genes involved in oxidative phosphorylation, such as peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha (PGC‐1α), nuclear respiratory factor‐1 (NRF1), and mtDNA transcription factor A (TFAM). Cumulatively, these effects result in increased mtDNA content and mitochondrial activity, suggesting a corresponding increase in mitochondrial biogenesis.11 Conversely, low BCAA levels are associated with impaired mitochondrial biogenesis and reduced energy production.9, 10 Finally, isoleucine and leucine catabolism in muscle causes increased levels of NADH and FADH2, which are essential cofactors for ATP synthesis.8 Incidentally, glycerophospholipids—specifically a subset of phosphatidylcholines—were significantly lower levels in participants with low muscle quality compared with controls.
Figure 1.
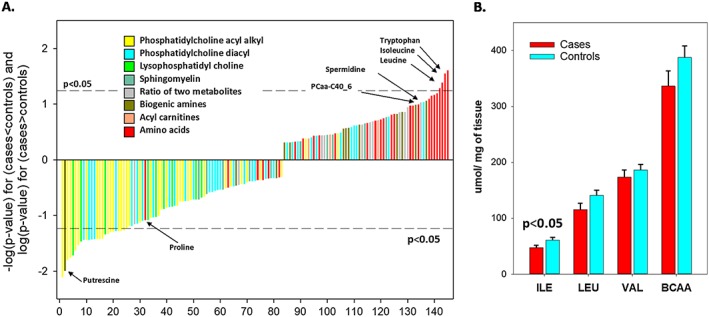
Low muscle quality is characterized by impaired transport of amino acids, especially branched chain amino acids. (A) Metabolomics plasma metabolites are ordered by expression differences between age, sex, and height matched pairs of cases (low muscle quality) and controls (high muscle quality) from the Baltimore Longitudinal of Aging. Participants with low muscle quality presented higher plasma concentrations of most amino acids, particularly isoleucine and leucine, compared with the control group (adapted from Moaddel et al.8) (N= 79 cases and 79 controls). (B) Concentration of BCAAs in biopsy specimens collected from the vastus lateralis was lower in participants with low muscle quality compared with those with high muscle quality (unpublished results) (N= 9 cases and 9 controls). ILE, isoleucine; LEU, leucine; VAL, valine; BCAA, branched chain amino acids.
In summary, these findings demonstrate that impaired transport of BCAAs and other amino acids into muscle negatively affects protein recycling and energy availability, which often corresponds to alterations in phospholipid metabolism. The exact reasons for why amino acid uptake is reduced in older persons with low muscle quality are unknown, and further work is required to identify putative intervention/therapeutic targets. Physiologically, amino acid uptake in muscle cells is regulated by three fundamental mechanisms: insulin signalling, BCAA (primarily leucine) blood concentration, and physical activity.12, 13, 14 Previous studies have also suggested that these ‘anabolic’ signals cause increased amino acid entry by dynamically enhancing muscle perfusion, and all three signals exhibit a dose–response relationship that is steeper in younger than in older persons.15 In other words, older persons tend to develop an ‘anabolic resistance’ to the three stimuli. Since muscle perfusion adaptation is mediated by endothelial reactivity, which is hampered by a pro‐inflammatory state, this hypothesis can also explain why inflammation is such a strong correlate and predictor of age‐related sarcopenia.16, 17, 18 In accordance with this hypothesis, we found that the protein intake that is required to maintain good muscle strength with ageing is increased in the presence of high interleukin‐6 levels.19 Also, consistent with this hypothesis, previous studies have shown that insulin resistance and diabetes are associated with an accelerated decline in muscle strength, mass, and quality with ageing20, 21, 22, 23 and also that muscle mass affects insulin sensitivity.24, 25 Surprisingly, in our metabolomics study presented above, blood leucine and isoleucine levels significantly correlated with BMI but not with insulin.
Mitochondrial oxidative capacity and metabolic flexibility
Multiple distinct and pre‐packaged adaptive strategies and signalling pathways in muscle allow boosting of energy production when demand increases, such as during prolonged exercise.26, 27, 28 For example, studies in human and animal models have revealed that the ability for the mitochondrial electron transport system to respond to increased demand declines with ageing, and this may account for some of the observed decrease in fitness in older persons.29, 30, 31 We have recently reported that when permeabilized muscle fibres are exposed to incremental concentrations of ADP, the ability of the mitochondria in vitro to produce energy as ATP increases linearly in samples from younger individuals, while these levels tend to remain stable above a certain threshold in samples from older individuals.31
These findings suggest that during ageing, mitochondria lose the ability to produce energy during maximal efforts but not when the energetic demand is lower. The loss in metabolic flexibility in skeletal muscle also occurs in the early stages of type 2 diabetes in nondiabetic adults.32 This impaired mitochondrial function could be due to inadequate perfusion or reduced muscle blood flow, resulting in lower oxygen delivery in skeletal muscle and diminished aerobic capacity.33 This hypothesis is interesting because it connects both energetic and anabolic deficits to the same mechanism. In accordance with this hypothesis, we have recently shown that muscle mitochondrial capacity is a strong independent correlate of lower extremity performance, especially in tasks that require walking at fast speeds or for long distances, and that long‐term exposure to some adiposity‐related factors may adversely affect mitochondrial function in humans.34 We have also found that muscle strength significantly mediates the relationship of mitochondrial function with walking performance.28 Overall, these results indicate that oxidative phosphorylation is progressively impaired with ageing; it is unclear whether this is because the number of mitochondria per muscle volume is diminished, the intrinsic capacity of mitochondria to generate ATP is impaired, or the availability of oxygen and nutrients at different levels of effort is compromised. Future studies that rely on protein content assessment, high resolution microscopic examination of muscle specimens, and techniques for dynamically assessing muscle perfusion during contraction are needed to address this question.
Mitochondrial damage, autophagy, and repair
Oxidative stress and defective mitophagy (mitochondrial autophagy) are potentially involved in the decline of muscle quality with ageing and need to be considered. Dysfunctional mitochondria are characterized by reduced oxidative phosphorylation efficiency and excessive production of reactive oxygen species, which oxidize and damage macromolecules.35 Normally, stressed/damaged mitochondria can be repaired by alternating cycles of fission and fusion, but if this mechanism is insufficient, defective mitochondria then can be removed by mitophagy or induced cellular apoptosis.36, 37
The hypothesis that oxidative stress causes degenerative changes in tissues that are highly metabolically active, such as the brain and the muscle, has been proposed for many years.38, 39 Reactive oxygen species can damage macromolecules in mitochondria, such as the oxidation of cardiolipins and mitochondrial DNA or damage of enzymatic and contractile proteins, which further accelerate energetic dysfunction and functional damage.39 However, evidence in support of this theory is limited, and some experiments in animal models have even shown that inducing oxidative stress can improve function.40 A recent study demonstrated that, when mitochondrial function of skeletal muscle samples is stimulated by increasing ADP concentration in vitro, the compensatory antioxidant capacity is hindered in muscle tissue from older adults compared with younger persons and may negatively affect respiration at high ADP levels.29 Oxidative stress may also affect satellite cells or muscle stem cell pools in skeletal muscle. In mice, satellite cells are able to reprogram their daily rhythmic functions to adapt to stress, suggesting that these cells might be evolutionarily prepared to reprogram their functions in response to metabolic changes, such as caloric restriction or exercise.41
There is still limited data suggesting that defective autophagy has a role in sarcopenia with ageing. In mice, defective autophagy has been related to neuromuscular dysfunction and the mechanisms of cyclic denervation and reinnervation that occurs with ageing,42 but this hypothesis has never been confirmed in humans. Reestablishment of autophagy has been shown to restore regenerative functions in old satellite cells in mice.43 Therefore, it seems that autophagy is essential to maintain the quiescent state in satellite cells to support muscle regeneration in sarcopenia. Overall, the role of oxidative stress and mitophagy in the decline of muscle quality remains largely unexplored and there is robust, ongoing research in this area.
Mitochondrial function and defects in the neuromuscular junction.
Defective mitochondrial function has been studied in regard to the neuromuscular junction (NMJ) remodelling that occurs with ageing, producing cycles of denervation–innervation that lead to motor unit loss, specifically in type II fibres, as well as muscle fibre atrophy.44 However, it is not clear whether these changes in the NMJ precede or follow the observed decline in muscle mass and strength that is observed with ageing. Some studies have reported altered mitochondria morphology in the plaque region of the NMJ that produce increased levels of oxidative stress, decreased enzymatic activity and ATP production, and impaired calcium buffering.45 The combination of these biological changes may have a strong negative impact on excitation–contraction coupling and eventually lead to the loss of motor units. In sarcopenic rats with altered NMJ integrity, the expression of genes and proteins implicated in mitochondrial energy metabolism is downregulated.46 Recent studies have suggested that overexpression of PGC‐1α, a transcription factor that promotes mitochondrial biogenesis, could help to maintain NMJ integrity during ageing.47, 48 Mice lacking autophagy and ubiquitin proteasome proteins, such as the ubiquitin ligases muscle atrophy f‐box (Atrogin1/MAFbx) and muscle ring finger‐1 (MuRF1), are resistant to atrophy induced by denervation.49, 50 Interventions, such as caloric restriction and exercise, seem to preserve the morphology and integrity of the NMJ with ageing in mice.51, 52
Overall, low muscle quality seems to be associated with (i) metabolic impairments that lead to reduced incorporation of the three major BCAAs, which are used by muscle as energy sources and are associated with muscle strength and endurance; (ii) fat accumulation in muscle tissue that ultimately leads to architectural disruption and loss of function; and (iii) high concentration of lipid species that are associated with impaired mitochondrial function and unrecycled mitochondrial proteins, potentially due to defective mitophagy or proteostasis. The extent and complexity to which these mechanisms are interconnected is unknown and should be examined in future studies. In addition, other factors that impact ageing muscle could also modulate mitochondrial function, such as (i) defects in the NMJ that leads to myofiber denervation—due to reduced capacity in motor neurons to reinnervate muscle fibres—consequently causing fibres to become atrophied; (ii) the age‐associated decline in the satellite cell pool, reducing muscle regeneration after injury; and (iii) ‘inflammaging’, the chronic low‐grade inflammation observed in older persons.
Conclusions
An exciting component of gerontology research relates to understanding the changes in energetics that occur as individuals age, especially regarding the biological underpinnings of ageing, such as the role of nutritional intake and the multifaceted relationships to mitochondrial function. The biological pathways that lead to accelerated decline in muscle quality and muscle strength with ageing are actively being investigated and explored, with many questions unanswered. Altered concentrations of specific metabolites could have important translational implications for early identification of subjects that are at high risk for developing sarcopenia, as well as identifying targets for new preventive strategies and treatments.
Conflict of interest
Marta Gonzalez‐Freire, Fatemeh Adelnia, Ruin Moaddel, and Luigi Ferrucci declare that there are no conflicts of interest regarding this work.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publication in the Journal of Cachexia, Sarcopenia and Muscle.53 This work was supported by the National Institutes of Health (NIH) and the Intramural Research Program (IRP), National Institute on Aging (NIA). The authors wish to thank Adam Cornish for editing the manuscript and for his suggestions that substantially improved the quality and readability of this work.
Gonzalez‐Freire, M. , Adelnia, F. , Moaddel, R. , and Ferrucci, L. (2018) Searching for a mitochondrial root to the decline in muscle function with ageing. Journal of Cachexia, Sarcopenia and Muscle, 9: 435–440. doi: 10.1002/jcsm.12313.
References
- 1. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006. Oct;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 2. Moore AZ, Caturegli G, Metter EJ, Makrogiannis S, Resnick SM, Harris TB, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr So 2014. Feb;62:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sipilä S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol 1994. Jul;14:433–442. [DOI] [PubMed] [Google Scholar]
- 4. Sipilä S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand 1996. Apr;156:457–464. [DOI] [PubMed] [Google Scholar]
- 5. Rantanen T, Guralnik JM, Izmirlian G, Williamson JD, Simonsick EM, Ferrucci L, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil 1998. Jul‐Aug;77:299–305. [DOI] [PubMed] [Google Scholar]
- 6. Fabbri E, Chiles Shaffer N, Gonzalez‐Freire M, Shardell MD, Zoli M, Studenski SA, et al. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle 2017. Jun;8:490–499, Epub 2017 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006. Dec 14;444:881–887. [DOI] [PubMed] [Google Scholar]
- 8. Moaddel R, Fabbri E, Khadeer MA, Carlson OD, Gonzalez‐Freire M, Zhang P, et al. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2016. Oct;71:1266–1272, Epub 2016 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valerio A, D'Antona G, Nisoli E. Branched‐chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging (Albany NY) 2011. May;3:464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zanchi NE, Lancha AH Jr. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 2008. Feb;102:253–263, Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- 11. D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched‐chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle‐aged mice. Cell Metab 2010. Oct 6;12:362–372. [DOI] [PubMed] [Google Scholar]
- 12. Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr 2006. Jan;136:277S–280S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brooks DC, Bessey PQ, Black PR, Aoki TT, Wilmore DW. Insulin stimulates branched chain amino acid uptake and diminishes nitrogen flux from skeletal muscle of injured patients. J Surg Res 1986. Apr;40:395–405. [DOI] [PubMed] [Google Scholar]
- 14. Blomstrand E, Saltin B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am J Physiol Endocrinol Metab 2001. Aug;281:E365–E374. [DOI] [PubMed] [Google Scholar]
- 15. Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care 2018. Apr;24:124–130. [DOI] [PubMed] [Google Scholar]
- 16. Mauvais‐Jarvis F. Novel link between inflammation, endothelial dysfunction, and muscle insulin resistance. Diabetes 2013. Mar;62:688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis 2013. Dec;23:S44–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium‐dependent dilatation in humans. Circulation 2000. Aug 29;102:994–999. [DOI] [PubMed] [Google Scholar]
- 19. Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini A, Palli D, et al. Protein intake and muscle strength in older persons: does inflammation matter? J Am Geriatr Soc 2013. Mar;60:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalyani RR, Tra Y, Egan JM, Ferrucci L, Brancati F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J Nutr Health Aging 2014;18:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalyani RR, Tra Y, Yeh H‐C, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc 2013;61:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015;38:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci 2012;67(1)74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramachandran R, Gravenstein KS, Metter EJ, Egan JM, Ferrucci L, Chia CW. Selective contribution of regional adiposity, skeletal muscle, and adipokines to glucose disposal in older adults. J Am Geriatr Soc 2012;60:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogasawara R, Fujita S, Hornberger TA, Kitaoka Y, Makanae Y, Nakazato K, et al. The role of mTOR signaling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep 2016. Aug 9;6:31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilegaard H, Saltin B, Darrell Neufer P. Exercise induces transient transcriptional activation of the PGC‐1α gene in human skeletal muscle. J Physiol 2003. Feb 1;546:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr 2006. Feb;136:533S–537S. [DOI] [PubMed] [Google Scholar]
- 28. Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 2017;16:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJC. Age‐associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep 2018. Mar 13;22:2837–2848. [DOI] [PubMed] [Google Scholar]
- 30. Stolle S, Ciapaite J, Reijne AC, Talarovicova A, Wolters JC, Aguirre‐Gamboa R, et al. Running‐wheel activity delays mitochondrial respiratory flux decline in aging mouse muscle via a post‐transcriptional mechanism. Aging Cell 2018. Feb;17:e12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez‐Freire M, Scalzo P, D'Agostino J, Moore ZA, Diaz‐Ruiz A, Fabbri E, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 2018. Apr;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fabbri E, Chia CW, Spencer RG, Fishbein KW, Reiter DA, Cameron D, et al. Insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31P‐magnetic resonance spectroscopy in participants without diabetes from the Baltimore Longitudinal Study of Aging. Diabetes 2017;66:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AlGhatrif M, Zane A, Oberdier M, Canepa M, Studenski S, Simonsick E, et al. Lower mitochondrial energy production of the thigh muscles in patients with low‐normal ankle‐brachial index. J Am Heart Assoc 6:e006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi S, Reiter DA, Shardell M, Simonsick EM, Studenski S, Spencer RG, et al. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2016;71:1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Picca A, Calvani R, Bossola M, Allocca E, Menghi A, Pesce V, et al. Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia. Biol Chem 2018. Feb 19. pii: /j/bchm.ahead‐of‐print/hsz‐2017–0331/hsz‐2017–0331.xml; [Epub ahead of print]399:421–436. [DOI] [PubMed] [Google Scholar]
- 36. Drake JC, Yan Z. Mitophagy in maintaining skeletal muscle mitochondrial proteostasis and metabolic health with ageing. J Physiol 2017. Oct 15;595:6391–6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim Y, Triolo M, Hood DA Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev 2017;2017:3165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013. Jun;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gonzalez‐Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, et al. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci 2015. Nov;70:1334–1342, Epub 2015 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II, et al. Mitochondrial ROS regulate oxidative damage and mitophagy but not age‐related muscle fiber atrophy. Sci Rep 2016. Sep 29;6:33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solanas G, Peixoto FO, Perdiguero E, Jardí M, Ruiz‐Bonilla V, Datta D, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 2017. Aug 10;170:678, e20–692. [DOI] [PubMed] [Google Scholar]
- 42. Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 2014. Sep 11;8:1509–1521, Epub 2014 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. García‐Prat L, Martínez‐Vicente M, Perdiguero E, Ortet L, Rodríguez‐Ubreva J, Rebollo E, et al. Autophagy maintains stemness by preventing senescence. Nature 2016. Jan 7;529:37–42. [DOI] [PubMed] [Google Scholar]
- 44. Gonzalez‐Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci 2014. Aug 11;6:208, eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia ML, Fernandez A, Solas MT. Mitochondria, motor neurons and aging. J Neurol Sci 2013. Jul 15;330:18–26. [DOI] [PubMed] [Google Scholar]
- 46. Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cel Biol 2013;33:194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gouspillou G, Picard M, Godin R, Burelle Y, Hepple RT. Role of peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha (PGC‐ 1alpha) in denervation‐induced atrophy in aged muscle: facts and hypotheses. Longev Healthspan 2013;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arnold AS, Gill J, Christe M, Ruiz R, McGuirk S, St‐Pierre J, et al. Morphological and functional remodeling of the neuromuscular junction by skeletal muscle PGC‐1alpha. Nat Commun 2014;5:3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001;294:1704–1708. [DOI] [PubMed] [Google Scholar]
- 50. Furlow JD, Watson ML, Waddell DS, Neff ES, Baehr LM, Ross AP, et al. Altered gene expression patterns in muscle ring finger 1 null mice during denervation‐ and dexamethasone‐induced muscle atrophy. Physiol Genomics 2013. Dec 1;45:1168–1185, Epub 2013 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stockinger J, Maxwell N, Shapiro D, deCabo R, Valdez G. Caloric restriction mimetics slow aging of neuromuscular synapses and muscle fibers. J Gerontol A Biol Sci Med Sci 2017. Dec 12;73:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, et al. Attenuation of age‐related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A 2010. Aug 17;107(33):14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


