Abstract
Non‐inferiority in the cumulative castration rate of the 3‐month formulation of degarelix compared with the 3‐month formulation of goserelin was evaluated in subjects with prostate cancer. A phase III, open‐label, parallel‐arm study was carried out. An initial dose of 240 mg degarelix or 3.6 mg goserelin was given s.c.; after day 28, a maintenance dose of 480 mg degarelix or 10.8 mg goserelin was given once every 84 days. Non‐inferiority in castration rate and safety of degarelix to goserelin were evaluated. The primary end‐point was the cumulative castration rate from day 28 to day 364 and the non‐inferiority margin was set to be 10%. A total of 234 subjects with prostate cancer were randomized to the degarelix group (n = 117) and the goserelin group (n = 117). The cumulative castration rate was 95.1% in the degarelix group and 100.0% in the goserelin group. As there were no events in the goserelin group, an additional analysis was carried out using 95% confidence intervals of the difference in the proportion of subjects with castration. Analyses indicated the non‐inferiority of the 3‐month formulation of degarelix to goserelin. Degarelix showed more rapid decreases in testosterone, luteinizing hormone, follicle stimulating hormone, and prostate‐specific antigen levels compared with goserelin. The most common adverse events in the degarelix group were injection site reactions. Non‐inferiority of the 3‐month formulation of degarelix to goserelin was shown for testosterone suppression. The 3‐month formulation of degarelix was also found to be tolerated as an androgen deprivation therapy for patients with prostate cancer. This trial was registered with ClinicalTrials.gov (identifier NCT01964170).
Keywords: androgen deprivation therapy, degarelix, goserelin, non‐inferiority, prostate cancer
Abbreviations
- ADR
adverse drug reaction
- ADT
androgen deprivation therapy
- AE
adverse event
- C‐FAS
completers full analysis set
- CI
confidence interval
- FAS
full analysis set
- FSH
follicle stimulating hormone
- GnRH
gonadotropin releasing hormone
- LH
luteinizing hormone
- PSA
prostate‐specific antigen
- SAE
serious adverse event
- SAF
safety analysis set
1. INTRODUCTION
Prostate cancer is the second most common cancer, accounting for 15% of all cancers in men; an estimated 1.1 million men worldwide were diagnosed in 2012.1 Androgen deprivation therapy is the primary systemic therapy in advanced disease or as neoadjuvant/concomitant/adjuvant therapy in combination with radiation in localized or locally advanced prostate cancers.2 For prognosis, a castrate level of serum testosterone of <0.5 ng/mL is recommended.2, 3 International guidelines recommend the use of GnRH agonists or GnRH antagonists as possible alternatives for ADT.2, 4 However, GnRH agonists have been shown to associate with an initial testosterone surge which, in advanced disease, can produce a flare in symptoms and metastatic manifestations.5 The European Association of Urology guidelines recommend concomitant anti‐androgens such as bicalutamide for selected patients in the initial 2 weeks of GnRH agonist therapy to mitigate flare effects.4
Degarelix, a GnRH antagonist newly developed by Ferring Pharmaceuticals, has been developed to achieve effective long‐term medical castration without the risk of testosterone surge and its associated flare.6, 7, 8 After s.c. administration, degarelix immediately forms a gel depot at the injection site, leading to sustained release of the drug into the circulation. The once‐monthly formulation of degarelix has been approved in the USA (2008), Europe (2009), and Japan (2012). Previous phase III studies showed degarelix was superior to leuprolide, a GnRH agonist, in the control of PSA and alkaline phosphatase in patients with prostate cancer.9, 10 In 2015, a post‐marketing surveillance of Japanese patients with prostate cancer showed component ratios of patients treated with degarelix were 29.6% in localized cancer, 17.2% in locally advanced cancer, and 52.5% in metastatic cancer, indicating degarelix is increasingly used in advanced cases.11
Three‐month formulations of GnRH agonists have been launched and are commonly used in patients with prostate cancer in clinical practice. A phase II study for the 3‐month dosing regimen of degarelix was undertaken in Japanese prostate cancer patients without a history of endocrine treatment. Patients were randomized to treatment with degarelix given s.c. at a maintenance dose of 360 mg or 480 mg every 84 days for 12 months after receiving an initial dose of 240 mg. The cumulative probability of a serum testosterone level of ≤0.5 ng/mL was 88.3% and 97.2% in the 360 mg and 480 mg groups, respectively. Both 3‐month dosing regimens were well tolerated and the optimal clinical dosage for phase III trials was determined to be 480 mg.12 These findings were comparable to those from the Japanese phase II study of the 1‐month regimen of degarelix. Following these clinical trials, a phase III study for the 3‐month formulation of degarelix was carried out in Japanese subjects with prostate cancer with an aim to evaluate non‐inferiority in the cumulative castration rate of the 3‐month formulation of degarelix compared with the 3‐month formulation of goserelin, a GnRH agonist.
2. MATERIALS AND METHODS
2.1. Study design
An open‐label, parallel‐arm study comparing the 3‐month formulation of degarelix with the 3‐month formulation of goserelin was carried out in prostate cancer subjects with coverage across 46 sites in Japan. This study consisted of two parts (Figure 1). The primary end‐point for part 1 was the cumulative castration rate based on testosterone levels from 4 to 52 weeks after treatment. In part 1, eligible patients were randomized to either the degarelix or the goserelin group, and non‐inferiority based on the cumulative castration rate, proportion of subjects with castration, chronological changes in serum testosterone, LH, FSH, and PSA, and safety were evaluated. In part 2, the long‐term safety of the maintenance dose of degarelix treatment was assessed in subjects who had completed study part 1 in the degarelix group. This report is based on data as of the cut‐off date of December 25, 2015.
Figure 1.
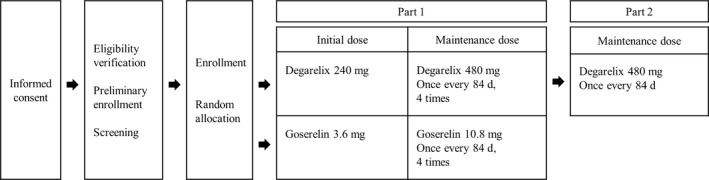
Design of this phase III study of Japanese prostate cancer patients treated with degarelix or goserelin
This study was approved by the institutional review board at each study site. Written informed consent was obtained from all subjects before enrollment. This study was undertaken in accordance with the ethical principles of the Declaration of Helsinki and the ethical guidelines for clinical studies by the Ministry of Health, Labour and Welfare of Japan and in compliance with Good Clinical Practice guidance by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (NCT01964170).
2.2. Subjects
The target number of subjects was set to be 230 in total (115 per group). Subjects who met the following inclusion criteria were enrolled: histopathological diagnosis of prostate cancer, judged to be in need of ADT, serum testosterone >2.2 ng/mL, an ECOG performance status ≤2, serum PSA ≥2 ng/mL, age ≥20 years, and had submitted written informed consent. Subjects who met the following criteria were excluded: a history of ADT against prostate cancer, scheduled for curative therapy such as total prostatectomy or radiation within 12 months, and previous treatment with degarelix or goserelin.
2.3. Intervention
Before starting study part 1, subjects were randomly allocated into a degarelix or goserelin group using a minimization method of adjusting age, cancer stage, pretreatment, and serum PSA. In the degarelix group, an initial dose of 240 mg (40 mg/mL) degarelix was s.c. administered; after day 28, a maintenance dose of 480 mg (60 mg/mL) was given once every 84 days. In the goserelin group, an initial dose of 3.6 mg goserelin was s.c. administered; after day 28, a maintenance dose of 10.8 mg was given once every 84 days. In part 2, a maintenance dose of 480 mg degarelix was given once every 84 days. The treatment period started on the date of initial administration of the study drug and lasted until the date of the decision made for treatment discontinuation. Discontinuation was decided either by predefined discontinuation criteria or by the sponsor's decision according to the recommendation from the data monitoring committee. The following drugs were prohibited during the study: GnRH agonists, GnRH antagonists, anti‐androgen drugs, estrogens, 5α‐reductase inhibitors, and antitumor drugs. The following treatments for prostate cancer were prohibited during the treatment period: surgery, radiotherapy, thermotherapy, and high intensity focused ultrasound. Concomitant administration of bicalutamide in the goserelin group from day 0 up to day 14 was allowed in subjects with renal dysfunction, cord compression or urinary obstruction or in subjects having the possibility of these problems.
2.4. End‐points
The primary end‐point was the cumulative castration rate of degarelix compared to goserelin. The non‐inferiority comparison was based on the cumulative castration rate from day 28 to day 364. Castration was defined as a decreased serum testosterone level to ≤0.5 ng/mL.4 The cumulative castration rate was calculated using the Kaplan–Meier method and the 95% CI was estimated using the Greenwood Formula. The non‐inferiority margin was set to be 10% and non‐inferiority was defined as the lower limit of 95% CI of the difference in the cumulative castration rate between degarelix and goserelin exceeding −10%. Secondary end‐points were as follows: (i) proportion of subjects with castration from day 28 to day 364; (ii) proportion of subjects with castration at day 3, 7, and 28; (iii) chronological changes in serum testosterone, LH, FSH, and PSA; and (iv) proportion of subjects with serum PSA relapse. Levels of serum testosterone, serum PSA, serum LH, and serum FSH were measured by SRL (Tokyo, Japan) in accordance with Good Laboratory Practice. Serum testosterone levels were measured by a centralized measurement using a validated method for low‐range detection of testosterone levels.
2.5. Safety
For the safety analysis, the incidence of AEs, SAEs, and ADRs were collected and graded according to Common Terminology Criteria for Adverse Events version 4.0.
2.6. Statistics
The target sample size for part 1 of the study was calculated to be 230 subjects (115 per group) after consideration of the following conditions: an estimated cumulative castration rate of 95% based on serum testosterone levels from day 28 to day 364 for both groups, a 10% non‐inferiority margin, a 15% discontinuation rate, a confidence coefficient of 0.95, and 90% power. The non‐inferiority margin of 10% was based on the phase III study of a 1‐month regimen of degarelix,8 deemed reasonable from a clinical perspective.
The FAS was defined as all subjects who were diagnosed with prostate cancer, received at least one dose of the study drug, and had post‐dose data of at least one efficacy variable (either primary or secondary). The C‐FAS was defined as subjects who had completed the study until day 364 or had serum testosterone ≥0.5 ng/mL during the period from day 28 to day 364. The SAF was defined as all subjects who had received the study drug.
Midway through the study, the possibility of a “no event” (no cases with serum testosterone levels >0.5 ng/mL) occurrence in the goserelin group during part 1 became evident. As there is no appropriate method to calculate the CI of cumulative castration rates for a no event factor, verification of non‐inferiority using a proportion of the subjects with castration was considered. Unlike in the case using the cumulative castration rate, there are practical methods to calculate the CI of the proportion of castration subjects. Considering the verification of the robustness of the result, a 95% CI of the difference in the proportion of the subjects with castration from day 28 to day 364 between the two groups was carried out for the FAS using 13 different methods13 and non‐inferiority was evaluated with respect to each method.
The AEs and ADRs were categorized according to the Preferred Terms of the MedDRA and their frequency tabulated.
3. RESULTS
3.1. Subject disposition
Two hundred and thirty‐four subjects were randomly allocated to the degarelix group (n = 117) or the goserelin group (n = 117; Figure 2). Nineteen subjects in the degarelix group and 23 subjects in goserelin group withdrew from part 1 of the study. The most common reason for discontinuation was an AE (n = 8) in the degarelix group and progressive disease (n = 10) in the goserelin group. Ninety‐eight subjects in the degarelix group and 94 subjects in the goserelin group completed part 1. Eighty subjects in the degarelix group were enrolled in part 2, 65 subjects of which continued until the data cut‐off date of December 25, 2015. The FAS and SAF in part 1 consisted of 117 subjects from each group. The C‐FAS in part 1 was comprised of 100 subjects in the degarelix group and 94 subjects in the goserelin group. The SAF in part 2 consisted of 80 subjects.
Figure 2.
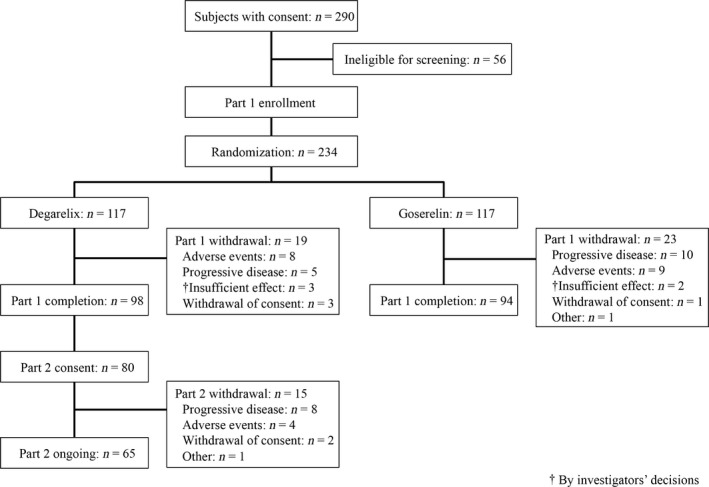
Flow diagram for study participants, consisting of Japanese prostate cancer patients treated with degarelix or goserelin
The mean duration of exposure (SD) at the cut‐off date including part 1 and 2 studies was 597.8 (196.8) in the degarelix group. Bicalutamide as flare protection was concomitantly given to 34 patients (29.1%) in the goserelin group.
3.2. Subjects characteristics
The mean age was 75.5 years in the degarelix group and 75.9 years in the goserelin group (Table 1). Nine (7.7%) subjects in the degarelix group and 12 (10.3%) subjects in the goserelin group had received pretreatments. For the degarelix group, pretreatments included watchful waiting (n = 5), radiation (n = 3), total prostatectomy (n = 2), and neoadjuvant/adjuvant therapies (n = 1). For the goserelin group, pretreatments included watchful waiting (n = 9), radiation (n = 3), and total prostatectomy (n = 1). Sixty‐two (53.0%) subjects in each group were classified as having localized cancer. Mean serum testosterone levels were 4.98 ng/mL in the degarelix group and 4.94 ng/mL in the goserelin group. Mean serum PSA levels were 66.04 ng/mL in the degarelix group and 61.08 g/mL in the goserelin group. No apparent differences between groups were found for age, rate of pretreatment, cancer stage, serum testosterone, or PSA level.
Table 1.
Characteristics of in Japanese subjects with prostate cancer (n = 234)
| Degarelix | Goserelin | |
|---|---|---|
| FAS, n | 117 | 117 |
| Age, years; mean ± SD | 75.5 ± 6.1 | 75.9 ± 5.9 |
| Classification, n (%) | ||
| <75 | 41 (35.0) | 42 (35.9) |
| ≥75 | 76 (65.0) | 75 (64.1) |
| Height, cm; mean ± SD | 163.48 ± 6.27 | 163.13 ± 5.64 |
| Body weight, kg; mean ± SD | 62.83 ± 10.05 | 62.93 ± 8.18 |
| ECOG PS, n (%) | ||
| 0 | 110 (94.0) | 114 (97.4) |
| 1 | 6 (5.1) | 3 (2.6) |
| 2 | 1 (0.9) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) |
| Pretreatment, n (%) | 9 (7.7%) | 12 (10.3) |
| Total prostatectomy | 2 (1.7) | 1 (0.9) |
| Radiation | 3 (2.6) | 3 (2.6) |
| Neoadjuvant/adjuvant therapy | 1 (0.9) | 0 (0.0) |
| Watchful waiting | 5 (4.3) | 9 (7.7) |
| Cancer stage, n (%) | ||
| Localized | 62 (53.0) | 62 (53.0) |
| Locally advanced | 30 (25.6) | 33 (28.2) |
| Metastasized | 23 (19.7) | 21 (17.9) |
| Unclassifiable | 2 (1.7) | 1 (0.9) |
| Serum testosterone level, ng/mL; mean ± SD | 4.98 ± 1.41 | 4.94 ± 1.59 |
| Classification, n (%) | ||
| <3.5 ng/mL | 16 (13.7) | 20 (17.1) |
| ≥3.5 to <5 ng/mL | 50 (42.7) | 45 (38.5) |
| ≥5 ng/mL | 51 (43.6) | 52 (44.4) |
| Serum PSA level, ng/mL; mean ± SD | 66.04 ± 140.33 | 61.08 ± 121.28 |
| Classification, n (%) | ||
| <10 ng/mL | 42 (35.9) | 43 (36.8) |
| ≥10 ng/mL to <20 ng/mL | 26 (22.2) | 23 (19.7) |
| ≥20 ng/mL to <50 ng/mL | 20 (17.1) | 21 (17.9) |
| ≥50 ng/mL | 29 (24.8) | 30 (25.6) |
FAS, full analysis set; PS, performance status; PSA, prostate‐specific antigen.
3.3. Primary end‐point
Cumulative castration rates from day 28 to day 364 in the FAS were 95.1% and 100.0% in the degarelix group and the goserelin group, respectively (Figure 3). The difference in the castration rate between groups was estimated to be −4.9%. Additional analyses using the proportion of subjects with castration from day 28 to day 364 showed that the lower limit of 95% CI of the difference between groups exceeded −10% of the predefined non‐inferiority margin in 11 out of 13 methods (Table 2). After an evaluation of the accuracy for each calculation method considering coverage probability, we concluded that the results indicated the non‐inferiority of the 3‐month formulation of degarelix to goserelin.
Figure 3.
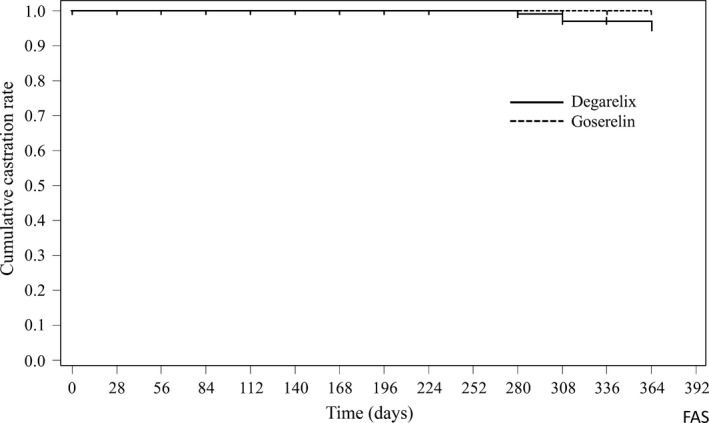
Kaplan–Meier curve of the cumulative castration rate in the full analysis set of Japanese prostate cancer patients treated with degarelix (n = 117) or goserelin (n = 117)
Table 2.
Confidence intervals for differences in the proportion of prostate cancer subjects, treated with degarelix (n = 117) or goserelin (n = 117), with castration
| No. | Statistical method | Difference in proportion of subjects with castration (95%CI) | Non‐inferiority |
|---|---|---|---|
| 1 | Wald, single parameters | −4.3 (−7.94, −0.61) | Yes |
| 2 | Wald, multiple parameters | −4.3 (−8.80, 0.25) | Yes |
| 3 | Beal's Haldane | −4.3 (−7.83, −0.55) | Yes |
| 4 | Beal's Jeffreys‐Perks | −4.3 (−8.16, −0.22) | Yes |
| 5 | Mee | −4.3 (−9.62, −0.97) | Yes |
| 6 | Miettinen & Nurminen | −4.3 (−9.63, −0.96) | Yes |
| 7 | EXACT | −4.3 (−17.19, 8.84) | No |
| 8 | EXACT: FM score | −4.3 (−9.84, −0.72) | Yes |
| 9 | Newcombe | −4.3 (−9.62, −0.23) | Yes |
| 10 | Newcombe (continuity correction) | −4.3 (−10.18, 0.57) | No |
| 11 | Farrington–Manning | −4.3 (−8.01, −0.54) | Yes |
| 12 | Hauck–Anderson | −4.3 (−8.39, −0.16) | Yes |
| 13 | Agresti–Caffo | −4.3 (−8.46, 0.08) | Yes |
3.4. Secondary end‐points
The proportion of subjects with castration from day 28 to day 364 in the C‐FAS was 95.0% in the degarelix group and 100.0% in the goserelin group. For the FAS in the degarelix group, the proportion of subjects with castration were 99.1% at day 3, 100.0% at day 7, and 100.0% at day 28 (Table 3). In the goserelin group, the proportion of subjects with castration were 0% at day 3, 0% at day 7, and 100.0% at day 28. Figure 4 shows the changes in median serum testosterone, LH, FSH, and PSA rate of change in the FAS. In the degarelix group, median serum testosterone level decreased to a castration level of ≤0.5 ng/mL at day 3 and ranged under 0.2 ng/mL from day 7 to day 364 (Figure 4A). In the goserelin group, serum testosterone rapidly increased by 52.74% at day 3 and then decreased to a castration level at day 28 and remained at <0.2 ng/mL from day 28 to day 364. In the degarelix group, median serum LH levels had decreased by 92.5% at day 3 and median lowering rates ranged from 94.56% to 97.88% from day 28 to day 364 (Figure 4B). In the goserelin group, median serum LH levels had increased by 108.53% at day 3 and then decreased, and the median lowering rates ranged from 92.36% to 98.59% from day 28 to day 364. Median lowering rates of serum FSH from day 28 to day 364 ranged from 84.66% to 95.32% in the degarelix group and from 65.31% to 87.79% in the goserelin group (Figure 4C). Median lowering rates of serum PSA at day 3 were 18.02% in the degarelix group and 1.95% in the goserelin group, with similar transitions after day 56 (Figure 4D). The proportion of subjects with serum PSA relapse by day 364 were 2.6% in the degarelix group and 0.9% in the goserelin group, with no apparent differences between groups.
Table 3.
Proportion of prostate cancer subjects with castration
| Degarelix | Goserelin | |
|---|---|---|
| FAS, n | 117 | 117 |
| Day 3, n | 117 | 116 |
| Number of patient with castrationa | 116 | 0 |
| Proportion, % (95% CI) | 99.1 (95.3, 100.0) | 0 (0.0, 3.1) |
| Day 7, n | 117 | 117 |
| Number of patient with castration | 117 | 0 |
| Proportion, % (95% CI) | 100 (96.9, 100.0) | 0 (0.0, 3.1) |
| Day 28, n | 116 | 115 |
| Number of patient with castration | 116 | 115 |
| Proportion, % (95% CI) | 100 (96.9, 100.0) | 100 (96.8, 100.0) |
Serum testosterone level ≤0.5 ng/mL. CI, confidence interval; FAS, full analysis set.
Figure 4.
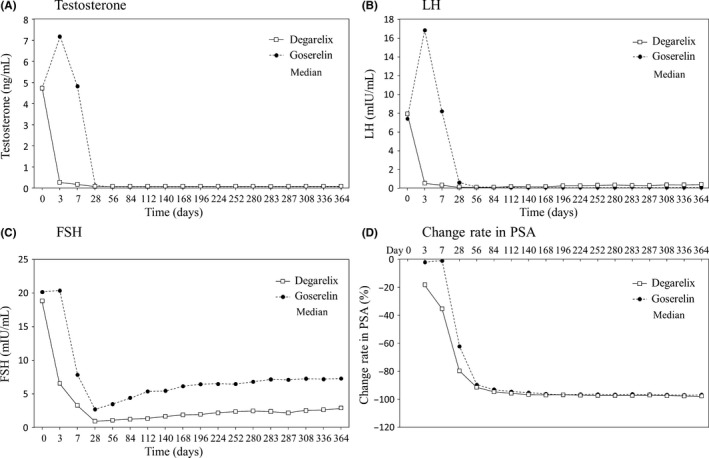
Changes in testosterone (A), luteinizing hormone (LH) (B), follicle stimulating hormone (FSH) (C), and prostate‐specific antigen (PSA) in the full analysis set of Japanese prostate cancer patients treated with degarelix (n = 117) or goserelin (n = 117)
3.5. Safety
In study part 1, AEs were found in 117 (100.0%) subjects in the degarelix group and in 106 (90.6%) subjects in the goserelin group (Table 4). The number of subjects with SAEs for degarelix versus goserelin was 15 (12.8%) versus 16 (13.7%), respectively. The number of subjects with grade ≥3 AEs was 23 (19.7%) vs 18 (15.4%), respectively. The most common AEs in the degarelix group were injection site reaction including injection site pain (n = 88, 75.2%), injection site erythema (n = 81, 69.2%) and injection site induration (n = 77, 65.8%). A grade ≥3 AE caused by an injection site reaction was found in one subject (0.9%) in the degarelix group. Common AEs other than injection site reaction were nasopharyngitis (n = 34, 29.1%), hot flush (n = 27, 23.1%), pyrexia (n = 18, 15.4%), and constipation (n = 12, 10.3%) in the degarelix group. The most common AEs in the goserelin group were hot flush (n = 38, 32.5%), nasopharyngitis (n = 25, 21.4%), and anemia (n = 12, 10.3%). No apparent differences between groups were found in terms of investigations, vital signs, electrocardiograms, or change in body weight. Regarding the long‐term safety of the maintenance dose of degarelix in part 2, AEs were found in 71 (88.8%) subjects (Table 5). The number of subjects with SAEs was 6 (7.5%). Most of the AEs were grade 1 or 2 and the number of subjects with grade ≥3 AEs was 5 (6.3%). The most common AE in part 2 was injection site pain (n = 44, 55.0%) followed by injection site induration (n = 42, 52.5%) and injection site erythema (n = 26, 32.5%). The most common AE in part 2, as with part 1, was injection site reaction, but its incidence did not tend to increase compared to part 1. On the whole, the types of AEs and incidence found in part 2 were similar to those found in part 1.
Table 4.
Adverse events (AEs) in Japanese prostate cancer subjects treated with degarelix (n = 117) and goserelin (n = 117): Study part 1
| Degarelix, n (%) | Goserelin, n (%) | |
|---|---|---|
| SAF, n | 117 | 117 |
| Total AEs, n (%) | 117 (100.0) | 106 (90.6) |
| Grade | ||
| 1 | 28 (23.9) | 31 (26.5) |
| 2 | 66 (56.4) | 57 (48.7) |
| 3 | 20 (17.1) | 12 (10.3) |
| 4 | 3 (2.6) | 5 (4.3) |
| 5 | 0 (0.0) | 1 (0.9) |
| ADRs | 114 (97.4) | 73 (62.4) |
| SAEs | 15 (12.8) | 16 (13.7) |
| AE incidence ≥5% (in either group), n (%) | ||
| Anemia | 3 (2.6) | 12 (10.3) |
| Constipation | 12 (10.3) | 11 (9.4) |
| Injection site erythema | 81 (69.2) | 1 (0.9) |
| Injection site induration | 77 (65.8) | 1 (0.9) |
| Injection site pain | 88 (75.2) | 7 (6.0) |
| Injection site pruritus | 17 (14.5) | 1 (0.9) |
| Injection site swelling | 26 (22.2) | 1 (0.9) |
| Injection site warmth | 7 (6.0) | 0 (0.0) |
| Malaise | 10 (8.5) | 4 (3.4) |
| Pyrexia | 18 (15.4) | 1 (0.9) |
| Nasopharyngitis | 34 (29.1) | 25 (21.4) |
| ALT increased | 7 (6.0) | 5 (4.3) |
| AST increased | 6 (5.1) | 5 (4.3) |
| Weight gain | 11 (9.4) | 7 (6.0) |
| Back pain | 6 (5.1) | 5 (4.3) |
| Hot flush | 27 (23.1) | 38 (32.5) |
| Hypertension | 7 (6.0) | 2 (1.7) |
ADR, adverse drug reaction; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event; SAF, safety analysis set.
Table 5.
Adverse events (AEs) in Japanese prostate cancer subjects treated with degarelix (n = 117) and goserelin (n = 117): Study part 2
| n (%) | |
|---|---|
| SAF, n | 80 |
| Total AEs, n (%) | 71 (88.8) |
| Grade | |
| 1 | 26 (32.5) |
| 2 | 40 (50.0) |
| 3 | 5 (6.3) |
| 4 | 0 (0.0) |
| 5 | 0 (0.0) |
| ADRs | 64 (80.0) |
| SAEs | 6 (7.5) |
| AE incidence ≥5%, n (%) | |
| Injection site erythema | 26 (32.5) |
| Injection site induration | 42 (52.5) |
| Injection site pain | 44 (55.0) |
| Injection site pruritus | 5 (6.3) |
| Injection site swelling | 14 (17.5) |
| Malaise | 5 (6.3) |
| Pyrexia | 10 (12.5) |
| Nasopharyngitis | 13 (16.3) |
| Upper respiratory tract infection | 6 (7.5) |
| Weight gain | 6 (7.5) |
| Back pain | 4 (5.0) |
ADR, adverse drug reaction; SAE, serious adverse event; SAF, safety analysis set.
4. DISCUSSION
For this study, non‐inferiority in castration rate and safety of the 3‐month formulations of degarelix compared with goserelin were evaluated in subjects with prostate cancer. The cumulative castration rate was 95.1% in the degarelix group and 100.0% in the goserelin group. As there were no events in the goserelin group, the original statistical method planned for confirming non‐inferiority was considered to be inappropriate, as mentioned above in “Statistics”. Hence, additional analyses were carried out using the 95% CI of the difference in the proportion of subjects with castration. Additional analyses showed that the lower limit of the 95% CI of difference between groups exceeded −10% of the predefined non‐inferiority margin in 11 of 13 methods. Of the 13 methods, the following four methods were considered to potentially underestimate the CI of the difference of the proportion of subjects with castration due to no event in the goserelin group, and thus were excluded from the evaluation: Wald test using single parameters (method 1), Wald test using multiple parameters (method 2), Hauck–Anderson (method 12), and Agresti–Caffo (method 13). The lower limit of 95% CI of difference between groups fell below −10% in the EXACT method (7) and in the Newcombe (continuity correction) method (10). The coverage probability was 0.994 and 0.993 for the EXACT and the Newcombe (continuity correction) methods, respectively, and scores were the highest among the nine methods. These two methods have been reported to tend to calculate an unduly conservative CI.14 In fact, the coverage probability greatly exceeded the nominal value of 0.95 and the CIs were considered to be overly conservative. With the exception of the six methods mentioned above, the lower limit of 95% CI of difference between groups exceeded −10% in all of the other seven methods. Considering the above, we concluded that the non‐inferiority of the 3‐month formulation of degarelix to goserelin had been established.
Degarelix showed a more rapid decrease in testosterone, LH, FSH, and PSA levels compared with goserelin. Our results showed that degarelix reduced testosterone, LH, FSH, and PSA levels more rapidly with no initial testosterone surge compared with goserelin, which is consistent with previously reported findings.11, 15 Gonadotropin releasing hormone agonists work by overstimulating GnRH receptors that causes receptor desensitization and, consequently, a reduction in LH, FSH, and testosterone.16, 17, 18 Testosterone suppression is achieved after an initial LH surge that not only delays the testosterone from reaching a castrate level but also stimulates the overproduction of testosterone. This potentially results in transient tumor expansion and a resultant flare in clinical symptoms including worsened bone pain, urinary obstruction, and spinal cord compression.19, 20, 21 Hence, the study protocol allowed concomitant administration of bicalutamide in the goserelin group only. Thirty‐four patients were treated with bicalutamide in the goserelin group. The concomitant administration could alter testosterone, LH, FSH, and PSA levels; however, because of the short duration of treatment in a small number of patients, the effect of bicalutamide on the outcome would be minimal. Degarelix showed none of these undesirable hormonal changes and thus was assumed to be clinically favorable. An experimental study has shown that the binding of FSH to FSH receptors in granulosa cells induces hypoxic conditions22 leading to upregulation of vascular endothelial growth factor, a pro‐angiogenic factor secreted during cancer growth. Although future studies are necessary, FSH suppression of degarelix could contribute to better anti‐angiogenesis in cancer treatment. Five patients treated with degarelix in part 1 did not maintain castration level with the value being 0.54‐0.80 ng/mL. None of these patients discontinued treatment because of PSA relapse and had distinctive characteristics compared to the others who maintained castration level. Testosterone nadir and baseline values were 0.05‐0.06 ng/mL and 2.80‐7.19 ng/mL, respectively, in those patients, whereas the median baseline value in all patients in the degarelix group was 4.73 ng/mL (2.59‐9.09 ng/mL).
The most common AEs in the degarelix group were injection site reactions with an incidence of 94.9% with no increase in incidence relative to duration. Most cases were grade 1 or 2; an injection site reaction of grade ≥3 was found in one subject (0.9%). The incidence of injection site reactions was more common after the initial injection compared to after each maintenance dose (once every 3 months), a trend consistent with that of the once‐monthly regimen of degarelix. Most injection site reactions disappeared within 2 months, indicating they would recover before the next administration of the 3‐month formulation of degarelix. Adverse events other than injection site reactions in the degarelix group were mainly nasopharyngitis (29.1%), hot flush (23.1%), pyrexia (15.4%), and malaise (8.5%). Most of the AEs observed in the degarelix group were those commonly reported with the once‐monthly formulation of degarelix. Considering the above, the present study indicates that the 3‐month formulation of degarelix would be tolerated as the ADT for subjects with prostate cancer.
Taking into consideration all of the above findings, we concluded that the non‐inferiority of the 3‐month formulation of degarelix to goserelin was shown to be effective for testosterone suppression and the 3‐month formulation of degarelix was tolerated as the ADT for patients with prostate cancer. In the current therapeutic strategy for prostate cancer, long‐term survival of ≥10 years is expected for quite a number of patients and ADT has been the mainstay of treatment for prostate cancer. The once‐monthly regimen of degarelix, a GnRH antagonist, has been approved and is widely used in clinical practice. However, a therapeutic option of a 3‐month regimen would potentially reduce the mental, physical, and social burden on patients and the labor burden on health‐care providers.
This study has several limitations. The number of subjects was calculated to evaluate non‐inferiority of degarelix to goserelin without consideration of the evaluation of other end‐points. In addition, only Japanese patients who met the eligibility criteria were enrolled and antiprostate cancer therapy other than the study drugs was restricted.
Non‐inferiority of the 3‐month formulation of degarelix to goserelin was indicated and the 3‐month formulation of degarelix can be safely used as the ADT for patients with prostate cancer.
CONFLICT OF INTEREST
Yasuo Ohashi has stock ownership of Statcom. Seiichiro Ozono has received honoraria from Astellas Pharma and Abbott. Seiji Naito has received honoraria from Astellas Pharma, Takeda Pharmaceutical, and Janssen Pharmaceutical. Shigeo Horie has received honoraria from Astellas Pharma and AstraZeneca and research funding from Astellas Pharma. Yasuo Ohashi has received honoraria from Chugai Pharmaceutical, Sanofi, Daiichi Sankyo, and YakultHonsha, and research funding from Eisai. Hiroji Uemura has received honoraria from Bayer Yakuhin, Takeda Pharmaceutical, Janssen Pharmaceutical, and Astellas Pharma. Hideyuki Akaza has received honoraria from Takeda Pharmaceutical, Janssen Pharmaceutical, and Astellas Pharma. Yasuo Ohashi is an employee of Statcom. Hidehito Kusuoka, Rio Akazawa, and Masako Saito are employees of Astellas Pharma. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
The authors wish to thank Springer Healthcare in Science Communications and Tetsuhiko Yokoyama for providing medical writing support, which was funded by Astellas Pharm. The authors also thank the following investigators for participating in the study (all in Japan): Hisanobu Adachi (Tohoku University, Miyagi), Shin Ebara (Okayama University, Okayama), Kohei Edamura (Hiroshima City Hospital, Hiroshima), Hideki Enokida (Kagoshima University, Kagoshima), Yutaka Enomoto (Mitsui Memorial Hospital, Tokyo), Kiyohide Fujimoto (Nara Medical University, Nara), Hiroshi Fukuhara (University of Tokyo,Tokyo), Hiroshi Furuse (Hamamatsu University School of Medicine, Shizuoka), Takahiko Hara (Yamaguchi University, Yamaguchi), Katsuyoshi Hashine (Shikoku Cancer Center, Ehime), Tomohiko Ichikawa (Chiba University, Chiba), Tsukasa Igawa (Nagasaki University, Nagasaki), Kazuyoshi Iijima (Nagano Municipal Hospital, Nagano), Takahiro Inoue (Kyoto University, Kyoto), Akihiro Ito (Tohoku University, Miyagi), Hirofumi Izaki (Tokushima University, Tokushima), Sadanori Kamikawa (Osaka City General Hospital, Osaka), Toshiyuki Kamoto (Miyazaki University, Miyazaki), Takashi Kasahara (Niigata University, Niigata), Masashi Kato (Nagoya University, Aichi), Koji Kawai (Tsukuba University, Ibaraki), Kiyotaka Kawashima (Tochigi Cancer Center, Tochigi), Hidefumi Kinoshita (Kansai Medical University, Osaka), Takeo Kosaka (Keio University, Tokyo), Naoya Masumori (Sapporo Medical University, Hokkaido), Hiroaki Matsumoto (Yamaguchi University, Yamaguchi), Hisashi Matsushima (Tokyo Metropolitan Police Hospital, Tokyo), Kenta Miki (Jikei University School of Medicine, Tokyo), Yasutomo Nakai (Osaka University, Osaka), Takashige Namima (Tohoku Rosai Hospital, Miyagi), Masami Nantani (Hokkaido Memorial Hospital of Urology, Hokkaido), Kazuo Nishimura (Osaka International Cancer Institute, Osaka), Tsutomu Nishiyama (Niigata University, Niigata), Shuji Nishizawa (Nagano Municipal Hospital, Nagano), Masashi Niwakawa (Shizuoka Cancer Center, Shizuoka), Kenji Numahata (Yamagata Prefectural Central Hospital, Yamagata), Takatsugu Okegawa (Kyorin University, Tokyo), Koji Okihara (Kyoto Prefectural University of Medicine, Kyoto), Takashi Saika (Hiroshima City Hospital, Hiroshima), Hideki Sakai (Nagasaki University, Nagasaki), Fuminori Sato (Oita University, Oita), Mikio Sugimoto (Kagawa University, Kagawa), Hiroyoshi Suzuki (Toho University Sakura Medical Center, Chiba), Kazuhiro Suzuki (Gunma University, Gunma), Satoshi Tamada (Osaka City University, Osaka), Akito Terai (Kurashiki Central Hospital, Okayama), Motohide Uemura (Osaka University, Osaka), Yoshiaki Wakumoto (Juntendo University, Tokyo), Akito Yamaguchi (Harasanshin Hospital, Fukuoka), Kunihisa Yamaguchi (Tokushima University, Tokushima), Akira Yokomizo (Kyushu University, Fukuoka), and Kazuhiro Yoshimura (Kindai University, Osaka).
Ozono S, Tsukamoto T, Naito S, et al. Efficacy and safety of 3‐month dosing regimen of degarelix in Japanese subjects with prostate cancer: A phase III study. Cancer Sci. 2018;109:1920–1929. https://doi.org/10.1111/cas.13600
Funding Information
Astellas Pharm Inc.
REFERENCES
- 1. GLOBOCAN 2012 v1.0 . Estimated cancer incidence, mortality, and prevalence worldwide. http://globocan. Accessed November 27, 2017.
- 2. NCCN Clinical Practice Guidelines in Oncology . Prostate cancer. version 2.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 28, 2017.
- 3. Klotz L, O'Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen‐deprivation therapy (ADT) predicts for time to castration‐resistant progression: a secondary analysis of the PR‐7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33:1151‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The European Association of Urology Prostate cancer guidelines . http://uroweb.org/guideline/prostate-cancer. Accessed April 28, 2017.
- 5. Thompson IM. Flare associated with LHRH‐agonist therapy. Rev Urol. 2001;3(Suppl 3):S10‐S14. [PMC free article] [PubMed] [Google Scholar]
- 6. Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, Olesen K. Degarelix: a novel gonadotropin‐releasing hormone (GnRH) receptor blocker–results from a 1‐yr, multicentre, randomised, phase 2 dosage‐finding study in the treatment of prostate cancer. Eur Urol. 2008;54:805‐813. [DOI] [PubMed] [Google Scholar]
- 7. Gittelman M, Pommerville PJ, Persson BE, Jensen JK, Olesen TK, Degarelix Study Group . A 1‐year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J Urol. 2008;180:1986‐1992. [DOI] [PubMed] [Google Scholar]
- 8. Klotz L, Boccon‐Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12‐month, comparative, randomized, open‐label, parallel‐group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531‐1538. [DOI] [PubMed] [Google Scholar]
- 9. Tombal B, Miller K, Boccon‐Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol. 2010;57:836‐842. [DOI] [PubMed] [Google Scholar]
- 10. Schroder FH, Tombal B, Miller K, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12‐month, comparative, phase III study. BJU Int. 2010;106:182‐187. [DOI] [PubMed] [Google Scholar]
- 11. Hyodo S, Ue E, Terada I, Takeda K, Yoshiyasu T. Safety and efficacy of degarelix in patients with prostate cancer: interim analysis of long–term post–marketing surveillance. Ther Res. 2015;36:1083‐1096. (Japanese). [Google Scholar]
- 12. Ozono S, Tsukamoto T, Naito S, et al. Efficacy and safety of a 3‐month dosing regimen of degarelix in Japanese patients with prostate cancer: a phase II maintenance‐dose‐finding study. Jpn J Clin Oncol. 2017;47:438‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873‐890. Erratum in: Stat Med 1999 May 30;18(10):1293. [DOI] [PubMed] [Google Scholar]
- 14. Iwasaki M, Hashigaki S. Confidence intervals for the difference between two independent binomial proportions. J Fac Eng Seikei Univ. 2004;41:9‐40. (Japanese). [Google Scholar]
- 15. Ozono S, Ueda T, Hoshi S, et al. The efficacy and safety of degarelix, a GnRH antagonist: a 12‐month, multicentre, randomized, maintenance dose‐finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol. 2012;42:477‐484. [DOI] [PubMed] [Google Scholar]
- 16. Van Poppel H, Klotz L. Gonadotropin‐releasing hormone: an update review of the antagonists versus agonists. Int J Urol. 2012;19:594‐601. [DOI] [PubMed] [Google Scholar]
- 17. Bruchovsky N, Goldenberg SL, Akakura K, Rennie PS. Luteinizing hormone‐releasing hormone agonists in prostate cancer. Elimination of flare reaction by pretreatment with cyproterone acetate and low‐dose diethylstilbestrol. Cancer. 1993;72:1685‐1691. [DOI] [PubMed] [Google Scholar]
- 18. Rick FG, Block NL, Schally AV. An update on the use of degarelix in the treatment of advanced hormone‐dependent prostate cancer. Onco Targets Ther. 2013;6:391‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waxman J, Man A, Hendry WF, et al. Importance of early tumour exacerbation in patients treated with long acting analogues of gonadotrophin releasing hormone for advanced prostatic cancer. Br Med J (Clin Res Ed). 1985;291:1387‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson IM, Zeidman EJ, Rodriguez FR. Sudden death due to disease flare with luteinizing hormone‐releasing hormone agonist therapy for carcinoma of the prostate. J Urol. 1990;144:1479‐1480. [DOI] [PubMed] [Google Scholar]
- 21. Boccon‐Gibod L, Laudat MH, Dugue MA, Steg A. Cyproterone acetate lead‐in prevents initial rise of serum testosterone induced by luteinizing hormone‐releasing hormone analogs in the treatment of metastatic carcinoma of the prostate. Eur Urol. 1986;12:400‐402. [DOI] [PubMed] [Google Scholar]
- 22. Alam H, Weck J, Maizels E, et al. Role of the phosphatidylinositol‐3‐kinase and extracellular regulated kinase pathways in the induction of hypoxia‐inducible factor (HIF)‐1 activity and the HIF‐1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle‐stimulating hormone. Endocrinology. 2009;150:915‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]


