Abstract
Infantile hemangioma (IH) is a benign tumor that is formed by aberrant angiogenesis and that undergoes spontaneous regression over time. Propranolol, the first‐line therapy for IH, inhibits angiogenesis by downregulating activation of the vascular endothelial growth factor (VEGF) pathway, which is hyperactivated in IH. However, this treatment is reportedly ineffective for 10% of tumors, and 19% of patients relapse after propranolol treatment. Both pro‐angiogenic and anti‐angiogenic factors regulate angiogenesis, and pigment epithelium‐derived factor (PEDF) is the most effective endogenous anti‐angiogenic factor. PEDF/VEGF ratio controls many angiogenic processes, but its role in IH and the relationship between this ratio and propranolol remain unknown. Results of the present study showed that the PEDF/VEGF ratio increased during the involuting phase of IH compared with the proliferating phase. Similarly, in hemangioma‐derived endothelial cells (HemEC), which were isolated with magnetic beads, increasing the PEDF/VEGF ratio inhibited proliferation, migration, and tube formation and promoted apoptosis. Mechanistically, the VEGF receptors (VEGFR1 and VEGFR2) and PEDF receptor (laminin receptor, LR) were highly expressed in both IH tissues and HemEC, and PEDF inhibited HemEC function by binding to LR. Interestingly, we found that propranolol increased the PEDF/VEGF ratio but did so by lowering VEGF expression rather than by upregulating PEDF as expected. Furthermore, the combination of PEDF and propranolol had a more suppressive effect on HemEC. Consequently, our results suggested that the PEDF/VEGF ratio played a pivotal role in the spontaneous regression of IH and that the combination of PEDF and propranolol might be a promising treatment strategy for propranolol‐resistant IH.
Keywords: angiogenesis, infant hemangioma, pigment epithelium‐derived factor, propranolol, vascular endothelial growth factor
Abbreviations
- co‐IP
co‐immunoprecipitation
- EC
endothelial cell
- HemEC
hemangioma‐derived endothelial cell
- IH
infant hemangioma
- LR
laminin receptor
- MVD
microvessel density
- non‐HemEC
non‐hemangioma‐derived endothelial cell
- PEDF
pigment epithelium‐derived factor
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2
- VEGF
vascular endothelial growth factor
- VM
venous malformation
- α‐SMA
alpha smooth muscle actin
- β‐AR
β‐adrenergic receptor
1. INTRODUCTION
Infantile hemangioma is one of the most common vascular tumors and occurs in approximately 5%‐10% of infants.1, 2 Although IH is a benign tumor, IH can severely affect the appearance and psychology of a child because 60% of IH occur around the head and neck. Moreover, serious cases can become life‐threatening when the IH affect specific regions containing functional organs.3, 4 This unique tumor comprises an increased number of unique EC that lead to rapid angiogenesis.5 IH usually forms within the first week after birth and grows rapidly during the first 3‐6 months, known as the proliferating phase of IH.6 Subsequently, spontaneous regression occurs from 12 months to 5 years of age and beyond.7, 8 Currently, the specific pathogenesis and molecular mechanisms underlying this spontaneous involution are largely unknown. The VEGF pathway is a well‐known regulatory mechanism.9 VEGF and VEGF receptors are overactivated during the proliferating phase of IH. As its name implies, VEGF stimulates EC proliferation, mitogenesis, and migration and subsequently initiates rapid angiogenesis.10, 11 However, both physiological and pathological angiogenesis is regulated by a balance of pro‐angiogenic and anti‐angiogenic factors. The roles of anti‐angiogenic factors and their mechanisms in IH progression have received little attention.
PEDF is a multifunctional secreted protein that has anti‐angiogenic, anti‐tumorigenic, and neurotrophic functions. In addition, PEDF is the most active endogenous anti‐angiogenic factor and can directly target EC to inhibit EC proliferation and promote cell apoptosis.12, 13 Recent studies have found that PEDF is highly expressed in the involuting phases of IH.14, 15 Previous investigations, including ours, have confirmed that the balance between PEDF and VEGF plays a crucial role in the processes of vascular development and angiogenesis.13, 16, 17, 18, 19, 20, 21 Nevertheless, whether and how the PEDF/VEGF ratio plays a role in IH is unclear. Herein, we found that the PEDF/VEGF ratio was increased during the involuting phase of IH compared to during the control proliferating phase of IH and compared to VM. To investigate the role of the PEDF/VEGF ratio in vitro, we separated HemEC with magnetic beads. Accordingly, the PEDF/VEGF ratio clearly inhibited HemEC proliferation, migration, and tube formation and induced HemEC apoptosis in a specific receptor‐dependent way.
Although IH can disappear without treatment, IH regression takes many years, and a significant proportion of lesions require some form of therapy as a result of their specific locations. In 2008, Leaute‐Labreze serendipitously found that propranolol, a β‐AR antagonist, could effectively inhibit IH growth and, thereafter, propranolol has been the first‐line treatment for IH.22 Nevertheless, the use of propranolol is not without problems. Approximately 10% of tumors do not respond,23 whereas 19% of patients experience relapsed growth within the first year of life.24, 25 Indeed, current researchers are mostly focusing on the effects of propranolol on the expression of pro‐angiogenic factors, especially VEGF. Multiple studies have shown that VEGF expression is downregulated after giving propranolol.26, 27, 28 However, the possible impact of propranolol on anti‐angiogenic factors and the balance in the PEDF/VEGF ratio remain unknown to date. Surprisingly, we found that propranolol increased the PEDF/VEGF ratio primarily by reducing VEGF expression but also by reducing PEDF expression in vivo and in vitro. However, when we artificially modified the PEDF/VEGF ratio by combining PEDF with propranolol, we observed a better inhibitory effect in HemEC. This study highlights the potential utility of natural medicine in the treatment of propranolol‐resistant IH.
2. MATERIALS AND METHODS
2.1. Immunohistochemistry
To investigate whether the PEDF/VEGF ratio contributed to IH progression, we carried out immunohistochemistry to examine PEDF and VEGF expression in 22 IH. This study was approved by the medical ethics committee of Sun Yat‐sen University for medical sciences, Guangzhou. All clinical tissue sections (from the Guangzhou Women and Children's Hospital) were stained with H&E using a standard protocol for histology, and the sections were pathologically confirmed. The sections were deparaffinized and rehydrated per standard methods and pretreated with 0.01 mol/L citrate buffer (pH 6.0) for 2 minutes at 100°C in an autoclave. After the sections were naturally cooled, they were immersed in 3% H2O2 and 100% methanol for 20 minutes to quench endogenous peroxidases. Next, the sections were treated with normal goat serum to block non‐specific background staining and washed 3 times in PBS with Tween 20 (PBST; pH 7.2) between each step. The slides were incubated with different antibodies: PEDF (1:100; Merck Millipore, La Jolla, CA, USA), VEGF‐A (1:100; Santa Cruz Biotechnology, Dallas, TX, USA), VEGFR1 (1:100; Abcam, Cambridge, MA, USA), VEGFR2 (1:100; Abcam), LR (1:200; Abcam), and β‐AR (1, 2, and 3) (1:100; Abcam) at 4°C overnight. Next, the slides were incubated with a biotin‐conjugated secondary antibody (DAKO, Glostrup, Denmark) for 40 minutes at room temperature, followed by counterstaining with hematoxylin. We compared the immunohistochemistry scores, which were calculated as the stained area (0‐3 points) multiplied by the staining intensities (0‐4 points) of each case for the different phases of IH or for VM.29
2.2. Immunofluorescence
To examine the microvascular morphology, density and localization of specific receptors in the tissue sections, we carried out immunofluorescence to detect proteins and cell markers. Tissue sections were pretreated as described above for immunohistochemistry and then blocked with normal donkey serum at room temperature for 1 hour. We used a CD31 monoclonal antibody (1:100; CST, Danvers, MA, USA) to detect EC and α‐SMA polyclonal antibody (1:200; Sigma Chemical Co., St Louis, MA, USA) to detect pericytes. To detect receptor expression, we used the following antibodies: LR (1:100; Abcam), VEGF‐A (1:100; Santa Cruz Biotechnology), VEGFR1 (1:100; Abcam), and VEGFR2 (1:100; Abcam). After overnight incubations at 4°C, appropriate fluorescent secondary antibodies were used (1:200 dilutions in blocking buffer, 1 hour, 37°C). After immunolabeling, sections were washed and stained with DAPI (Sigma Chemical Co.) for 10 minutes at room temperature. Sections were visualized under a confocal microscope (ZEISS, Jena, Germany).
2.3. Cell culture
To establish an in vitro cell model of IH to examine whether the increased PEDF/VEGF ratio could affect IH, we collected fresh IH tissues from Guangzhou Women and Children's Hospital under a human subject protocol that was approved by the medical ethics committee of Sun Yat‐sen University for Medical Sciences, Guangzhou. Informed consent was obtained for the specimens, according to the Declaration of Helsinki. Briefly, hemangioma tissues were extracted from the patients, washed with normal saline, soaked in tissue storage solution (Miltenyi Biotec, Bergisch Gladbach, Germany) and delivered to the lab within 3 hours. The tissues were isolated according to mechanical cutting and collagen digestion methods, as described previously30 with minor modifications. The digested tissues were centrifuged at 425 g for 3 minutes The pellet was resuspended in Endothelial Growth Media‐2 (EGM‐2; Lonza, Walkersville, MD, USA) and seeded in 60‐mm dishes in preparation for cell sorting. HemEC were selected using anti‐CD31‐coated magnetic beads (Miltenyi Biotec). CD31‐selected cells were cultured on fibronectin‐coated (1 μg/cm2) plates with 15% fetal bovine serum (Gibco, Gaithersburg, MD, USA) in EGM‐2, and CD31‐negative cells were designated HemEC(−). HUVEC were isolated from umbilical veins according to a protocol described previously.31 These cells were cultured in EGM‐2 with 100 U/mL streptomycin (Gibco) in an atmosphere of 5% CO2 at 37°C.
2.4. Cell viability assay
Recombinant PEDF was expressed and purified as described previously.12 Recombinant human VEGF165 protein (VEGF) was purchased from (R&D Systems, Minneapolis, MN, USA). HemEC and HUVEC were seeded in 96‐well culture plates at 5000 cells per well. After the cells attached to the plates, the culture medium was replaced with serum‐free medium containing various concentrations of PEDF or VEGF. Cell viability was assessed with CCK‐8 (Dojindo, Kyushu, Japan), according to the manufacturer's instructions. Samples were assessed at 450 nm using a Sunrise microplate reader (TECAN, Männedorf, Switzerland).
2.5. Apoptosis assay
HemEC were seeded at 5 × 104 per well in 6‐well plates and were allowed to attach overnight. The cell cultures were treated with PEDF at different concentrations for 48 hours. Cells were harvested according to the manufacturer's recommendations. Flow cytometry was carried out to measure apoptosis using the Annexin V‐FITC Apoptosis Assay Kit (Bender Med Systems, Vienna, Austria). Cells treated with 25 μmol/L colchicine were used as positive controls, and cells treated with PBS were used as negative controls.
2.6. Migration assay
Migration abilities of HemEC and HUVEC were assessed in 24‐well Transwell plates (Corning, Corning, NY, USA). Cell suspensions (3 × 104 cells/100 μL) were aliquoted into the upper chambers of the Transwell, and after the cells had attached, the culture medium was replaced with serum‐free medium containing different ratios of PEDF/VEGF, as described above. The lower chambers were filled with 600 μL of the corresponding medium containing 10% FBS. Cells were allowed to migrate for 8‐16 hours. Then, the cells on the top sides of the filters were removed with a cotton swab. The cells that reached the lower surfaces of the filters were fixed with 3.5% paraformaldehyde and stained with 0.1% crystal violet, and images were obtained using a digital camera coupled with a microscope. Last, relative migration was quantified using ImageJ software.
2.7. Tube formation assay
Tube formation abilities of HemEC and HUVEC were assessed in vitro. Matrigel (BD Biosciences, San Jose, CA, USA) was thawed at 4°C overnight, and 100 μL Matrigel was evenly added to each well of a precooled 24‐well plate. The plate was then incubated at 37°C for 30 minutes. Cells (2 × 105) in 1‐mL suspensions were seeded to the top of the matrix in the 24‐well plate and incubated at 37°C for 12 hours. The cells were subjected to different treatments, as described above. Then, the capillary‐like structures were photographed under a light microscope using an Inverted Fluorescence Microscope (1 × 71; Olympus, Tokyo, Japan), and the tubes were scanned and quantified using ImageJ software.
2.8. Immunoblotting (western blotting)
Cells were harvested and lysed for total protein extraction using SDS sample buffer, followed by boiling for 30 minutes. Protein concentrations were measured using a BCA protein assay kit (KeyGEN BioTECH, Nanjing, China) according to the manufacturer's protocol. SDS‐PAGE was carried out to resolve each sample (25 μg), and 8%‐15% gradient gels were used. The proteins were transferred to 4.5‐μm PVDF membranes (Millipore Corp., Billerica, MA, USA), and the membranes were incubated overnight with primary antibodies, namely, PEDF (1:1000), VEGF‐A (1:1000), VEGFR1 (1:1000), VEGFR2 (1:2000), LR (1:1000), caspase3 (1:1000), GAPDH (1:5000) and β‐actin (1:5000). Densitometry was carried out using ImageJ software.
2.9. Co‐immunoprecipitation
We carried out a co‐IP analysis to determine whether PEDF could bind to LR in vitro. We first washed 1 × 107 HemEC with cold PBS and lysed the cells in RIPA lysis buffer (Applygen, Beijing, China) containing 1× phosphatase inhibitor and 1× protease inhibitor cocktails (Sigma‐Aldrich, St Louis, MO, USA) and 1 mmol/L PMSF (Sigma‐Aldrich) at 4°C for 4 hours. Lysates were pre‐cleared, quantitated as having 1000 μg protein each, and incubated with PEDF (1:100; Millipore) or LR (1:100; Abcam) antibody at 4°C overnight, followed by the addition of protein A/G‐Sepharose beads (Invitrogen, Carlsbad, CA, USA) for 4 hours at 4°C. Immunoprecipitates were washed twice with RIPA lysis buffer. Precipitated proteins were lysed with 1× SDS buffer and then subjected to western blot analysis.
2.10. siRNA transfection
LR siRNA and a non‐specific siRNA (control) were purchased from RayBio (Guangzhou, China). According to the manufacturer's instructions, transfections were carried out at approximately 60% confluency using Lipofectamine 3000 (Invitrogen). For each transfection reaction, 20 nmol/L LR siRNA or control siRNA was used for the preparation of siRNA transfection complexes at room temperature for 20 minutes. Transfections were carried out in 0.5 mL (12‐well plates) or 1.5 mL (6‐well plates) of serum‐free medium for 8 hours. After incubation, the medium containing the transfection complexes was removed and replaced with fresh medium. Western blotting analysis was carried out to determine the transfection efficiency in HemEC (80%‐90% cells attached). The cells were used for experiments at 24‐72 hours after transfection.
2.11. Enzyme‐linked immunosorbent assay
To measure protein expression in the serum before and after propranolol treatment, we collected paired peripheral blood serum samples from the 20 IH patients and stored these samples in liquid nitrogen until use. Informed consent was provided by all study participants. The participants were clinically diagnosed with IH at the Guangzhou Women and Children's Hospital between 2015 and 2016. Finally, serum PEDF and VEGF levels were measured using human PEDF (R&D Systems) and VEGF‐A (R&D Systems) ELISA kits, respectively, according to the manufacturer's protocols.
2.12. Quantitative real‐time PCR (qPCR)
We carried out a qPCR assay to evaluate PEDF and VEGF expression at the mRNA level. Total RNA was extracted from HemEC by Trizol Reagent (Invitrogen). Total RNA (500 ng) was used for reverse transcription and qPCR. Target mRNA was determined using the capillary‐based Light Cycler 2.0 Systems (Roche Diagnostics Corporation, Indianapolis, IN, USA). β‐Actin was used as an internal control. cDNA was amplified with special primers for PEDF (forward, 5′‐TGGCTTACTTCAAGGGGCAG‐3′; reverse, 5′‐CATCATGGGGACTCTCACGG‐3′), VEGF (forward, 5′‐GAGCCTTGCCTTGCTGCTCTA‐3′; reverse, 5′‐CACCAGGGTCTCGATTGGATG‐3′) and β‐actin (forward, 5′‐GTTGGCGTACAGGTCTTTGC‐3′; reverse, 5′‐GCACTCTTCCAGCCTTCCTT‐3′).
2.13. Statistical analyses
Standard t tests were used to compare 2 groups; 1‐way analysis of variance was used to compare more than 2 groups. Paired t tests were used to compare paired samples. Statistical analysis was carried out using SPSS 13.0 software. P < .05 indicated a statistically significant difference.
3. RESULTS
3.1. PEDF/VEGF ratio is increased during the involuting phase of IH
The proliferating phase of IH is characterized by immature vessels that are formed as a result of densely packed tumor cells. Features of the involuting phase include blurred boundaries between EC, mature and expanded blood vessels and the gradual apoptosis of EC.32 These features are obvious by HE staining (Figure 1A, top row). VM is a subtype of vascular malformation that affects the venous vasculature and is usually congenital and found at birth. Because the EC of VM arise from a non‐proliferative quiescent state in contrast to hemangioma EC,33 we used VM tissues as a relative control. Many reports have confirmed that pro‐angiogenic factors, especially VEGF, are excessively expressed to facilitate the proliferation of IH in an autocrine method.34 We found that VEGF expression was increased in all IH specimens compared to VM specimens (Figure 1A, bottom row). In contrast, PEDF staining was strongest during the involuting phase of IH (Figure 1A, middle row) and higher than in the proliferating phase (Figure 1B). We calculated the relative ratio of PEDF/VEGF and found that the ratio was decreased during the proliferating phase and increased during the involuting phase (Figure 1B). These results indicated that the PEDF/VEGF ratio may play a crucial role in the spontaneous regression of IH. In addition, by immunofluorescence staining of the endothelium and of pericyte markers, we found that the microvascular density was reduced and that the vessel structures were expanded and matured during the involuting phase of IH (Figure 1C). These features of EC regression occurred simultaneously to the increase in the PEDF/VEGF ratio, thus indicating that upregulation of the PEDF/VEGF ratio may influence EC function to induce IH regression.
Figure 1.
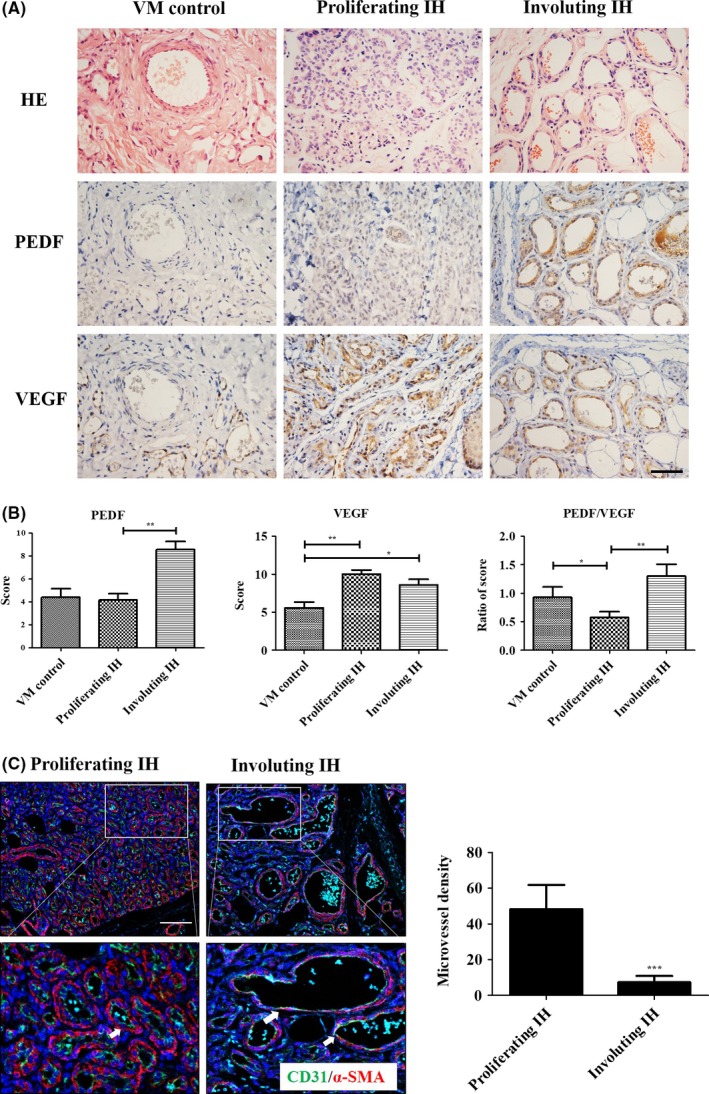
Pigment epithelium‐derived factor/vascular endothelial growth factor (PEDF/VEGF) ratio is increased during the involuting phase of infant hemangioma (IH). A, Paraffin tissue sections representative of different phases of IH and venous malformation (VM) were subjected to immunohistochemistry with the specific anti‐PEDF (middle row) and anti‐VEGF‐A (bottom row) antibodies. H&E staining indicates histopathological structures (top row); magnification: 400×; scale bar: 50 μm. B, Histogram indicates average immunohistochemistry scores for the different groups: VM (n = 5), proliferating phase of IH (n = 12), and involuting phase of IH (n = 10). Columns indicate mean ± SEM, *P < .05, **P < .01. C, Immunofluorescence staining of endothelial cell (EC) marker CD31 (green) and pericyte marker α‐smooth muscle actin (α‐SMA) (red) for the different phases of IH. Nuclei were stained with DAPI (blue). White arrows indicate the blood vessels that are expanding and maturing; scale bar: 100 μm. Histogram represents the microvessel density of each field, and the columns indicate the mean ± SEM, ***P < .001
3.2. Upregulation of the PEDF/VEGF ratio inhibits HemEC viability and induces apoptosis
Infantile hemangioma comprises unique EC but lacks corresponding cell lines for investigation.5 Thus, we digested cells from 3 IH patients (Table S1), mixed the cells, and then separated the HemEC with CD31‐positive magnetic beads. Morphologies of non‐HemEC (CD31−) and HemEC (CD31+) are presented in Figure 2A. To show that the HemEC were, indeed, EC, we carried out western blot analysis (Figure 2B) and immunofluorescence staining (Figure S1) of EC markers (CD31, VE‐cadherin or von Willebrand factor [vWF]). HUVEC were used as a positive control for EC. As observed with HUVEC, HemEC highly expressed EC characteristics; however, non‐HemEC showed high expression levels of a pericyte/fibroblast marker (α‐SMA) (Figure 2B; Figure S1). We set the effective VEGF concentration to 50 ng/mL based on preliminary experiments and increased the PEDF/VEGF ratio by gradually increasing the PEDF concentration. PEDF‐treated groups of HemEC and HUVEC showed dose‐dependent inhibition in cell viability, and HemEC were more sensitive than HUVEC (Figure 2C). Moreover, increasing the PEDF/VEGF ratio induced HemEC apoptosis (Figure 2D,E). We treated HemEC with PEDF alone and found that the pro‐apoptotic effect was mainly achieved by PEDF (Figure 2F,G) and resulted in the cleavage of apoptotic protein caspase3 (Figure 2H). These results suggested that increasing the PEDF/VEGF ratio induced HemEC apoptosis to promote IH regression.
Figure 2.
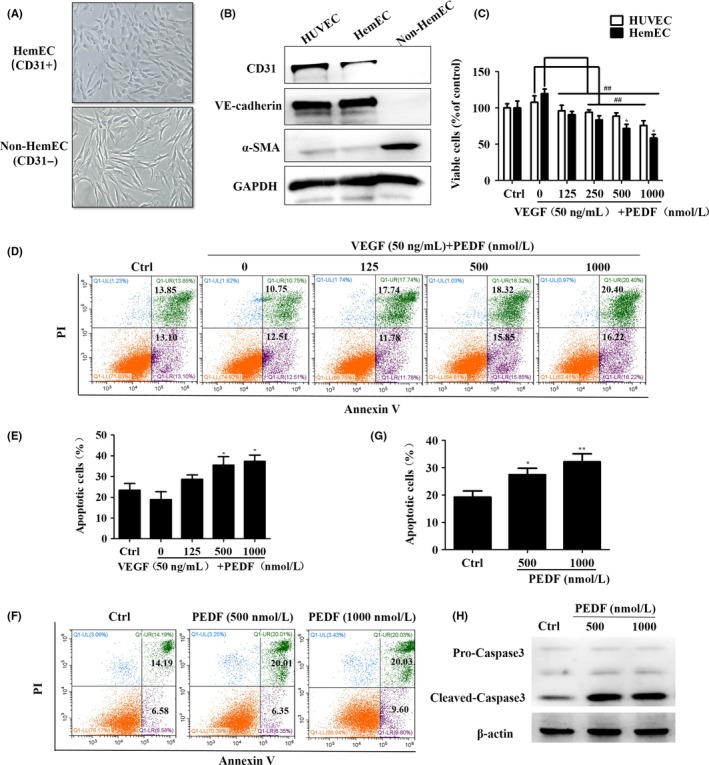
Increasing the pigment epithelium‐derived factor/vascular endothelial growth factor (PEDF/VEGF) ratio inhibits hemangioma‐derived endothelial cell (HemEC) viability and induces apoptosis. A, HemEC were sorted with CD31‐positive magnetic beads, and the morphologies of non‐HemEC (CD31‐) and HemEC (CD31+) are shown; magnification: 200×. B, Western blot analysis to evaluate endothelial cell (EC) markers (CD31 and VE‐cadherin) and a pericyte/fibroblast marker (α‐smooth muscle actin [α‐SMA]) in the indicated cells. GAPDH, control protein. C, HemEC and HUVEC were treated with PEDF and VEGF at the indicated concentrations for 24 h in the absence of serum. Cell viability was determined by the CCK‐8 assay. Data represent absorbance at 450 nm and indicate mean ± SD. ## P < .01 vs the 0‐concentration group; *P < .05 vs HUVEC. D, HemEC were subjected to Annexin V‐FITC/propidium iodide (PI) staining after 48 h of treatment, and quantitative analysis of the apoptotic cells was carried out using flow cytometry. E, Histogram indicates apoptotic cells (%) per the flow cytometry analysis, and the results are shown as mean ± SD of triplicates. *P < .05 vs the 0‐concentration group. F, Diagrams of Annexin V‐FITC/PI flow cytometry analysis of PEDF‐treated HemEC are shown. G, Histogram indicates the apoptotic cells (%) per flow cytometry analysis, and the results are shown as mean ± SD of triplicates. *P < .05 ,**P <.01 vs the control group. H, Western blotting analysis of the expression of apoptosis‐related proteins in HemEC
3.3. Increasing the PEDF/VEGF ratio inhibits HemEC migration and tube formation
Given the importance of VEGF and PEDF in endothelial migration,35 we carried out Boyden cell migration assays to determine whether the PEDF/VEGF ratio affected HemEC migration. The results showed that PEDF reduced HemEC migration in a concentration‐dependent method compared to VEGF alone (Figure 3A,B). This indicated that increasing the PEDF/VEGF ratio inhibited HemEC migration because PEDF might have antagonized the promoting effect of VEGF on cell migration. Moreover, increasing the PEDF/VEGF ratio hindered tube formation ability of HemEC (Figure 3C,D). We obtained similar results in normal EC; increasing the PEDF/VEGF ratio inhibited HUVEC migration and tube formation (Figure S2). Furthermore, increasing the PEDF/VEGF ratio with a gradient decrease in the VEGF concentration also resulted in the inhibition of cell viability, migration and tube formation (Figure S3). Together, these results indicated that the elevation of PEDF/VEGF ratio can affect IH progression by inhibiting HemEC migration and tube formation.
Figure 3.
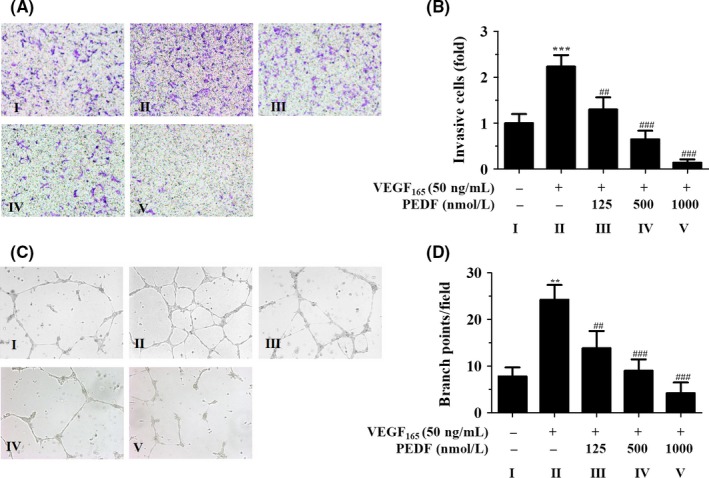
Increasing the pigment epithelium‐derived factor/vascular endothelial growth factor (PEDF/VEGF) ratio inhibits hemangioma‐derived endothelial cell (HemEC) migration and tube formation. A, HemEC migration in the presence of VEGF (50 ng/mL) and different concentrations of PEDF, as evaluated with Boyden chamber assays for 12 h. Cells were stained with crystal violet; magnification: 200×. B, Fold‐changes of migrating cells relative to the control group were calculated, and the plots indicate the mean ± SD of triplicates. ***P < .001 vs control; ## P < .01 and ### P < .001 vs the 0‐concentration group. C, HemEC tube formation in the presence of VEGF (50 ng/mL) and different concentrations of PEDF, as evaluated in 3‐D tube formation assays for 12 h; magnification: 200×. D, Quantifications of the number of branch points per field are shown as the mean ± SD of triplicates. **P < .01 vs control; ## P < .01 and ### P < .001 vs the 0‐concentration group
3.4. VEGFR1, VEGFR2, and LR are overexpressed in IH tissues
VEGF mainly binds to VEGFR‐1 (Flt‐1) and VEGFR‐2 (KDR/Flk‐1) and triggers downstream signals to promote angiogenesis.36, 37 Multiple studies have confirmed that the hyperactivation of VEGF‐VEGFR‐2 pathways in IH induces downstream signaling to promote IH angiogenesis.10, 34 Consistent with these reports, we found that the expression levels of both VEGFR1 and VEGFR2 were significantly higher in IH tissues than in VM tissues (Figure 4). PEDF is a well‐known secreted protein that regulates EC functions, depending on specific receptors in an autocrine or paracrine method.35 In recent years, several PEDF receptors (ATGL/LR/LRP6) have been reported, where LR and LRP6 mediate the anti‐angiogenic effects of PEDF in EC and cancer cells.38, 39 However, until now, the expression of PEDF receptor in IH tissues has not been addressed. We found that LR staining was obviously stronger in both proliferating and involuting phases of IH than in VM (Figure 4). Nevertheless, LRP6 was nearly undetectable in IH tissue sections after staining with a specific antibody (Figure 4A). Moreover, in serial sections that were examined by immunofluorescence, we found that the receptors for VEGF and PEDF were mainly located in EC and merged with CD31 staining (Figure 5, denoted by white arrows; the red arrows indicate that some non‐HemEC also express LR and VEGFR1). Here, we were primarily concerned with HemEC and hypothesized that VEGF exerted its pro‐angiogenic effects through its receptors (VEGFR1 and VEGFR2) in the EC of IH as previously reported, whereas PEDF carried out its anti‐angiogenic actions primarily through LR.
Figure 4.
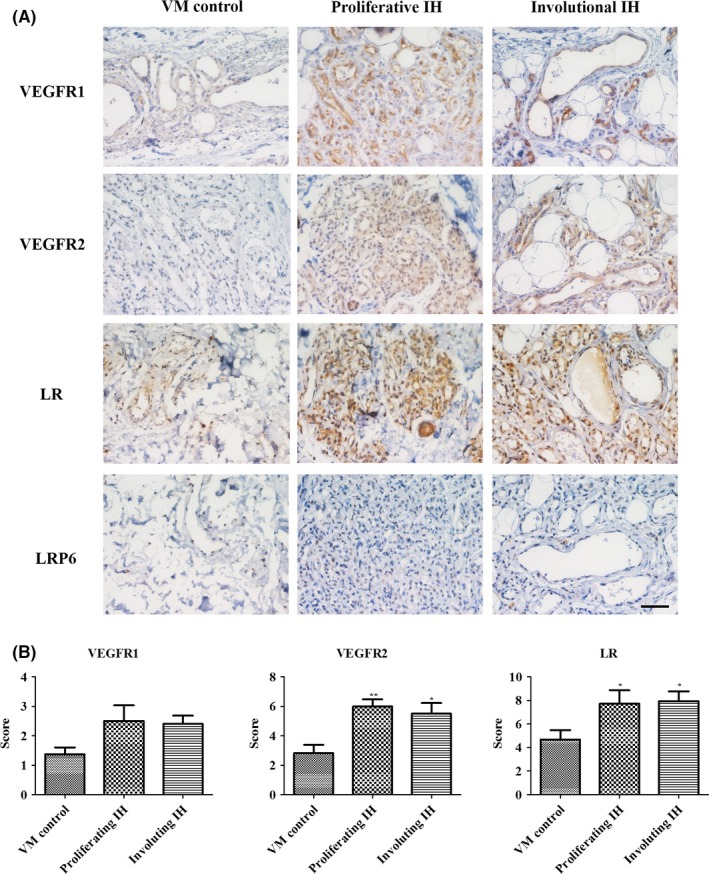
Vascular endothelial growth factor receptor 1 (VEGFR1), vascular endothelial growth factor receptor 2 (VEGFR2), and laminin receptor (LR) were overexpressed in infant hemangioma (IH) tissues. A, Immunohistochemical staining of VEGF receptors (VEGFR1 and VEGFR2) and pigment epithelium‐derived factor (PEDF) receptors (LR and LRP6) in different tissue sections comprising the proliferating phase (n = 9), involuting phase (n = 7) or venous malformation (VM; n = 5); magnification: 400×; scale bar: 50 μm. B, Histograms indicate immunohistochemistry scores; *P < .05, **P < .01. Results are presented as mean ± SEM
Figure 5.
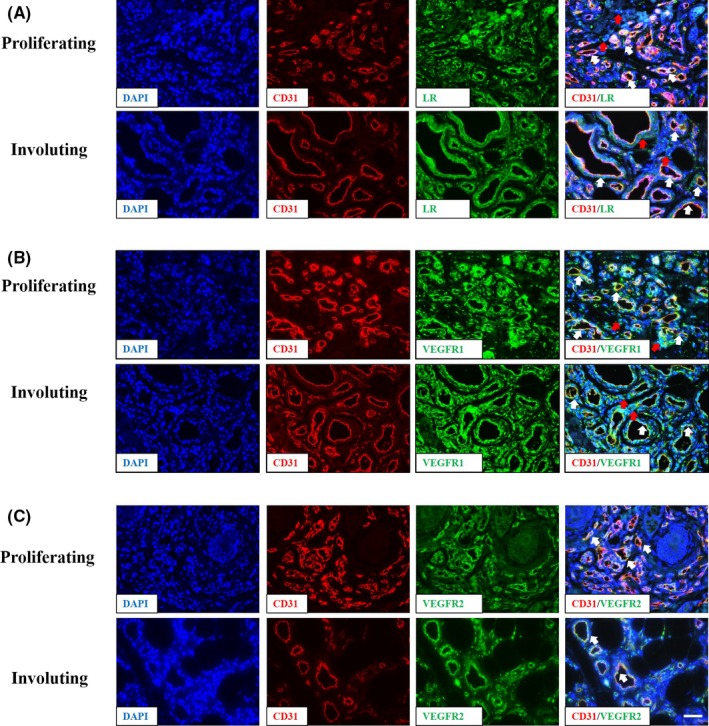
Vascular endothelial growth factor receptor 1 (VEGFR1), vascular endothelial growth factor receptor 2 (VEGFR2), and laminin receptor (LR) are mainly expressed in endothelial cells (EC). Immunofluorescence staining of VEGFR1, VEGFR2, and LR in infant hemangioma tissue sections. The image showed LR in part A, VEGFR1 in part B and VEGFR2 in part C, these three receptors were indicated in green. EC are stained with CD31 (red), and nuclei are stained with DAPI (blue). Magnification: 400×. White arrows indicate merged EC; red arrows indicate some of the non‐hemangioma‐derived endothelial cells
3.5. PEDF inhibits HemEC functions by binding to LR
To further test the above hypothesis in vitro, we examined the expression levels of the above‐mentioned receptors in HemEC. Expression levels of VEGFR1, VEGFR2, and LR were obviously increased in HemEC compared to HUVEC (Figure 6A). Our results by co‐IP indicated that PEDF bound with high specificity and affinity to LR in HemEC (Figure 6B). Meanwhile, we knocked down LR with siRNA and analyzed cell viability after treatment with PEDF. The results showed that PEDF‐induced inhibition of HemEC activity was partially reversed by the knockdown of LR (Figure 6C,D). Furthermore, an LR neutralization antibody also blocked the inhibitory function of PEDF in HemEC (Figure 6E). Similarly, PEDF‐mediated upregulation of cleaved‐caspase3 was also reversed by the LR neutralization antibody (Figure 6F). These results suggested that PEDF mainly carried out its anti‐angiogenic actions through LR in HemEC.
Figure 6.
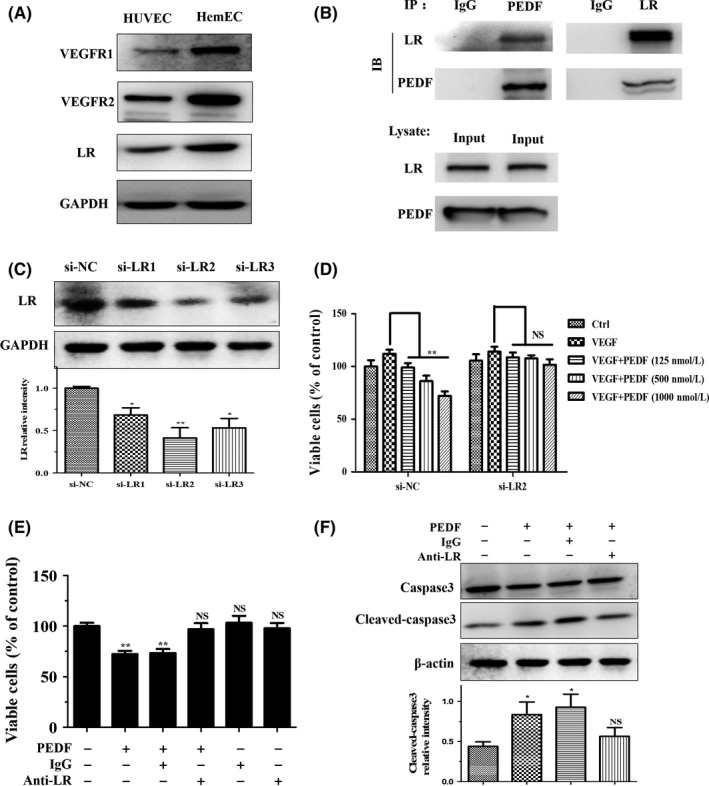
Pigment epithelium‐derived factor (PEDF) inhibits hemangioma‐derived endothelial cell (HemEC) function by binding to laminin receptor (LR). (A) Western blots were used to detect vascular endothelial growth factor receptor 1 (VEGFR1), vascular endothelial growth factor receptor 2 (VEGFR2), and LR in hemangioma‐derived endothelial cells (HemEC) and HUVEC. GAPDH, control protein. B, Co‐immunoprecipitation analysis of the binding activity of PEDF and LR in HemEC. C, After 48 h, the interfering effects of LR siRNAs (si‐LR1/2/3) were analyzed by western blotting. The histogram below shows the relative intensity of LR, and the results are shown as the mean ± SD of triplicates, *P < .05, **P < .05. D, CCK‐8 assays show that the effect of PEDF on endothelial cell (EC) activity was blocked after LR knockdown with siRNA (si‐LR2) in HemEC after 48 h. E, Regulatory effect of PEDF on EC activity was also blocked by LR neutralization antibody treatment for 48 h. Results are presented as mean ± SD, **P < .01 NS, non‐significant. F, HemEC were treated with PEDF (500 nmol/L) for 24 h, and the upregulation of apoptosis‐related protein cleaved‐caspase3 was reversed by LR neutralizing antibody, as shown by western blot analysis. The histogram below indicates the relative intensity of cleaved‐caspase3, and the results are shown as mean ± SD of triplicates, *P < .05; NS, no significant difference
3.6. PEDF and propranolol combination achieves greater suppression of HemEC
Propranolol has recently emerged as the first‐line drug for IH and causes tumor regression.22, 27 Propranolol induces vasoconstriction through its effect on nitric oxide, blocks angiogenesis through its effect on VEGF and induces apoptosis. We examined the expression levels of 3 β‐AR. As observed in previous studies,40 all 3 receptors were overexpressed in IH (Figure S4), and the majority of these receptors were merged with CD31 (Figure S5). Many published articles have indicated that propranolol inhibits VEGF expression.26, 27, 41 However, the mechanism through which propranolol affects the most active anti‐angiogenesis factor, PEDF, has not been explored. Here, we collected paired blood sera from 20 IH patients before and after oral propranolol treatment and measured the expression levels of VEGF and PEDF by ELISA. Expression levels of serum VEGF were significantly reduced after 1 month of oral propranolol treatment (Figure 7A). Surprisingly, the PEDF levels were also lower, and this decrease was statistically significant (Figure 7B) and consequently resulted in a slightly higher relative PEDF/VEGF ratio (Figure 7C). These results indicated that propranolol caused regression mainly through its effect on VEGF rather than on PEDF. We verified these results in cell‐based experiments and found that HemEC proliferation was inhibited by propranolol after 24 hours (Figure 7D). Similarly, propranolol downregulated the expression of PEDF in a dose‐dependent method at the mRNA (Figure 7E) and protein levels (Figure 7F), in contrast to the presumed notion that propranolol upregulated PEDF. The relative increase in the PEDF/VEGF ratio was mainly as a result of a more pronounced decrease in VEGF expression in several of the propranolol treatment groups. This may be a possible explanation for the relapse after propranolol dosage in 19% of patients. Moreover, HemEC proliferation was better inhibited by the combination of PEDF and propranolol (Figure 7G). Therefore, these data provide a promising new therapeutic strategy for propranolol‐resistant IH.
Figure 7.
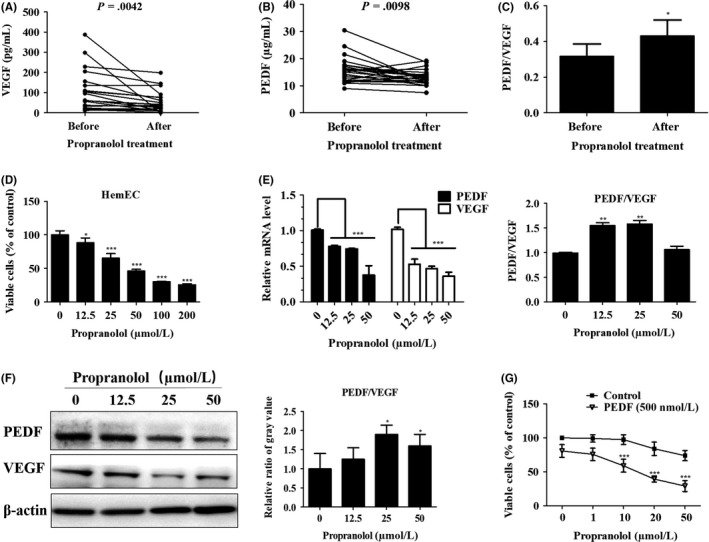
Pigment epithelium‐derived factor (PEDF) and propranolol combination treatment achieves greater suppression of hemangioma‐derived endothelial cell (HemEC). A,B, Paired blood serum samples of 20 patients before and after 1 mo of oral propranolol treatment. A, Vascular endothelial growth factor (VEGF) and (B) PEDF expression in the serum was determined using human PEDF and VEGF‐A ELISA kits and paired t test analysis was carried out. C, Relative PEDF and VEGF ratio was calculated in absolute terms and analyzed with paired t tests. Results are presented as the mean ± SEM, *P < .05. D, Different concentrations of propranolol were added to HemEC for 24 h, and CCK‐8 assays were carried out to measure cell vitality. *P < .05,***P < .001 vs the 0‐concentration group. E, qRT‐PCR analysis of VEGF and PEDF expression in HemEC after treatment with propranolol at the indicated concentrations for 24 h. Histogram indicates the relative PEDF and VEGF ratio in terms of mRNA levels, and results are shown as the mean ± SD of triplicates.**P < .01, ***P < .001 vs the 0‐concentration group. F, Western blotting analysis of VEGF and PEDF production in HemEC after treatment with propranolol at the indicated concentrations for 48 h. β‐actin, control protein. Histogram indicates the relative ratio of the PEDF and VEGF gray values from the western blots, and the results are shown as the mean ± SD of triplicates. *P < .05 vs the 0‐concentration group. G, PEDF/VEGF ratio was artificially modified with treatments comprising combinations of PEDF (500 nmol/L) and different concentrations of propranolol in HemEC for 48 h, and cell viability was measured with CCK‐8 assays. Results are shown as the mean ± SD. ***P < .001 vs the control group
4. DISCUSSION
In the present study, we found that the development of IH was regulated by the PEDF/VEGF ratio in the abnormal endothelium through specific receptors. This study has extended our understanding of the pathogenesis of IH and indicates that spontaneous regression is regulated by a balance between pro‐angiogenic factors and anti‐angiogenic factors. Multiple investigators have found that hyperactivation of VEGF‐VEGFR signaling in HemEC is essential for stimulating angiogenesis and accelerating vascular tumor growth, whereas we have shown that not only VEGF receptors (VEGFR1, VEGFR2) but also PEDF receptors (LR) are highly expressed in IH tissues and HemEC. These results indicate that anti‐angiogenic pathways might also play crucial roles in IH. The involuting of IH appears to be regulated by PEDF through its specific receptor, LR. Interestingly, we found that propranolol increased the PEDF/VEGF ratio mainly by decreasing VEGF expression while also lowering PEDF levels in vivo and in vitro. In this way, the artificial modification of the PEDF/VEGF ratio with the PEDF and propranolol combination can achieve a better effect toward HemEC inhibition.
Pigment epithelium‐derived factor is a 50‐kDa secreted glycoprotein that belongs to the non‐inhibitory serpin group and has been described as a potent anti‐angiogenic factor.13, 42 Until now, PEDF has been regarded as a suppressor of ischemia‐induced retinal neovascularization, VEGF‐induced migration and growth,43and EC migration and proliferation.13 Consistent with studies published in 2014 and 2017,14, 15 we found that the increased PEDF/VEGF ratio during the involuting phase of IH may be caused by both a decrease in VEGF and an increase in PEDF and that this increase is accompanied by a lower microvascular density and expanded blood vessel structures (Figure 1). Furthermore, our results indicated that an increased PEDF/VEGF ratio could impact IH progression by inhibiting HemEC processes, including proliferation, apoptosis, migration, and tube formation (Figures 2 and 3). However, the molecules that shift the balance toward PEDF and away from VEGF in the spontaneous regression of IH are still unknown. Reports have shown that hypoxia, or low oxygen conditions, led to VEGF upregulation and PEDF downregulation.16 Another mechanism might involve adipose cells. PEDF is widely expressed in plasma and most organs, especially in the liver and adipose tissue.42, 44 During IH regression, a large number of fat cells fill the vacant areas. Thus, this fatty tissue may secrete PEDF to promote EC apoptosis in a paracrine way. Alternatively, the increased PEDF may induce differentiation of hemangioma stem cells into adipocytes and induce extinction of the hemangioma. As with the question regarding the chicken and the egg, we cannot know whether PEDF or the adipose tissue came into being first; this mechanism is a possible explanation that should be confirmed in subsequent studies.
Although we have not yet identified the upstream regulatory mechanisms, we have established the downstream receptors of the PEDF/VEGF ratio adjustment. Consistent with previous studies,34, 41 our results showed that the expression levels of VEGF and VEGFR were upregulated in the EC of hemangiomas compared with the relatively quiescent EC. In contrast to previous studies, we found a major anti‐angiogenic pathway in IH. We first showed that LR was highly expressed in IH and could be merged with EC (Figures 4 and 5) and found that PEDF bound to LR, and selectively inhibited HemEC through LR (Figure 6). A previous study reported that PEDF promoted simulated‐EC apoptosis through activating JNK/NFAT signaling,45 and other researchers showed that PEDF could induce phosphorylation of JNK through LR and promote EC apoptosis by caspase3 activation.39 Therefore, we examined the levels of p‐JNK and total JNK in HemEC after increasing the PEDF/VEGF ratio. We also found that PEDF promoted HemEC apoptosis by increasing the phosphorylation of JNK and cleavage of caspase3 (Figure S6A), which was consistent with previous reports.39 VEGF can induce the PI3K/AKT/eNOS pathway to promote angiogenesis.46 Additionally, PEDF can inhibit VEGF‐induced migration and tube formation by blocking VEGF‐induced PI3K/Akt phosphorylation in bovine retinal EC.47 As shown in Figure S6B,C, we found that the level of p‐AKT was higher in the proliferation phase of IH than in the involuting phase and, in vitro, PEDF could also inhibit the VEGF‐induced AKT/eNOS pathway transduction in HemEC. This indicated that PEDF and VEGF have important regulatory effects on the signaling pathways in HemEC through their respective receptors.
Although we have determined the functions of VEGF and PEDF on HemEC, the effects on other cells need further exploration. During our analysis, we noticed that the receptors for VEGF and PEDF were also located on some non‐HemEC and are denoted by red arrows in Figure 5. There are reportedly many types of cells in IH other than EC, including pericytes, hemangioma‐stem cells, mesenchymal stem cells, fibroblasts, and adipocytes.32 VEGFR1 and VEGFR2 have also been detected in hemangioma‐stem cells.32 However, we are the first to report the expression of LR in IH. LR is also located in the cells of other cancers, such as cervical cancer and lung cancer, and in vascular stem cells, functioning in angiogenesis and cancer cell apoptosis.48, 49 Furthermore, in a western blot analysis, we found that LR was expressed in non‐HemEC derived from hemangioma tissues, even though the level was lower in non‐HemEC than in HemEC (Figure S7). Even so, we still suggest that PEDF mainly inhibits the progression of IH by acting directly on EC as PEDF can selectively induce the apoptosis of stimulated EC.45 Furthermore, PEDF cannot inhibit proliferation of the majority of non‐EC such as pericytes50 and tumor cells.12, 51, 52 However, PEDF can reduce the expression of VEGF in tumor cells to suppress angiogenesis in a paracrine way. Additionally, PEDF may indirectly inhibit angiogenesis in IH by the downregulation of proangiogenic factors in some stromal cells, which needs further research in the future.
Propranolol, a non‐selective β‐AR blocker, has been the first‐line treatment for IH since 2008.22 We and others have found that β‐AR are overexpressed in IH.40 Indeed, in studies of the pharmacological mechanism, multiple researchers have focused on the effects of propranolol on pro‐angiogenic factors. The data indicated that VEGF levels were reduced by propranolol in a dose‐dependent method in vitro,53 and significantly decreased expression levels of serum VEGF have been shown in IH after 1 and 2 months of propranolol treatment.26, 54 Our data showed the same expression trend. However, we are the first to indicate the impact of propranolol on anti‐angiogenic factors. Surprisingly, serum PEDF level was also lowered by oral propranolol treatment, and the same results were observed in cell‐based experiments (Figure 7). These results may explain the rebound growth after the cessation of propranolol that is observed in 19% of cases during the first year after birth.25 However, propranolol increased the PEDF/VEGF ratio mainly by decreasing VEGF expression. Whether the reduced expression of PEDF after propranolol treatment is related to propranolol resistance remains unknown. Hopefully, more studies will be conducted to address this problem. Our report presents a promising solution involving the application of a PEDF and propranolol combination treatment where the artificial increase in the PEDF/VEGF ratio is more remarkable and the inhibition of HemEC proliferation is more significant than those of propranolol alone. This study presents several options for a future approach to IH treatment by applying anti‐angiogenic factors and indicates that PEDF may be a promising candidate for IH treatment.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
5. ACKNOWLEDGMENTS
This study was supported by National Nature Science Foundation of China, Grant Numbers: 81572342, 81770808, 81471033, 81600641, 81370945, 81400639, 81570871, 81570764; National Key Sci‐Tech Special Project of China, Grant Numbers: 2013ZX09102‐053, 2015GKS‐355; Key Project of Nature Science Foundation of Guangdong Province, China, Grant Numbers: 2015A030311043, 2016A030311035, 2016A020214001; Guangdong Natural Science Fund, Grant Numbers: 2014A020212023, 2014A030313073, 2015A030313029, 2015A030313103; Guangdong Science Technology Project, Grant Number: 2017A020215075; Initiate Research Funds for the Central Universities of China (Youth Program), Grant Numbers: 13ykpy06, 14ykpy05, 16ykpy24; Key Sci‐tech Research Project of Guangzhou Municipality, China, Grant Numbers: 201508020033, 201510010052, 201707010084, 201803010017; Pearl River Nova Program of Guangzhou Municipality, China, Grant number: 201610010186; 2017 Milstein Medical Asian American Partnership Foundation Research Project Award in Translational Medicine.
Zhu L, Xie J, Liu Z, et al. Pigment epithelium‐derived factor/vascular endothelial growth factor ratio plays a crucial role in the spontaneous regression of infant hemangioma and in the therapeutic effect of propranolol. Cancer Sci. 2018;109:1981–1994. https://doi.org/10.1111/cas.13611
Funding information
Pearl River Nova Program of Guangzhou Municipality, China (Grant/Award Number: ‘201610010186’), National Nature Science Foundation of China (Grant/Award Numbers: ‘81572342, 81770808, 81471033, 81600641, 81370945, 81400639, 81570871, 81570764’), Guangdong Natural Science Fund (Grant/Award Numbers: ‘2014A020212023, 2014A030313073, 2015A030313029, 2015A030313103’), Key Sci‐Tech Research Project of Guangzhou Municipality, China (Grant/Award Numbers: ‘201508020033, 201510010052, 201707010084, 201803010017’), Key Project of Nature Science Foundation of Guangdong Province, China (Grant/Award Numbers: ‘2015A030311043, 2016A030311035, 2016A020214001’), National Key Sci‐Tech Special Project of China (Grant/Award Numbers: ‘2013ZX09102‐053, 2015GKS‐355’), Initiate Research Funds for the Central Universities of China (Youth Program) (Grant/Award Numbers: ‘13ykpy06, 14ykpy05, 16ykpy24’), Guangdong Science Technology Project (Grant Award Number: ‘2017A020215075’)
Liuqing Zhu, Jinye Xie, and Zhenyin Liu contributed equally to this work.
Contributor Information
Guoquan Gao, Email: gaogq@mail.sysu.edu.cn.
Jing Zhang, Email: fejr@foxmail.com.
Xia Yang, Email: yangxia@mail.sysu.edu.cn.
REFERENCES
- 1. Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173‐181. [DOI] [PubMed] [Google Scholar]
- 2. Kanada KN, Merin MR, Munden A, Friedlander SF. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240‐245. [DOI] [PubMed] [Google Scholar]
- 3. Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:e1060‐e1104. [DOI] [PubMed] [Google Scholar]
- 4. Pandey A, Gangopadhyay AN, Upadhyay VD. Evaluation and management of infantile hemangioma: an overview. Ostomy Wound Manage. 2008;54:16‐18. [PubMed] [Google Scholar]
- 5. North PE, Mihm MC Jr. Histopathological diagnosis of infantile hemangiomas and vascular malformations. Facial Plast Surg Clin North Am. 2001;9:505‐524. [PubMed] [Google Scholar]
- 6. Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics. 2012;130:e314‐e320. [DOI] [PubMed] [Google Scholar]
- 7. Bauland CG, Luning TH, Smit JM, Zeebregts CJ, Spauwen PH. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg. 2011;127:1643‐1648. [DOI] [PubMed] [Google Scholar]
- 8. Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360‐367. [DOI] [PubMed] [Google Scholar]
- 9. Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jinnin M, Medici D, Park L, et al. Suppressed NFAT‐dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye X, Abou‐Rayyah Y, Bischoff J, et al. Altered ratios of pro‐ and anti‐angiogenic VEGF‐A variants and pericyte expression of DLL4 disrupt vascular maturation in infantile haemangioma. J Pathol. 2016;239:139‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang H, Cheng R, Liu G, et al. PEDF inhibits growth of retinoblastoma by anti‐angiogenic activity. Cancer Sci. 2009;100:2419‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium‐derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245‐248. [DOI] [PubMed] [Google Scholar]
- 14. Kim KJ, Yun JH, Heo JI, et al. Role of pigment epithelium‐derived factor in the involution of hemangioma: autocrine growth inhibition of hemangioma‐derived endothelial cells. Biochem Biophys Res Commun. 2014;454:282‐288. [DOI] [PubMed] [Google Scholar]
- 15. Li M, Chen Y, Guo Z, et al. The pigment epithelium‐derived factor (PEDF): an important potential therapeutic agent for infantile hemangioma. Arch Dermatol Res. 2017;309:169‐178. [DOI] [PubMed] [Google Scholar]
- 16. Gao G, Li Y, Gee S, et al. Down‐regulation of vascular endothelial growth factor and up‐regulation of pigment epithelium‐derived factor: a possible mechanism for the anti‐angiogenic activity of plasminogen kringle 5. J Biol Chem. 2002;277:9492‐9497. [DOI] [PubMed] [Google Scholar]
- 17. Fan W, Crawford R, Xiao Y. The ratio of VEGF/PEDF expression in bone marrow mesenchymal stem cells regulates neovascularization. Differentiation. 2011;81:181‐191. [DOI] [PubMed] [Google Scholar]
- 18. Jeng KS, Sheen IS, Jeng WJ, Su JC. PEDF effectively decreases VEGF to PEDF messenger RNA ratio of the inner edge of rat hepatocellular carcinoma induced by diethyl nitrosamine—an “in vivo” study. Hepatogastroenterology. 2012;59:1484‐1490. [DOI] [PubMed] [Google Scholar]
- 19. Yang H, Xu Z, Iuvone PM, Grossniklaus HE. Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model. Mol Vis. 2006;12:511‐517. [PubMed] [Google Scholar]
- 20. Zhang Y, Ma A, Wang L, Zhao B. Nornicotine and nicotine induced neovascularization via increased VEGF/PEDF ratio. Ophthalmic Res. 2015;55:1‐9. [DOI] [PubMed] [Google Scholar]
- 21. Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia‐induced retinal neovascularization. FEBS Lett. 2001;489:270‐276. [DOI] [PubMed] [Google Scholar]
- 22. Leaute‐Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649‐2651. [DOI] [PubMed] [Google Scholar]
- 23. Causse S, Aubert H, Saint‐Jean M, et al. Propranolol‐resistant infantile haemangiomas. Br J Dermatol. 2013;169:125‐129. [DOI] [PubMed] [Google Scholar]
- 24. Bagazgoitia L, Hernandez‐Martin A, Torrelo A. Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol. 2011;28:658‐662. [DOI] [PubMed] [Google Scholar]
- 25. Shehata N, Powell J, Dubois J, et al. Late rebound of infantile hemangioma after cessation of oral propranolol. Pediatr Dermatol. 2013;30:587‐591. [DOI] [PubMed] [Google Scholar]
- 26. Chen XD, Ma G, Huang JL, et al. Serum‐level changes of vascular endothelial growth factor in children with infantile hemangioma after oral propranolol therapy. Pediatr Dermatol. 2013;30:549‐553. [DOI] [PubMed] [Google Scholar]
- 27. Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF‐1alpha‐mediated inhibition of VEGF‐A. Ann Surg. 2012;256:146‐156. [DOI] [PubMed] [Google Scholar]
- 28. Pan WK, Li P, Guo ZT, Huang Q, Gao Y. Propranolol induces regression of hemangioma cells via the down‐regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer. 2015;62:1414‐1420. [DOI] [PubMed] [Google Scholar]
- 29. Charafe‐Jauffret E, Tarpin C, Bardou VJ, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J Pathol. 2004;202:265‐273. [DOI] [PubMed] [Google Scholar]
- 30. Khan ZA, Melero‐Martin JM, Wu X, et al. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood. 2006;108:915‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji Y, Chen S, Li K, Li L, Xu C, Xiang B. Signaling pathways in the development of infantile hemangioma. J Hematol Oncol. 2014;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol. 1997;24:701‐710. [DOI] [PubMed] [Google Scholar]
- 34. Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF‐A in infantile hemangioma‐derived stem cells. N Engl J Med. 2010;362:1005‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia‐induced angiogenic diseases. Clin Sci (Lond). 2015;128:805‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598‐5605. [DOI] [PubMed] [Google Scholar]
- 37. Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor‐2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003‐2012. [DOI] [PubMed] [Google Scholar]
- 38. Bernard A, Gao‐Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti‐angiogenic activity of pigment epithelium‐derived factor. J Biol Chem. 2009;284:10480‐10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konson A, Pradeep S, D'Acunto CW, Seger R. Pigment epithelium‐derived factor and its phosphomimetic mutant induce JNK‐dependent apoptosis and p38‐mediated migration arrest. J Biol Chem. 2017;292:8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema‐McKenney AE. beta‐Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446‐1451. [DOI] [PubMed] [Google Scholar]
- 41. Przewratil P, Kobos J, Wnek A, et al. Serum and tissue profile of VEGF and its receptors VGFR1/R2 in children with infantile hemangiomas on systemic propranolol treatment. Immunol Lett. 2016;175:44‐49. [DOI] [PubMed] [Google Scholar]
- 42. Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi‐functional serpin family protein. J Cell Biochem. 2009;106:769‐775. [DOI] [PubMed] [Google Scholar]
- 43. Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium‐derived factor suppresses ischemia‐induced retinal neovascularization and VEGF‐induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821‐829. [PubMed] [Google Scholar]
- 44. Tombran‐Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium‐derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411‐414. [DOI] [PubMed] [Google Scholar]
- 45. Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk‐1/KDR is involved in sphingosine 1‐phosphate‐stimulated phosphorylation of Akt and endothelial nitric‐oxide synthase (eNOS). J Biol Chem. 2002;277:42997‐43001. [DOI] [PubMed] [Google Scholar]
- 47. Elayappan B, Ravinarayannan H, Pasha SP, Lee KJ, Gurunathan S. PEDF inhibits VEGF‐ and EPO‐ induced angiogenesis in retinal endothelial cells through interruption of PI3K/Akt phosphorylation. Angiogenesis. 2009;12:313‐324. [DOI] [PubMed] [Google Scholar]
- 48. Branca M, Giorgi C, Ciotti M, et al. Relationship of up‐regulation of 67‐kd laminin receptor to grade of cervical intraepithelial neoplasia and to high‐risk HPV types and prognosis in cervical cancer. Acta Cytol. 2006;50:6‐15. [DOI] [PubMed] [Google Scholar]
- 49. Moodley K, Weiss SF. Downregulation of the non‐integrin laminin receptor reduces cellular viability by inducing apoptosis in lung and cervical cancer cells. PLoS One. 2013;8:e57409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amano S, Yamagishi S, Inagaki Y, et al. Pigment epithelium‐derived factor inhibits oxidative stress‐induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res. 2005;69:45‐55. [DOI] [PubMed] [Google Scholar]
- 51. Yang J, Chen S, Huang X, et al. Growth suppression of cervical carcinoma by pigment epithelium‐derived factor via anti‐angiogenesis. Cancer Biol Ther. 2010;9:967‐974. [DOI] [PubMed] [Google Scholar]
- 52. Hong H, Zhou T, Fang S, et al. Pigment epithelium‐derived factor (PEDF) inhibits breast cancer metastasis by down‐regulating fibronectin. Breast Cancer Res Treat. 2014;148:61‐72. [DOI] [PubMed] [Google Scholar]
- 53. Ji Y, Li K, Xiao X, Zheng S, Xu T, Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma‐derived endothelial cells. J Pediatr Surg. 2012;47:2216‐2223. [DOI] [PubMed] [Google Scholar]
- 54. Yuan WL, Jin ZL, Wei JJ, Liu ZY, Xue L, Wang XK. Propranolol given orally for proliferating infantile haemangiomas: analysis of efficacy and serological changes in vascular endothelial growth factor and endothelial nitric oxide synthase in 35 patients. Br J Oral Maxillofac Surg. 2013;51:656‐661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


