Abstract
AMD is the leading cause of blindness in developed countries. The dry form of AMD, also known as atrophic AMD, is characterized by the death of RPE and photoreceptors. Currently, there are no treatments for this form of the disease due in part to our incomplete understanding of the mechanism causing AMD. Strong experimental evidence from studies of human donors with AMD supports the emerging hypothesis that defects in RPE mitochondria drive AMD pathology. These studies, using different experimental methods, have shown disrupted RPE mitochondrial architecture and decreased mitochondrial number and mass, altered content of multiple mitochondrial proteins, increased mitochondrial DNA damage that correlates with disease severity, and defects in bioenergetics for primary RPE cultures from AMD donors. Herein, we discuss a model of metabolic uncoupling that alters bioenergetics in the diseased retina and drives AMD pathology. These data provide the rationale for targeting the mitochondria in the RPE as the most efficacious intervention strategy if administered early, before vision loss and cell death.
Keywords: retinal metabolism, retinal pigment epithelium, photoreceptors, mitochondria, human donor tissue
AMD is a challenging disease to study due to the involvement of the macula, an anatomical feature unique to primates, and an age-dependent onset that requires more than 55 years to develop. Although animal models have provided valuable insight into how disruption of specific pathways affects retinal function, they do not faithfully replicate all of the cardinal features of AMD. Use of human donor eyes to investigate AMD disease mechanism overcomes these challenges. A critical component of this approach is that donor eyes must be evaluated for the presence and severity of the disease. Our laboratory and others have been using the Minnesota Grading System to determine the donor's stage of AMD.1 Evaluation is performed from high-resolution color fundus photographs of the RPE after removal of the neural retina. Disease severity is based on pigmentary changes in the RPE, size and location of drusen, and the presence of geographic atrophy or choroidal neovascularization.2,3 The Alabama Grading System is another scale used to evaluate AMD in donor eyes that have been processed for histological analysis.4 Histopathological grades for AMD severity are based on RPE and photoreceptor appearance, and the presence of retinal deposits, drusen, and choroidal neovascularization. Other research groups use clinical records from human donors to verify the presence or absence of AMD.5,6 Because human donor tissue graded for AMD severity captures key features of the disease, their use has helped define disease mechanism and identify potential targets for therapy.
This article summarizes data from human donor tissue that supports the emerging idea that mitochondria in the RPE are a site of primary pathology in dry AMD. The mitochondria are the cells' primary source of energy that produce ATP through several routes. The main pathway for ATP generation is oxidative phosphorylation (OxPhos), which involves the transfer of electrons between multisubunit complexes (I to IV) in the electron transport chain (ETC). The production of an electrochemical gradient across the inner mitochondrial membrane drives conversion of ADP to ATP by Complex V. The Citric Acid Cycle (TCA) and β-oxidation are two additional energy-producing pathways that are found in the mitochondria. Acetyl CoA, a product of TCA and β-oxidation, is used to produce the energy substrates NADH and FADH2, which feed into OxPhos. Unlike other cells in the retina, RPE also have the ability to metabolize fatty acids to produce β-hydroxybutarate as an alternative energy source.7 Hence, mitochondrial bioenergetics is highly coordinated between multiple energy-producing pathways.
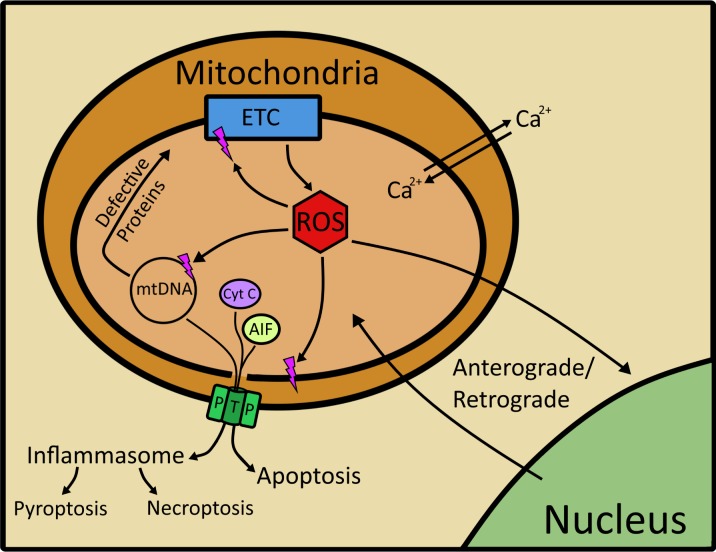
In addition to energy production, the mitochondria act as a signaling platform that communicates changes in the mitochondrial environment to the nucleus in a process referred to as retrograde/anterograde signaling (Fig. 1).8,9 Under normal conditions, reactive oxygen species (ROS) that are produced as a by-product of electron transfer and the systematic reduction of oxygen serve as an important signaling molecule between the mitochondria and nucleus. In addition to ROS, mitochondrial changes in the electrochemical gradient, unfolded protein response, and levels of ATP and calcium coordinate communication between the mitochondria and nucleus.9 This communication via multiple signaling mechanisms allows for adjustments of gene expression in both the nucleus and mitochondria to accommodate changing energy demands. The positive effects of ROS can be undermined when excessive ROS are produced, which can damage lipids, proteins, and mitochondrial DNA (mtDNA), altering both mitochondrial and cellular function.
Figure 1.
Mitochondria as a signaling platform. The mitochondria contribute to a number of cellular functions, including energy production, regulation of intracellular calcium levels, and production of ROS, all of which contribute to anterograde and retrograde signaling. Excessive ROS can damage mtDNA, proteins, and lipids. Damage to the mitochondrial genome can produce defective proteins of the ETC and translation machinery. ROS can also directly damage mitochondrial proteins, including those in the respiratory chain, thereby adversely affecting energy production. Mitochondrial membrane damage can lead to opening of mitochondrial permeability transition pores, decreasing membrane potential, and releasing mtDNA, and the proteins apoptosis-inducing factor and cytochrome c. Release of these mitochondrial molecules initiates cell death via apoptosis and activates the inflammasome, potentially leading to pyroptosis or necroptosis.
The mitochondrial genome, a potential target of ROS, is a circular approximately 16-kb double-stranded DNA that is localized to the inner matrix in close proximity to the ETC, the major source of mitochondrial ROS. Although not protected by histones like the nuclear genome, mitochondrial transcription factor A (TFAM) and nucleoid complexes of multiple proteins provide some protection for mtDNA from ROS-induced damage.10 With limited DNA repair available in the mitochondria, mtDNA damage can be long lasting. Damage to nearly any part of the mitochondrial genome can have a significant effect on function, as approximately 98% of the sequence encodes for 37 genes, including 13 proteins that are subunits of the ETC, and all of the machinery (tRNAs, 16S and 12S ribosomal RNA) required to produce those proteins. The remaining 2% of the genome is in the D-loop, which controls DNA replication and translation. Thus, the combined effects of environment, decreased protection, and limited repair are responsible for the increased damage observed in mtDNA compared with nuclear DNA reported for multiple cell types.11
Consequences of ROS-induced damage include double- and single-strand breaks, as well as the formation of DNA adducts, such as reactive aldehydes produced from lipid peroxidation, which can disrupt translation and replication of the mitochondrial genome.12,13 One of the lasting repercussions of double-strand break repair is the deletion of a specific 4977-bp region of the mitochondrial genome, referred to as the “Common Deletion.” This damage accumulates with age in post-mitotic tissue, including brain, skeletal muscle, and heart.14 We also have observed an age-dependent increase of the Common Deletion in RPE from healthy and AMD donors.15 However, donors with AMD had additional genome-wide damage, suggesting a mechanism of damage specific to AMD that goes beyond age-related changes to the mitochondrial genome.
Extensive mitochondrial damage can initiate cell death via the release of mitochondrial proteins (i.e., cytochrome c, apoptosis-inducing factor) and mtDNA through opening of the mitochondrial permeability transition pore.16,17 The presence of these mitochondrial proteins and mtDNA in the cytoplasm triggers apoptosis and inflammasome activation, with the later process leading to necroptosis or pyroptosis.18–20 The release of mtDNA into the cytoplasm activates the inflammasome and triggers sterile inflammation.16,18 Thus, mitochondria form the central hub for multiple cellular signaling events, including cell death.
Evidence for RPE Mitochondrial Damage in AMD
In the early 2000s, the role for oxidative stress in the etiology of AMD was beginning to emerge.21 Data supporting this notion included evidence from human donor tissue that showed increased advanced glycation end products and ω-(2-carboxyethyl)pyrroles, which are products of protein oxidation, in the retinas of donors with AMD.22–31 Oxidative stress can elicit a compensatory response by upregulating expression of proteins that counteract ROS or protect against ROS-induced damage. Data showing elevated levels of multiple antioxidant enzymes and heat shock proteins in RPE from donors with AMD provided indirect evidence for increased oxidative stress.3 Positive clinical outcomes from the Age-Related Eye Disease Study also supported a link between oxidative stress and AMD.32 This clinical trial sponsored by the National Eye Institute showed supplements of antioxidants plus zinc slowed progression of the disease. Although these studies helped direct the focus to oxidative stress, they did not provide the mechanistic details required to understand the pathogenic changes driving the disease.
During this decade, multiple “discovery-based” approaches, such as proteomics and genomics, were being used to gain insight into disease mechanism. Our early efforts to elucidate the molecular details of AMD used an unbiased proteomics approach to determine how the proteome was altered with AMD progression in both the neural retina and RPE. These analyses used quantitative two-dimensional gel electrophoresis followed by mass spectrometry to identify proteins with altered content. Investigation of the neural retina found 26 proteins exhibited differential content with AMD progression. Most of these proteins were involved in microtubule regulation and in protein folding/chaperone activities.33 In contrast to the results from the neural retina, a global proteome analysis of the RPE found 12 proteins were changing with AMD and, importantly, most of these proteins were localized to the mitochondria.34 Decreased content was revealed for multiple subunits of the ETC and mitochondrial heat shock proteins, suggesting potential defects in energy production and the process of translocation and refolding of nuclear-encoded proteins that reside in the mitochondria. These early results provided the first indication that the mitochondria in RPE are a potential site of AMD pathology and supported the rationale for performing a more in-depth analysis of the mitochondrial proteome. This second analysis of mitochondria isolated from RPE showed multiple subunits of the ETC and mtHSP70 exhibited decreased content, thereby reconfirming the initial hypothesis that defects in energy production and mitochondrial protein import and refolding occurs with AMD.35
Two additional proteins, mitofilin and mitochondrial translation factor Tu (Tufm), were increased in RPE from donors at an early stage of AMD. Mitofilin is involved in maintaining mitochondrial cristae stability and, therefore, increased mitofilin content may act to combat the destabilizing effects of AMD. Consistent with this idea, a study of RPE mitochondrial ultrastructure reported AMD donors had a significantly greater loss of cristae and matrix density compared with their age-matched controls.36 Furthermore, the finding that AMD donors had a significant reduction in the number and area of mitochondria per cell supports the notion that defects in the RPE mitochondria contribute to AMD pathology.
Tufm delivers aminoacylated tRNAs to the mitochondrial ribosome for production of the 13 mitochondrial-encoded proteins. Previous studies showed that overexpression of Tufm rescued mitochondrial phenotypes caused by a MELAS (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes) tRNA mutation37 and a translation defect resulting in an OxPhos enzyme deficiency.38 Thus, the dramatic 4-fold upregulation in Tufm content could be a compensatory response in an effort to counter defects in mitochondrial translation that occur early in AMD.
A process that could disrupt mitochondrial translation involves damage to mtDNA in the form of strand breaks and/or addition of DNA adducts. To directly test whether mtDNA damage was increased in macular RPE from AMD donors, damage was measured using a long-extension PCR. This assay is based on the principle that strand breaks or covalent DNA modifications can slow down or block amplification by DNA polymerase. Consequently, the amount of amplified product is proportional to the amount of undamaged endogenous template. Analysis of two separate cohorts that included a total of 53 age-matched controls and 88 donors with AMD showed mtDNA damage correlated with AMD severity.15,39 A more refined analysis of the mitochondrial genome localized the sites of increased damage to regions encoding the 16S and 12S ribosomal RNA and 8 of the 22 tRNAs.39 Defects in these genes could adversely affect translation of the 13 mitochondrial-encoded proteins. Other significantly damaged regions included genes for OxPhos subunits and the D-loop, potentially leading to reduced ATP production and disruptions in translation and replication.
As previously outlined, mitochondria can also regulate cell death via apoptosis, necroptosis, and pyroptosis. Cell death is particularly relevant to the geographic atrophy observed in advanced dry AMD. The presence of TUNEL staining in the nuclei of photoreceptor and RPE located near the edges of atrophy provides evidence for DNA cleavage and impending cell death. However, the exact mechanism of cell death is not known because DNA cleavage is a shared outcome of apoptosis and necroptosis. Upregulated inflammasome proteins in AMD-affected eyes were observed near drusen and the edges of geographic atrophy.40–42 These data suggest the involvement of either necroptosis or pyroptosis in AMD cell death because both pathways are activated by the inflammasome.19,20 As a caveat to these data, a recent article reporting extensive characterization of inflammasome antibodies called into question the validity of these results.43 However, it is conceivable that the pathogen-associated molecular patterns derived from RPE mitochondria could activate inflammasomes in microglial cells, where the presence of inflammasomes is indisputable.44
In summary, strong evidence from analysis of human donors with AMD supports the emerging hypothesis that defects in RPE mitochondria drives AMD pathology. These studies, using different experimental methods, have shown disrupted RPE mitochondrial architecture and decreased mitochondrial number and mass, altered content of multiple mitochondrial proteins, and increased mtDNA damage that correlates with disease severity. Importantly, damage to the mitochondria would have a significant negative impact on ATP production, leading to a bioenergetic crisis in the RPE.
Rapid postmortem changes that occur in the mitochondria negate the possibility of directly measuring mitochondrial function in human donor tissue. To overcome this limitation, we generated primary cultures of RPE from human donors with and without AMD. These cultures retain many of the prototypic characteristics of RPE in vivo, including cobblestone morphology, the presence of pigment and prototypic RPE proteins, and the ability to phagocytose outer segments.45 To directly test if there were differences in bioenergetics associated with AMD, the bioenergetic profiles of two major energy pathways, glycolysis and mitochondrial OxPhos, were measured using the Seahorse Extracellular Flux Analyzer. Results showed that in RPE from AMD donors both glycolysis and mitochondrial OxPhos were significantly decreased.45 In primary cultures from AMD donors, Golestaneh and colleagues46 also found reduced ATP production via OxPhos, but ATP produced via glycolysis was increased in their experimental system. These results are consistent with the hypothesis that RPE mitochondria are damaged with AMD and, consequently, the ensuing bioenergetic crisis drives AMD pathology.
A Model Explaining How a Bioenergetic Crisis in the RPE Drives AMD Pathology
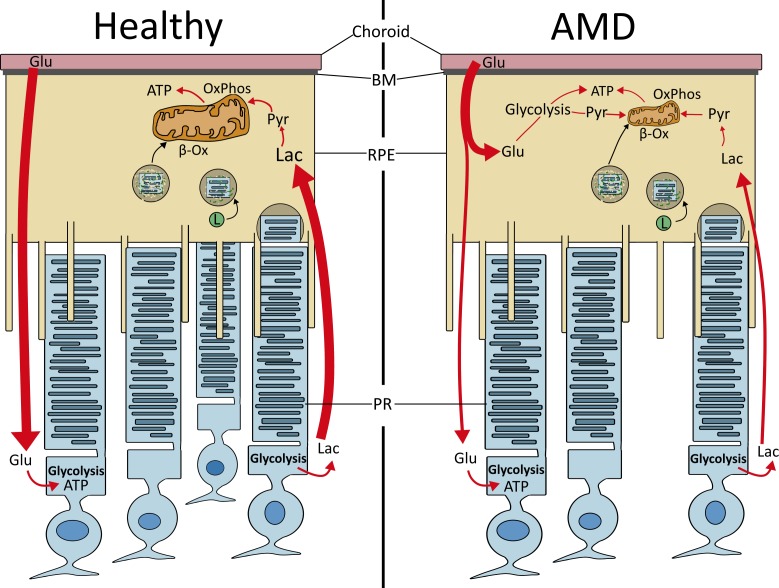
The work thus far implicates the mitochondria, and overall alterations to bioenergetics in RPE, as a potential mechanism driving AMD pathology. Recent publications by the laboratories of Hurley et al.47 and Philp7 have promoted the idea of metabolic coupling between the RPE and retina. This concept provides a potential explanation for how reduced RPE mitochondrial function could have a global effect on the retina. Based on a series of elegant studies, Kanow and colleagues48 propose that the neural retina and RPE are part of a “metabolic ecosystem,” whereby each cell is co-dependent on the other cell for survival. In this working model (Fig. 2), glucose from the blood is largely unused by the RPE and is transported to the photoreceptors.48 Photoreceptors use glucose through glycolysis to produce energy and the by-product lactate, which is transported to the RPE where it is used for OxPhos. An important part of this highly regulated process is the suppression of glycolysis in the RPE by lactate, thereby preserving glucose as an energy source for the photoreceptors.48 Photoreceptors also supply the RPE with a large amount of lipids through daily phagocytosis and digestion of the photoreceptor outer segments. These lipids are substrates for β-oxidation, which produces acetyl CoA, feeding into the TCA and producing β-hydroxybutarate, an alternative energy source for the retina.7,49 Although other energy sources, such as glutamine,50 proline,51 and glycogen52 are not specifically mentioned here, they would likely contribute to the flow of energy substrates between the retina and RPE.
Figure 2.
Bioenergetics of the retinal ecosystem. In a healthy retina (left), glucose (Glu) from the choroid remains unused by the RPE and is shuttled to the photoreceptors (PRs). PRs use glucose in glycolysis to produce ATP and the by-product lactate (Lac). Lactate is taken up by the RPE, converted to pyruvate (Pyr), and used in OxPhos. PRs also supply RPE with an influx of lipids through phagocytosis of the outer segments and degradation on fusion with the lysosome (L). These lipids enter the mitochondria and are used in β-oxidation (β-Ox) to generate ATP. In AMD (right), the RPE's damaged mitochondria lose the ability to generate ATP, causing the cell to use Glu from the choroid, due to its new reliance on glycolysis. This lowers the concentration of Glu transported to the PRs, and consequently lowering lactate supplied to the RPE. This cell-specific shift in reliance on alternative bioenergetics upsets the metabolic ecosystem and leads to death of both PRs and RPE. Thus, metabolic uncoupling that alters retinal bioenergetics may be the central defect in AMD. Adapted from Kanow et al.48
AMD disrupts this metabolic ecosystem. As mitochondria are the site of OxPhos and β-oxidation, damage to this organelle in the RPE would reduce ATP production. RPE would begin to rely on glycolysis to maintain the cell's energy requirement, thereby reducing the flow of glucose to the photoreceptors. Decreased photoreceptor glycolysis could have multiple effects, including reduced production of lactate for RPE to use as an energy source. Limited suppression of glycolysis due to decreased lactate promotes glucose utilization by the RPE, starving photoreceptors, leading to degeneration and cell death. It has been well documented in AMD that rod death precedes the loss of cones.40,53,54 This observation is relevant to the metabolic co-dependence that cones have with rods. Rods secrete an inactive thioredoxin, coined rod-derived cone viability factor, which promotes glucose uptake by the cones and stimulates glycolysis.55 In the context of changes in RPE metabolism due to AMD-induced mitochondrial damage, the reduced flow of glucose to the photoreceptors coupled with rod death would accelerate the loss of macular cones, a hallmark of advanced AMD.
Mitochondria as a Therapeutic Target
Genetic analysis of AMD patients revealed associations between increased risk for AMD and more than 30 loci involved in multiple pathways, including complement/innate immune system, lipid/cholesterol regulation, cell survival, extracellular matrix remodeling, and angiogenesis.56,57 Although this information has been exceptionally useful in formulating specific hypotheses about mechanisms that may be involved in disease development, they imply that a single treatment may not be universally effective. Therefore, we need to develop methods to decipher which aberrant pathway or process is contributing to disease progression in AMD patients, and then use drugs to interrupt or supplement these defects. Additionally, the most efficacious treatment will be one that is administered early in the disease, before retina/RPE cell death and vision loss.
Based on our analysis of RPE mtDNA damage, we found significant mitochondrial damage occurred at an early disease stage preceding when vision loss would occur in AMD patients.58 This was an exciting finding, as it suggests an early intervention targeting the mitochondria could prevent vision loss in individuals with the greatest mitochondrial damage. With this goal in mind, we used human donor tissue, phenotyped for the presence and severity of AMD and genotyped for AMD risk alleles, to determine if the greatest damage was associated with a specific high-risk genotype.58 Our analysis found that donors carrying the high-risk allele for the Complement Factor H (CFH) single nucleotide polymorphism (rs1061170) that causes the Y402H mutation had significantly more mtDNA damage compared with donors harboring the CFH wild-type allele. CFH, a component of the innate immune system, prevents inappropriate complement activation thereby keeping inflammation in check. The reduced function of the Y402H variant permits the occurrence of retinal inflammation and tissue damage, promoting invasion of immune cells that release ROS and cytokines. These extracellular signaling molecules upregulate cell pathways (i.e., nuclear factor κB) that increase intracellular oxidative stress in the surrounding tissue. These events could ultimately cause the observed RPE mtDNA damage early in AMD.
This fortuitous discovery linking the CFH high-risk variant with increased mtDNA damage, as well as our earlier result using donors with a mixture of genotypes, was potentially influenced by our sample population from Minnesota, where a high percentage of the ancestry is from Northern Europe. This group has the highest risk for AMD. Notably, approximately 60% of our donors harbored the CFH high-risk allele, whereas in the general population 30% to 50% of all AMD patients carry this risk variant.59 We postulate that individuals with the CFH risk allele would have the greatest positive response to a mitochondrial-targeted therapy aimed at enhancing or protecting mitochondrial function. Results from this study also provide a roadmap for using donor tissue, coupled with an accessible biomarker (i.e., genotype, clinical phenotype) in patients, as a way to define defective pathways in a specific patient population and then develop therapies targeting the primary defect. This strategy would be one way to move toward “personalized medicine” in treating AMD.
Bioenergetics, Metabolic Uncoupling, and Critical Unanswered Questions
One of the preeminent questions in AMD research is “Why the macula?” Specifically, why is there preferential death of macular RPE and photoreceptors when both clinical (peripheral drusen and geographic atrophy)60,61 and biochemical (protein changes in peripheral RPE and retina)33–35 evidence shows that the detrimental effects of AMD impacts the entire retina? A potential explanation involves the higher bioenergetic demand in the macula and metabolic uncoupling of the retinal ecosystem with AMD. Results from analysis of RPE mtDNA damage showed damage was present throughout the retina of AMD donors,39 suggesting a global bioenergetic crisis in the RPE that would eventually starve the overlying photoreceptors. The significantly greater loss of macular photoreceptors could be due to the higher density of cones in this region. As discussed previously, rods die first in AMD and cones depend on rods to secrete a neuroprotective factor that improves their glucose uptake. Death of macular rods would have a more significant impact on neighboring cones, as fewer nearby rods are available to support cone metabolism. The higher overall metabolic activity of the macula, combined with death of rods, would contribute to the accelerated loss of both RPE and photoreceptors in the macula with AMD.
We also have observed that although RPE mtDNA damage was increased with AMD, damage to the neural retina was low and did not change with disease progression.39 This raises the question “Why is mitochondrial damage limited to the RPE?” The answer may involve cell-specific differences in metabolism. RPE rely almost exclusively on mitochondria as an energy source, whereas photoreceptors depend on glycolysis. Recall that a by-product of mitochondrial OxPhos is ROS, which can damage mtDNA. Thus, the lower mtDNA damage observed in the retina is consistent with a reliance on glycolysis rather than OxPhos. The link between the amount of mtDNA mutations and reliance on a specific metabolic pathway was also observed when comparing tumor cells with adjacent nontumor cells.62 A lower mutation rate was found in tumor cells that had undergone a shift in metabolism from OxPhos to aerobic glycolysis, a process known as the “Warburg effect.” Hence, the mtDNA mutation frequency in these cells appears to be inversely proportional to the cells' dependence on glycolysis. Of note, the Warburg effect was first described by Otto Warburg in two tissues, tumors and retinas.63 However, his reported result from the neural retina was largely ignored until the recent resurgence in studies of retinal metabolism. It is interesting to consider that pathobiology could be precipitated by the contributions of the photoreceptor's unique metabolism and the metabolic uncoupling that occurs with AMD.
Summary
This perspective provides evidence for the hypothesis that a bioenergetics crisis in the RPE underlies the pathobiology of AMD. Although our focus has been on metabolism, the potential ramifications that mitochondrial dysfunction would have on cell signaling, calcium handling, and gene expression that would adversely affect RPE cell function should also be considered. Targeting the mitochondria in the RPE may provide the most efficacious intervention strategy if administered early, before vision loss and cell death.
Acknowledgments
The authors thank James Hurley and members of the Mitochondria Task Group at the 2016 meeting of the Ryan Initiative for Macula Research for their insightful discussions about retinal metabolism.
Supported by National Institutes of Health/National Eye Institute Grants RO1 EY026012, RO1 EY028554 (DAF), and National Institute of Aging T32-AG029796 (CRF); an anonymous benefactor for AMD research; the Lindsay Family Foundation; the Elaine and Robert Larson Endowed Vision Research Chair; and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Neuroscience.
Disclosure: C.R. Fisher, None; D.A. Ferrington, None
References
- 1. Bonilha VL, Shadrach KG, Rayborn ME,et al. . Retinal deamination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD). Exp Eye Res. 2013; 111: 71– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olsen TW, Feng X. . The Minnesota grading system of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004; 45: 4484– 4490. [DOI] [PubMed] [Google Scholar]
- 3. Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW. . Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2007; 143: 607– 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curcio CA, Medeiros NE, Millican CL. . The Alabama age-related macular degeneration grading system for donor eyes. Invest Opthalmol Vis Sci. 1998; 39: 1085– 1096. [PubMed] [Google Scholar]
- 5. Whitmore SS, Braun TA, Skeie JM,et al. . Altered gene expression in dry age-related macular degeneration suggests early loss of choroidal endothelial cells. Mol Vis. 2013; 19: 2274– 2297. [PMC free article] [PubMed] [Google Scholar]
- 6. Bird AC, Phillips RL, Hageman GS. . Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014; 132: 338– 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adijanto J, Du J, Moffat C, Seirfert EL, Hurley JB, Philp NJ. . The retinal pigment epithelium utilizes fatty acids for ketogenesis. J Biol Chem. 2014; 289: 20570– 20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nita M, Grzybowski A. . The role of reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016; 2016: 3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Cunha FM, Torelli NQ, Kowaltowski AJ. . Mitochondrial retrograde signaling: triggers, pathways, and outcomes. Oxid Med Cell Longev. 2015; 2015: 482582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vernochet C, Mourier A, Bezy O,et al. . adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab. 2012; 16: 765– 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yakes MF, Van Houten B. . Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Aad Sci U S A. 1997; 94: 514– 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zsurka G, Peeva V, Kotlyar A, Kunz WS. . Is there still any role for oxidative stress in mitochondrial DNA-dependent aging? Genes (Basel). 2018; 9: E175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cline SD. . Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim Biophys Acta. 2012; 1819: 979– 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meissner C, Bruse P, Mohamed SA,et al. . The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp Gerontol. 2008; 43: 645– 652. [DOI] [PubMed] [Google Scholar]
- 15. Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. . Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010; 51; 5470– 5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, Gaziev A. . Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell Mol Life Sci. 2004; 61: 3100– 3103. [DOI] [PubMed] [Google Scholar]
- 17. Wang C, Youle RJ. . The role of mitochondria in apoptosis. Annu Rev Genet. 2009; 43: 95– 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimada K, Crother TR, Karlin J,et al. . Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012; 36: 401– 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu J, Nagasu H, Murakami T,et al. . Inflammasome activation leads to caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci U S A. 2014; 111: 15514– 15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conos SA, Chen KW, De Nardo D,et al. . Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017; 114: E961– E969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beatty S, Koh H, Phil M, Henson D, Boulton M. . The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115– 134. [DOI] [PubMed] [Google Scholar]
- 22. Ishibashi T, Murata T, Hangai M,et al. . Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol. 1998; 116: 1629– 1632. [DOI] [PubMed] [Google Scholar]
- 23. Handa JT, Verzijl N, Matsunaga H,et al. . Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci. 1999; 40: 775– 779. [PubMed] [Google Scholar]
- 24. Hammes HP, Hoerauf H, Alt A,et al. . N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999; 40: 1855– 1859. [PubMed] [Google Scholar]
- 25. Howes KA, Liu Y, Dunaief JL,et al. . Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004; 45: 3713– 3720. [DOI] [PubMed] [Google Scholar]
- 26. Yamada Y, Ishibashi K, Ishibashi K,et al. . The expression of advanced glycation end product receptors in RPE cells associated with basal deposits in human maculas. Exp Eye Res. 2006; 82: 840– 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ni J, Yuan X, Gu J,et al. . Plasma protein pentosidine and carboymethyllysine, biomarkers for age-related macular degeneration. Mol Cell Proteomics. 2009; 8: 1921– 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan X, Gu X, Crabb J,et al. . Quantitative proteomics: comparison of the macular Bruch membrane/choroid complex from age-related macular degeneration and normal eyes. Mol Cell Proteomics. 2010; 9: 1031– 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crabb JW, Miyagi M, Gu X,et al. . Drusen proteome analysis: an approach to the etiology of age-related macular degnereation. Proc Natl Acad Sci U S A. 2002; 99: 14682– 14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu X, Meer SG, Miyagi M,et al. . Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003; 278: 42027– 42035. [DOI] [PubMed] [Google Scholar]
- 31. Gu J, Pauer GJ, Yue X,et al. . Proteomic and genomic biomarkers for age-related macular degeneration. Adv Exp Med Biol. 2010; 664: 411– 417. [DOI] [PubMed] [Google Scholar]
- 32. Age-related Eye Disease Study Research Group. . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417– 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ethen CM, Reilly C, Feng X, Olsen TW, Ferrington DA. . The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 2280– 2290. [DOI] [PubMed] [Google Scholar]
- 34. Nordgaard CL, Berg KM, Kapphahn RJ,et al. . Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 815– 822. [DOI] [PubMed] [Google Scholar]
- 35. Nordgaard CL, Karunadharma PP, Feng X, Olsen TW, Ferrington DA. . Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 2848– 2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. . Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006; 27: 983– 993. [DOI] [PubMed] [Google Scholar]
- 37. Feuermann F, Francisci S, Rinaldi T,et al. . The yeast counterparts of human ‘MELAS' mutations cause mitochondrial dysfunction that can be rescued by overexpression of the mitochondrial translation factor EF-Tu. EMBO Rep. 2003; 4: 53– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smeitink JA, Elpeleg O, Antonicka H,et al. . Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am J Hum Genet. 2006; 79: 869– 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terluk MR, Kapphahn RJ, Soukup LM,et al. . Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci. 2015; 35: 7204– 7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dunaief JL, Dentchev T, Ying GS, Milam AH. . The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002; 120: 1435– 1442. [DOI] [PubMed] [Google Scholar]
- 41. Tarallo V, Hirano Y, Gelfand BD,et al. . DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012; 149: 847– 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tseng WA, Thein T, Kinnunen K,et al. . NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 110– 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kosmidou C, Efstathiou NE, Hoang MV,et al. . Issues with the specificity of immunological reagents for NLRP3: implications for age-related macular degeneration. Sci Rep. 2018; 8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scheiblich H, Schlutter A, Golenbock DT,et al. . Activation of the NLRP3 inflammasome in microglia: the role of ceramide. J Neurochem. 2017; 143: 534– 550. [DOI] [PubMed] [Google Scholar]
- 45. Ferrington DA, Ebeling MC, Kapphahn RJ,et al. . Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol. 2017; 13: 255– 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. . Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017; 8: e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hurley JB, Lindsay KJ, Du J. . Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res. 2015; 93: 1079– 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanow MA, Giarmarco MM, Jankowski CS,et al. . Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife. 2017; 6: e28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reyes-Reveles J, Dhingra A, Alexander D, Bragin A, Philp NJ, Boesze-Battaglia K. . Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J Biol Chem. 2017; 292: 8038– 8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du J, Yanagida A, Knight K,et al. . Reductive carboxylation is a major metabolic pathway in the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2016; 113: 14710– 14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chao JR, Knight K, Engel AL,et al. . Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J Biol Chem. 2017; 292: 12895– 12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Senanayake Pd, Calabro A, Hu JG,et al. . Glucose utilization by the retinal pigment epithelium: evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res. 2006; 83: 235– 246. [DOI] [PubMed] [Google Scholar]
- 53. Curcio C, Medeiros ANE, Millican CL. . Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236– 1249. [PubMed] [Google Scholar]
- 54. Curcio CA. . Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond). 2001; 15: 376– 383. [DOI] [PubMed] [Google Scholar]
- 55. Ait-Ali N, Fridlich R, Millet-Puel G,et al. . Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015; 161: 817– 832. [DOI] [PubMed] [Google Scholar]
- 56. Fritsche LG, Igi W, Bailey JN,et al. . A large genome-wide associated study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134– 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ratnapriya R, Chew EY. . Age-related macular degeneration-clinical review and genetics update. Clin Genet. 2013; 84: 160– 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrington DA, Kapphahn RJ, Leary MM, Atilano SR, Terluk MR, Karunadharma P. . Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp Eye Res. 2016; 145: 269– 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. . A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch Ophthalmol. 2007; 125: 55– 62. [DOI] [PubMed] [Google Scholar]
- 60. Domalpally A, Clemons TE, Danis RP,et al. ; Writing Committee for the OPTOS Peripheral Retina (OPERA) Study. Peripheral retinal changes associated with age-related macular degeneration in the age-related eye disease study 2: age-related eye disease study 2 report number 12 by the age-related eye disease study 2 Optos PEripheral RetinA (OPERA) study research group. Ophthalmology. 2017; 124: 479– 487. [DOI] [PubMed] [Google Scholar]
- 61. Postel EA, Agarwal A, Schmidt S,et al. . Comparing age-related macular degeneration phenotype in probands from singleton and multiplex families. Am J Ophthalmol. 2005; 139: 820– 825. [DOI] [PubMed] [Google Scholar]
- 62. Ericson NG, Kulawiec M, Vermulst M,et al. . Decreased mitochondrial DNA mutagenesis in human colorectal cancer. PLoS Genet. 2012; 8: e1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Warburg O, Posener K, Negelein E. . On the metabolism of carcinoma cells. Bioschem Z. 1924; 152: 309– 344. [Google Scholar]